Abstract
Determining the contribution of organelle genes to plant phenotype is hampered by several factors, including the paucity of variation in the plastid and mitochondrial genomes. To circumvent this problem, evolutionary divergence between maize (Zea mays ssp. mays) and the teosintes, its closest relatives, was utilized as a source of cytoplasmic genetic variation. Maize lines in which the maize organelle genomes were replaced through serial backcrossing by those representing the entire genus, yielding alloplasmic sublines, or cytolines were created. To avoid the confounding effects of segregating nuclear alleles, an inbred maize line was utilized. Cytolines with Z. mays teosinte cytoplasms were generally indistinguishable from maize. However, cytolines with cytoplasm from the more distantly related Z. luxurians, Z. diploperennis, or Z. perennis exhibited a plethora of differences in growth, development, morphology, and function. Significant differences were observed for 56 of the 58 characters studied. Each cytoline was significantly different from the inbred line for most characters. For a given character, variation was often greater among cytolines having cytoplasms from the same species than among those from different species. The characters differed largely independently of each other. These results suggest that the cytoplasm contributes significantly to a large proportion of plant traits and that many of the organelle genes are phenotypically important.
THE phenotype of a eukaryote is determined primarily, but not entirely, by its nuclear genome. In plants, literally thousands of nuclearly controlled phenotypes have been described in a wide variety of agronomic and nonagronomic species. The genes underlying these phenotypes are almost always inherited in a Mendelian fashion, so that it is possible to discover, investigate, and manipulate them in a fairly easy and straightforward manner. Genomes of plastids and mitochondria are also known to play a role in the growth, development, and well-being of a plant, but this role is generally presumed to be small or invariant. Despite the genetic simplicity of organelles, the actual extent of this role and what effect cytoplasmic genes have on plant phenotype is largely undetermined. The cytoplasmically associated variation that has been observed in plants is in fact limited, in part because organelle DNA sequence is very highly conserved within plant species and often within genera (Wolfe et al. 1987). Since plant organelles are in most cases strictly uniparentally inherited (Soliman et al. 1987; Reboud and Zeyl 1994), and thus not subject to Mendelian assortment, cytoplasmic effects are usually notable only in comparisons of reciprocal crosses. Furthermore, because multiple organelle genomes are inherited within each organelle, the effect that any new mutation might engender is typically not exposed because it is masked by dozens to thousands of nonmutant genomes.
Most described cytoplasmically inherited phenotypes are limited to the obvious, such as lack of greening—generally due to nuclear-plastid incompatibility (Kirk and Tilney-Bassett 1978)—or cytoplasmic male sterility (CMS)—exclusively associated with nuclear-mitochondrial incompatibility (Leaver et al. 1988; Hanson 1991). Plants that suffer lack of greening are, not unexpectedly, often subject to growth and development deficiencies, whereas plants that exhibit CMS, while failing to produce or shed functional pollen, are usually otherwise lacking in gross phenotypic abnormalities (e.g., Laughnan and Gabay-Laughnan 1983).
Additional cytoplasmically inherited effects, such as tissue-culture regeneration ability (e.g., Ekiz and Konzak 1991), pathogen resistance (e.g., Voluevich and Buloichik 1992), seed starch type (e.g., Pooni et al. 1993b), yield (e.g., Loessl et al. 2000), tolerance of cold (e.g., Hutton and Loy 1992) and heat (e.g., Shonnard and Gepts 1994), and high-salt (Hou et al. 2000) or low-water availability (e.g., Uprety and Tomar 1993), have been discovered sporadically in recent years. Many of these phenotypes were discovered through serendipity (e.g., nonchromosomal stripe 5; Newton et al. 1990) or through focused searches for specific traits (e.g., screens for cytoplasms conferring CMS; e.g., Isshiki and Kawajiri 2002). These methods tend to uncover only those mutations that have severe phenotypes. This severity allows for their detection, but can also preclude obtaining homoplasmic plants. Microarray analyses of the interaction of nuclear and cytoplasmic gene transcription have revealed that a subset of organelle genes is affected by various stresses, but these were not specifically associated with phenotypes (Yu et al. 2001).
An alternative approach takes advantage of the evolutionary genetic differences that exist between closely related taxa. Over evolutionary time, cytoplasmic genomes accumulate mutations and nuclear genomes accumulate compensatory mutations and vice versa. By juxtaposing nuclear and cytoplasmic genomes that are evolutionarily diverged and thus not subject to such mutual evolution, novel nuclear-cytoplasmic combinations can be created. These cytoplasmic “evolutionary mutations” might be expected to affect phenotype if incompatibilities exist between the products of the mismatched nuclear and organelle genomes. A popular way to accomplish this juxtaposition for many genes is through somatic hybridization (e.g., utilizing protoplast manipulation), either intragenerically, e.g., Nicotiana (Belliard et al. 1979) or intergenerically, e.g., Nicotiana and Petunia (Glimelius and Bonnett 1986). However, most such experiments that have been reported have involved at least one of several factors that limits their usefulness for precise investigations of nuclear-cytoplasmic interactions, such as mixed or recombinant organelle genomes (e.g., Kirti et al. 1995), CMS cytoplasms and their restorers (e.g., Wang et al. 1998), polyploidy (e.g., Berbec 2001), hybridity (e.g., Mumba and Galwey 1999), or considerable nuclear genome heterogeneity (e.g., Berbec 1994).
Standard serial backcrossing offers a possibly less expeditious, but easy and reliable, way to juxtapose the cytoplasmic genomes of one plant with the nuclear genome of another (Tsunewaki 1980; Allen et al. 1989). The nuclear genome of the cytoplasm donor can theoretically be replaced essentially completely by performing a sufficient number of backcrosses. Since plastids and mitochondria are strictly maternally inherited in most angiosperms and in all studied grasses (e.g., Conde et al. 1979; Soliman et al. 1987), the cytoplasmic genomes in each resulting generation will be derived solely from the individual plant rooting the maternal lineage, making the lines homoplasmic as well as alloplasmic. If the nuclear genome is well defined and homogeneous, phenotypic differences between the reference (native cytoplasm) and the alloplasmic plants will presumably be the result only of (evolutionary) differences between the native and alien organellar genomes. The relatively small evolutionary distances between taxa that can be juxtaposed by such backcrossing can be expected to limit the genotypic divergence of the organelle to a level that is usually short of lethal, but that can still provide a detectable phenotypic change. Unlike the situation encountered with new cytoplasmic mutations, any phenotypically important “mutation” uncovered by this technique would almost certainly be homoplasmic by virtue of its residence time in the donor species.
Regardless of the methodology utilized, minimal nuclear heterozygosity is desirable because segregating nuclear alleles can confound the interpretation of observed phenotype changes. This is most easily achieved through the use of inbred lines. In both maize and wheat, alloplasmic inbred lines have been utilized successfully to uncover nuclear-cytoplasmic effects. For example, in maize, plants and seeds having cytoplasms from certain Zea species are miniature (not dwarf) in the absence of nuclear genes that are present in teosinte and most maize inbred lines. This is referred to as teosinte-cytoplasm-associated miniature (TCM; Allen et al. 1989). In wheat alloplasmic lines, each of many characters observed, including fertility and growth and development, was found to have a significant cytoplasmic component to its expression, despite sometimes exceedingly limited sample sizes (e.g., Tsunewaki 1980). As with TCM, many of the changes were observed only with certain nuclear genomes, which underscores the nuclear component of nuclear-cytoplasmic interactions.
The fact that essentially all of the characters observed in the wheat study were subject to cytoplasmic control suggested that the suite of cytoplasmically influenced characters described to date is far from complete. Whereas the wheat studies were undertaken in the context of crop production, the studies reported here utilizing maize, a genetically well-characterized diploid organism, originated from a desire to expand our understanding of the extent to which morphological, developmental, and functional characters can be affected by, or are attributable to, the cytoplasmic genomes.
MATERIALS AND METHODS
Cytoline development:
Cytoplasm replacement lines that have the nuclear genome of a single maize inbred line (Z. mays ssp. mays inbred line A619) and the cytoplasmic genomes of the closest relatives of maize, the teosintes, which include all members of the genus Zea except maize, were created (Table 1). Cytoplasm donor information is presented in Table 2. Cytoplasm replacement was accomplished via standard backcrossing (Figure 1), which was possible because maize is interfertile with all other Zea taxa (Allen et al. 1989). Because the resulting lines are defined by their cytoplasms, they are termed cytolines. Cytoline development for inbred line A619 is essentially identical to that previously described for cytolines having an inbred line W23 nuclear genome (Allen et al. 1989). Where possible, for each accession utilized, replicate lines were generated from two teosinte plants (Table 2). A minimum of eight crosses by maize ensured that the teosinte component of the nuclear genome was (theoretically) reduced to <0.5%.
TABLE 1.
Zea taxonomy
| Section Zea |
|---|
| Zea mays |
| ssp. mays |
| ssp. mexicana |
| ssp. parviglumis |
| ssp. huehuetenangensis |
| Section Luxuriantes |
| Z. luxurians |
| Z. diploperennis |
| Z. perennis |
TABLE 2.
Cytoplasms used in this study
| Taxon | Collection | Planta | Cytotypeb |
|---|---|---|---|
| Z. mays ssp. mays | Inbred line A619 | 0 | |
| Z. mays ssp. mexicana R Nobogame | Galinat Nobogamec | G132 | 1 |
| Z. mays ssp. mexicana R Nobogame | Galinat Nobogamec | G133 | 1 |
| Z. mays ssp. mexicana R Nobogame | Galinat Nobogamec | 1 | |
| Z. mays ssp. mexicana R Chalco | Wilkes Amecameca | 2 | |
| Z. mays ssp. mexicana R Chalco | Wilkes Chalco | 2 | |
| Z. mays ssp. mexicana R Central Plateau | Wilkes 45121 | 4 | |
| Z. mays ssp. mexicana R Central Plateau | Wilkes 48703 | 3 | |
| Z. mays ssp. parviglumis | Wilkes 47890 | 4 | |
| Z. mays ssp. parviglumis | Wilkes 47335 | 5 | |
| Z. mays ssp. parviglumis | Beadle El Salado | 5 | |
| Z. mays ssp. parviglumis | Beadle Kato 2 and 3 | 5 | |
| Z. mays ssp. parviglumis | Guzman La Huert. | G120 | 6 |
| Z. mays ssp. parviglumis | Guzman La Huert. | G121 | 6 |
| Z. mays ssp. parviglumis | Wilkes 47711 | 4 | |
| Z. mays ssp. huehuetenangensis | Wilkes San Antonio Huista | 7 | |
| Z. luxurians | Wilkes 51186 | P181 | 8 |
| Z. luxurians | Wilkes 51186 | P182 | 8 |
| Z. luxurians | Iltis et al. G-36 | G105 | 9 |
| Z. luxurians | Iltis et al. G-36 | G106 | 9 |
| Z. luxurians | Galinat Hondurasc | G127 | 10 |
| Z. luxurians | Galinat Hondurasc | G129 | 10 |
| Z. luxurians | Mazotid | 11 | |
| Z. diploperennis | Guzman 777 | G112 | 12 |
| Z. diploperennis | Guzman 777 | G113 | 13 |
| Z. diploperennis | Iltis et al. 1190 | G114 | 14 |
| Z. diploperennis | Iltis et al. 1190 | G116 | 13 |
| Z. diploperennis | Iltis et al. 1250 | G117 | 12 |
| Z. diploperennis | Iltis et al. 1250 | G119 | 14 |
| Z. perennis | Beckett W23e | 15 | |
| Z. perennis | Beckett N6e | 15 | |
| Z. perennis | Wisconsin 1e | 15 | |
| Z. perennis | Wisconsin 2e | 15 | |
| Z. perennis | Iltis et al. 1050 | 16 |
Plant identification is given only where two plants per accession were utilized.
Cytotypes are based on mtDNA RFLP patterns (see text).
Teosinte propagated by W. Galinat before use in this study.
Via E. H. Coe; history obscure, but pollinated by maize for several generations.
From G. N. Collins and J. H. Kempton; all cytolines ultimately derived from the same teosinte clone.
Figure 1.—
Experimental design. Plants grown from teosinte seeds were pollinated with maize pollen. The resulting seeds were grown, and the hybrid plants were also pollinated with maize pollen. Continued backcrossing always used the plant tracing back to teosinte as female, ensuring that the progeny had teosinte cytoplasm. Thus, for each cytoline, all plants in the lineage trace back ultimately to a single teosinte seed. Twelve seeds from each plant in the penultimate generation were used to produce families of 10 progeny for observation (only plant-six pedigree is shown), except that for those cytotypes showing substantially elevated defective kernel proportions (cytotypes 8–13; see results), 10 sixth-backcross families were grown. The first five plants in each seventh-generation family were manually backcrossed to obtain ear and kernel data.
Experimental layout:
Data for each cytoline were obtained from 7 (Z. mays cytolines) or 10 (most Section Luxuriantes cytolines) replicate families. Each replicate family was grown from 12 seeds from a single, different ear; each of those 7 (or 10) ears was itself the progeny of a single ear harvested the previous generation (Figure 1). All families were planted in a total random array. Measurements were taken of the first 10 plants in each family (or all plants, if only 10 or fewer were present). The first 5 plants were hand pollinated with the standard inbred line A619 and the remaining ears were open pollinated.
Plant phenotypes:
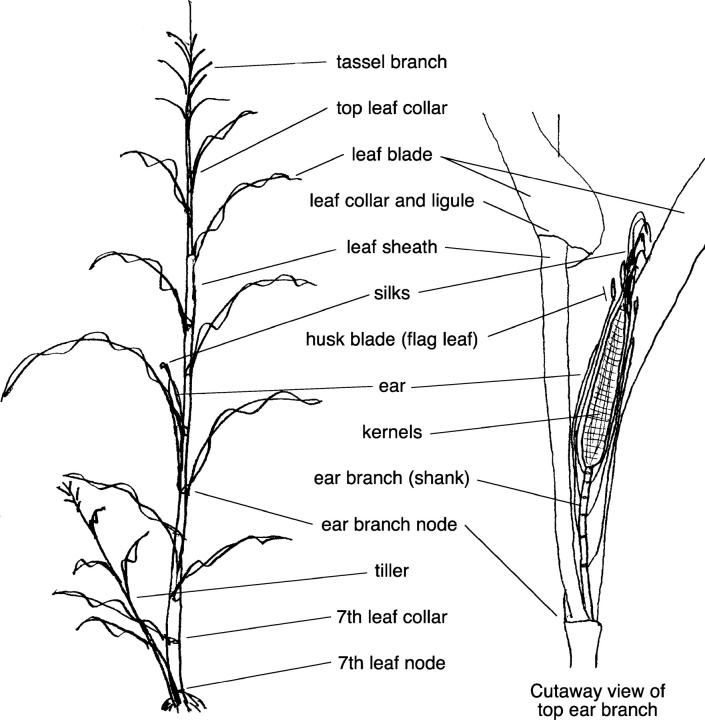
The characters studied were chosen to cover as many aspects of plant growth, development, and function as possible in the context of this study. In addition, all characters that appeared to be variable among cytolines in casual observations during cytoline development were observed in the study. Cytoline plants were observed for primary characters, such as plant height at various time points, time to developmental milestones, organ morphology, and male fertility (Table 3). Observation of developmental milestones continued only until 100 days postplanting, by which time >99.5% of the plants that should have reached these milestones had done so. Other characters, such as proportion of kernels that were defective or relative timing of events, were calculated from two or more of the observation data (Table 3). Husk blade length, which was particularly variable after silk emergence, was measured only at the time of silk emergence because at that time the ears of the first five plants in each family were trimmed (i.e., the ends of the husks were cut off) to facilitate hand pollination. Relevant plant parts are diagrammed in Figure 2. Not all characters are discussed in the text.
TABLE 3.
Characters observed and calculated in this study
| Observed characters | Calculated characters |
|---|---|
| No. of kernels germinating (per family) | Proportion of kernels germinating (per family) |
| 6-wk height (cm to top of visible leaf collar) | Proportion of 14-wk height at 6 wk |
| 8-wk height (cm to top of visible leaf collar) | Proportion of 14-wk height at 8 wk |
| 10-wk height (cm to top of visible leaf collar) | Proportion of 14-wk height at 10 wk |
| 12-wk height (cm to top of visible leaf collar) | Proportion of 14-wk height at 12 wk |
| 14-wk height (cm to top of visible leaf collar) | Ear height (ear branch node height + ear branch length) |
| Days postplanting to top of ear shoot emergencea (eo) | Ear length as a proportion of mature height |
| Days postplanting to top of leaf collar emergencea (tlc) | Shank length as a proportion of mature height |
| Days postplanting to first silk emergence (so) | No. of leavesb |
| Days postplanting to first pollen shed (ps) | Days between eo and tlc (eo → tlc) |
| Fertility (fertile, semifertile, sterile; see text) | Days between eo and ps (e → ps) |
| No. of primary tassel branches | Days between eo and so (eo → so) |
| No. of leaves above the top ear node | Days between tlc and ps (tlc → ps) |
| No. of leaves below the top ear nodeb | Days between tlc and so (tlc → so) |
| No. of tillers | Days between ps and so (ps → so) |
| No. of nascent ear branchesb | eo as a proportion of tlc (eo/tlc) |
| No. of mature ears | eo as a proportion of ps (eo/ps) |
| Husk blade length (cm; longest at time of silk emergence) | eo as a proportion of so (eo/so) |
| Height of ear branch node (cm) | tlc as a proportion of ps (tlc/ps) |
| Ear length (cm) | tlc as a proportion of ps (tlc/ps) |
| Ear branch (shank) length (cm to base of mature ear) | ps as a proportion of so (ps/so) |
| Plant lodging (none, <45°, >45°) | Proportion of normal kernels |
| Ear tip emergent from husk (+/−) | Proportion of mildly defective kernelsc |
| No. of normal kernels | Proportion of severely defective kernelsc |
| No. of mildly defective kernelsc | No. of kernels on the top ear |
| No. of severely defective kernelsc | Proportion of normal kernels on parent ear |
| No. of normal kernels on parent ear | Proportion of mildly defective kernelsc on parent ear |
| No. of mildly defective kernelsc on parent ear | Proportion of severely defective kernelsc on parent ear |
| No. of severely defective kernelsc on parent ear | No. of kernels on parent ear |
Underlining indicates data that are presented and discussed in this article. Italic indicates data that are discussed in this article but presented in supplemental figures.
From under the ligule at time of top ear silk emergence.
Above the sixth node (see Figure 2).
Defective in endosperm production: mildly defective kernels had at least some pericarp wrinkling, and severely defective kernels had no smooth (attached) pericarp.
Figure 2.—
Relevant parts of the maize plant. Both illustrations show the plant shortly after silk emergence. The plant's morphology changes little after that point, with the exception of substantial growth of the ear and its associated structures, such as the husk. The first six leaf nodes are underground and below the roots illustrated.
RFLP analyses:
mtDNA was prepared, digested with XhoI and BamHI, and electrophoresed (Conde et al. 1979) on 20-cm agarose gels at 40 V for either 18 or 28 hr to resolve either short (50–5000 bp) or long (500–20,000 bp) digestion fragments. The fragment profiles of the cytolines were grouped into cytotypes on the basis of shared mobility patterns. Profiles for all cytolines composing a cytotype were identical, except for Z. mays cytotypes 2, 4, 5, and 7.
Data pooling:
All cytolines initially were analyzed individually. Cytolines having a single mitochondrial RFLP type, which constituted a cytotype (J. O. Allen, unpublished results), were statistically indistinguishable (not shown), which allowed the data for all of the cytolines of each cytotype to be pooled. (For cytotype 9 some of the characters were divergent; see results.) Therefore the results for the 33 cytolines are presented for the resulting 16 cytotypes (Table 2). In most cases, results are presented graphically as the proportional difference of the cytolines from the recurrent parent inbred line, A619. Standard error bars are included when they are not too small to be discerned in the figure.
ANOVA tables were calculated with Statview 512+ (Brainpower) using Scheffé criteria for determining levels of significance. Correlations were calculated for those character pairs for which they would be meaningful. Principal components analyses were conducted using SAS (SAS Institute).
RESULTS
Cytoplasm replacement lines that have the nuclear genome of a single maize inbred line (Zea mays ssp. mays inbred line A619) and the cytoplasmic genomes of maize's closest relatives, the teosintes, were created. Teosinte includes all members of Zea except maize. This genus is divided taxonomically into Section Luxuriantes, which contains the annual Z. luxurians and the perennials Z. diploperennis and Z. perennis, and Section Zea, which contains the single annual species Z. mays (Table 1). Z. mays is further divided into four subspecies: mexicana, parviglumis, and huehuetenangensis—which are also teosintes—and mays, the cultigen maize (Doebley and Iltis 1980; Iltis and Doebley 1980).
Section Zea cytolines:
Cytolines possessing cytoplasmic genomes from Z. mays teosintes were not significantly different in phenotype from the maize reference inbred line for all but two of the characters observed or calculated: seedlings were shorter at 6 weeks in three cytotypes (ρ < 0.01) and the number of tassel branches was reduced in two (ρ < 0.02).
Section Luxuriantes cytolines:
In contrast, substitution with cytoplasmic genomes from Section Luxuriantes teosintes yielded cytolines that expressed myriad differences in phenotype. Almost all of the characters were significantly different from the inbred line in at least one cytotype. Furthermore, in six of the eight cytotypes most of the characters were significantly different from the inbred line. Data are presented here or in supplemental figures at http://www.genetics.org/supplemental/ for only a subset of the 58 characters (see Table 3).
Plant characters
Growth rate:
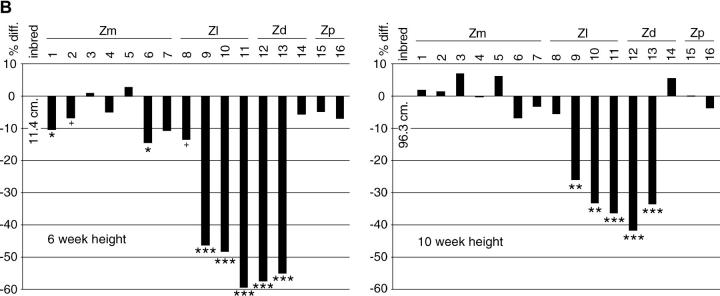
Growth retardation was one of the most obvious effects of alien cytoplasmic genomes and was greatest at early stages (Figure 3). At 6 weeks postplanting, average plant height was reduced 50–60% in Z. luxurians cytotypes 9, 10, and 11 and in Z. diploperennis cytotypes 12 and 13. Plants of the other cytotypes were almost as tall as those of inbred line A619. At later time points (e.g., at 10 weeks) the retardation was less pronounced, and by maturity the height differences were not significant.
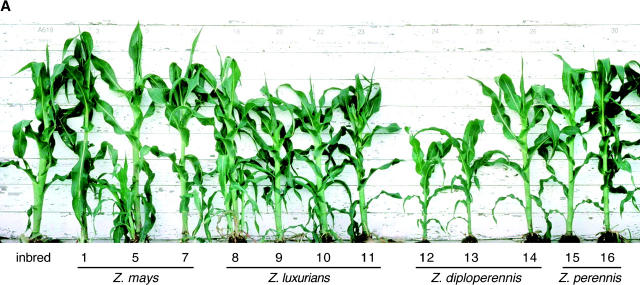
Figure 3.—
Plant height. (A) Plants at 10 weeks postplanting. These plants were grown expressly for photographic purposes and thus the heights do not necessarily match the data in the 10-week height graph. Numbers indicate cytotypes (see Table 2). (B) Average plant heights at 6 and 10 weeks postplanting. Data are shown as the proportional difference from the reference inbred line (A619). The actual value for the inbred line is given under “inbred line” on the chart. There is much-reduced growth in most Z. luxurians and Z. diploperennis cytotypes, but not in Z. perennis and Z. mays cytotypes. The scale is the same for both graphs. Standard errors are too small to be seen in the figure. Cytotypes are labeled below their cytoplasm donor species; Zm, Z. mays; Zl, Z. luxurians; Zd, Z. diplolperennis; Zp, Z. perennis. Statistical significance is indicated as appropriate (***ρ < 0.0001, **ρ < 0.001, *ρ < 0.01, +ρ < 0.02).
Development:
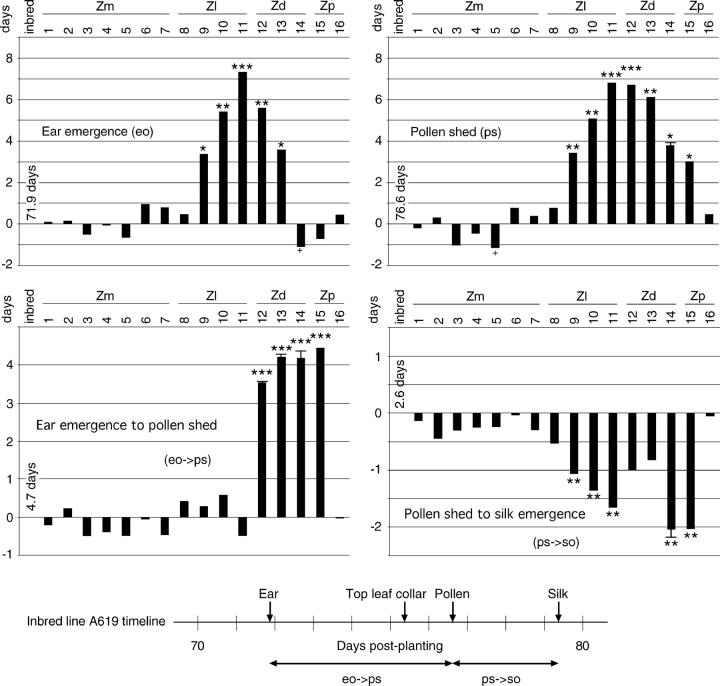
The five cytotypes exhibiting slow growth also exhibited slow development, but the delays in development did not parallel the retardation in growth (Figure 4 and supplemental Figure 1 at http://www.genetics.org/supplemental/). Furthermore, pollen shed was affected in two cytotypes not affected for the other characters. Delays were highly variable, ranging from less than a day to more than a week. The four Z. luxurians cytotypes were markedly different from each other, but consistent from character to character. In contrast, the Z. diploperennis cytotypes and Z. perennis cytotype 15 varied independently both among cytotypes and among characters.
Figure 4.—
Difference from the inbred line to two developmental milestones in two times separating developmental milestones (in days). The developmental time line for the reference inbred line is shown at the bottom. Scales are not all the same. Standard error bars are included where they are large enough to be seen. See Figure 3 for general information.
Differences in the times between pairs of developmental milestones might have been expected to parallel those for the four developmental milestones themselves, but none of those six characters did (Figure 4 and supplemental Figure 1 at http://www.genetics.org/supplemental/). In contrast to both growth and development characters, for three of the spacing characters none of the Z. luxurians cytotypes was different from the inbred line, whereas the Z. diploperennis and Z. perennis cytotypes were very different.
Tassel branches:
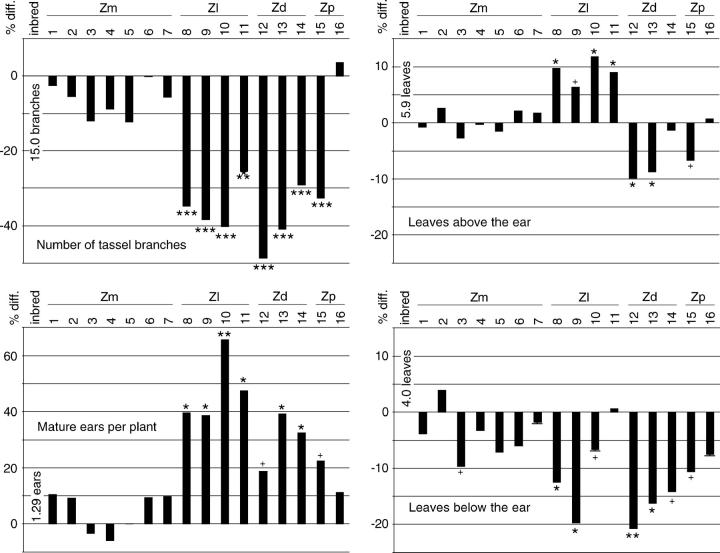
A maize plant from an inbred line typically has a narrowly defined number of organs or plant parts that it produces. Inbred line A619 plants had an average of 15 ± 3 tassel branches, including the central spike (Figure 5). In cytolines having any of the cytoplasms, the number of tassel branches was only 50–70% of the inbred line number, or ∼9 branches. The alteration in this character was unusual in that it was similar among all of the Section Luxuriantes cytolines.
Figure 5.—
Proportional difference from the inbred line in numbers of plant parts. “Number of tassel branches” includes only primary tassel branches. “Mature ears per plant” is equivalent to “branches per mature plant,” since each branch had an ear. Scales are the same for both leaf graphs. See Figure 3 for general information.
Ears and ear branches:
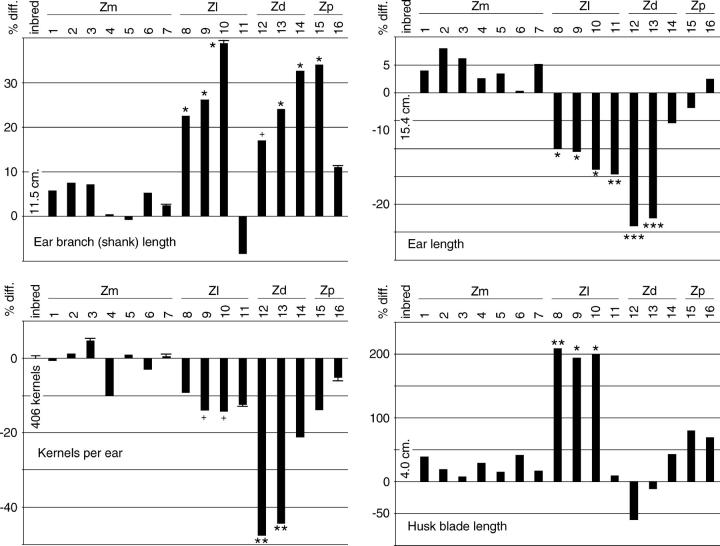
Maize ears develop as the termini of ear branches, with the top ear branch normally developing the largest ear. Corn-belt maize inbred lines universally have only one developed ear per ear branch, but all branches have an ear. In the cytolines the average number of branches per mature plant was higher than that per inbred line plant, largely because a greater proportion of second, third, and fourth ear branches proceeded through development (Figure 5). Only 13% of Z. luxurians cytotype 10 second-ear branches aborted, and the highest proportion among the cytolines was 45% in Z. diploperennis cytotype 12. However, in the inbred line 66% of second-ear branches aborted, and no plants had more than two mature ears. Interestingly, the greater the number of nascent ear branches (above one), the lower the proportion that aborted. In addition to producing more branches, occasionally plants with Z. luxurians cytoplasm had two, and in two cases three, ears on a single branch. Multiple mature ears were never observed on a single branch of any of several thousand healthy inbred line A619 plants. The increase in the number of ears in Z. luxurians cytolines, in particular, more than made up for the decrease in the number of kernels per ear (see below), so that the plants produced more seed than ears in the inbred line.
Cytoline ear branches were longer than those in inbred line A619 in all cytotypes except cytotype 11 (Figure 6). However, although the ear is the terminal extension of the ear branch, cytoline ears were shorter and had fewer kernels than inbred line ears (Figure 6).
Figure 6.—
Proportional difference from the inbred line in sizes of plant parts and in kernels per ear. Scales are all different. See Figure 3 for general information.
Leaves:
Inbred line A619 had an average of 10 ± 0.5 leaves above the sixth node (see Figure 2); six leaves were above the top ear node and four were below. In all Section Luxuriantes cytolines the number of leaves below the top ear node was reduced (Figure 5). In Z. diploperennis and Z. perennis cytolines the numbers above the ear were also reduced, leading to plants with fewer total leaves. However, in Z. luxurians cytolines there were more upper leaves and, as a result, even though the plants were shorter, the total number of leaves remained roughly unchanged from the reference inbred line.
Ear morphology:
Perhaps the most striking differences occurred in organ morphology. But they were also the most difficult to quantify. The most pronounced changes were observed in the ear, the organ that most defines the difference between maize and teosinte. Not only were the cytolines distinctly different from the inbred line, but also the annual teosinte (Z. luxurians) cytolines were markedly different from the perennial teosinte (Z. diploperennis and Z. perennis) cytolines (Figure 7B, Table 4). Occasionally the tips of Z. diploperennis and Z. perennis cytoline ears were so tight and tough that the ears were not able to extrude silks, and the plants were thus functionally female sterile. The blades of Z. luxurians husks were very long (Figure 6) and continued to grow well after silk emergence (unlike maize husk blades), with some eventually exceeding 60 cm in length (Figure 7, B and C). The blades were also unusual in being longitudinally ridged and oriented along the axis of the ear branch rather than angling away from it, as in normal A619 ears. Curiously, Z. luxurians cytotype 11 was indistinguishable from the reference inbred line for most ear characters.
Figure 7.—

Reproductive-structure morphology. Phenotypes were consistent within a taxon, with the exception of cytotype 11 (see Table 4). (A) Tassels 2 days after the onset of pollen shed, except for Z. diploperennis, which never shed pollen. Sterile branches are thin because anthers have not exserted. (B) Ears the day after the onset of silk emergence. Z. diploperennis and Z. luxurians ear branches are longer than Z. mays ear branches and are angled away from the stalk. Z. diploperennis husks are pointedly conical with virtually no blades. Z. luxurians husks are cylindrical with long blades that hide the silks from view. Z. mays plant is unusual in having two fully developed ears, whereas the other two cytotypes are typical in having at least that many. (C) Example of very long husk blades (flag leaves). Cross section of ears showing tight packing of Z. diploperennis husks. Both ears were cut at the very tip of the cob, seen as white at the center of each ear. Contemporary photo of Z. luxurians ear was not taken.
TABLE 4.
Ear and ear branch characters
| Cytotypea
|
|||
|---|---|---|---|
| Character | Z. mays | Z. luxurians | Z. diploperennis, Z. perennis |
| Ear branch angleb,c | Small (∼5°–10°) | Large (∼15°–20°) | Large (∼15°–20°) |
| Branch length | Short | Long | Long |
| Ears per branch | One | One or more | One |
| External ear shapeb,c | Bullet | Cylindrical | Conical |
| Silk productionb | Normal | Normal | Reduced |
| Husk/silk packingb,c | Intermediate | Loose | Tight |
| Husk blade lengthc | Intermediate | Very long | Short |
Z. mays: A619, cytotypes 1–7; Z. luxurians: cytotypes 8–10; Z. diploperennis, Z. perennis: cytotypes 12–15; Z. luxurians cytotype 11 and Z. perennis cytotype 16 had Z. mays morphology.
Not quantified.
See Figure 7.
Fertility:
Inbred line A619 sheds functional pollen well under most conditions, although occasional plants are less than fully prolific; 88% of A619 plants shed pollen at a normal level and 12% shed reduced amounts during the season this study was conducted. Z. mays cytoline plants were similar: 90.0% were fully fertile, 9.6% were partially fertile (reduction visually apparent without quantification), and 0.4% were sterile (no detectable pollen). In contrast, only 4% of Z. diploperennis cytotype 13 plants were fully fertile and 75% were completely male sterile (Figures 7A and 8). Furthermore, as noted above, many of the plants failed to extrude silks, which rendered them functionally both male and female sterile. In the most affected Z. luxurians cytotype (cytotype 9), 30% of the plants were fully fertile and 11% were completely sterile.
Figure 8.—
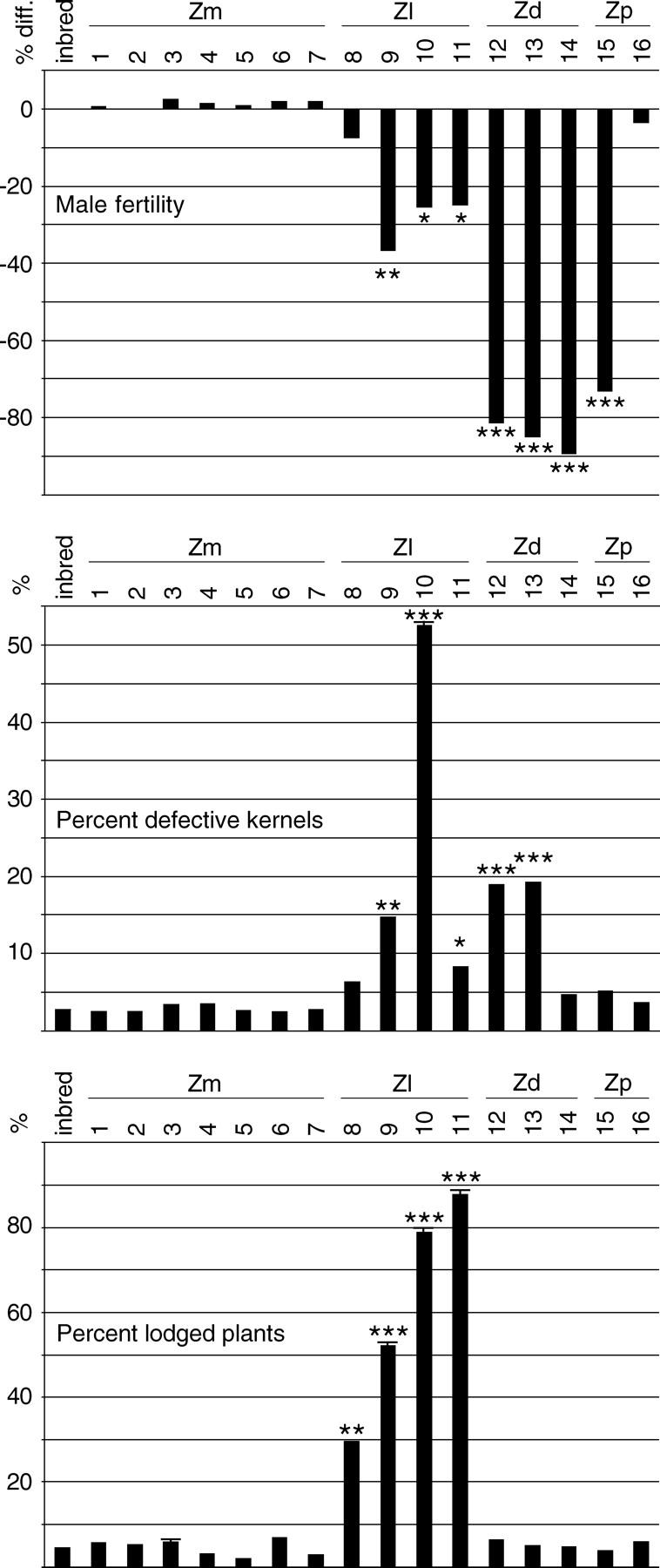
Male fertility, kernel defects, and plant lodging. Fertility was determined as a function of pollen quantity and is shown as the proportional difference from the reference inbred line. Z. diploperennis and Z. perennis cytoplasms had a severe effect on male fertility but essentially none on resistance to lodging, while the reverse was true for Z. luxurians cytoplasm. “Percent defective kernels” is the sum of the proportion of mildly defective and severely defective kernels (see Table 3). See Figure 3 for general information.
The viability of the pollen that was shed did not appear to be affected. Observations with a field microscope did not detect excess abnormal pollen, and crosses using plants shedding minimal pollen were successful (see below). However, even for plants that were classified as fully fertile, the total amount of pollen produced per plant was reduced because the number of tassel branches was reduced.
Cytotype 3 (Z. mays ssp. mexicana, Wilkes 48703) has mitochondrial DNA identical at the RFLP level to subtype M of a common cytoplasmic male sterile cytoplasm, CMS-S, yet is completely fertile and indistinguishable in that regard from the other Z. mays cytotypes. Thus it is not the unusual organization of its mtDNA that confers male sterility to the CMS-S cytotype.
Endosperm production:
Seed development was also affected, sometimes dramatically. Normal inbred line ears have occasional defective kernels that are completely flat and contain no endosperm; inbred line A619 had ∼3% such kernels. In Section Luxuriantes cytolines, rather than kernels being either fully normal or fully defective, there was a continuum of affectedness, from fully normal to completely defective (Figure 9), and a larger proportion of kernels was affected. All the cytolines were affected, but the most affected were cytotypes 9, 12, and 13, with about six times as many affected kernels (∼20%; Figure 8), and cytotype 10, in which more than half of the kernels were defective for endosperm production. Despite the defects in endosperm production in all of the Section Luxuriantes cytolines, the embryos in all kernels, except those totally lacking endosperm, appeared to be viable, indicated by the fact that almost all of the kernels tested, regardless of size, germinated under favorable laboratory conditions (data not shown).
Figure 9.—

Defective endosperm production. (A) Severely and mildly affected ears from Z. luxurians cytotype 10 (upper two ears) and from cytotype 9. Only about a dozen normal kernels are visible on the top ear. (B) Close-up of mildly affected cytotype 10 ear.
Lodging:
Under wet soil conditions, strong winds can dislodge a maize plant, causing it to tilt or even fall over completely, both of which constitute lodging. It usually does not kill the plant, but can retard its growth and development and make the ears difficult to harvest. Conditions that induce lodging were encountered during this study. Only 4% of inbred line A619 plants lodged, a proportion similar to Z. diploperennis and Z. perennis cytolines (Figure 8). In the presence of Z. luxurians cytoplasm, even in the best of the cytotypes, 30% of the plants lodged, and in the worst case, cytotype 11, almost 90% of the plants were affected. The lodging occurred after most of the plants had reached their full height and the four developmental milestones, so it did not affect the data for most of the characters observed.
Cytotype differences are not due to the nuclear genome
Reciprocal crosses:
At the time that the phenotype measurements were taken, each of the cytolines had been crossed by maize a minimum of eight times. Theoretically this would have reduced the residual nuclear teosinte genome to <1% (1/28 < 0.004). To check for residual nuclear teosinte genes that might nevertheless contribute to the observed phenotypic effects in the present study, crosses in the reverse direction were performed; i.e., inbred line A619 was used as the female parent and plants from each Z. luxurians and Z. diploperennis cytotype were used as pollen parents. Progeny from these crosses were grown in two successive growing seasons and observed as in the main study. The characters that were quantified—plant height at four time points, times to reach the four developmental milestones, defective endosperm development, and male fertility—were chosen because of the large effects seen in them in the main study. These reciprocal-cross progeny were indistinguishable from the inbred line for each of the characters (ρ > 0.05; data not shown). Unquantified observations of other, obvious characters such as husk morphology, tillering, or tassel branch number also did not uncover differences between these plants and the reference inbred line.
Lack of segregation:
In the main study, either 7 or 10 replicate families for each cytoline were used. Each replicate used seed from a different ear, and each of those ears was derived the year before from the single ear that was part of the backcrossing lineage. There were no significant differences between replicate families (not shown); i.e., there was no indication of alleles segregating among ears. In addition, there was no indication of bimodality in the data on individual families that could have been indicative of alleles segregating among plants.
Lack of selection:
Seed set was full, with no indication of selection against segregating alleles. In those cytolines in which defective endosperm was present in any ears at levels >10%, plants from defective kernels were compared to plants from normal kernels, as for the reciprocal-cross tests. With the exception of delayed growth resulting from slow germination, plants from defective kernels were indistinguishable from those grown from normal kernels (data not shown).
Chromosome number:
Root-tip cells from all four cytoline species had the expected 10 chromosome pairs of the inbred line A619 paternal parent, discounting the possibility of teosinte supernumerary chromosomes. This includes Z. perennis cytolines, which were derived from a tetraploid. Chromosomes were not identified other than by approximate length.
RFLP:
Perhaps the most important evidence is the correspondence of genotype and phenotype. Cytolines having different mitochondrial DNA restriction fragment length polymorphism (mtDNA RFLP) patterns, i.e., different cytotypes, had substantially different overall phenotypes. On the other hand, independently derived cytolines having the same mtDNA RFLP patterns were, with the one exception discussed below, statistically indistinguishable for almost all characters.
From these results it is concluded that remnant teosinte nuclear genes, at least in conjunction with maize cytoplasm, contributed insignificantly, if at all, to the changes in phenotype observed in the cytolines. The changes in phenotype observed in this study are therefore solely a result of the change of cytoplasm.
Of the two cytolines constituting Z. luxurians cytotype 9, more often than expected by chance, one of them was similar to cytotype 8 while the other was similar to cytotype 10. This association was borne out in cluster analyses and principal components analysis on individual cytolines (not shown). The cytotype assignments are correct, as indicated by mitochondrial typing, cytotype associations, and the fact that the relationships among the phenotypes were consistent in an identical study conducted at the same time and location utilizing inbred line W23 (Allen 1992). Plastid types are currently being characterized.
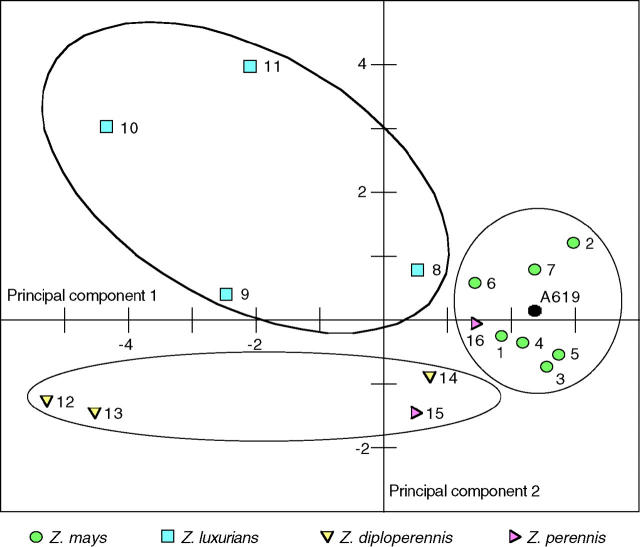
Principal components analyses
The array of changes and the seeming incongruities among cytotypes prompted the question of whether there was order or consistency in the way in which the nuclear genome interacted with the various cytoplasms and, in particular, if any such consistency followed taxonomy. This was addressed using principal components analysis on nonautocorrelated characters. The first two principal components accounted for 57% (37 and 20%, respectively) of the variation. In a plot of the first two principal components, Z. mays cytolines formed a distinct cluster around the inbred line (Figure 10), but their arrangement did not correspond to subspecific taxonomy. Rather, the subspecies were interspersed. Z. luxurians cytotypes formed a dispersed, yet still discrete, group that ranged from very maize-like to quite un-maize-like, but did not overlap with any other group. The two perennial teosintes formed an overlapping group composed of two pairs of cytotypes. Z. perennis cytotype 15 and Z. diploperennis cytotype 14 were adjacent and near to the Z. mays cluster, whereas the other two Z. diploperennis cytotypes fell together as about the least maize-like cytolines on the plot. Z. perennis cytotype 16 lay solidly within the Z. mays cluster (see below).
Figure 10.—
Plot of the first two principal components in analyses of non-auto-correlated characters by cytotype. Principal components 1 and 2 accounted for 37 and 20% of the variance, respectively. Cytoplasm species clusters are circled. Z. mays cytotypes form a tight, discrete group with the inbred line at its approximate center. Cytotypes from Z. luxurians and from the perennial teosintes form another two dispersed but discrete groups.
If the perennial teosintes are treated as a single taxon (see below), all of the cytotypes except 16 were parts of nonoverlapping groups that corresponded to the species from which the cytoplasms were derived. Z. perennis cytotype 16 lies at the edge of the Z. mays cluster nearest to the Z. perennis cytotype 15. This cytoplasm has been shown to have a Z. mays plastid genome (Doebley 1989). Thus its placement within the Z. mays cluster is not particularly surprising.
The distribution indicates an underlying species-specific consistency to the variation observed, despite occasional apparent phenotypic alliances between species rather than within species. These unifying similarities as well as the discriminating differences can be seen in more detail in comparisons of the character summaries for each cytotype (supplemental Figure 3 at http://www.genetics.org/supplemental/).
DISCUSSION
It has long been known that the cytoplasmic genomes of plants (plastid and mitochondrial) play a role in plant phenotype. For example, cytoplasmic male sterility is a common cytoplasmically controlled character that is widely utilized in breeding programs. Other phenotypes such as chlorosis, salt tolerance, and tissue-culture regeneration ability have been individually reported to have cytoplasmic components. However, despite a wide variety of reports detailing the individual contributions of the cytoplasm to plant phenotype, it is still commonly assumed operationally that cytoplasmic genes contribute minimally to most plant characters. It has been difficult to rigorously test this assumption because of the highly conserved nature of plant cytoplasmic genomes, the presence of numerous copies of those genomes within cells, and their (usually) strictly uniparental inheritance. In the present study, the cytoplasmic genomes of maize inbred line A619 were replaced with those of the entire range of teosintes, the closest relatives of maize, to yield cytolines defined solely by their cytoplasms. This approach used the otherwise problematic uniparental inheritance as an advantage in that the replaced cytoplasms were homoplasmic for both plastids and mitochondria and utilized the evolutionary divergence between maize and the other Zea taxa as a source of genetic variation in the organelle genomes. The decision to backcross the cytoplasm into an inbred line was driven by the desire to minimize the contribution of segregating or interacting nuclear alleles. Thus the cytoplasm is the only factor differentiating the cytoline and the inbred line.
The only truly comparable studies have been carried out using wheat, where a considerable cytoplasmic contribution to phenotype was detected. Triticum/Aegilops is an old, geographically widespread and species-rich genus pair; because Zea is a young, geographically limited, and species-poor genus (Doebley and Iltis 1980), the effects were expected to be few and generally subtle. Few and subtle was indeed the result for cytolines having cytoplasms from Z. mays teosintes; they were almost completely indistinguishable from the inbred line itself. In contrast, despite the relative youth of the genus, cytolines having cytoplasms of teosintes from Section Luxuriantes (Z. luxurians, Z. diploperennis, and Z. perennis) exhibited extensive divergence from the reference A619 maize inbred line, and the effects were varied and diverse.
Many characters are affected:
The characters studied were chosen to cover as many aspects of plant growth, development, and function as feasible, including rates of growth and development, form, profligacy, vigor, and function. Regardless of the type of character observed, the cytoplasm exchanges led to an abundance of phenotypic differences. All but one of the 27 characters reported here, as well as virtually all of those that are not, differed significantly from the reference inbred line in at least one nuclear-cytoplasmic combination. In fact, most characters were affected in a majority of the eight cytotypes. These results are consistent with those reported for wheat, the most-studied organism, where most of >20 characters were also significantly altered (e.g., Endo 1980; Tsunewaki et al. 1980). The majority of those characters were analogous to those observed in this study, although the way or the degree to which they were altered was not necessarily the same as in this study. Furthermore, the current study has uncovered more cytoplasmically influenced phenotypes, either in absolute numbers or as a proportion of observed characters, than any other study reported to date. This is in part because more characters were observed, but is also probably an increase in resolution due to the larger sample sizes utilized, which ranged between 70 and 270 plants per cytotype, vs. the 2–30 plants per nuclear-cytoplasmic combination that were observed in the wheat studies.
Of course, some of these differences were not unexpected. Given that the organelle genomes of the cytolines are evolutionarily diverged from those that they replaced and are assumed to be a less optimal match to the maize nuclear genome, it was anticipated that characters such as growth rate would be negatively affected. Mazoti (1954) observed that maize into which teosinte cytoplasm had been substituted had slower growth and development. Consistent with this, growth was retarded in several Z. luxurians and Z. diploperennis cytotypes. However, it is also significant that retarded growth was not a necessary consequence of such cytoplasmic substitution, in that some cytolines within each of the three species were not significantly shorter, despite significant changes in other characters, such as tassel branch number. Thus, at least some of the effects are not simply the result of general incompatibility or simple epistatic effects.
Other characters were also negatively affected, resulting in plants that were, for instance, less fertile or less resistant to lodging. But again this cannot be taken as an indication of simply a general nuclear-cytoplasmic incompatibility, since each of those characters varied independently among cytotypes and was not necessarily associated with other negative effects, such as delays in either growth or development. In fact, many of the changes did not involve negative effects on the plants at all. There were characters for which the changes were seemingly neutral, such as husk blade length, which was longer in Z. luxurians cytolines and shorter in Z. diploperennis cytolines. Some were even positive, including greater ear branch initiation and longer ear branches. This last pair of characters is in contrast to what has been observed in wheat, where shorter plants were associated with both shorter ear branches and shorter husk blades (flag leaves; Tsunewaki 1993). Although it is not known if those characters were altered in wheat simply as a result of general incompatibility, it is clear that such is not the case in the Zea cytolines.
The cytoplasmic genomes appear to affect not just a few plant characters, but most of the ones that could be observed. Furthermore, it appears unlikely that all of the characters whose expression is affected by the cytoplasmic genomes have been discovered. The fact that virtually all of the 58 characters in this study were affected suggests that other characters remain to be discovered even within this experimental system. For instance, characters such as salt tolerance, heat tolerance, and disease resistance, which have been reported to have a cytoplasmic component in other systems, depend on specific conditions to be observed, and those conditions were not present in this study. The variation in resistance to lodging, which requires strong winds when soils are very wet and was discovered serendipitously in this study, is an example of such a trait. Other nuclear genomes will also undoubtedly reveal many additional phenotypes. Preliminary backcrossing of these cytolines with other inbred lines has revealed several new phenotypes, including prodigious tillering with certain cytoplasm types (J. O. Allen, personal observation). There are also considerable effects of different environments and different nuclear genomes on the expression of some of the traits in this study (J. O. Allen, unpublished results).
On the other hand, other obvious morphological and developmental phenotypes, such as chlorosis, stunted plants, necrosis, and the elevated generation of haploids, have been observed in other systems and attributed to nuclear-cytoplasmic incompatibility. These have also been observed in maize, and the first three have examples there that are cytoplasmically determined. They would have been observed had they occurred here, but they were not. That the plants in those studies carry essentially the same array of plastid and mitochondrial genes as are in the Zea organelle genomes utilized here again highlights the large number of nuclear-cytoplasmic interactions that are possible.
While it is true that mitochondrial genomes can have large effects on suites of characters, such as flower morphology, these effects are typically the result of alterations in the regulation of regulatory genes. The resulting phenotypic changes, in the case of flower morphology, tend to be global alterations in flower composition (Kubko 2004). Almost all of the characters in the current study varied significantly, and, of critical importance, most of them did so independently of the other characters. This is not particularly consistent with only a few organelle genes acting in a global manner. In addition, the nuclear genome is that of the homogeneous inbred line A619, and thus it cannot account for differences among characters or cytolines. Rather, these results suggest that there are many phenotypically important genes among the ∼60 protein-coding genes in the organellar genomes whose interactions with the nuclear genome, or with the genome of the other type of organelle, are varied and extensive.
The effects of cytoplasmic substitution can be substantial:
Fifty-eight characters were observed or calculated in this study. More than 90% were different at a significance of at least ρ < 0.01 in at least one cytotype and more than half at ρ < 0.0001. The height reductions are in the latter category and are similar to those observed in a variety of studies on cytoplasmic substitution in two other grasses, wheat and rice (e.g., Tsunewaki 1980, 1993; Panayotov 1983). A similar effect was reported for TCM, which occurs with these same Section Luxuriantes cytoplasms in another nuclear background (inbred line W23; Allen et al. 1989). In TCM, in the absence of a rectifying nuclear allele of the Rcm locus, the plants may attain only a small fraction of their normal height, even at maturity. This is similar to the effects of scs and Vi in wheat cytolines, where extreme lack of vigor occurs in the absence of rectifying nuclear alleles (Maan 1992). However, in a sampling of six diverse inbred lines no nuclear genes were found that affected the reduced growth rate in these A619 cytolines (data not shown). Extreme growth reduction has also been observed in dicots, an example being the “stunted” phenotype in Capsicum alloplasmic lines (Inai et al. 1993).
As is often the case, some of the most striking effects of nuclear-cytoplasmic interaction involved reproduction. Negative effects on male fertility are common in plants, as they were here. More unusual were the major effects on female reproductive structures. Whereas only ∼3% of the kernels on inbred line A619 ears were defective, >50% were affected in the most-affected cytotype, Z. luxurians cytotype 10 (and as many as 99% on some ears). The degree of defectiveness on progeny ears was not correlated with that on the parent ear, but only with the particular cytoplasm that the plants carried. Thus the average level of defectiveness in progeny ears was predicted by the average level of defectiveness among the population of parent ears. The nature of this trait thus appears to be stochastic, as is seen in other mitochondrially associated mutants, such as NCS6, which also involves defective endosperm production (Lauer et al. 1990). The kernel defects in the cytolines were also like those of NCS6 in that they did not detectably affect the embryo. This is distinct from the case with nuclear defective kernel mutants, which are almost always embryo lethal in planta (Neuffer and Sheridan 1980; Sheridan and Neuffer 1980; Neuffer et al. 1996). It may be that the level of kernel defectiveness in the cytolines is related to the number of organelles that a given kernel inherits. In keeping with that idea, very few mitochondria were obtained from liquid-stage kernels that were destined to be severely defective, whereas they were abundant in normal kernels from the same ear (J. O. Allen, personal observation).
In interspecific crosses in some other plant systems, such as sunflower or soybean, the alien genomes were sufficiently incompatible with the nuclear genome to entirely preclude sexual reproduction or even to prove lethal (Jan 1992; Palmer and Minor 1994). Although the effect of the cytoplasmic genomes on phenotype was substantial for most of the characters in Zea, all of the cytoplasms were still sufficiently compatible with the maize nuclear genome that cytolines that were fully viable in the field as well as reproductively competent, albeit only unisexually in some cases, could be developed.
Does teosinte cytoplasm make a maize plant teosintoid?
Because these alien cytoplasms came from teosinte, it is reasonable to ask if the cytoplasms made the cytoline plants at all teosintoid. The primary differences between maize and teosinte are the presence of the ear and the prevalence and form of lateral branches on which inflorescences are found (Wilkes 1967; Iltis 1986; Doebley et al. 1997). These defining features of maize are attributed in most part to five linkage groups in the nuclear genome (Beadle 1980; Doebley and Stec 1991).
The cobs and kernels of cytoline plants were thoroughly typical of maize ears, and thus for them the answer is no. However, the ear is such a “monstrous or teratological development” (Hackel 1890) or “hopeful female tassel monster” (Gould 1977), governed by strongly dominant alleles (Doebley and Stec 1993), that any cytoplasmic contribution may well be completely overwhelmed by nuclear factors, even in this system.
The external inflorescence morphology, such as of husks, of Z. diploperennis and Z. perennis cytolines was even more unlike teosinte than maize was, and the plants had fewer, or even no, tillers (ground-level branches). Although Z. diploperennis cytolines did have more ear branches that were longer than maize ear branches, overall they appeared even less teosintoid than maize.
In contrast, Z. luxurians cytoline ears were more like those of teosinte, the husks being soft and loose, with long blades. The plants also had four to five times as many tillers as the inbred line, consistent with the increased branching of teosinte. They also had more ear branches that were longer than maize ear branches and occasionally had more than one ear per branch and thus more ears per plant. Overall, Z. luxurians cytolines did have a more teosintoid appearance. It would be interesting to compare teosinte cytolines, i.e., teosinte plants with identical nuclear genomes and native teosinte vs. alien maize cytoplasms, to see if this initial impression holds up.
Variation among cytotypes:
One of the more surprising results of this study was that substantial variation occurs not only among cytotypes between cytoplasm-donor species, but also among the cytotypes within each of the donor species. This variation was, more often than not, greater among cytotypes within at least one of the Section Luxuriates species than between species. Furthermore, which of the species harbored the greater diversity, and which cytotypes within the more-variable species were most affected, depended on the specific character being observed; the relative differences between cytotypes either within or between species were character specific. Finally, associations or correlations that occurred between two characters in one cytotype did not hold in at least one other cytotype. Taken together, these observations suggest, first, that there are many phenotypically important genes and/or alleles within the Zea cytoplasmic genomes and that these interact in a multitude of ways with the A619 inbred line nuclear genome. Second, the variation among cytotypes for individual characters suggests that there is substantial variation in these genes. There are multiple, significantly different alleles in different populations of the same species.
For three-fourths of the characters, the range of variation within at least one of the species ranged from insignificant to highly significant. The presence of broad and significant variation within one species for the same characters for which there was a paucity or absence of variation in another species gives the impression that this variation arose episodically for at least a subset of phenotypically important genes. It appears that rapid diversification occurred in one species but not in another for certain characters or genes, while the reverse occurred for other characters or genes. Given the conserved nature of plant cytoplasmic genomes, it is tempting to postulate that this diversity is being driven by the need to adjust to the variation in the much-less-conserved nuclear genome, since most organelle-encoded proteins constitute only a part of much larger multimeric enzymes or structures into which they must fit productively.
Inconsistency between phenotype and other data:
That the cytolines of each of the three Section Luxuriantes species contain a broad range of phenotypic diversity is also somewhat surprising in light of the apparent consistency within the group by a variety of other measures. For instance, in this study Z. luxurians cytolines were the most heterogeneous phenotypically, yet Z. luxurians teosinte itself is the most homogeneous of the Zea taxa both phenotypically and isoenzymatically (Doebley et al. 1984). Looking more broadly, phenotypic variation within and among Section Luxuriantes cytolines was widespread and substantial, but variation within Section Zea cytolines was minimal. In contrast, variation in plastid DNA in Section Luxuriantes was minimal, but variation in Section Zea was substantial. In an analysis in which roughly half of the plastid genome was surveyed with 22 restriction enzymes, only three differences separated the annual from the perennial teosintes, only one restriction site polymorphism distinguished the two perennial species Z. diploperennis and Z. perennis, and all three taxa were monotypic (Doebley et al. 1987b). On the other hand, within Z. mays at least five types were observed.
The only relevant published data for the mitochondrial sequences, which are generally thought to be even more highly conserved than the plastid sequences (Wolfe et al. 1987), come from two Z. diploperennis mitochondrial genes: cox2 and a ribosomal RNA gene (rrn26). Compared to their maize counterparts, no nucleotide differences were present in either the coding region of cox2 or the rDNA (Gwynn et al. 1987). Unfortunately, it could not be determined from which of the three Z. diploperennis mitochondrial cytotypes the sequenced genes were derived.
Given the apparently highly conserved nature of plant organelle genomes, it is surprising that cytoline phenotypes should be so diverse within species. There have been no reports of this level of cytoplasmically influenced phenotypic diversity within any other species in which cytoplasmic effects have been investigated using non-CMS cytoplasms. Intraspecific phenotypic variation has been observed in alloplasmic CMS rice lines, but that variation was ascribed, at least in part, to the actions of their nuclear fertility restorer genes (Yang and Lu 1989; Wang et al. 1998). Clearly the genetic diversity must be present in the cytolines, despite the seemingly minimal diversity indicated by Zea, wheat, and rice plastid DNA studies and by the sequence comparison of the two mitochondrial genes from maize and Z. diploperennis. Either there is more variation within these genomes than is currently appreciated (i.e., the important portions of the genome were not surveyed) or the effect of the known variation is greater than is currently appreciated (e.g., regions important for gene regulation were not recognized as such). Zea mitochondrial and plastid genome sequencing should shed a more precise light on sequence divergence rates among the taxa involved in this study as well as provide candidate genes and alleles to account for specific phenotypic differences.
Phenotypic radiation:
Cytotypes within both Z. luxurians and Z. diploperennis ranged from quite un-maize-like to quite maize-like either for individual characters or in the principal components summary. Z. luxurians cytotype 8 was so maize-like that it is actually more similar to Z. mays cytotype 6 than were the other Z. mays cytotypes. This result is not consistent with studies of plastid DNA (Doebley et al. 1987b) or with the mitochondrial RFLP groupings used in this study. In both cases, the Z. mays organelle genomes are distinctly different from the other two species, which are either monotypic (plastid) or cohesively different (mitochondria). This would lead to expectations of cytoline phenotypes that were at least consistent in their being different from Z. mays cytolines. There is little to compare to because there have been very few reports of phenotypic variation caused by different non-CMS cytoplasms from the same species, and none of those studies yielded results comparable to those in the current study (Panayotov 1983; Pooni et al. 1993a,b; Chen and Line 1995; Voluevich et al. 1995). Furthermore, Panayotov surmised that the variation that he observed was due to an alien cytoplasm in the source and not to inherent differences within the species. The existence of such “phenotypic series” in the Zea cytolines could be explained by roughly simultaneous divergence of all three species from a common ancestor, with only some Z. luxurians and Z. diploperennis cytoplasms subsequently accumulating phenotypically important mutations (at least in the context of this study). Alternatively, because all of the Zea species are interfertile, there may be interspecific mitochondrial gene flow. Native Mexicans occasionally cross maize with Z. diploperennis for crop improvement purposes (Benz et al. 1990), and the reciprocal cross can also occur. Mitochondria, but not plastids, are known to take up exogenous DNA, and such a mechanism may also be responsible for the surprising diversity in these genomes and perhaps for the seemingly chimeric mitochondrial genome of Z. perennis cytotype 16 (J. O. Allen, personal observation).
Nuclear-cytoplasmic concordance:
The Z. perennis teosinte plants from which the seeds for these studies were collected were phenotypically indistinguishable from each other (Doebley 1989). The same was true for the Z. diploperennis plants (Iltis et al. 1979) and for their plastid DNA as well (Doebley et al. 1987b). In other words, each of the three Z. diploperennis cytotypes was apparently completely compatible with the range of nuclear genomes that it was likely to encounter. The same seems to be true for the two Z. perennis cytoplasms. But coupling those same cytoplasms with a single, different nuclear genome led to substantially altered phenotypes that differed among cytotypes; this result serves as a reminder of the nuclear part of nuclear-cytoplasmic interactions.
The phenotypic variation observed in the cytolines may or may not be due to mitochondrial DNA RFLP diversity, but it highlights and reinforces the desirability of a broad sampling of collections within species when studying an organism's cytoplasmic genome(s) or when using organelle genomes for phylogenetic studies. That extensive phenotypic and mitochondrial RFLP variation was observed even within individual seed collections of both Z. diploperennis and Z. perennis (which is in itself surprising for such geographically limited endemic species) suggests that even replication within a collection is desirable. This sampling advice may be especially applicable to monocot species, which have shown more cytoplasmic diversity in molecular genetic studies than dicot species have (e.g., Zurawski et al. 1984; Qiu et al. 2001).
Z. mays cytolines:
A lack of taxonomic resolution within Z. mays cytolines is apparent in that the distribution of cytotypes from all four subspecies completely overlapped. Previous authors (Doebley et al. 1987a,b) have commented on the lack of subspecific resolution in Z. mays plastid DNA data, where most of the RFLP types are present in multiple subspecies. This suggests that the subspecies are phylogenetically reticulate (Doebley 1990). Still, the placement of inbred line A619 centrally within the Z. mays cluster in the principal components analysis is yet more evidence in support of the contention that maize arose from Z. mays teosinte. In a principal components analysis that was performed on individual cytolines (as opposed to cytotypes; not shown), three of the four cytolines most similar to maize had cytoplasms from Z. mays ssp. parviglumis, the presumed immediate ancestor of maize. Interestingly, the distribution of Z. mays ssp. parviglumis cytolines was bimodal, with those cytolines most similar to maize derived from plants from the Balsas River area, where maize is thought to have arisen (Iltis and Doebley 1984), whereas those that were least similar were derived from plants collected from locations farther to the south.
Conclusion:
The fact that this broad range of 58 characters varied among cytolines, which presumably differ from one another only in their cytoplasmic genomes, points out the wide-ranging effects of the cytoplasmic genomes on plant phenotype. The large number of characters that varied significantly and independently of each other among cytotypes within each species suggests the existence of a large number of phenotypically important, independent genes and/or alleles in the cytoplasmic genomes. This suggests the presence of abundant and substantial cytoplasmic diversity that is compatible with teosinte nuclear genomes but that is only sometimes compatible with maize nuclear genomes.
Acknowledgments
The author thanks Jerry Kermicle, Christine Chase, Susan Gabay-Laughnan, and Christiane Fauron for critical reading of the manuscript and Jeffrey Palmer, Peter Kuhlman, and Kathleen Newton for helpful comments on the organization of the data and the manuscript. The author also thanks those students of teosinte who supplied seed and stocks. This research was supported in part by a Department of Energy grant FG02-86ER13539.
References
- Allen, J. O., 1992 Teosinte cytoplasmic genomes: interaction with maize nuclear genomes and molecular genetic characterization of the mitochondria. Ph.D. Thesis, University of Wisconsin, Madison, WI.
- Allen, J. O., G. K. Emenhiser and J. L. Kermicle, 1989. Miniature kernel and plant: interaction between teosinte cytoplasmic genomes and maize nuclear genomes. Maydica 34: 277–290. [Google Scholar]
- Beadle, G. W., 1980. The ancestry of corn. Sci. Am. 242: 112–119. [Google Scholar]
- Belliard, G., F. Vedel and G. Pelletier, 1979. Mitochondrial recombination in cytoplasmic hybrids of Nicotiana tabacum by protoplast fusion. Nature 281: 401–403. [Google Scholar]
- Benz, B. F., L. R. Sanchez-Velasquez and F. J. Santana Michel, 1990. Ecology and ethnobotany of Zea diploperennis: preliminary investigations. Maydica 35: 85–98. [Google Scholar]
- Berbec, A., 1994. Variation among offspring of alloplasmic tobacco Nicotiana tabacum L. cv ‘Zamojska 4’ with the cytoplasm of N. knightiana Goodspeed. Theor. Appl. Genet. 89: 127–132. [DOI] [PubMed] [Google Scholar]
- Berbec, A., 2001. Floral morphology and some other characteristics of iso-genomic alloplasmics of Nicotiana tabacum L. Beitraege zur Tabakforschung Int. 19: 309–314. [Google Scholar]
- Chen, X., and R. F. Line, 1995. Gene action in wheat cultivars for durable, high-temperature, adult-plant resistance and interaction with race-specific, seedling resistance to Puccinia striiformis. Phytopathology 85: 567–572. [Google Scholar]
- Conde, M. F., D. R. Pring and C. S. Levings, III, 1979. Maternal inheritance of organelle DNA in Zea mays-Zea perennis reciprocal crosses. J. Hered. 70: 2–4. [Google Scholar]
- Doebley, J. F., 1989. Molecular evidence for a missing wild relative of maize and the introgression of its chloroplast genome into Zea perennis. Evolution 43: 1555–1559. [DOI] [PubMed] [Google Scholar]
- Doebley, J. F., 1990. Molecular evidence for gene flow among Zea species. Bioscience 40: 443–448. [Google Scholar]
- Doebley, J. F., and H. H. Iltis, 1980. Taxonomy of Zea (Gramineae). 1. A subgeneric classification with key to taxa. Am. J. Bot. 67: 982–993. [Google Scholar]
- Doebley, J. F., and A. Stec, 1991. Genetic analysis of the morphological differences between maize and teosinte. Genetics 129: 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebley, J. F., and A. Stec, 1993. Inheritance of the morphological differences between maize and teosinte: comparison of results for two F2 populations. Genetics 134: 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebley, J. F., M. M. Goodman and C. W. Stuber, 1984. Isoenzymatic variation in Zea (Gramineae). Syst. Bot. 9: 203–218. [Google Scholar]
- Doebley, J. F., M. M. Goodman and C. W. Stuber, 1987. a Patterns of isozyme variation between maize and Mexican annual teosinte. Econ. Bot. 41: 234–246. [Google Scholar]
- Doebley, J. F., W. Renfroe and A. Blanton, 1987. b Restriction site variation in the Zea chloroplast genome. Genetics 117: 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebley, J. F., A. Stec and L. Hubbard, 1997. The evolution of apical dominance in maize. Nature 386: 485–488. [DOI] [PubMed] [Google Scholar]
- Ekiz, H., and C. F. Konzak, 1991. Nuclear and cytoplasmic control of anther culture response in wheat I. Analyses of alloplasmic lines. Crop Sci. 31: 1421–1427. [Google Scholar]
- Endo, T. R., 1980 Genetic constancy of the cytoplasm, pp. 13–48 in Genetic Diversity of the Cytoplasm in Triticum and Aegilops, edited by K. Tsunewaki. Japan Society for the Promotion of Science, Tokyo.
- Glimelius, K., and H. T. Bonnett, 1986. Nicotiana tabacum cultivar Turkish-samsun cybrids with Petunia hybrida cultivar comanche chloroplasts. Theor. Appl. Genet. 72: 794–798. [DOI] [PubMed] [Google Scholar]
- Gould, S. J., 1977. The return of hopeful monsters. Nat. Hist. 86: 22–30. [Google Scholar]
- Gwynn, B., R. E. Dewey, R. R. Sederoff, D. H. Timothy and C. S. Levings, III, 1987. Sequence of the 18S–5S ribosomal gene region and the cytochrome oxidase II gene from mt-DNA of Zea diploperennis. Theor. Appl. Genet. 74: 781–788. [DOI] [PubMed] [Google Scholar]
- Hackel, E., 1890 The True Grasses. Henry Holt, New York.
- Hanson, M. R., 1991. Plant mitochondrial mutations and male sterility. Annu. Rev. Genet. 25: 461–486. [DOI] [PubMed] [Google Scholar]
- Hou, N., Y. W. Wu, C. G. Liu, C. L. Zhang and Y. Zhang, 2000. Studies of salt tolerance of alloplasmic wheat. Acta Genet. Sinica 27: 325–330. [PubMed] [Google Scholar]
- Hutton, M. G., and J. B. Loy, 1992. Inheritance of cold germinability in muskmelon. HortScience 27: 826–829. [Google Scholar]
- Iltis, H. H., 1986 Maize evolution and agricultural origins, pp. 195–213 in Grass Systematics and Evolution, edited by T. R. Soderstrom. Smithsonian Institution Press, Washington, DC.
- Iltis, H. H., and J. F. Doebley, 1980. Taxonomy of Zea (Gramineae). 2. Subspecific categories in the Zea mays complex and a generic synopsis. Am. J. Bot. 67: 994–1004. [Google Scholar]
- Iltis, H. H., and J. F. Doebley, 1984 Zea—a biosystematical odyssey, pp. 587–616 in Plant Biosystematics, edited by W. F. Grant. Academic Press, San Diego/New York/London.
- Iltis, H. H., J. F. Doebley, M. R. Guzman and B. Pazy, 1979. Zea diploperennis (Gramineae): a new teosinte from Mexico. Science 203: 186–188. [DOI] [PubMed] [Google Scholar]
- Inai, S., K. Ishikawa, O. Nunomura and H. Ikehashi, 1993. Genetic analysis of stunted growth by nuclear-cytoplasmic interaction in interspecific hybrids of Capsicum by using RAPD markers. Theor. Appl. Genet. 87: 416–422. [DOI] [PubMed] [Google Scholar]
- Isshiki, S., and N. Kawajiri, 2002. Effect of cytoplasm of Solanum violaceum ort. on fertility of eggplant (S. melongena L.). Sci. Hortic. 93: 9–18. [Google Scholar]
- Jan, C. C., 1992. Cytoplasmic-nuclear gene interaction for plant vigor in Helianthus species. Crop Sci. 32: 320–323. [Google Scholar]
- Kirk, J. T. O., and R. A. E. Tilney-Bassett, 1978 The Plastids: Their Chemistry, Structure, Growth and Inheritance, pp. 460–486. Elsevier, Amsterdam.
- Kirti, P. B., T. Mohapatra, A. Baldev, S. Prakash and V. L. Chopra, 1995. A stable cytoplasmic male-sterile line of Brassica juncea carrying restructured organelle genomes from the somatic hybrid Trachystoma ballii + B. juncea. Plant Breed. 114: 434–438. [Google Scholar]
- Kubko, M. K., 2004. Mitochondrial tuning fork in nuclear homeotic functions. Trends Plant Sci. 9: 61–64. [DOI] [PubMed] [Google Scholar]
- Lauer, M., C. Knudsen, K. J. Newton, S. Gabay Laughnan and J. R. Laughnan, 1990. A partially deleted mitochondrial cytochrome oxidase gene in the NCS6 abnormal growth mutant of maize. New Biol. 2: 179–186. [PubMed] [Google Scholar]
- Laughnan, J. R., and S. Gabay-Laughnan, 1983. Cytoplasmic male sterility in maize. Annu. Rev. Genet. 17: 27–48. [DOI] [PubMed] [Google Scholar]
- Leaver, C. J., P. G. Isaac, I. D. Small, J. Bailey Serres, A. D. Liddell et al., 1988. Mitochondrial genome diversity and cytoplasmic male sterility in higher plants. Philos. Trans. R. Soc. Lond. B Biol. Sci. 319: 165–176. [Google Scholar]
- Loessl, A., M. Goetz, A. Braun and G. Wenzel, 2000. Molecular markers for cytoplasm in potato: male sterility and contribution of different plastid-mitochondrial configurations to starch production. Euphytica 116: 221–230. [Google Scholar]
- Maan, S. S., 1992. The scs and Vi genes correct a syndrome of cytoplasmic effects in alloplasmic durum wheat. Genome 35: 780–787. [DOI] [PubMed] [Google Scholar]
- Mazoti, L. B., 1954. Caracteres citoplasmáticos heredables derivados del híbrido de Euchlaena por Zea. Revista Invest. Agr. Buenos Aires 8: 175–183. [Google Scholar]
- Mumba, L. E., and N. W. Galwey, 1999. Compatibility between wild and cultivated common bean (Phaseolus vulgaris L.) genotypes of the Mesoamerican and Andean gene pools: evidence from the inheritance of quantitative characters. Euphytica 108: 105–119. [Google Scholar]
- Neuffer, M. G., and W. F. Sheridan, 1980. Defective kernel mutants of maize. I. Genetic and lethality studies. Genetics 95: 929–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuffer, M. G., E. H. Coe and S. R. Wessler, 1996 The Mutants of Maize. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Newton, K. J., C. Knudsen, S. Gabay-Laughnan and J. R. Laughnan, 1990. An abnormal growth mutant in maize has a defective mitochondrial cytochrome oxidase gene. Plant Cell 2: 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer, R. G., and V. C. M. Minor, 1994. Nuclear-cytoplasmic interaction in chlorophyll-deficient soybean, Glycine max (Fabaceae). Am. J. Bot. 81: 997–1003. [Google Scholar]
- Panayotov, I., 1983 The cytoplasm in Triticinae, pp. 481–497 in The Sixth International Wheat Genetics Symposium, edited by S. Sakamoto. Plant Germplasm Institute, Faculty of Agriculture, Kyoto University, Kyoto, Japan.
- Pooni, H. S., I. Kumar and G. S. Khush, 1993. a Genetical control of amylose content in a diallel set of rice crosses. Heredity 71: 603–613. [Google Scholar]
- Pooni, H. S., I. Kumar and G. S. Khush, 1993. b Genetical control of amylose content in selected crosses of indica rice. Heredity 70: 269–280. [Google Scholar]
- Qiu, Y. L., J. Lee, B. A. Whitlock, Q. F. Bernasconi and O. Dombrovska, 2001. Was the ANITA rooting of the angiosperm phylogeny affected by long-branch attraction? Mol. Biol. Evol. 18: 1745–1753. [DOI] [PubMed] [Google Scholar]
- Reboud, X., and C. Zeyl, 1994. Organelle inheritance in plants. Heredity 72: 132–140. [Google Scholar]
- Sheridan, W. F., and M. G. Neuffer, 1980. Defective kernel mutants of maize. II. Morphological and embryo culture studies. Genetics 95: 945–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonnard, G. C., and P. Gepts, 1994. Genetics of heat tolerance during reproductive development in common bean. Crop Sci. 34: 1168–1175. [Google Scholar]
- Soliman, K., G. Fedak and R. W. Allard, 1987. Inheritance of organelle DNA in barley and Hordeum × Secale intergeneric hybrids. Genome 29: 867–872. [Google Scholar]
- Tsunewaki, K., 1980 Genetic Diversity of the Cytoplasm in Triticum and Aegilops. Japan Society for the Promotion of Science, Tokyo.
- Tsunewaki, K., 1993. Genome-plasmon interactions in wheat. Jpn. J. Genet. 68: 1–34. [Google Scholar]
- Tsunewaki, K., Y. Mukai and T. R. Endo, 1980 Detailed studies of the alloplasmic wheats produced in Kyoto University, pp. 49–100 in Genetic Diversity of the Cytoplasm in Triticum and Aegilops, edited by K. Tsunewaki. Japan Society for the Promotion of Science, Tokyo.
- Uprety, D. C., and V. K. Tomar, 1993. Photosynthesis and drought resistance of Brassica carinata and its parent species. Photosynthetica 29: 321–327. [Google Scholar]
- Voluevich, E. A., and A. A. Buloichik, 1992. Nuclear-cytoplasmic interactions in wheat resistance to fungi pathogens: V. Quantitative resistance of alloplasmic line seedlings of Penjamo 62 variety to powdery mildew. Genetika 28: 82–88.1427060 [Google Scholar]
- Voluevich, E. A., A. A. Buloichik and A. N. Palilova, 1995. Effects of alien and intraspecies cytoplasms on expression of major nuclear genes for wheat resistance to brown rust. III. Intraline heterogeneity of reciprocal hybrids in F2. Genetika 31: 947–957. [Google Scholar]
- Wang, C., S. Tang and Y. Tang, 1998. Effects of male sterile cytoplasm on yield and agronomic characters in Japonica hybrid rice, Oryza sativa L. Breed. Sci. 48: 263–271. [Google Scholar]
- Wilkes, H. G., 1967 Teosinte: The Closest Relative of Maize. The Bussey Institution of Harvard University, Cambridge, MA.
- Wolfe, K. H., W. H. Li and P. M. Sharp, 1987. Rates of nucleotide substitution vary greatly among plant mitochondrial chloroplast and nuclear DNA. Proc. Natl. Acad. Sci. USA 84: 9054–9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, R., and H. Lu, 1989. A study on effects of nucleo-cytoplasmic interaction in Oryza L. Scientia Agric. Sinica 22: 56–63. [Google Scholar]
- Yu, J., R. Nickels and L. McIntosh, 2001. A genome approach to mitochondrial-nuclear communication in Arabidopsis. Plant Physiol. Biochem. Paris 39: 345–353. [Google Scholar]
- Zurawski, G., M. T. Clegg and A. H. D. Brown, 1984. The nature of nucleotide sequence divergence between barley and maize chloroplast DNA. Genetics 106: 735–750. [DOI] [PMC free article] [PubMed] [Google Scholar]