Abstract
We show that, contrary to claims in the literature, “sterile” males resulting from the cross of the Bogota and USA subspecies of Drosophila pseudoobscura are weakly fertile. Surprisingly, these hybrid males produce almost all daughters when crossed to females of any genotype (pure Bogota, pure USA, hybrid F1). Several lines of evidence suggest that this sex ratio distortion is caused by sex chromosome segregation distortion in hybrid males. We genetically analyze this normally cryptic segregation distortion and show that it involves several regions of the Bogota X chromosome that show strong epistatic interactions with each other. We further show that segregation distortion is normally masked within the Bogota subspecies by autosomal suppressors. Our analysis shows that the genetic basis of hybrid segregation distortion is similar to that of hybrid male sterility between the same subspecies. Indeed the severity of segregation distortion is correlated with the severity of sterility among hybrids. We discuss the possibility that hybrid sterility in this paradigmatic case of incipient speciation is caused by segregation distortion.
SELFISH genetic elements may play a role in the origin of species (Grun 1976, pp. 352–354; Cosmides and Tooby 1981; Hurst and Pomiankowski 1991; Hurst and Werren 2001; Coyne and Orr 2004). While this idea has appeared in many guises, involving transposable elements, infectious endosymbionts like Wolbachia, and mitochondrial mutations that cause cytoplasmic male sterility, one version has proved particularly attractive. According to this idea, alleles that cause meiotic drive might systematically give rise to postzygotic isolation generally and to hybrid sterility specifically (Frank 1991; Hurst and Pomiankowski 1991; Henikoff et al. 2001; Tao et al. 2001; Henikoff and Malik 2002; Tao and Hartl 2003).
Mutations that cause meiotic drive distort Mendelian ratios to their own advantage, usually by inactivating sperm that carry a homologous chromosome. X chromosome-bearing sperm might, for instance, inactivate Y chromosome-bearing sperm. While obviously advantageous for the driving mutation, meiotic drive imposes a fertility cost on its bearers (since many gametes are rendered nonfunctional) as well as a fitness cost on most other genes in the genome (Lyttle 1991). When residing on the sex chromosomes, mutations causing meiotic drive also bias sex ratios away from the 50:50 favored by Fisherian selection. For all these reasons, there will usually be strong selection to suppress meiotic drive (Sandler and Novitski 1957; Lyttle 1991; Jaenike 2001).
It is easy to imagine how mutations causing meiotic drive could ultimately give rise to intrinsic postzygotic isolation between taxa: two allopatric populations might each be invaded by different meiotic drive mutations; if each mutation later becomes suppressed, both populations will return to normal segregation ratios; if, however, these populations later come into secondary geographic contact and hybridize, this normally cryptic meiotic drive could become reexpressed (assuming that the suppressors of meiotic drive are less than fully dominant). In the simplest (although not the only) scenario, X-linked drive alleles might inactivate Y-bearing sperm in hybrids, while Y-linked alleles would inactivate X-bearing sperm, rendering XY hybrids sterile. Such a scenario might even help to explain “Haldane's rule,” the preferential sterility or inviability of hybrids of the heterogametic (XY) sex, an idea that was proposed independently by Frank (1991) and Hurst and Pomiankowski (1991).
Tao and Hartl (2003), Henikoff et al. (2001), and Henikoff and Malik (2002) have recently suggested variations on the meiotic drive theory of postzygotic isolation. In Tao and Hartl's scenario, struggles over sex ratio are especially acute in the heterogametic sex as different portions of the genome in heterogametic individuals “prefer” different sex ratios (the Y chromosome in Drosophila, for instance, prefers more sons). In Henikoff and colleagues' scenario, struggles over which chromosome segregates into an egg (and not into a polar body) in female meiosis give rise to bouts of meiotic drive followed by suppression of drive; these bouts, they suggest, involve evolution at centromeric sequences and at the special histones that bind these sequences. Despite their differences, these models all share a central theme: segregation distorters appear and are then suppressed within species, only to be reexpressed in species hybrids.
Although the meiotic drive theory of postzygotic isolation is attractive—especially as segregation distortion occurs in a wide variety of organisms, including insects, mammals, plants, and fungi (Lyttle 1991)—it fell out of favor in the early 1990s. The main reason was that experiments by Coyne (1986), Johnson and Wu (1992), and Coyne and Orr (1993) found no segregation distortion in hybrids between several pairs of Drosophila species. (These hybridizations produce partially fertile F1 hybrids, allowing tests of segregation distortion in hybrid gametogenesis.) These findings were widely viewed as fatal to the meiotic drive theory of speciation (a view once shared by the senior author).
More recent studies, however, suggest that Coyne, Orr, Johnson, and Wu may have been unlucky in their choice of species pairs or of hybrid genotypes. Several cases of normally cryptic segregation distortion have now been described. All occur in Drosophila, presumably reflecting the intense genetic scrutiny of this genus. By far the best studied of these cases involves the species pair Drosophila simulans and D. mauritiana. Tao et al. (2001) showed that otherwise D. simulans males that are homozygous for a small region of the D. mauritiana third chromosome suffer sex ratio segregation distortion, producing ∼80% daughters. Tao et al. suggest that the D. simulans genome carries an X-linked meiotic drive factor(s) that is normally suppressed within species by a dominant autosomal suppressor on the D. simulans third chromosome. When this dominant suppressor is replaced by recessive autosomal material from D. mauritiana, sex chromosome meiotic drive results. Tao et al. (2001) map this autosomal suppressor to <80 kb of DNA; they call the putative suppressor gene residing in this region too much yin (tmy). Similarly, Dermitzakis et al. (2000) showed that certain hybrid introgression lines between D. simulans and D. sechellia suffer male meiotic drive, which causes sex ratio distortion among their progeny. Although several different introgression lines show such distortion, complementation tests suggested that the same autosomal region is involved in all lines (Dermitzakis et al. 2000). While the above cases involve hybrids between named “good” species, other cases involve hybrids between populations or strains within species. In one, D. simulans flies produced by crossing individuals from Tunisia with individuals from Seychelles or New Caledonia suffer meiotic drive, while pure-population individuals do not (Mercot et al. 1995; Cazemajor et al. 1997; Montchamp-Moreau and Joly 1997). In another, hybrids between certain stocks of D. subobscura suffer meiotic drive, while pure-stock individuals do not (Hauschteck-Jungen 1990).
Here we report the discovery of segregation distortion in hybrids between the Bogota and USA subspecies of D. pseudoobscura, taxa that have often been viewed as paradigmatic of the earliest stages of speciation (e.g., Lewontin 1974). The Bogota subspecies is restricted to high elevations near Bogota, Colombia and is geographically isolated from the USA subspecies of North and Central America by >2000 km (Prakash 1972). The Bogota-USA system represents an especially young hybridization: DNA sequence analysis shows that the Bogota and USA subspecies may have separated as recently as 155,000–230,000 years ago (Schaeffer and Miller 1991; Wang et al. 1997; Machado et al. 2002; Machado and Hey 2003). Not surprisingly, the Bogota and USA subspecies are incompletely reproductively isolated: they show little or no prezygotic isolation (Prakash 1972) or conspecific sperm precedence (Dixon et al. 2003) and produce completely fertile female hybrids. Male hybrids are also fertile in one direction of the hybridization (USA mothers), while male hybrids from the reciprocal direction of the hybridization (Bogota mothers) have traditionally been described as completely sterile. This hybrid male sterility has been the subject of several genetic studies (Prakash 1972; Dobzhansky 1974; Orr 1989a,b; Orr and Irving 2001).
Here we show that hybrid males having Bogota mothers are not, in fact, completely sterile; instead these hybrids become weakly fertile when aged. Surprisingly, these F1 hybrid males produce almost all daughters. The results presented below suggest that this sex ratio distortion reflects normally cryptic segregation distortion in hybrid males. We also present the results of a preliminary genetic analysis of this distortion.
MATERIALS AND METHODS
Stocks and crosses:
Our methods generally follow those of Orr (1989a)(b) and Orr and Irving (2001). These articles also describe many of the stocks used. The Sex Ratio (SR) and Standard (ST) arrangement stocks were kindly provided by John Jaenike and were collected in Tucson, Arizona. The wild-type iso-female lines of D. pseudoobscura USA were kindly provided by Mohamed Noor. All crosses were performed at room temperature unless otherwise indicated. All map positions are from Anderson and Norman (1977), except for those of the X chromosome, which are from Orr (1995a).
Male fertility:
Male fertility was measured by assessing sperm motility, which is standard in studies of hybrid male sterility in Drosophila (e.g., Coyne 1985; Vigneault and Zouros 1986; Orr 1987; Orr and Coyne 1989; Davis and Wu 1996). Testes were dissected from 4- day-old virgin males and examined under a compound microscope with dark field optics. Males were classified into three sperm motility classes: Many, wherein a male had a large number of motile sperm that filled the field of vision; Few, wherein small pockets of motile sperm were seen; and None, wherein no motile sperm were seen. While sperm motility is not equivalent to fertility, the two are strongly correlated (Orr 1987). In one large cross described below, male fertility was measured by counting number of offspring produced, as these offspring were produced for other reasons.
Egg to adult lethality:
The frequency of lethality among offspring of hybrid males was measured by aging hybrid F1 males for 8–9 days and then single-pair mating them to 3- to 4-day-old virgin Bogota females. When a pair of flies began to produce first instar larvae, the pair was transferred to an egg-counting vial. This vial contained a small plastic spoon filled with standard media dyed purple to ease visualization of eggs. The adult pair was left in this vial for 24 hr and then transferred to a new egg-counting vial for another 24 hr. Because hybrid males are almost completely sterile, almost all eggs are unfertilized. It is thus impractical to measure hatch rates among the very rare fertilized eggs. Instead, we simply counted the number of dead offspring. In particular, each egg-counting vial was scored for number of dead eggs and dead first instar larvae 24 and 48 hr after the parents were removed. (Dead eggs and larvae of D. pseudoobscura are necrotic and unmistakably brown.) The media from the egg-counting vials were transferred to fresh vials and maintained at room temperature. Vials were scored for further larval lethality 7 days after the parental pair was removed. Vials were later scored for number of emerging adults. After 3 consecutive days in which no adults emerged, pupae were scored for lethality. (Empty pupal cases are easily distinguished from those containing a dead individual.) Pupal cases containing dead individuals were dissected to score sex; this is typically possible as D. pseudoobscura males have bright orange testes.
Statistics:
When comparing sex ratios produced by males of different genotypes, we treat each father as a single data point; i.e., each father produces a percentage of daughters. This is much more conservative than treating each offspring as a single data point. The null hypothesis of no difference in sex ratio between genotypes was tested with unpaired t-statistics on arcsin square-root transformed proportion daughters (Sokal and Rohlf 1981). Nonparametric tests (Mann-Whitney U-test) on untransformed proportions usually yielded similar results. In our large X chromosome mapping experiments, the effect of chromosome regions on sex ratio was tested by comparing the sex ratios produced by all fathers that differ at a marker; only fathers producing 10 or more offspring were included in these mapping experiments, to ensure some accuracy in sex ratio measurements.
RESULTS
A hybrid fertility rescue mutation:
Our study began as a search for a hybrid fertility “rescue mutation.” It is well known that certain single mutations can rescue the viability of normally lethal hybrids in the D. melanogaster group (Watanabe 1979; Hutter and Ashburner 1987; Hutter et al. 1990; Sawamura et al. 1993a,b,c; Orr and Irving 2000; Coyne and Orr 2004, Chap. 8). We hoped to recover a similar mutation that would rescue the fertility of normally sterile hybrid males produced in the cross of Bogota females to USA males. We screened 97 wild-type iso-female USA lines for fertility rescue. In particular, we mass mated wild-type Bogota-ER females to males from each of the 97 USA lines, establishing multiple vials of each cross. We scored the number of emerging hybrid F1 females and males and transferred all F1 hybrids to fresh vials, testing for the appearance of F2 hybrid (larval, pupal, or adult). As we expect F1 males to be sterile, the appearance of F2 hybrids shows that F1 male fertility has been at least partially rescued.
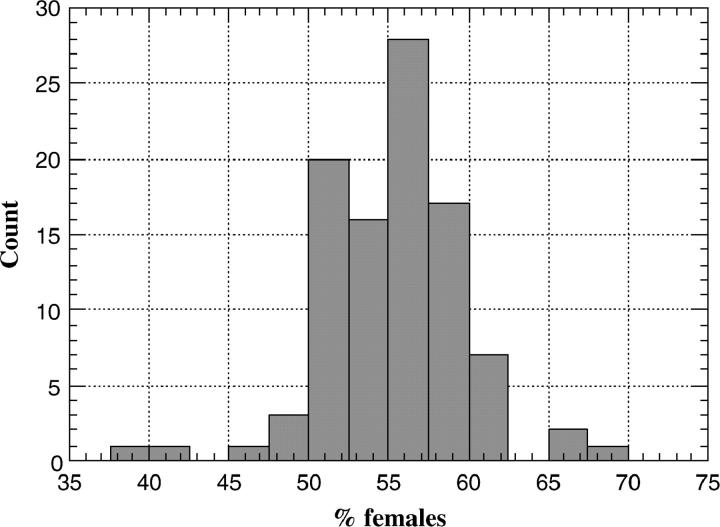
The sex ratio among adult F1 hybrids was close to even, although there was a slight excess of females (mean ± SD, 55.1 ± 4.6%; Figure 1), suggesting mild hybrid male inviability in the F1 generation. Surprisingly, however, 18 of 97 lines produced some F2 hybrids. These cases did not reflect contamination as, in all instances, crosses were extremely difficult and very few F2 hybrids appeared (sometimes only a single fly). Several USA iso-female lines that produced F2 hybrids were retested; most consistently produced a few F2 offspring (results not shown). As the “Flagstaff-5” iso-female line produced the largest number of F2 hybrids, this line was divided into sublines and each subline was tested further. We chose the subline that produced the most F2 hybrids for further analysis. (The number of F2 hybrids produced by this subline varied with the Bogota stock to which it was crossed. In the best case, an average of 1.7 F2 progeny were produced per F1 male; in the worst case, an average of 0.02 F2 progeny were produced per F1 male; see below and some results not shown.) As the results presented below suggest that this subline carries a Mendelizing hybrid fertility rescue mutation, we refer to this stock as Hybrid male fertile (Hmf).
Figure 1.—
Percentage of females among F1 offspring from the cross Bogota-ER females × USA males. Each cross involved a different USA iso-female line; 97 lines were tested.
Hmf represents a USA allele; it is not a Bogota contaminant. This is confirmed by several lines of evidence. First, as expected, the cross of Hmf female × USA ct sd y se sp male produces all fertile F1 males (sperm motility: 81 Many, 0 Few, 0 None). Second, also as expected, the cross of Bogota-ER female × Hmf male produces almost all sterile F1 males (4 Many, 96 Few, 101 None). Hmf thus only weakly rescues the fertility of F1 males.
We crossed hybrid F1 males carrying Hmf to many different genotypes of D. pseudoobscura females. The results are shown in Table 1. Remarkably, these normally sterile hybrid F1 males produce almost all daughters (88–99%). This is true regardless of the Bogota strain to which Hmf was initially crossed and regardless of whether the resulting F1 males were subsequently crossed to pure USA females, to pure Bogota females, or to hybrid F1 females (having USA or Bogota cytoplasm). The reciprocal class of Hmf F1 males—those having a USA mother—do not produce distorted sex ratios. Instead, these normally fertile F1 males produce offspring having nearly even sex ratios (Table 2).
TABLE 1.
Hybrid males carryingHmf produce almost all daughters
| Hybrid fathera | Mother | No. of daughters |
No. of sons |
% females |
|---|---|---|---|---|
| Bog-ER × Hmf | Bog-ER | 93 | 4 | 95.9 |
| Bog-ER × Hmf | Hmf | 155 | 21 | 88.1 |
| Bog-ER × Hmf | Bog-ER × Hmf | 433 | 21 | 95.4 |
| Bog-ER × Hmf | Hmf × Bog-ER | 82 | 1 | 98.8 |
| Bog-ER × Hmf | USA y gl or inc | 117 | 2 | 98.3 |
| Bog Toro-1 × Hmf | Bog Toro-1 | 464 | 26 | 94.7 |
| Bog Toro-1 × Hmf | Hmf | 190 | 26 | 88.0 |
| Bog Toro-1 × Hmf | Bog Toro-1 × Hmf | 335 | 35 | 90.5 |
| Bog Potosi-1 × Hmf | Bog Potosi-1 | 26 | 2 | 92.9 |
| Bog Potosi-3 × Hmf | Bog Potosi-3 | 45 | 1 | 97.8 |
The cross is that used to produce the hybrid male; the female in the cross is listed first.
TABLE 2.
Reciprocal hybrid males carrying a USAXproduce even sex ratios
| Hybrid fathera | Mother | No. of daughters |
No. of sons |
% females |
|---|---|---|---|---|
| Hmf × Bog-ER | Bog-ER | 223 | 195 | 53.3 |
| Hmf × Bog-ER | Hmf | 909 | 724 | 55.7 |
| Hmf × Bog-ER | Hmf × Bog-ER | 1685 | 1357 | 55.3 |
| Hmf × Bog-ER | Bog-ER × Hmf | 1245 | 1154 | 51.9 |
The cross is that used to produce the hybrid male; the female in the cross is listed first.
Because normally sterile F1 males carrying Hmf do not receive their X chromosome or cytoplasm from USA, the gene(s) underlying fertility rescue must reside on the USA autosomes or Y. This was confirmed in a large analysis involving chromosome substitution between the (rescuing) Hmf stock and (nonrescuing) USA stocks containing balancer chromosomes on the second (Ba; 2-62.1, associated with an inversion) or third (L; 3, associated with medial Santa Cruz inversion chromosomes). Briefly, 130 single hybrid males that either did or did not carry a second or third chromosome from Hmf were crossed to Bogota-ER females. The results show that hybrid males must carry a second chromosome from Hmf to produce offspring (Table 3; Fisher's exact test, P = 0.025). The Hmf fertility rescue mutation thus appears to reside on the second chromosome. Importantly, fertility-rescued F1 males again showed distorted sex ratios among their offspring. Indeed only daughters appeared (79 females,0 males; Table 3).
TABLE 3.
Mapping of the autosomal hybrid fertility rescue mutation
| Hybrid father | Vials set up |
Vials with progeny |
Total progeny (female:male) |
|---|---|---|---|
| XBog/YUSA; 2Hmf/2Bog | 37 | 6 | 41:0 |
| XBog/YUSA; 2Ba/2Bog | 36 | 0 | 0:0 |
| XBog/YUSA; 3Hmf/3Bog | 27 | 4 | 28:0 |
| XBog/YUSA; 3L/3Bog | 30 | 4 | 10:0 |
The second chromosome experiment involved crossing y; Ba/Delta; or females × Hmf males and crossing the resulting phenotypically y Ba males to Bogota-ER females, producing two genotypes of males (rows 1 and 2). The third chromosome experiment involved crossing or L/or L+; spa females × Hmf males and crossing the resulting phenotypically L males to Bogota-ER females, producing two genotypes of males (rows 3 and 4).
Finally, we tested whether Hmf also rescues hybrid male fertility between the more evolutionarily distant species pair, D. pseudoobscura and D. persimilis. It does not, although only a single line of D. persimilis was tested (Table 4).
TABLE 4.
Test of whetherHmf rescues the fertility ofD. pseudoobscura- D. persimilis hybrid males
| F1 male fertility
|
||||
|---|---|---|---|---|
| Female | Male | Many | Few | None |
| D. pseudo y; gl; or; inc | D. persimilis or | 0 | 0 | 91 |
| D. persimilis or | D. pseudo y; gl; or; inc | 0 | 0 | 41 |
| D. pseudo Hmf | D. persimilis or | 0 | 0 | 102 |
| D. persimilis or | D. pseudo Hmf | 0 | 0 | 58 |
The top two rows confirm that “normal” F1 hybrid males between D. pseudoobscura and D. persimilis are sterile in both directions of the hybridization. The bottom two rows show that the D. pseudoobscura Hmf stock does not rescue this sterility. Hybrid male fertility was assessed cytologically in testis squash preparations.
Evidence for hybrid segregation distortion:
The fact that hybrid males with Bogota mothers produce almost all daughters could be an artifact of Hmf. After all, we know nearly nothing about this mutation except that it is zygotically acting, dominant, and resides on the second chromosome. To our surprise, however, we discovered that “normal” hybrid males between arbitrary strains of Bogota and standard marker strains of USA become weakly fertile when aged for several weeks. Although these F1 males almost never produce offspring within the first 2 weeks of a cross, they often produce offspring after several weeks of repeated transfers to fresh vials (Table 5; although we did not perform precise timing experiments, we did not notice a sharp critical period after which hybrids produce progeny). Cytological examination of hybrid testes confirms an effect of age on hybrid sperm motility (Table 6): although “normal” hybrid F1 males sometimes produce a few motile sperm at day 2, they produce significantly more at day 14. This age effect is also seen in hybrid males that carry the Hmf mutation (Table 6).
TABLE 5.
“Normal” hybrid males also produce almost all daughters
| Hybrid father | Mother | No. of daughters |
No. of sons |
% females |
|---|---|---|---|---|
| Bog Poto-1 × y gl or inc | Bog Potosi-1 | 32 | 0 | 100.0 |
| Bog Poto-3 × y gl or inc | Bog Potosi-3 | 12 | 1 | 92.3 |
| Bog Toro-1 × y gl or inc | Bog Toro-1 | 69 | 2 | 97.2 |
| Bog Toro-1 × y gl or inc | y gl or inc | 71 | 26 | 73.2 |
| Bog Toro-1 × y gl or inc | Bog Toro-1 × y gl or inc | 27 | 6 | 81.8 |
| Bog Toro-1 × USA Tempe-5 | Bog Toro-1 | 348 | 34 | 91.1 |
| Bog w × y gl or inc | Bog w × y gl or inc | 83 | 9 | 90.2 |
TABLE 6.
Effect of age on hybrid male fertility
| F1 male fertility
|
||||||
|---|---|---|---|---|---|---|
| Mother | Father | Day | Many | Few | None | χ2 |
| Bogota-ER | USA y gl or inc | 2 | 0 | 24 | 177 | |
| 14 | 0 | 77 | 125 | 37.7*** | ||
| Bogota w | USA y gl or inc | 2 | 0 | 1 | 206 | |
| 14 | 4 | 80 | 199 | 70.8*** | ||
| Bogota-ER | USA Hmf | 2 | 4 | 96 | 101 | |
| 14 | 22 | 158 | 22 | 78.0*** | ||
| Bogota w | USA Hmf | 2 | 7 | 75 | 120 | |
| 14 | 20 | 136 | 47 | 55.8*** | ||
The χ2 values reflect comparing sperm motility within a genotype after males were aged 2 vs. 14 days. In cases in which a cell value equaled 0, it was set to 0.1 to allow calculation of a χ2 statistic; other small values do not substantially change the above probabilities.
P < 0.0001.
Remarkably, hybrid F1 males between “normal” stocks of Bogota and USA also produce almost all daughters. Indeed F1 males from all stock combinations showed sex ratio distortion among their offspring (Table 5). Once again, distortion occurs whether F1 males are crossed to pure Bogota females, to pure USA females, or to hybrid F1 females. The sex ratio distortion seen among the offspring of hybrid males is not, therefore, an artifact of Hmf—distortion seems to always occur among the offspring of hybrid males, regardless of which stocks are used. (Additional examples involving other stock combinations appear below.) The key question is: Why do hybrid F1 males produce almost all daughters?
There are at least three possibilities. First, sex transformation may occur among the progeny of hybrid males, with genetic males transformed into somatic females. Second, hybrid inviability may occur among the progeny of F1 males, with most sons dying. Third, segregation distortion may occur in F1 males, such that most zygotes derive from sperm that carry an X chromosome. The sex transformation hypothesis is more plausible than it might first seem: partial or complete sex transformation has been observed in species hybrids both in Drosophila (Sturtevant 1946) and in Caenorhabditis (Baird 2002). We were, however, able to rule out this possibility by using X-linked visible markers: we crossed Hmf-rescued hybrid F1 males to USA females carrying the X-linked mutation, yellow (1-74.5). As expected, almost all offspring were again female (Table 1, line 5). These daughters were all phenotypically y+, while the few emerging sons were phenotypically y. Sex transformation does not, therefore, occur among the offspring of F1 males.
We tried to distinguish the hybrid inviability and segregation distortion hypotheses in two ways. The first was indirect: with hybrid inviability, we expect the fitness of the sons of F1 males to depend on their genotype, e.g., on whether sons carry a Bogota or a USA X chromosome. As emphasized, however, F1 males produce almost all daughters regardless of whether F1 males are crossed to pure Bogota, pure USA, or hybrid females—and so regardless of whether their sons carry a pure Bogota X, a pure USA X, or a recombinant X chromosome. This pattern differs qualitatively from that expected with hybrid inviability.
The second approach was more direct: if the absence of sons reflects hybrid inviability, massive lethality must occur among the offspring of hybrid F1 males. To assess this, we measured egg, larval, and pupal lethality in a large cross involving hybrid F1 males, as described in materials and methods and in Table 7. Once again, we found that hybrid F1 males produced almost all daughters (485 females, 29 males; Table 7). Not surprisingly, some lethality was seen in this cross (which does, after all, involve subspecific hybridization). The observed lethality was, however, far too rare to explain the near-absence of sons. Although ∼456 sons are “missing” (485 − 29 = 456), we observed very few dead embryos or larvae and only 37 dead pupae (Table 7). Importantly, we could score the sex of 21 of these dead pupae and almost all were female (17 females, 4 males). Sex ratio distortion thus appears before the pupal stage, but there is very little embryonic or larval lethality (Table 7).
TABLE 7.
Lethality among offspring of hybrid F1 males
| Stage | Number |
|---|---|
| Dead eggs (24 hr) | 15 |
| Dead eggs (48 hr) | 19 |
| Dead larvae | 6 |
| Dead pupae (unemerged female) | 17 |
| Dead pupae (unemerged male) | 4 |
| Dead pupae (sex undetermined) | 16 |
| Total dead offspring | 77 |
| Emerged adult females | 485 |
| Emerged adult males | 29 |
Offspring resulted from the cross of F1 males (Bogota Toro-1 females × USA Hmf males) × Bogota Toro-1 females. A total of 50 F1 males were single-pair mated to Bogota females. See materials and methods for detailed protocol.
Taking these facts together—(i) hybrid males produce almost all daughters regardless of whom they are crossed to and (ii) hybrid males produce few dead offspring—it appears that hybrid F1 males show sex chromosome segregation distortion. We do not, however, yet know the functional basis of this distortion (see below).
Genetic basis of hybrid segregation distortion—X chromosome mapping:
We would like to understand the genetic basis of hybrid segregation distortion. Given, however, that even “fertility-rescued” hybrid males remain highly sterile, all genetic analyses proved extraordinarily difficult and our sample sizes were, consequently, often less than ideal. Nonetheless, we were able to establish several facts, which we describe for the remainder of this article.
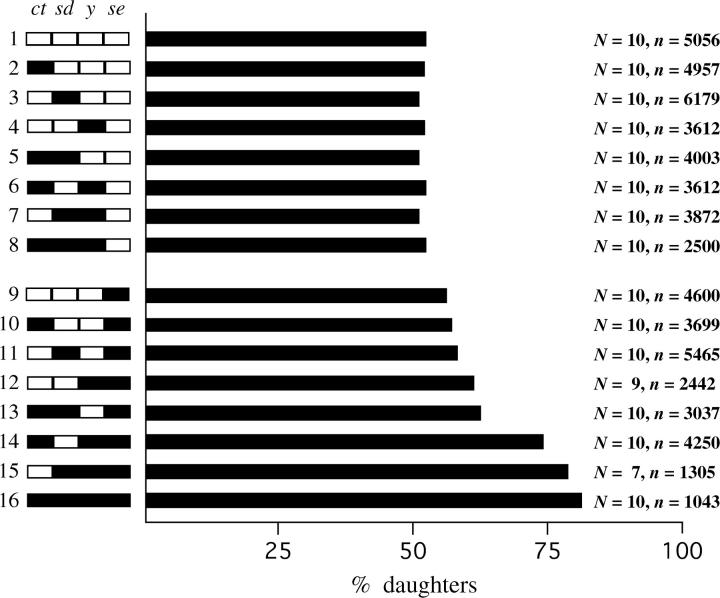
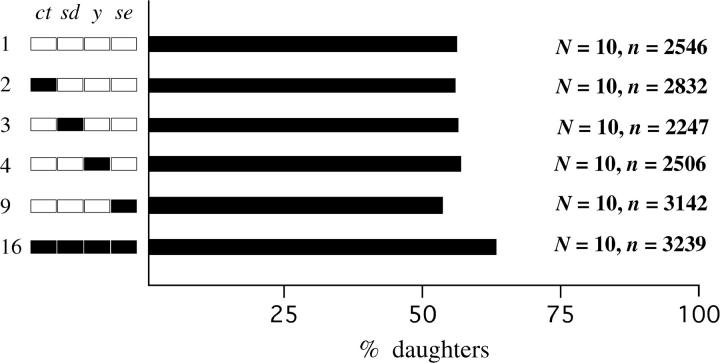
Given that hybrid segregation distortion occurs in F1 males that carry a Bogota X and a USA Y and that almost all daughters result, it seems likely that the Bogota X chromosome carries gene(s) that cause segregation distortion. To confirm this and to roughly map these putative X-linked genes, we produced backcross hybrid males that carried recombinant X chromosomes, a USA Y chromosome, and mostly USA autosomes. In particular, we backcrossed F1 females from the cross of Bogota Toro-1 females × USA ct (1-22.5) sd (1-43.0) y (1-74.5) se (1-156.5) males to USA Hmf males. Recombinant backcross males of known X chromosome genotype were then singly mated to wild-type Bogota Toro-1 females and the sex ratio of the resulting progeny was scored. The results are shown in Figure 2, which is arranged to match Figure 2 of Orr and Irving (2001).
Figure 2.—
Mapping of X chromosome segregation distortion genes. The x-axis shows the average percentage of daughters produced by hybrid backcross males of a given genotype; the y-axis shows the X chromosome genotypes studied. Backcross males resulted from the cross of F1 females (Bogota Toro-1 females × USA ct sd y se males) × USA Hmf males. Data derive from backcross males of known genotype that successfully produced offspring when singly mated to Bogota Toro-1 females. N is the number of fathers of a given genotype that produced progeny; n is the total number of progeny that they produced. A total of 59,632 offspring were scored for sex. The modest sample sizes reflect the extreme difficulty of some of the crosses. All backcross hybrid males carry a recombinant X chromosome, a USA Y chromosome, and mostly USA autosomes. White chromosome regions represent USA material, while black chromosome regions represent Bogota material.
As expected, segregation distortion depends on X chromosome genotype. Males that have a USA-like X genotype (ct sd y se; genotype 1) produce nearly even sex ratios, while males that have a Bogota-like genotype (ct+ sd+ y+ se+; genotype 16) produce mostly daughters. Figure 2 also shows that gene(s) tightly linked to se are essential for segregation distortion: genotypes 1–8, which carry USA material at se, produce nearly even sex ratios, while genotypes 9–16, which carry Bogota material at se, often produce biased sex ratios (t = 8.08, P < 0.0001). Perhaps most remarkably, ct+ sd+ y+ se males (genotype 8), which carry Bogota material at all markers except se, produce nearly even sex ratios, while ct+ sd+ y+ se+ males (genotype 16), which also carry Bogota material at se, produce very biased sex ratios. Segregation distortion thus requires Bogota material near se. Restricting our attention to hybrids that carry se+, Figure 2 also shows that the y and sd regions have large effects on sex ratio (y: t = 5.02, P < 0.0001; sd: t = 2.65, P = 0.0099). The ct region has a lesser effect on sex ratio (t = 1.53, P = 0.13, although this contrast is significant with a nonparametric test). Looking across Figure 2, it appears that genes residing on both the left arm (XL: sd, y, and perhaps ct) and the right arm (XR: se) of the X chromosome affect offspring sex ratio.
The X-linked genes causing segregation distortion also show strong conspecific epistasis: comparisons among genotypes 1–4 and 9 reveal that no single X chromosome region from Bogota has any effect on sex ratio by itself. Instead, sex ratio distortion appears only when several X chromosome regions from Bogota are jointly introgressed into a USA background. It also appears that at least one gene causing segregation distortion is loosely linked to our X-linked markers, since the most Bogota-like of our backcross genotypes (ct+ sd+ y+ se+; genotype 16) does not suffer full F1-male-like levels of segregation distortion.
Hybrid segregation distortion and hybrid male sterility:
Although crude, the above mapping results resemble those from our previous work on hybrid male sterility between the Bogota and USA subspecies (Orr and Irving 2001). Indeed the same regions of the Bogota X chromosome are involved in both hybrid male sterility and hybrid segregation distortion and these regions show a similar pattern of complex conspecific epistasis for both phenotypes. Moreover, the region near se plays a large—and necessary—role in both hybrid segregation distortion and hybrid male sterility. Our findings are, then, at least consistent with the idea that the same genes cause both phenomena. Indeed throughout many of the above crosses we noticed that hybrid males that show segregation distortion produce few progeny, while males that do not show segregation distortion produce many progeny.
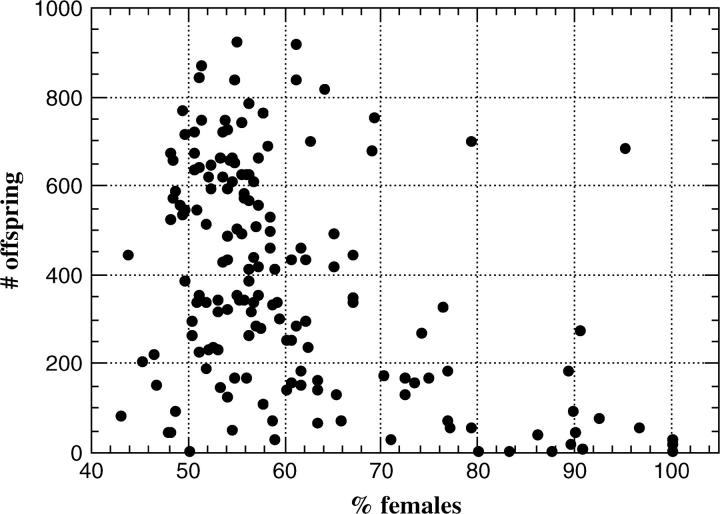
To better assess this possible association between hybrid segregation distortion and hybrid male sterility, we scored the number of offspring produced by each recombinant backcross male from the above X chromosome mapping experiment. The results are shown in Figure 3. There is a highly significant correlation between sex ratio among progeny and the number of offspring produced by a male (r = −0.472, P < 0.0001; Kendall's τ = −0.297, P < 0.0001). This negative correlation is not an artifact of any inviability of sons, as sex ratio and number of daughters produced by a hybrid male are also strongly correlated (r = −0.372, P < 0.0001; Kendall's τ = −0.205, P < 0.0001). Although the evidence presented in this section certainly does not prove that the same genes cause both hybrid segregation distortion and hybrid male sterility, we cannot exclude this possibility.
Figure 3.—
Scatterplot of percentage of daughters vs. number of offspring produced by the hybrid backcross males studied in Figure 2. Because these backcross males were produced through F1 females (that undergo recombination), they are genetically heterogeneous. This plot includes data from all backcross fathers, even if they produced very few offspring.
Genetic basis of hybrid segregation distortion—autosomal suppressors:
The genes on the Bogota X chromosome that cause segregation distortion do so only in hybrids, not within the Bogota subspecies. The Bogota genome must therefore carry suppressors of segregation distortion. Moreover, these suppressors must be Y linked and/or autosomal and must be incompletely dominant (as a single dose of Bogota autosomes does not fully suppress distortion in F1 hybrid males).
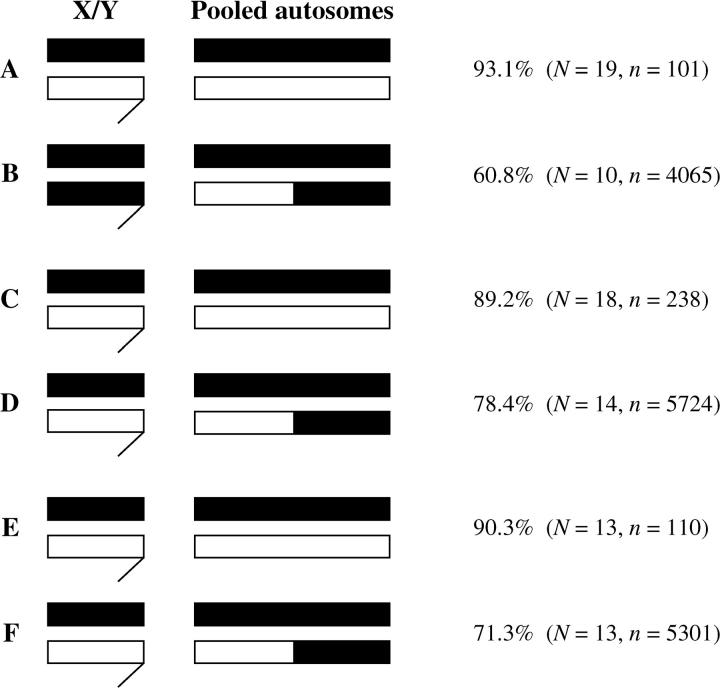
The existence of Bogota suppressors of segregation distortion is confirmed in the top panel of Figure 4. Genotypes A and B both carry an unrecombined X chromosome from Bogota as well as pure Bogota cytoplasm. But genotype A carries a Y chromosome from USA and only half of its autosomes from Bogota and shows strong segregation distortion; genotype B, on the other hand, carries a Y chromosome from Bogota and three-fourths of its autosomes (on average) from Bogota and shows little segregation distortion (t = 4.58, P < 0.0001). Replacing the Y chromosome and/or autosomes from USA with those from Bogota thus suppresses distortion. The middle panel of Figure 4 confirms that at least some of the suppressors of segregation distortion reside on the Bogota autosomes. Genotypes C and D both carry an unrecombined X chromosome from Bogota and a Y chromosome from USA; they differ only in the fraction of the autosomes that, on average, derive from Bogota (one-half in genotype C and three-fourths in genotype D). Genotype C shows strong segregation distortion, while genotype D shows weaker distortion (although this difference has borderline significance: t = 1.92, P = 0.065). While this contrast involved the Hmf stock, the bottom panel in Figure 4 shows that these findings do not depend on Hmf. Instead, hybrid males that carry fewer autosomes from Bogota (genotype E) show significantly stronger segregation distortion than do those that carry more autosomes from Bogota (genotype F), even when Hmf is not used (t = 2.63, P = 0.015).
Figure 4.—
Evidence for autosomal suppressors of segregation distortion in Bogota. Plot shows percentage of females produced by hybrid males of various genotypes. The short chromosomes at the left represent the sex chromosomes (X on top, Y on bottom; Y shown with hook). The long chromosomes at the right represent haploid sets of autosomes. Black chromosomes derive from Bogota and white from USA. Genotype A resulted from the cross of Bogota Toro-1 females × USA Hmf males; genotype B resulted from Bogota Toro-1 female × F1 male (Hmf female × Toro-1 male). Genotype C again resulted from Bogota Toro-1 females × USA Hmf males; genotype D resulted from Bogota Toro-1 female × F1 male (Toro-1 female × Hmf male). Genotype E resulted from Bogota Toro-1 females × USA Tempe-5 males; genotype F resulted from Bogota Toro-1 female × F1 male (Toro-1 female × Tempe-5 male). N is the number of fathers of a particular genotype that produced progeny; n is the total number of progeny produced.
Further crosses involving repeated backcrosses to Bogota show that the severity of segregation distortion gradually decreases as the autosomes become more Bogota. In particular, we backcrossed hybrid males for three generations to pure Bogota females. All backcross males in each generation carried an unrecombined X from Bogota, unrecombined autosomes (as backcrossing proceeded through males), a USA Y chromosome, and Bogota cytoplasm. Table 8 shows that increasing the fraction of autosomes from Bogota causes the sex ratio among the progeny of backcross males to become progressively more even (eventually leveling off at ∼60% females). While the top half of Table 8 involves Hmf, the bottom half does not. In both cases, the autosomes affect the strength of hybrid segregation distortion.
TABLE 8.
Sex ratio among the offspring of F1 and repeated backcross males
| Cross | % daughters | N | n |
|---|---|---|---|
| Bogota Toro-1 × USA Hmf | |||
| F1 | 89.2 | 18 | 238 |
| BC1 | 78.4 | 14 | 5724 |
| BC2 | 65.0 | 22 | 3782 |
| BC3 | 62.3 | 15 | 2874 |
| Bogota Toro-1 × USA Tempe-5 | |||
| F1 | 90.3 | 13 | 110 |
| BC1 | 71.3 | 13 | 5301 |
| BC2 | 60.4 | 23 | 4314 |
| BC3 | 60.1 | 17 | 1954 |
Hybrid males were backcrossed to Bogota Toro-1 females each generation. The sex ratios shown were calculated by averaging the percentage of daughters produced by individual hybrid fathers. The number of fathers (N) producing offspring, as well as the total number of offspring scored each generation (n), is shown. Due to hybrid sterility, many more fathers were typically set up than produced offspring, especially among F1 males. “BC1” represents first-generation backcross hybrids and so on.
While the results of this section show that the Bogota autosomes carry suppressors of hybrid segregation distortion, we have not yet succeeded in mapping these suppressors to particular autosomes. The necessary crosses involved passing dominantly marked balancer chromosomes through F1 males that carry a USA Y chromosome and that are therefore nearly completely sterile; these crosses were extremely difficult and mostly failed.
Genetic basis of hybrid segregation distortion—Y chromosome:
It appears that segregation distortion is strongest when hybrid males carry a USA Y chromosome. We performed a number of crosses that were essentially identical to many described above but in which hybrid males carried a Bogota Y chromosome. In all cases, we observed little sex ratio distortion among offspring. One example is shown in Figure 5. The backcross males shown there are similar to those shown in Figure 2. The key difference is that the males in Figure 2 carry a USA Y chromosome (and show strong sex ratio distortion), while the males in Figure 5 carry a Bogota Y chromosome (and show little sex ratio distortion; indeed all sex ratios are within 7% of each other). It is especially interesting to note that males having a Bogota-like X chromosome genotype (ct+ sd+ y+ se+) produce ∼65% daughters when carrying a Bogota Y chromosome (Figure 5); the same genotype produces ∼85% daughters when carrying a USA Y chromosome (Figure 2). Thus while some segregation distortion may occur on a Bogota Y genetic background, it is weaker than that on a USA Y background.
Figure 5.—
Segregation distortion is weaker on a Bogota Y genetic background. The backcross males shown are analogous to those in Figure 2 except that these males carry a Bogota Y chromosome (genotypes are numbered to match those in Figure 2). Backcross males resulted from the cross of F1 females (USA ct sd y se female × Bogota Toro-1 male) × Bogota Toro-1 males. Data derive from backcross males of known genotype that successfully produced offspring when singly mated to Bogota Toro-1 females. N is the number of fathers of a given genotype that produced progeny; n is the total number of progeny that they produced. A total of 16,512 offspring were scored. All other details are as in Figure 2.
Connection to Sex Ratio rearrangement:
Finally, we tested whether the segregation distortion seen in Bogota-USA hybrids is connected to the meiotic drive caused by the Sex Ratio (SR) chromosome, an X-linked rearrangement that segregates in some USA populations of D. pseudoobscura. [SR is associated with three inversions on XR; see review in Jaenike (2001).] Dobzhansky et al. (1963) showed that the SR inversions are not present in Bogota; we confirmed this by salivary gland preparations of Bogota-USA F1 hybrids (not shown). Nonetheless, hybrid segregation distortion and SR drive might still be connected as the segregation distortion seen in SR males is almost certainly due to genes within the SR inversions, not to the inversions per se (Wu and Beckenbach 1983; Jaenike 2001). We also know, though, that hybrid segregation distortion involves genes on both XL and XR (Figure 2), whereas SR drive involves genes on XR only. At most, then, the genetic bases of the two types of segregation distortion might be partly overlapping.
To test this, we asked whether the SR chromosome causes segregation distortion on a largely Bogota genetic background, i.e., on a background that contains some suppressors of hybrid segregation distortion. In particular, we crossed USA SR females to Bogota Toro-1 males; the resulting F1 males carry an SR X chromosome, but a Y chromosome and haploid complement of autosomes from Bogota. As a control, we crossed USA SR females to USA Standard arrangement males; the resulting F1 males carry an SR X chromosome on an entirely USA genetic background. As expected, SR causes strong segregation distortion in control USA F1 males: scoring offspring from 52 F1 fathers (singly mated to USA Standard females), the average sex ratio was 96.5% daughters. The experimental hybrid F1 males also showed segregation distortion, although not as strong: scoring offspring from 75 F1 fathers (singly mated to USA Standard females), the average sex ratio was 87.4% daughters. While highly significant (t = 7.26, P < 0.0001), this difference is small and SR segregation distortion clearly still occurs in hybrid males. We also attempted to produce backcross hybrid males that carry an SR X chromosome on a homozygous autosomal Bogota background. Unfortunately, these crosses proved extremely difficult and we could not recover progeny from a meaningful number of SR backcross males.
It thus appears that SR may be slightly less effective on a Bogota genetic background. However, SR still causes strong segregation distortion when paired with a Bogota Y chromosome, unlike the X-linked hybrid segregation distortion genes described above. The hybrid and SR meiotic drive systems thus appear mostly independent.
DISCUSSION
We can draw three main conclusions from our study. First, male hybrids between the Bogota and USA subspecies of D. pseudoobscura are not completely sterile. Although F1 hybrid males having Bogota mothers have been described as sterile throughout several decades of study (Prakash 1972; Dobzhansky 1974; Orr 1989a,b; Orr and Irving 2001), our results reveal that this is incorrect. It is clear, however, why the fertility reported here went unnoticed by previous workers (including the authors of this study): hybrid fertility is very weak and hybrid males typically produce offspring only after being aged for several weeks. Our results further show that the extent of hybrid male fertility varies somewhat with the particular parental strains used. In particular, we isolated a subline of D. pseudoobscura USA deriving from Flagstaff, Arizona that allows the recovery of considerably more progeny from F1 males than is usually possible. Because our results suggest that the weak hybrid fertility rescue seen with this strain involves a factor(s) on the second chromosome, we refer to this strain as Hybrid male fertile (Hmf). The important point, however, is that—with enough effort—offspring can apparently be obtained from hybrid F1 males between essentially any arbitrary stocks of the Bogota and USA subspecies.
Our second main conclusion is that Bogota-USA hybrid males show segregation distortion. More precisely, Bogota-USA hybrid males having Bogota mothers produce almost all daughters (typically >90%). Several lines of evidence suggest that this sex ratio bias is caused not by male inviability or sex transformation, but by sex chromosome segregation distortion. This distortion occurs in crosses between all Bogota and USA strains tested. Reciprocal F1 hybrid males (those that have a USA mother and are highly fertile) do not produce distorted sex ratios. It is important to note that this case of hybrid segregation distortion, unlike those recently described in the D. simulans clade (e.g., Tao et al. 2001), affects not only later-generation hybrids but also F1 hybrids.
We do not yet know the precise functional basis of hybrid segregation distortion. The possibilities include “classic” meiotic drive in which X-bearing sperm inactivate Y-bearing sperm, which are not transferred to females (Lyttle 1991); failure of Y-bearing sperm to function properly in the female reproductive tract (e.g., failure to migrate to sperm storage organs); or failure of Y-bearing male pronuclei to fuse with X-bearing female pronuclei following fertilization (yielding eggs that suffer no obvious necrosis, etc.). We currently know only that hybrid males produce few necrotic eggs or dead larvae; that sex ratio distortion appears before the pupal stage; and that hybrid segregation distortion is largely independent of the meiotic drive caused by the Sex Ratio (SR) X chromosomal arrangement. Because F1 hybrid males are highly sterile—and show disrupted spermatogenesis—our preliminary work suggests that cytological approaches alone will not cleanly resolve the functional basis of segregation distortion. We are thus planning real-time PCR analyses to characterize the stage at which sex chromosome segregation distortion first appears (e.g., in hybrid males vs. in the uterus of females immediately after copulation vs. in sperm storage organs several hours after copulation).
There are at least two possible interpretations of the present—and other—cases of normally cryptic hybrid segregation distortion. The first is that described in the Introduction: a mutation causing segregation distortion appears within one of the parental taxa, is subsequently suppressed, and becomes reexpressed upon hybridization of two taxa. The second is that segregation distortion never appeared in the evolutionary histories of either lineage leading to the present species and instead represents a hybrid pathology (Dermitzakis et al. 2000; Orr and Presgraves 2000). Under this second interpretation, hybrid segregation distortion is a consequence of the inappropriate interaction of genes from two taxa and represents a special case of Dobzhansky-Muller incompatibilities between taxa (Orr 1995b). Unfortunately, we currently know of no way to distinguish between these possibilities.
We also report the results of a preliminary genetic analysis of Bogota-USA hybrid segregation distortion. Although the near-complete sterility of hybrid males showing segregation distortion obviously compromises any such analysis, several facts seem reasonably clear. For one thing, segregation distortion requires genes from several regions of the Bogota X chromosome. Also, the effect of these genes is suppressed within the Bogota subspecies by (incompletely dominant) autosomal alleles (we have not yet succeeded in localizing these autosomal genes). Moreover, segregation distortion is more extreme when the Bogota X chromosome is paired with a USA Y chromosome than with a Bogota Y chromosome.
These mapping experiments lead to our third and final main conclusion: the genetic basis of segregation distortion in the Bogota-USA hybridization is similar to that of hybrid male sterility between the same taxa. Our experiments confirm that the genes causing both phenomena map to the same regions of the X chromosome. More remarkably, both hybrid phenotypes show the same pattern of conspecific epistasis: both hybrid male segregation distortion and hybrid male sterility appear only when hybrids carry the appropriate combination of X-linked alleles from the Bogota subspecies, and no single X-linked region can, by itself, cause any hybrid segregation distortion or hybrid sterility (Orr and Irving 2001). Moreover, one or more genes tightly linked to sepia play a large and necessary role in both phenomena. Our results also show a strong correlation between the fertility of individual backcross hybrid males and the sex ratio of their offspring. (The fact that this correlation is imperfect is not surprising, as individual males often produced very few offspring, causing sex ratio to be measured with considerable error.)
Although these findings are suggestive, we do not claim that hybrid segregation distortion causes Bogota-USA hybrid male sterility. Indeed there are some reasons for thinking that segregation distortion cannot be the sole cause of hybrid male sterility. For one thing, the segregation distortion discovered here—if involving classic meiotic drive—would inactivate only half of all sperm (i.e., those carrying a Y chromosome), which could not explain the near-complete sterility of F1 males having a Bogota mother. Although additional meiotic drive systems might act within Bogota-USA hybrids, perhaps also inactivating many X-bearing as well as Y-bearing sperm, we presently have no evidence for such systems.
We also do not claim, however, that segregation distortion plays no role in Bogota-USA hybrid sterility. Instead, while our results suggest an association between hybrid segregation distortion and hybrid male sterility, they do not currently allow us to either accept or reject the hypothesis that segregation distortion causes hybrid sterility. Indeed it is worth noting that all of our findings can be accommodated by the more moderate hypothesis that hybrid segregation distortion contributes to, but is not the sole cause of, hybrid male sterility. Interestingly, Tao et al. (2001) arrived at a similar conclusion in their analysis of D. simulans-D. mauritiana hybrids. Through an impressively fine-scale genetic analysis, Tao et al. showed that the same 80-kb region that allows hybrid segregation distortion also causes partial hybrid male sterility. They further showed, however, that complete hybrid male sterility requires the action of at least one additional autosomal locus (which they mapped and named broadie). Indeed Tao et al. (2001) speculate that hybrid segregation distortion and hybrid male sterility may often involve partially (but not completely) overlapping sets of genes.
In conclusion, the most important unanswered question now confronting us is clear: Do the genes that cause hybrid segregation distortion between the Bogota and USA subspecies also contribute to hybrid male sterility? Fortunately, this question can be resolved in a straightforward way: one need only determine if, in some chromosome region of large effect on both phenotypes, the genes causing hybrid segregation distortion can be separated meiotically from those causing hybrid male sterility (e.g., Tao et al. 2001). We are now attempting to answer this question via a large introgression experiment in which the genes causing hybrid segregation distortion and hybrid male sterility in the sepia region of XR will be fine mapped using molecular markers. This analysis will obviously be facilitated by the availability of the complete D. pseudoobscura genome sequence.
Acknowledgments
We thank A. Betancourt, K. Dyer, L. Harshman, J. Jaenike, J. P. Masly, M. Noor, N. Phadnis, D. Presgraves, and S. Schaeffer for helpful discussions or comments. This work was supported by National Institutes of Health grant GM-51932.
References
- Anderson, W. W., and R. A. Norman, 1977. Brief descriptions and map positions of currently-available mutants of D. pseudoobscura linkage data. Dros. Inf. Serv. 52: 11. [Google Scholar]
- Baird, S. E., 2002. Haldane's rule by sexual transformation in Caenorhabditis. Genetics 161: 1349–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazemajor, M., C. Landre and C. Montchamp-Moreau, 1997. The sex-ratio trait in Drosophila simulans: genetic analysis of distortion and suppression. Genetics 147: 635–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmides, L. M., and J. Tooby, 1981. Cytoplasmic inheritance and intragenomic conflict. J. Theor. Biol. 89: 83–129. [DOI] [PubMed] [Google Scholar]
- Coyne, J. A., 1985. The genetic basis of Haldane's rule. Nature 314: 736–738. [DOI] [PubMed] [Google Scholar]
- Coyne, J. A., 1986. Meiotic segregation and male recombination in interspecific hybrids of Drosophila. Genetics 114: 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne, J. A., and H. A. Orr, 1993. Further evidence against meiotic-drive models of hybrid sterility. Evolution 47: 685–687. [DOI] [PubMed] [Google Scholar]
- Coyne, J. A., and H. A. Orr, 2004 Speciation. Sinauer Associates, Sunderland, MA.
- Davis, A. W., and C.-I Wu, 1996. The broom of the sorcerer's apprentice: the fine structure of a chromosomal region causing reproductive isolation between two sibling species of Drosophila. Genetics 143: 1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermitzakis, E. T., J. P. Masly, H. M. Waldrip and A. G. Clark, 2000. Non-Mendelian segregation of sex chromosomes in heterospecific Drosophila males. Genetics 154: 687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, S.M., J. A. Coyne and M. A. F. Noor, 2003. The evolution of conspecific sperm precedence in Drosophila. Mol. Ecol. 12: 1179–1184. [DOI] [PubMed] [Google Scholar]
- Dobzhansky, T., 1974. Genetic analysis of hybrid sterility within the species Drosophila pseudoobscura. Hereditas 77: 81–88. [DOI] [PubMed] [Google Scholar]
- Dobzhansky, T., A. S. Hunter, O. Pavlovsky, B. Spassky and B. Wallace, 1963. Genetics of natural populations: XXXI. Genetics of an isolated marginal population of Drosophila pseudoobscura. Genetics 48: 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, S. A., 1991. Divergence of meiotic drive-suppression systems as an explanation for sex-biased hybrid sterility and inviability. Evolution 45: 262–267. [DOI] [PubMed] [Google Scholar]
- Grun, P., 1976 Cytoplasmic Genetics and Evolution. Columbia University Press, New York.
- Hauschteck-Jungen, E., 1990. Postmating reproductive isolation and modification of the ‘sex ratio’ trait in Drosophila subobscura induced by the sex chromosome gene arrangement A2+3+5+7. Genetica 83: 31–44. [DOI] [PubMed] [Google Scholar]
- Henikoff, S., and H. S. Malik, 2002. Selfish drivers. Science 417: 227. [DOI] [PubMed] [Google Scholar]
- Henikoff, S., K. Ahmad and H. S. Malik, 2001. The centromere paradox: stable inheritance with rapidly evolving DNA. Science 293: 1098–1102. [DOI] [PubMed] [Google Scholar]
- Hurst, G. D. D., and J. H. Werren, 2001. The role of selfish genetic elements in eukaryotic evolution. Nat. Rev. Genet. 2: 597–606. [DOI] [PubMed] [Google Scholar]
- Hurst, L. D., and A. Pomiankowski, 1991. Causes of sex ratio bias may account for unisexual sterility in hybrids: a new explanation of Haldane's rule and related phenomena. Genetics 128: 841–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter, P., and M. Ashburner, 1987. Genetic rescue of inviable hybrids between Drosophila melanogaster and its sibling species. Nature 327: 331–333. [DOI] [PubMed] [Google Scholar]
- Hutter, P., J. Roote and M. Ashburner, 1990. A genetic basis for the inviability of hybrids between sibling species of Drosophila. Genetics 124: 909–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenike, J., 2001. Sex chromosome meiotic drive. Annu. Rev. Ecol. Syst. 32: 25–49. [Google Scholar]
- Johnson, N. A., and C.-I Wu, 1992. An empirical test of the meiotic drive models of hybrid sterility: sex ratio data from hybrids between Drosophila simulans and Drosophila sechellia. Genetics 130: 507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewontin, R. C., 1974 The Genetic Basis of Evolutionary Change. Columbia University Press, New York.
- Lyttle, T. W., 1991. Segregation distorters. Annu. Rev. Genet. 25: 511–557. [DOI] [PubMed] [Google Scholar]
- Machado, C. A., and J. Hey, 2003. The causes of phylogenetic conflict in a classic Drosophila species group. Proc. R. Soc. Lond. Ser. B 270: 1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado, C. A., R. M. Kliman, J. A. Markert and J. Hey, 2002. Inferring the history of speciation from multilocus DNA sequence data: the case of Drosophila pseudoobscura and close relatives. Mol. Biol. Evol. 19: 472–488. [DOI] [PubMed] [Google Scholar]
- Mercot, H., A. Atlan, M. Jacques and C. Montchamp-Moreau, 1995. Sex-ratio distortion in Drosophila simulans: co-occurrence of a meiotic drive and a suppressor of drive. J. Evol. Biol. 8: 283–300. [Google Scholar]
- Montchamp-Moreau, C., and D. Joly, 1997. Abnormal spermiogenesis is associated with the X-linked sex-ratio trait in Drosophila simulans. Heredity 79: 24–30. [Google Scholar]
- Orr, H. A., 1987. Genetics of male and female sterility in hybrids of Drosophila pseudoobscura and D. persimilis. Genetics 116: 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr, H. A., 1989. a Genetics of sterility in hybrids between two subspecies of Drosophila. Evolution 43: 180–189. [DOI] [PubMed] [Google Scholar]
- Orr, H. A., 1989. b Localization of genes causing postzygotic isolation in two hybridizations involving Drosophila pseudoobscura. Heredity 63: 231–237. [DOI] [PubMed] [Google Scholar]
- Orr, H. A., 1995. a A new linkage map of the D. pseudoobscura X chromosome. Dros. Inf. Serv. 76: 127–128. [Google Scholar]
- Orr, H. A., 1995. b The population genetics of speciation: the evolution of hybrid incompatibilities. Genetics 139: 1805–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr, H. A., and J. A. Coyne, 1989. The genetics of postzygotic isolation in the Drosophila virilis group. Genetics 121: 527–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr, H. A., and S. Irving, 2000. Genetic analysis of the Hybrid male rescue locus of Drosophila. Genetics 155: 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr, H. A., and S. Irving, 2001. Complex epistasis and the genetic basis of hybrid sterility in the Drosophila pseudoobscura Bogota-USA hybridization. Genetics 158: 1089–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr, H. A., and D. C. Presgraves, 2000. Speciation by postzygotic isolation: forces, genes and molecules. BioEssays 22: 1085–1094. [DOI] [PubMed] [Google Scholar]
- Prakash, S., 1972. Origin of reproductive isolation in the absence of apparent genic differentiation in a geographic isolate of Drosophila pseudoobscura. Genetics 72: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler, L., and E. Novitski, 1957. Meiotic drive as an evolutionary force. Am. Nat. 91: 105–110. [Google Scholar]
- Sawamura, K., T. Taira and T. K. Watanabe, 1993. a Hybrid lethal systems in the Drosophila melanogaster species complex. I. The maternal hybrid rescue (mhr) gene of Drosophila simulans. Genetics 133: 299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura, K., T. K. Watanabe and M.-T. Yamamoto, 1993. b Hybrid lethal systems in the Drosophila melanogaster species complex. Genetica 88: 175–185. [DOI] [PubMed] [Google Scholar]
- Sawamura, K., M.-T. Yamamoto and T. K. Watanabe, 1993. c Hybrid lethal systems in the Drosophila melanogaster species complex. II. The zygotic hybrid rescue (Zhr) gene of D. melanogaster. Genetics 133: 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer, S. W., and E. L. Miller, 1991. Nucleotide sequence analysis of Adh gene estimates the time of geographic isolation of the Bogota population of Drosophila pseudoobscura. Proc. Natl. Acad. Sci. USA 88: 6097–6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal, R. B., and F. J. Rohlf, 1981 Biometry. W. H. Freeman, San Francisco.
- Sturtevant, A. H., 1946. Intersexes dependent on a maternal effect in hybrids between Drosophila repleta and D. neorepleta. Proc. Natl. Acad. Sci. USA 32: 84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, Y., and D. L. Hartl, 2003. Genetic dissection of hybrid incompatibilities between Drosophila simulans and Drosophila mauritiana. III. Heterogeneous accumulation of hybrid incompatibilities, degree of dominance and implications for Haldane's rule. Evolution 57: 2580–2598. [DOI] [PubMed] [Google Scholar]
- Tao, Y., D. L. Hartl and C. C. Laurie, 2001. Sex-ratio distortion associated with reproductive isolation in Drosophila. Proc. Natl. Acad. Sci. USA 98: 13183–13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneault, G., and E. Zouros, 1986. The genetics of asymmetrical male sterility in Drosophila mohavensis and Drosophila arizonensis hybrids: interaction between the Y chromosome and autosomes. Evolution 40: 1160–1170. [DOI] [PubMed] [Google Scholar]
- Wang, R. L., J. Wakely and J. Hey, 1997. Gene flow and natural selection in the origin of Drosophila pseudoobscura and close relatives. Genetics 147: 1091–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, T. K., 1979. A gene that rescues the lethal hybrids between Drosophila melanogaster and D. simulans. Jpn. J. Genet. 54: 325–331. [Google Scholar]
- Wu, C.-I, and A. T. Beckenbach, 1983. Evidence for extensive genetic differentiation between the sex-ratio and the standard arrangement of Drosophila pseudoobscura and D. persimilis and identification of hybrid sterility factors. Genetics 105: 71–86. [DOI] [PMC free article] [PubMed] [Google Scholar]