Abstract
Transposable elements have proven to be invaluable tools for genetically manipulating a wide variety of plants, animals, and microbes. Some have suggested that they could be used to spread desirable genes, such as refractoriness to Plasmodium infection, through target populations of Anopheles gambiae, thereby disabling the mosquito's ability to transmit malaria. To achieve this, a transposon must remain mobile and intact after the initial introduction into the genome. Endogenous, active class II transposable elements from An. gambiae have not been exploited as gene vectors/drivers because none have been isolated. We report the discovery of an active class II transposable element, Herves, from the mosquito An. gambiae. Herves is a member of a distinct subfamily of hAT elements that includes the hopper-we element from Bactrocera dorsalis and B. cucurbitae. Herves was transpositionally active in mobility assays performed in Drosophila melanogaster S2 cells and developing embryos and was used as a germ-line transformation vector in D. melanogaster. Herves displays an altered target-site preference from the distantly related hAT elements, Hermes and hobo. Herves is also present in An. arabiensis and An. merus with copy numbers similar to that found in An. gambiae. Preliminary data from an East African population are consistent with the element being transpositionally active in mosquitoes.
DESPITE breakthroughs in the generation of transgenic mosquitoes that, in laboratory studies, are refractory to the transmission of rodent malaria (Ito et al. 2002; Moreira et al. 2002; Kim et al. 2004), a key remaining obstacle to the extension of this technology to the field remains the absence of gene vectors that can efficiently drive genes through mosquito populations. Thus, while the distribution and spread of P and hobo elements through field populations of Drosophila melanogaster is well documented (Anxolabehere et al. 1988; Daniels et al. 1990a,b), and while the distribution of mariner elements in arthropods suggests that these elements were once capable of spreading both within and between genomes (Robertson and MacLeod 1993), no evidence for the ability of transposable elements to move within mosquito populations exists. This is in contrast to recent developments in gene transfer technologies in mosquitoes in which several transposable elements have been used to genetically transform mosquito species. These include the Hermes element from Musca domestica (Jasinskiene et al. 1998; Allen et al. 2001), the piggyBac element from Trichoplusia ni (Grossman et al. 2001; Kokoza et al. 2001; Moreira et al. 2002; Perera et al. 2002), the Minos element from Drosophila hydei (Catteruccia et al. 2000), and the Mos1 element from Drosophila mauritiana (Coates et al. 1998). The remobilization properties of Hermes, piggyBac, and Mos1 in somatic and germ-line nuclei were examined in transgenic lines of the yellow fever mosquito, Aedes aegypti (O'Brochta et al. 2003; Wilson et al. 2003). Little or no evidence of germ-line remobilization was found for these transposable elements in these transgenic lines of mosquito, indicating that these elements may not be suitable agents for driving transgenes through insect populations. The basis for this low frequency of remobilization is not known; however, it is consistent with the behavior of mariner elements in transgenic lines of D. melanogaster in which remobilization of these modified elements is also low (Lohe and Hartl 1996; Lozovsky et al. 2002).
We were interested in determining if active transposable elements were present in the genome of the malaria vector Anopheles gambiae. This mosquito species is the principal vector of the pathogen of human malaria, Plasmodium falciparum, in Africa and so is the target for genetic control and population replacement strategies aimed at preventing the transmission of Plasmodium through the female mosquito. An active transposable element from An. gambiae might serve as a platform for constructing gene vectors from the target mosquito species itself and would also facilitate the study of transposable element movement and spread through An. gambiae populations. We report here the isolation of Herves, an active transposable element from An. gambiae. Herves is a member of the hAT superfamily of transposable elements but is only distantly related to the Hermes and hobo elements that also are found in insects and are active. This element was discovered in silico through the use of a unique algorithm designed to identify active hAT elements from the output of genome-sequencing projects on the basis of conserved structural features of these elements in addition to conserved amino acid sequences. We describe the successful use of this element in interplasmid transposition assays in a Drosophila cell line and embryos as well as its use as a genetic transformation vector in this species. We show that Herves is present in one east African population of An. gambiae sensu stricto. Initial observations of copy number and site-occupancy data from this population are consistent with this element being transpositionally active. Herves is perhaps an illustrative example of transposable element invasion and spreading through An. gambiae, which could serve as an example of how class II transposable elements will behave in this species when they are used as part of a genetic drive strategy aimed at introducing, and then spreading, beneficial genes through mosquito populations that vector human disease.
MATERIALS AND METHODS
Computer searches for active hAT elements in An. gambiae:
All 8987 scaffolds of the An. gambiae genome project (build 2, version 1; available at ftp.ncbi.nih.gov/genbank/genomes/Anopheles_gambiae/) were searched for nucleotide stretches with characteristics of recently active hAT elements (characteristics described below). All searches were performed using custom-written programming scripts in PERL v.5.8.0 (scripts available as online supplemental material at http://www.entomology.ucr.edu/people/atkinson.html). Nucleotide stretches were identified as those of potentially active hAT elements and saved for further analysis if they had the following characteristics: (1) they had to be <10,000 bp in length; (2) they had to have an 8-bp duplication at the beginning and end of the stretch (duplications >8 bp were not saved to avoid duplicated regions of the genome); (3) inverted terminal repeat sequences (ITRs) had to be adjacent to the 8-bp duplications and be at least 11 bp long with up to three mismatches; and (4) they had to have an ORF ≥ 200 aa. This list of potential elements was sorted further by isolating stretches in which the same ITR was found adjacent to at least two different 8-bp duplications. From the resulting list of nucleotide stretches those containing ORFs ≥200 aa were identified and the ORF compared to the “nr” peptide database in GenBank (http://www.ncbi.nih.gov) using the program BLASTP with expected value E ≤ 10−6 (Altschul et al. 1990) for similarities to known proteins. Finally, annotations from the BLASTP output were screened manually for references to known transposable elements.
Isolation of the Herves element from whole-genome extractions:
Intact copies of Herves were amplified using PCR primer pairs designed for sequences surrounding three apparently intact copies of the element identified by the computer searches (see results). We attempted to isolate Herves from the RSP-1 strain because it is closely related to the PEST strain from which the An. gambiae genome sequence was obtained and because the PEST strain is no longer available. Genomic DNA from a dozen pupae from the RSP strain was extracted using the Wizard Genomic DNA purification kit (Promega, Madison, WI). Primer pairs were 5′-TAA GTG TGG TGA CTC GGG AT-3′ (lgam8859) and 5′-CCC TGG ACA GTT GTA GTT GA-3′ (rgam8859), 5′-TGA GGG ACG CTA ATT GAT TC-3′ (lgam8964) and 5′-GCT TTG CGT TCC TTG TAT CT-3′ (rgam8964), and 5′-TCC TAC ACA GGG CTG CTT CTC-3′ (lgam8978) and 5′-CAG CGA GAG GAC TAA TTT GG-3′ (rgam8978). A first round of PCR was performed using Ventr DNA polymerase (New England Biolabs, Beverly, MA) under the following conditions: 94° for 3 min and 30 cycles of 94° for 30 sec, 65° for 30 sec, and 72° for 5 min. A second round of PCR, using the products of the first reaction as a DNA template, was performed to amplify internal sections of the element.
Transposition assays and Drosophila transformations:
The amplified Herves element was cloned into plasmids for use in an interplasmid transposition assay similar to the one described in Sarkar et al. (1997). Donor plasmids were created by cloning the entire Herves sequence into a pBluescriptSK+ vector (Stratagene, La Jolla, CA) and replacing the ORF with a gene conferring resistance to kanamycin, the ColE1 origin of replication, and a lacZ reporter gene (all three were obtained from the pKSacO∝ plasmid; Sarkar et al. 1997). The Herves ORF isolated from the RSP strain was cloned into a pK19 vector containing a heat-shock protein 70 promoter (pKhsp70) to create the helper plasmid pKhsp70Herves-RSP. The ORF from RSP differed from that found in the PEST genome in that the amino acid at position 505 was P instead of L. This variable amino acid was mutated so that the ORF from RSP was identical to that found in the PEST strain. This altered ORF was cloned into pKhsp70 to create pKhsp70Herves-PEST. The target plasmid was pGDV1.
Helper, donor, and target plasmids were introduced into D. melanogaster Schneider2 (S2) cells (Schneider 1972) using Cellfectin (Invitrogen, San Diego) following the manufacturer's suggested procedure. In each experiment 5 million cells were transfected with 2.5 μg each of donor and helper plasmids and 5.0 μg of target plasmid. Twenty-four hours after transfection cells were heat-shocked at 41° for 2 hr and allowed to recover at 23° for an additional 24 hr. Cells were then collected and DNA was extracted using the Wizard Genomic purification kit (Promega).
Helper, donor, and target plasmids were introduced into D. melanogaster Canton-S,w embryos by direct micro-injection. Injection plasmid mixtures were at 0.5, 0.5, and 1.0 mg/ml of donor, helper, and target plasmids, respectively. Drosophila preblastoderm embryos were injected between 30 and 60 min postoviposition. Eggs were allowed to develop at 26° for 16 hr at which time they were heat-shocked for 1 hr at 37°. After heat shock the embryos were allowed to recover at 26° at which time plasmids were recovered. Plasmid recovery was as described (Sarkar et al. 1997).
Plasmids recovered from both transfected cell lines and injected embryos were electroporated into competent Escherichia coli cells (DH10β, Invitrogen) and plated on Luria-Bertani (LB) plates containing kanamycin and chloramphenicol (25 and 10 μg/ml, respectively). Colonies from these plates were grown overnight in LB media containing kanamycin (25 μg/ml) and DNA was extracted using the Wizard Plus miniprep kit (Promega). DNA was digested with PstI restriction enzyme (New England Biolabs) to check for transposition. Because the Herves donor element and the target plasmid contain PstI restriction sites, recombinant plasmids resulting from the transposition of Herves into pGDV1 produce a characteristic pattern of three fragments following digestion with this enzyme. A 622-bp fragment arising from two PstI sites inside the lacZ gene in the Herves donor element is common to all recombinant plasmids. Two fragments totaling 6449 bp but varying in size as a function of the position of the integration site within the target plasmid permit the location of the integrated element to be determined. Transposition events were confirmed by DNA sequencing at the University of California Riverside Institute for Integrative Genome Biology.
Drosophila transformations were performed essentially as described (Rubin and Spradling 1982). A 1.3-kb BstXI-BglII fragment containing the enhanced green fluorescence (EFGP) gene placed under the control of the 3xP3 promoter from D. melanogaster was removed from plasmid pBac[3xP3-EGFP,afm] and the 5′ and 3′ overhangs were removed with the large fragment of Klenow DNA polymerase and cloned into the blunt-ended PstI site, also generated by the Klenow holoenzyme, in plasmid pBSHerves, creating plasmid pHerves[3xP3-EGFP], which contained 1.4 kb of Herves L end, 302 bp of Herves R-end sequence, and the 8-bp target-site duplication 5′-GTAGCAAC-3′ from An. gambiae. Plasmid pHerves [3xP3-EGFP] (250 mg/ml) was coinjected with the Herves helper plasmid pKhsp70Herves (300 mg/ml) into preblastoderm Drosophila Canton-S,w embryos. Surviving G0 adults were backcrossed to Canton-S,w and G1 progeny were examined for the expression of EGFP genetic marker. Homozygous lines were established by repeated backcrossing of transgenic individuals.
Phylogenetic analysis:
Transposase protein sequences from 11 known hAT elements and Herves were aligned using CLUSTALW v.1.83 and the PAM250 matrix (Thompson et al. 1994). Phylogenetic analyses aimed at establishing the placement of Herves on a tree of hAT element transposases were performed using programs based on maximum-likelihood optimality criteria [MRBAYES v.3.0b4 (Huelsenbeck and Ronquist 2001) and TREE-PUZZLE v.5.0 (Schmidt et al. 2002)] and on maximum parsimony (PAUP*4.0b10; Swofford 1998). Amino acid sequences of hAT element transposases were selected from prior publications to represent the diversity of the hAT superfamily with particular attention to sequences likely to be closely related to Herves (Calvi et al. 1991; Rubin et al. 2001; Robertson 2002; Handler 2003). Amino acid substitution rates were modeled using the JTT (Jones et al. 1992) and WAG (Whelan and Goldman 2001) substitution matrixes. Among-site rate variation was modeled by a discrete gamma parameter estimated from the data by MRBAYES and TREE-PUZZLE.
Mosquito samples:
Mosquito strains examined in this study were the G3 strain maintained at the University of Maryland Biotechnology Institute; Suakoko (SUA) from A. Crisanti, Imperial College, London, and A. Richman, Department of Veterans Affairs, Washington, DC.; RSP-2 from F. H. Collins, University of Notre Dame, Notre Dame, Indiana; KIL from P. J. Eggleston, Keele University, Keele, UK, and C. Curtis, London School of Hygiene and Tropical Medicine, London; and isofemale lines from recently caught field samples of An. gambiae from Mali from G. C. Lanzaro, University of California, Davis, California. Insects collected from Furvela, Mozambique, were provided by D. Charlwood, Danish Bilharziasis Institute, Charlottenlund, Denmark.
Herves transposon display analysis (TEDA):
The procedure used for transposon display has previously been described (Guimond et al. 2003) and was modified for use with the Herves element. Genomic DNA was isolated as described and processed for transposable element display after digesting with MseI (Guimond et al. 2003). For these experiments, the Herves-specific primers HervTEDAL1 5′-AAT TCG ACG GGT TCC TAC C-3′ (preselective PCR) and HervTEDAL2 Cy5/5′-GTT GAT TAG ATG AAC GTA GG-3′ (selective PCR) were used in addition to the adapter-specific primer MseIa GAC GAT GAG TCC TGA G previously described (Guimond et al. 2003). Preselective PCR conditions were 95° for 3 min; 25 cycles of 95° for 15 sec, 60° for 30 sec, and 72° for 1 min; and 72° for 5 min. Selective PCR conditions were 95° for 3 min; 5 cycles of 95° for 15 sec; 64°–60° at 1° per cycle for 30 sec; 72° for 1 min; 25 cycles of 95° for 15 sec; 60° for 30 sec; 72° for 1 min; and 72° for 5 min. Selective PCR products were separated on a 6% denaturing polyacrylamide gel. The gel was dried onto 3MM paper and scanned with a Typhoon phosphorimager (Amersham, Buckinghamshire, UK). Individual bands were excised from the dried gels, eluted, reamplified, and sequenced as previously described (Guimond et al. 2003).
RESULTS
hAT elements in the PEST genome:
The search for nucleotide stretches with characteristics of active hAT elements yielded 3553 nucleotide stretches that satisfied the structural criteria. Of these, 1536 also contained ORFs ≥200 aa with significant similarities (E ≤ 10−6) to a number of class I and II elements, some of which have previously been described, including P elements (Sarkar et al. 2003a) and Tc1-mariner elements (Holt et al. 2002). Three different ORF sequences had significant similarities to known hAT elements: hopper, Hermes, hobo, Ac, and Tam3. The first ORF was 603 aa long and was present three times within the genome (Figure 1). The second ORF was almost identical to the first and differed from it only by the insertion of a 328-bp section of a Topi transposable element (Grossman et al. 1999) at aa 231, causing a frameshift mutation. The third ORF was 297 aa long and was present only once within the genome sequence.
Figure 1.—
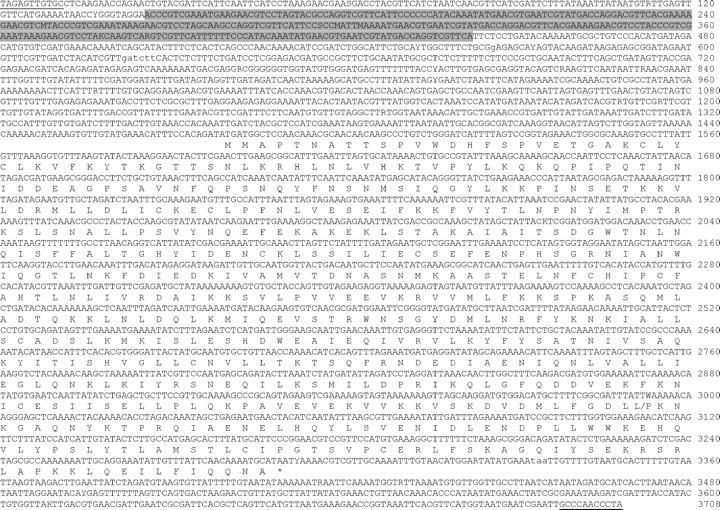
Consensus sequence of three Herves elements from the An. gambiae genome (see text) and amino acid translation of the transposase. IUPAC ambiguity codes indicate sites where the three copies differ; lowercase letters indicate a deletion in at least one copy. Inverted terminal repeats are underlined. A region containing three almost-perfect tandem repeats is shaded.
The presence of three identical ORFs within stretches identified as possible active hAT elements and a fourth almost identical sequence was encouraging and these sequences were investigated further. The three 603-aa ORFs were almost identical and were located between identical ITR sequences forming three putative transposable elements of 3699, 3702, and 3707 bp. These elements compose a new subfamily of transposable elements referred to as Herves. One copy of Herves was located on chromosome 2 and two copies on chromosome 3. The fourth copy, containing the Topi insertion, was located in an unmapped area of the genome. The Herves element was characterized by 11-bp ITRs containing two mismatched bases, 8-bp target-site duplications showing sequence similarity to the hAT element consensus sequence 5′-GTNNNNAC-3′, and a transposase with amino acid sequence similarity to hAT element transposases. Sequences identical to Herves have been reported by Jurka (2000).
Phylogenetic analyses:
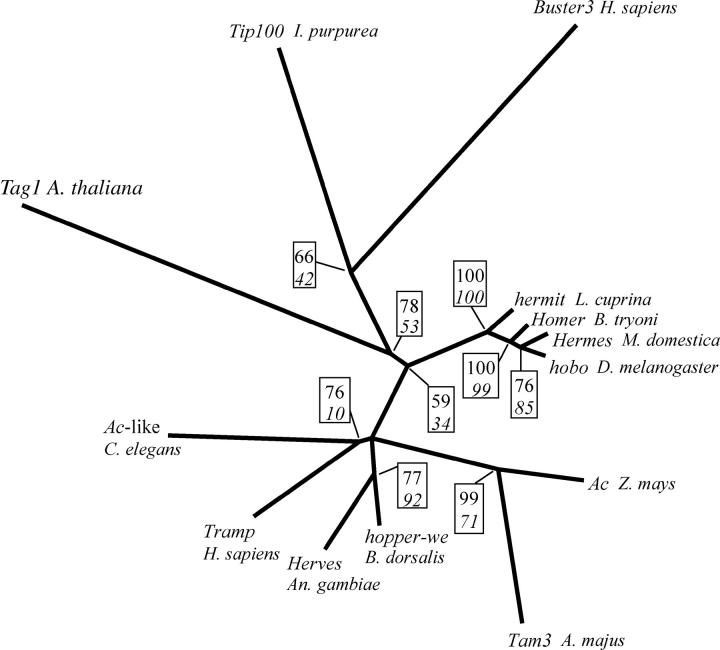
All phylogenetic analyses using either maximum-likelihood- or maximum-parsimony-based methods resulted in topologies consistent with the tree shown in Figure 2. Maximum-likelihood methods, using more sophisticated models of evolution, had generally higher nodal support for deep branches than did maximum parsimony. Among-site rate variation was low (the lowest estimated α-shape parameter was 2.64).
Figure 2.—
Phylogenetic relationships of transposase amino acid sequences from selected hAT elements (see text). Boxed numbers next to each node represent measures of nodal support. The top number is the quartet puzzling reliability percentage from TREE-PUZZLE using the WAG matrix substitution model and among-site rate variation estimated using a discrete gamma shape parameter. The bottom number in italics is the percentage of bootstrap support from unweighted maximum-parsimony analysis using PAUP*. Tree topology and branch lengths correspond to the tree obtained with TREE-PUZZLE from 100 maximum-likelihood quartets. All transposase sequences except that of Herves were obtained from GenBank (http://www.ncbi.nih.gov/): Tam3 (accession no. CAA38906), Ac (accession no. CAA29005), hobo (accession no. A39652), Hermes (accession no. AAC37217), Homer (accession no. AAD03082), hermit (accession no. AAA64851), Buster3 (accession no. NP_071373), Tip100 (accession no. BAA36225), Tag1 (accession no. T52187), Ac-like (accession no. NP 509255), hopper (accession no. AAL93203), and Tramp (accession no. CAA76545).
The Herves transposase sequence was most closely related to the hopper-we sequence (Handler 2003) and did not form a monophyletic group with the other insect elements hopper, Hermes, Homer, and Hermit. Corrected distance estimates indicated that Herves was as distantly related to these insect elements as to the human Tramp element (Esposito et al. 1999; Table 1). The close relationship of Herves and hopper-we was confirmed by high levels of transposase sequence similarity (28% amino acid identity, 53% amino acid similarity), overall nucleotide sequence similarity (40% nucleotide identity when aligned using ClustalW), and ITR sequence similarity.
TABLE 1.
Measurements of amino acid similarity and distance of 12hAT superfamily elements
|
hAT superfamily element
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transposable element |
Herves | hopper-we | Tramp | Ac-like | Activator | Hermes | hobo | Homer | Hermit | Buster3 | Tip100 | Tag1 |
| Herves | — | 1.90 | 3.09 | 4.23 | 4.31 | 5.29 | 4.64 | 5.02 | 4.99 | 8.15 | 7.93 | 8.54 |
| hopper-we | 0.30 (0.49) | — | 5.57 | 3.52 | 4.65 | 4.75 | 4.73 | 4.90 | 4.67 | 7.12 | 8.05 | 9.00 |
| Tramp | 0.25 (0.42) | 0.25 (0.43) | — | 4.32 | 6.16 | 5.65 | 4.99 | 5.44 | 5.57 | 6.99 | 9.00 | 9.00 |
| Ac-like | 0.21 (0.39) | 0.22 (0.44) | 0.21 (0.37) | — | 5.27 | 5.68 | 5.59 | 6.16 | 6.15 | 8.47 | 9.00 | 9.00 |
| Activator | 0.24 (0.43) | 0.23 (0.4) | 0.20 (0.38) | 0.21 (0.37) | — | 6.24 | 7.16 | 6.75 | 6.70 | 8.88 | 9.00 | 9.00 |
| Hermes | 0.21 (0.37) | 0.22 (0.43) | 0.20 (0.38) | 0.20 (0.37) | 0.21 (0.41) | — | 0.75 | 0.85 | 1.26 | 7.11 | 8.19 | 8.43 |
| hobo | 0.22 (0.38) | 0.25 (0.43) | 0.21 (0.4) | 0.20 (0.4) | 0.21 (0.4) | 0.53 (0.70) | — | 0.80 | 1.20 | 7.25 | 9.00 | 7.88 |
| Homer | 0.21 (0.37) | 0.20 (0.40) | 0.20 (0.36) | 0.19 (0.35) | 0.19 (0.37) | 0.51 (0.69) | 0.52 (0.69) | — | 1.09 | 6.66 | 8.36 | 8.12 |
| hermit | 0.22 (0.38) | 0.23 (0.44) | 0.22 (0.36) | 0.20 (0.38) | 0.23 (0.39) | 0.38 (0.58) | 0.40 (0.59) | 0.41 (0.59) | — | 7.24 | 8.63 | 7.17 |
| Buster3 | NA | NA | NA | NA | NA | NA | NA | NA | NA | — | 7.40 | 9.00 |
| Tip100 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | — | 9.00 |
| Tag1 | 0.20 (0.37) | 0.19 (0.38) | NA | NA | NA | 0.22 (0.37) | 0.23 (0.38) | 0.33 (0.61) | 0.36 (0.58) | NA | NA | — |
Numbers above the diagonal are maximum-likelihood distances calculated by TREE-PUZZLE using the WAG amino acid substitution matrix and the among-site variation estimated by a discrete gamma parameter (α = 2.64). Sequences were aligned using ClustalW. Numbers below the diagonal are the proportion of amino acid identity and, in parentheses, similarity (using the Blosum62 matrix). Amino acid identity and similarity were estimated from pairwise alignments generated by the BLASTP program (E ≤ 0.01). NA indicates that no significant similarity between sequences could be identified by BLASTP.
Herves is a functional element:
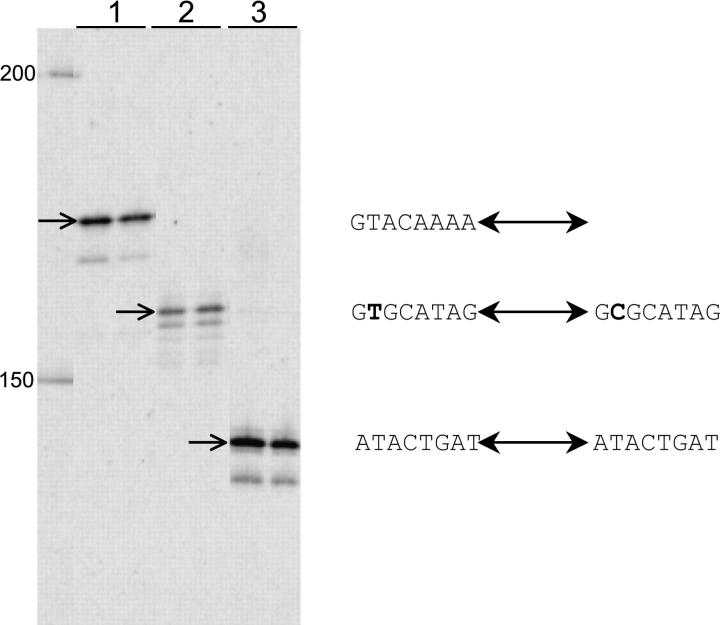
Three almost identical copies of Herves were identified in the published scaffolds of the An. gambiae genome-sequencing project and a fourth copy was also identified that differed by the insertion of 238 bp of the Topi transposable element inside its ORF. Circumstantial evidence of recent movement by Herves was discovered in the course of isolating Herves from the An. gambiae RSP strain. Three chromosomal sites within the genome of RSP that contained full-length Herves sequences in the PEST strain were amplified using PCR and sequenced. The genome of RSP differed from PEST at these three sites (Figure 3). The first site did not contain Herves but instead the observed sequence was consistent with what would be expected prior to transposition into that site, i.e., a single copy of the 8-bp host duplication. The second site also did not contain the Herves element, but instead the Herves sequence was replaced with a single A/T base pair. This was consistent with the predicted excision footprint of the Hermes hAT element following a recently proposed mechanism of Hermes excision and transposition (Zhou et al. 2004). The third site appeared to be heterozygous and PCR amplifications yielded two types of fragments: (1) a fragment with the expected nucleotide sequence prior to Herves insertion and (2) a fragment containing the full-length Herves element. These results suggest that (1) either some Herves sequences have mobilized since the PEST and RSP strains have become isolated or, alternatively, there has been differential assortment of an ancestral polymorphism of Herves insertions prior to the creation of these two strains; (2) Herves is capable of persisting in a single genome location for at least several generations; and (3) the RSP strain is not homozygous with respect to Herves integrations. It is unclear if this heterogeneity stems from the mating of individuals with and without the insertion, from movement of the element, or from both.
Figure 3.—
Evidence of past mobility of Herves in An. gambiae PEST and RSP strains. Sequences are grouped by locus under the heading of the scaffold from the An. gambiae sequencing project. PEST sequences were obtained from GenBank while RSP sequences were from PCR amplifications; the two RSP lines for scaffold AAAB01008978 represent different PCR amplification products. Herves ITRs are underlined, omitted Herves nucleotides are represented by (…), and dashes indicate insertions/deletions.
To test if the Herves element present in RSP was functional, interplasmid transposition assays were performed using two Herves ORF sequences as sources of transposases: first, Herves-PEST, with the amino acid sequence shown (Figure 1) and second, Herves-RSP, which has the same amino acid sequence but with L505 replaced with phenylalanine (see above). Assays performed in Drosophila S2 cells and Drosophila embryos demonstrated the transpositional activity of Herves (Table 2). Twenty-seven perfect transpositions and 13 imperfect transpositions of Herves were recorded (Table 2). All were dependent on the presence of Herves transposase since transposition events were not recovered in the absence of transposase. Perfect transpositions involved transposition of only the Herves element with the creation of an 8-bp target-site duplication at the point of insertion. The target-site selection of Herves, as reflected in the distribution of integrations in the pGDV1 target plasmid, differed from that of both the Hermes and hobo elements. From transposition assays performed in Drosophila Canton-S,w embryos, both Hermes and hobo show a very similar pattern of site selection within this plasmid, with insertion hotspots at nucleotides 736, 2154, 2271, and 2303 (Sarkar et al. 1997; Y.-J. Kim, D. A. O'Brochta and P. W. Atkinson, unpublished results). This was not the case for Herves. None of the 21 transpositions recovered from Drosophila embryos inserted at hotspots 736, 2154, and 2271 and only one insertion was seen at 2203. A new hotspot (8/21 insertions) was observed at nucleotide 476, at which no previous insertions of either hobo or Hermes have been recorded (Sarkar et al. 1997; Y.-J. Kim, D. A. O'Brochta and P. W. Atkinson, unpublished results).
TABLE 2.
Herves transposition in cell lines and embryos ofD. melanogaster
| Cell line name/strain | Transposase source | No. of experiments |
No. of plasmids screened |
No. of cut-and-paste transpositions |
No. of transposase- mediated eventsa |
Events per 104 plasmids screenedb |
|---|---|---|---|---|---|---|
| Cell line/S2 | hsp70Herves-PEST | 3 | 551,400 | 5 | 7 | 0.09 |
| Cell line/S2 | hsp70Herves-RSP | 2 | 306,200 | 1 | 4 | 0.03 |
| Cell line/S2 | 0 | 3 | 887,800 | 0 | 0 | 0 |
| Embryos/Canton-S,w | hsp70Herves-PEST | 4 | 649,000 | 21 | 2 | 0.32 |
| Embryos/Canton-S,w | 0 | 2 | 615,000 | 0 | 0 | 0 |
“Transposase-mediated events” were defined as the insertion of at least one ITR into new DNA with the breakpoint immediately outside the ITR.
Rates based on the number of “cut-and-paste” transpositions only.
To test whether Herves could be used to genetically transform insects, the plasmid pHerves[3xP3-EGFP] was co-injected with helper Herves plasmid into preblastoderm embryos of D. melanogaster. From two injection experiments, four independent transgenic lines were obtained with an overall transformation frequency of 30.1% (Table 3). This frequency of transformation is comparable to transformation frequencies obtained when other class II elements such as P, hobo, Hermes, and piggyBac are used to transform this species and is higher than predicted from the low frequency of transposition of Herves observed in Drosophila (Table 2). Three of these transgenic lines were selected for further analysis. Cut-and-paste integration of solitary Herves elements was confirmed by TEDA in the three lines examined (Figure 4). For each line, a single but different fragment was amplified. Purification and sequencing of each of these revealed that, for lines 2 and 3, at both ends, the Herves element insertion was delimited by the first nucleotide of Herves with flanking sequence from D. melanogaster. An 8-bp sequence consistent with the consensus insertion sites of hAT elements was present adjacent to each Herves element (Figure 4). For line 1, only the left-end integration site has been characterized and the insertion is delimited by the terminal nucleotide of Herves. The 8-bp site flanking Herves conforms to the target-site consensus of hAT elements. A BLAST search of the flanking sequences indicated that in line 1 Herves had inserted into chromosome 3L at 66A, in line 2 Herves had inserted into chromosome 3R at 95E, and in line 3 Herves had inserted into a sequence found in five locations throughout the genome.
TABLE 3.
Transformation ofD. melanogaster with pHerves[3xP3-EGFP] and pKhsp70Herves
| Experiment no. |
No. of embryos injected |
No. of G0 adults | No. of fertile matings | No. of G0 matings producing transgenic adults |
No. of transgenic G1's |
Transformation frequency (%) |
|---|---|---|---|---|---|---|
| 1 | 120 | 38 | 9 | 3 | 15 males, 25 females |
33 |
| 7 males, 17 females | ||||||
| 2 males, 1 female | ||||||
| 2 | 60 | 7 | 4 | 1 | 3 total | 25 |
| Total | 180 | 45 | 13 | 4 | 30.1 |
Figure 4.—
Transposable element display of the left end of Herves in transgenic D. melanogaster lines 1, 2, and 3. The results from two individuals from each line are shown along with the position of the molecular weight makers. Bands were excised, eluted, reamplified, and sequenced. Chromosomal position was determined by BLAST searching the D. melanogaster sequence database. Bands below the main bands (arrows) are identical in sequence to the main bands and are often associated with abundant PCR products <250 bp in length. The sequences adjacent to the right inverted terminal repeat were determined by direct amplification using primers specific for the Herves element and flanking genomic DNA. The 8-bp target-site duplications flanking each of the three independent transpositions are shown to the right and each corresponds to the arrowed band at the corresponding migration distance on the gel. For line 1, only sequence flanking the left end of Herves was obtained. For line 2, the 8-bp target-site duplication was imperfect with the single-base-pair difference (T vs. G) shown in boldface type.
Distribution of Herves:
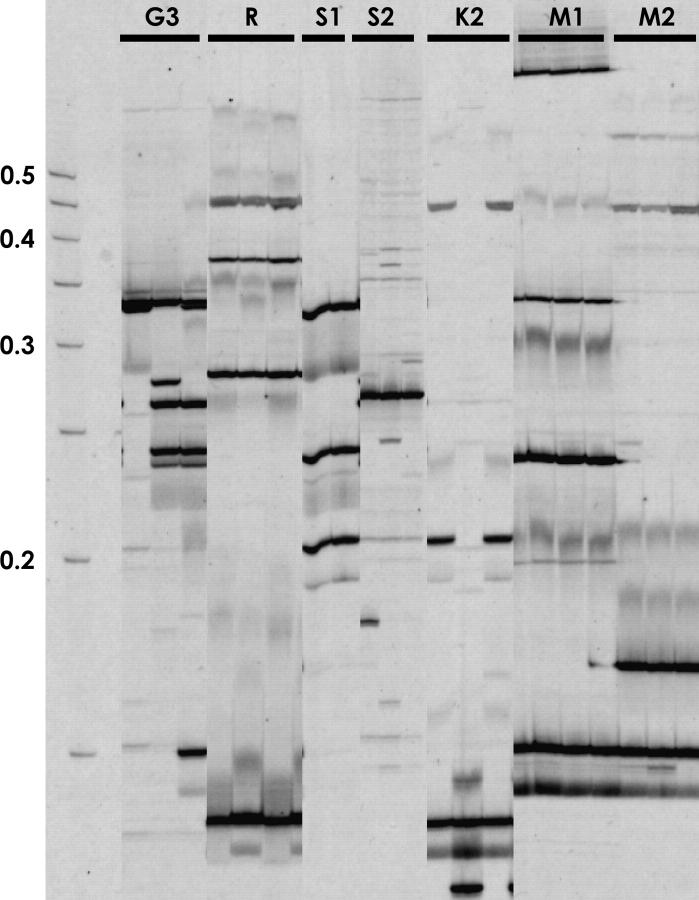
Seven laboratory lines of An. gambiae were tested and all contained Herves (Figure 5). Likewise, separate colonies of the laboratory line Suakoko also showed notable differences in the number and position of elements. The G3 and KIL (no. 2) lines showed intraline variation in element position and copy number. Twenty-five bands from Figure 5 were excised from the gel, reamplified, and sequenced. Ten of the elements were inserted in unique, single-copy DNA; 9 were inserted in sequences repeatedly found throughout the genome, precluding localization; and 6 were integrated in sequences that were not present in the An. gambiae sequence database. Seven of the localized elements were on the second chromosome, two were on the X chromosome, and one was inserted in a sequence that had not yet been placed within the existing scaffold structure of the An. gambiae genome database.
Figure 5.—
Comparison of the Herves content of laboratory lines of An. gambiae s.s. Transposable element display was performed on individuals from the laboratory lines G3, RSP (R), Suakoko obtained from two sources (S1 and S2), KIL obtained from two sources (K1 and K2), and two recently established isofemale lines from individuals collected from Mali (M1 and M2). Molecular weight markers are indicated.
Preliminary analysis of An. gambiae sensu lato collected from a village (Furvela) located along the southern coast of Mozambique revealed the presence of Herves not only in An. gambiae s.s. but also in Anopheles arabiensis and Anopheles merus (Figure 6). All insects analyzed contained at least one element (n = 35, An. gambiae s.s.; n = 45, An. arabiensis; n = 6, An. merus; Figure 7). Bands arising from transposable element display are dominant markers and copy numbers of elements were estimated to be 4x/3 where x = number of occupied sites (“bands”) and Hardy-Weinberg equilibrium was assumed. In the three species examined, copy numbers were 4.5 elements/genome in An. merus (range 1–7), 4.6 elements/genome in An. gambiae s.s. (range 1–8), and 7.3 elements/genome in An. arabiensis (range 1–11). Elements were observed in a variety of different positions (sites) within the genome. Twenty-five sites were detected in An. gambiae s.s., 31 sites in An. arabiensis, and 14 sites in An. merus.
Figure 6.—
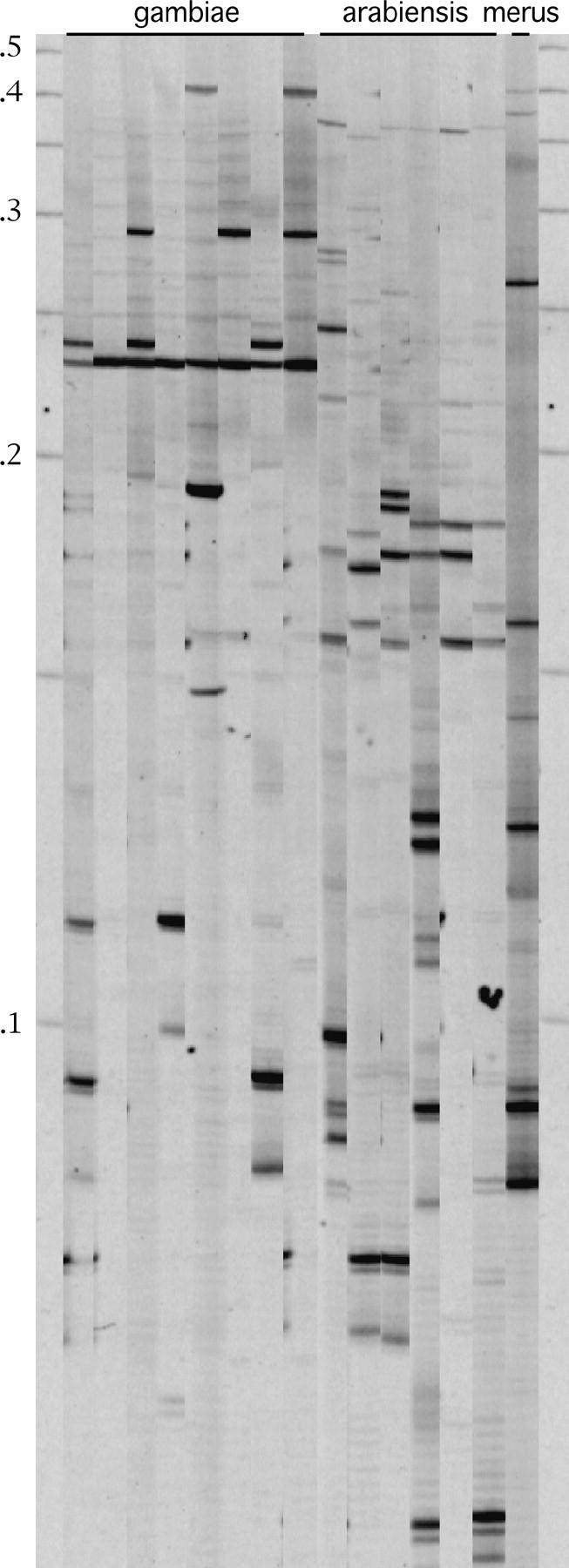
Transposable element display of individuals collected from a local population in Mozambique. Three species are represented: An. arabiensis, An. gambiae s.s., and An. merus. Molecular weight markers (in kilobase pairs) are shown.
Figure 7.—
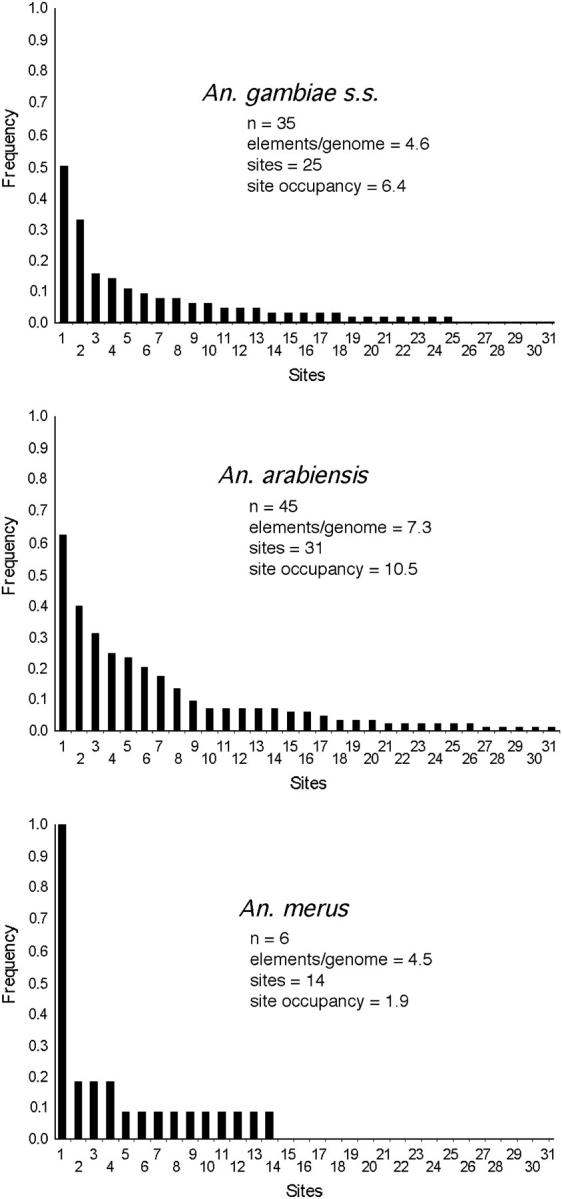
Herves in three species of Anopheles in Mozambique. Data were derived from transposable element displays on the number of individuals indicated (n). “Site” refers to a genomic position occupied by at least one Herves element in the sample and corresponds to a unique PCR product on a transposable element display. Sites are arbitrarily numbered and are not similar between species. “Site occupancy” refers to the total number of elements within a sample divided by the number of sites.
DISCUSSION
We have identified a functional class II element of the hAT element superfamily, called Herves, that displays variation in both copy number and site distribution in both laboratory and field populations of An. gambiae, consistent with it being active, or recently active, in these populations.
Originally, Holt et al. (2002) identified 15 hAT sequences in the genome of An. gambiae. Of these 15 sequences, 4 matched the three active and the one presumably inactive copy of Herves (with a Topi element insertion) described here. The remaining 11 hAT sequences had high amino acid identity and similarity to the Herves ORF (identity ranged from 24 to 51%, similarity ranged from 41 to 69%, and for sequences aligned using BLASTP, E ≤ 0.01). This suggests that these sequences were closely related to Herves and may be from inactive forms of this element.
Several features of the Herves transposable element placed it in the hAT superfamily. First, Herves contained several amino acid motifs shared among hAT elements. For example, Herves contains the well-conserved WwxxxxxxxPxLxxxAxxxL motif described by Calvi et al. (1991) with 12 of the 17 amino acids in this region being identical to those found in Ac, hobo, and Tam3. Also, of the six conserved hAT protein blocks described by Rubin et al. (2001), Herves, like hobo, contained five (blocks A–E). Second, Herves ITRs were consistent with a proposed consensus sequence of hAT ITRs (Warren et al. 1995). Third, the three active Herves copies identified in the An. gambiae genome and the seven observed “cut-and-paste” transposition events (Table 2) inserted by forming 8-bp target-site duplications, characteristic of hAT elements. These duplications had a NWNNNNAY 85% consensus sequence, similar to consensus sequences proposed for other hAT elements (e.g., NTNNNNAC proposed for hobo and Hermes; O'Brochta et al. 1996).
Within the hAT superfamily the Herves transposase sequence was most closely related to the hopper-we element from Bactrocera dorsalis and Bactrocera cucurbitae (Handler 2003) but was distantly related to transposase sequences from other insect elements (e.g., Hermes from M. domestica). Beyond their transposase sequences, Herves and hopper-we also shared similar ITR sequences. This suggests that Herves and hopper-we might form a new group of insect hAT transposable elements that diverged prior to the Brachycera-Nematocera divergence. This hypothesis would be supported by the discovery of closely related elements in other species within these suborders. BAC-end clones from the Ae. aegypti sequencing project (http://www.tigr.org/tdb/e2k1/aabe/) were searched using the TBLASTN program (E ≤ 10−20; Altschul et al. 1990) and the Herves transposase amino acid sequence. This revealed the presence of a 213-aa sequence in Ae. aegypti with high similarity to Herves and hopper-we (distance to Herves: 0.96; to hopper-we, 1.4; to all other ORFs, 2.1–2.9; distance calculated using the program PROTDIST; Felsenstein 1993). While more research will be necessary to confirm that this sequence is indeed from a transposable element, these results suggest the possibility of a new, unexplored group of insect transposable elements within the hAT superfamily.
Herves can transpose in Drosophila cell lines and in Drosophila embryos and can genetically transform Drosophila. Transposition rates are highest in Drosophila embryos but are ∼30-fold less than those seen with the related Hermes element in similar assays performed in the Canton-S,w strain (Sarkar et al. 1997). Nevertheless, Herves transforms Drosophila at frequencies of the same order of magnitude as the P, hobo, Hermes, and piggyBac elements, indicating that it will be an efficient gene vector in at least this species. As such, Herves is the first active class II element isolated from any mosquito species and is the first to be used as a gene vector in an insect species. The An. gambiae genome project has facilitated the identification of potentially active P and piggyBac elements (Sarkar et al. 2003a,b), while there has been, previous to the publication of this genome sequence, circumstantial evidence for activity of the class II element Ikirara (Romans et al. 1998) and the class I elements Moose (Biessmann et al. 1999) and mtanga (Rohr et al. 2002). Donor Herves plasmids used in both transposition assays and fly transformations contain identical amounts of Herves left (1.4 kb) and right (302 bp) sequences, indicating that these sequences are sufficient for transposition of the element. Why Herves should perform relatively poorly in interplasmid transposition assays conducted in embryos and cell culture, but not in transformation of Drosophila, is unknown. The value of transposition assays may be primarily qualitative rather than quantitative; alternatively, Herves may be more efficient at transposition in germ-line nuclei than in somatic nuclei, the latter being measured in transposition assays performed in both cell cultures and developing embryos. The DNA target is also different between the transposition assays and genetic transformations, being a plasmid in the former and chromatin in the later. This difference might lead to a difference in transposition frequency but it is difficult, at this stage, to identify the precise structural or functional basis of such a difference between the Herves and Hermes elements.
The difference in target-site specificity between Herves and both hobo and Hermes is intriguing. Within the constraints of generating an 8-bp target-site duplication conforming to the consensus sequence of hAT element insertions, our data show that Herves does not favor insertion at the major hobo/Hermes hotspots in pGDV1, but rather prefers its own unique hotspot for integration. The existence of at least three functional insect hAT elements, hobo, Hermes, and Herves, provides a unique opportunity to examine the role that element sequences within a transposable element family play in determining insertion site specificity. Our data show that Herves, while clearly being a hAT element, is both structurally and functionally distinct from the hobo and Hermes elements. We have preliminary evidence that Herves may be active in An. gambiae. This, combined with its mobility in D. melanogaster, opens up the possibility that Herves might be used to improve the efficiency of mosquito transformation, particularly of An. gambiae and perhaps of other species as well. Herves transposition into the anopheline chromosomes might occur at high frequencies if this element has already adapted to being able to move in this mosquito genome. A converse argument is that mechanisms to suppress Herves transposition may have already evolved in An. gambiae but, as shown above and discussed below, we have preliminary evidence that suggests that Herves is currently mobile in this species. We have shown that Herves is functionally different from Hermes and hobo, and it is of interest to determine if these differences will manifest themselves in the behavior of engineered Herves elements in transgenic mosquitoes. Genetic transformation of An. gambiae remains problematic with only three successful transformations reported since 1987 (Miller et al. 1987; Grossman et al. 2001; Kim et al. 2004). Furthermore, the difficult husbandry of An. gambiae demands that transformation rates be high so as to ensure that transformed genotypes can be propagated from adults surviving the micro-injection procedure used to introduce gene vectors into developing germ-line cells.
Herves is likely to be actively transposing in natural populations of An. gambiae, An. arabiensis, and An. merus on the basis of the abundance of chromosomal sites that are occupied by Herves elements at low frequencies within populations. Earlier studies of transposable elements in natural populations of D. melanogaster described similar distributions and indicated that element movement was probably responsible for producing the observed frequency spectrum (Kaplan and Brookfield 1983; Langley et al. 1983; Montgomery and Langley 1983). Given the generally small effective population sizes for An. gambiae and An. arabiensis (on the order of 103 individuals), the observed frequency spectrums of Herves in An. gambiae s.l. in Furvela, Mozambique, are consistent with the hypothesis that the element is active (Taylor et al. 1993; Lehmann et al. 1998; Taylor and Manoukis 2003). The question that arises is whether Herves is at equilibrium in these populations, equilibrium being when the number of Herves elements in the genomes of members of a population is constant through a balance between replicative transposition and forces, such as genetic drift and natural selection, that might eliminate these elements. If the element is at equilibrium, then the frequency spectrum reflects the ongoing transposition and deletion activity of Herves. If the element is not at equilibrium, then the observed frequency spectrum may reflect a recent invasion of An. gambiae s.l. by Herves. These alternative hypotheses cannot be resolved using the data presented here. There are two interesting implications of these observations. First, current natural populations of An. gambiae should be able to support transpositional activity of Herves. Therefore, gene vectors or genetic drive agents constructed from this element will be functional in An. gambiae. Second, the active transposition of Herves in An. gambiae provides an opportunity to examine the dynamics of transposable element movement in a species being considered as a target for genetic control using recombinant DNA technologies. Using transposable elements to carry and spread genes of interest through natural populations of An. gambiae will require an understanding of how such elements are likely to behave. Herves may provide a critical bridge between laboratory-based gene vector technology development and the natural history of An. gambiae.
Acknowledgments
We thank G. C. Lanzaro (University of California, Davis, CA) and J. D. Charlwood (Danish Bilharziasis Institute) for supplying field material, P. Eggleston (Keele University), and F. H. Collins (University of Notre Dame) for supplying laboratory material used in these studies. We thank J. F. Y. Brookfield (University of Nottingham) for helpful and stimulating discussions and A. Arensburger (University of Maryland) for help with algorithm design. This research was supported by U.S. Public Health Service National Institutes of Health grants GM48102 to D.A.O. and AI45741 to P.W.A.
Sequence data from this article have been deposited with the EMBL/GenBank Data Libraries under accession no. AY462096.
References
- Allen, M. L., D. A. O'Brochta, P. W. Atkinson and C. S. LeVesque, 2001. Stable germ-line transformation of Culex quinquefasciatus (Diptera: Culicidae). J. Med. Entomol. 38: 701–710. [DOI] [PubMed] [Google Scholar]
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers and D. J. Lipman, 1990. Basic local alignment search tool. J. Mol. Biol. 215: 403–410. [DOI] [PubMed] [Google Scholar]
- Anxolabehere, D., M. G. Kidwell and G. Periquet, 1988. Molecular characteristics of diverse populations are consistent with the hypothesis of a recent invasion of D. melanogaster by mobile P elements. Mol. Biol. Evol. 5: 252–269. [DOI] [PubMed] [Google Scholar]
- Biessmann, H., M. F. Walter, D. Le, S. Chuan and J. G. Yao, 1999. Moose, a new family of LTR-retrotransposons in the mosquito, Anopheles gambiae. Insect Mol. Biol. 8: 201–212. [DOI] [PubMed] [Google Scholar]
- Calvi, B. R., T. J. Hong, S. D. Findley and W. M. Gelbart, 1991. Evidence for a common evolutionary origin of inverted terminal repeat transposons in Drosophila and plants: hobo, Activator and Tam3. Cell 66: 465–471. [DOI] [PubMed] [Google Scholar]
- Catteruccia, F., T. Nolan, T. G. Loukeris, C. Blass, C. Savakis et al., 2000. Stable germline transformation of the malaria mosquito Anopheles stephensi. Nature 405: 959–962. [DOI] [PubMed] [Google Scholar]
- Coates, C. J., N. Jasinskiene, L. Miyashiro and A. A. James, 1998. Mariner transposition and transformation of the yellow fever mosquito, Aedes aegypti. Proc. Natl. Acad. Sci. USA 95: 3748–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels, S. B., A. Chovnick and I. A. Boussy, 1990. a Distribution of hobo transposable elements in the genus Drosophila. Mol. Biol. Evol. 7: 589–606. [DOI] [PubMed] [Google Scholar]
- Daniels, S. B., K. R. Peterson, L. D. Strausbaugh, M. G. Kidwell and A. Chovnick, 1990. b Evidence for horizontal transmission of the P transposable element between Drosophila species. Genetics 124: 339–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito, T., F. Gianfrancesco, A. Ciccodicola, L. Montanini, S. Mumm et al., 1999. A novel pseudoautosomal human gene encodes a putative protein similar to Ac-like transposases. Hum. Mol. Genet. 8: 61–67. [DOI] [PubMed] [Google Scholar]
- Felsenstein, J., 1993 PHYLIP version 3.5c. Department of Genetics, University of Washington, Seattle.
- Grossman, G. L., A. J. Cornel, C. S. Rafferty, H. M. Roberston and F. H. Collins, 1999. Tsessebe, Topi and Tiang: three distinct Tc-1 like transposable elements in the malaria vector, Anopheles gambiae. Genetica 105: 69–80. [DOI] [PubMed] [Google Scholar]
- Grossman, G. L., C. S. Rafferty, J. R. Clayton, T. K. Stevens, O. Mukabayire et al., 2001. Germline transformation of the malaria vector, Anopheles gambiae, with the piggyBac transposable element. Insect Mol. Biol. 10: 597–604. [DOI] [PubMed] [Google Scholar]
- Guimond, N. D., D. K. Bideshi, A. C. Pinkerton, P. W. Atkinson and D. A. O'Brochta, 2003. Patterns of Hermes element transposition in Drosophila melanogaster. Mol. Genet. Genomics 268: 779–790. [DOI] [PubMed] [Google Scholar]
- Handler, A. M., 2003. Isolation and analysis of a new hopper hAT transposon from the Bactrocera dorsalis white eye strain. Genetica 118: 17–24. [DOI] [PubMed] [Google Scholar]
- Holt, R. A., G. M. Subramanian, A. Halpern, G. G. Sutton, R. Charlab et al., 2002. The genome sequence of the malaria mosquito Anopheles gambiae. Science 298: 129–149. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck, J. P., and F. Ronquist, 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Ito, J., A. Ghosh, L. A. Moreira, E. A. Wimmer and M. Jacobs-Lorena, 2002. Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature 417: 387–388. [DOI] [PubMed] [Google Scholar]
- Jasinskiene, N., C. J. Coates, M. Q. Benedict, A. J. Cornel, C. Salazar-Rafferty et al., 1998. Stable, transposon-mediated transformation of the yellow fever mosquito, Aedes aegypti, using the Hermes element from the house fly. Proc. Natl. Acad. Sci. USA 95: 3743–3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D. T., W. R. Taylor and J. M. Thornton, 1992. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 8: 275–282. [DOI] [PubMed] [Google Scholar]
- Jurka, J., 2000. Repbase update: a database and an electronic journal of repetitive elements. Trends Genet. 16: 418–420. [DOI] [PubMed] [Google Scholar]
- Kaplan, N., and J. F. Y. Brookfield, 1983. Transposable elements in Mendelian populations. III. Statistical results. Genetics 104: 485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, W., H. Koo, A. M. Richman, D. Seeley, J. Vizioli et al., 2004. Ectopic expression of a cecropin transgene in the human malaria vector mosquito Anopheles gambiae (Diptera: Culicidae): effects on susceptibility to Plasmodium. J. Med. Entomol. 41: 447–455. [DOI] [PubMed] [Google Scholar]
- Kokoza, V., A. Ahmed, E. A. Wimmer and A. S. Raikhel, 2001. Efficient transformation of the yellow fever mosquito Aedes aegypti using the piggyBac transposable element vector pBac [3xP3-EGFPafm]. Insect Biochem. Mol. Biol. 31: 1137–1143. [DOI] [PubMed] [Google Scholar]
- Langley, C. H., J. F. Y. Brookfield and N. Kaplan, 1983. Transposable elements in Mendelian populations. I. A theory. Genetics 104: 457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, T., W. A. Hawley, H. Grebert and F. H. Collins, 1998. The effective population size of Anopheles gambiae in Kenya: implications for population structure. Mol. Biol. Evol. 15: 264–276. [DOI] [PubMed] [Google Scholar]
- Lohe, A. R., and D. L. Hartl, 1996. Reduced germline mobility of a mariner vector containing exogenous DNA: Effect of size or site? Genetics 143: 1299–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozovsky, E. R., D. I. Nurminsky, E. A. Wimmer and D. L. Hartl, 2002. Unexpected stability of mariner transgenes in Drosophila. Genetics 160: 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, L. H., R. K. Sakai, P. Romans, R. W. Gwadz, P. Kantoff et al., 1987. Stable integration and expression of a bacterial gene in the mosquito, Anopheles gambiae. Science 237: 779–781. [DOI] [PubMed] [Google Scholar]
- Montgomery, E. A., and C. H. Langley, 1983. Transposable elements in Mendelian populations. II. Distribution of three copia-like elements in a natural population. Genetics 104: 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira, L. A., J. Ito, A. Ghosh, M. Devenport, H. Zieler et al., 2002. Bee venom phosopholipase inhibits malaria parasite development in transgenic mosquitoes. J. Biol. Chem. 277: 40839–40843. [DOI] [PubMed] [Google Scholar]
- O'Brochta, D. A., W. D. Warren, K. J. Saville and P. W. Atkinson, 1996. Hermes, a functional non-drosophilid insect gene vector from Musca domestica. Genetics 142: 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brochta, D. A., N. Sethuramuran, R. Wilson, R. H. Hice, A. C. Pinkerton et al., 2003. Gene vector and transposable element behavior in mosquitoes. J. Exp. Biol. 203: 3823–3834. [DOI] [PubMed] [Google Scholar]
- Perera, O. P., R. A. I. Harrell and A. M. Handler, 2002. Germ-line transformation of the South American malaria vector, Anopheles albimanus, with a piggyBac/EGFP transposon vector is routine and highly efficient. Insect Mol. Biol. 11: 291–297. [DOI] [PubMed] [Google Scholar]
- Robertson, H. M., 2002 Evolution of DNA transposons in eukaryotes, pp. 1093–1110 in Mobile DNA II, edited by N. L. Craig, R. Craigie, M. Gellert and A. M. Lambowitz. ASM Press, Washington, DC.
- Robertson, H. M., and E. G. MacLeod, 1993. Five major subfamilies of mariner transposable elements in insects, including the Mediterranean fruit fly, and related arthropods. Insect Mol. Biol. 2: 125–139. [DOI] [PubMed] [Google Scholar]
- Rohr, C. J. B., H. A. Ranson, X. Wang and N. J. Besansky, 2002. Structure and evolution of mtange, a retrotransposon actively expressed on the Y chromosome of the African malaria vector Anopheles gambiae. Mol. Biol. Evol. 19: 149–162. [DOI] [PubMed] [Google Scholar]
- Romans, P., R. K. Bhattacharyya and A. Colavita, 1998. Ikirara, a novel transposon family from the malaria vector mosquito, Anopheles gambiae. Insect Mol. Biol. 7: 1–10. [DOI] [PubMed] [Google Scholar]
- Rubin, E., G. Lithwick and A. A. Levy, 2001. Structure and evolution of the hAT transposon superfamily. Genetics 158: 949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, G. M., and A. C. Spradling, 1982. Genetic transformation of Drosophila with transposable element vectors. Science 218: 348–353. [DOI] [PubMed] [Google Scholar]
- Sarkar, A., C. J. Coates, S. Whyard, U. Willhoeft, P. W. Atkinson et al., 1997. The Hermes element from Musca domestica can transpose in four families of cylorrhaphan flies. Genetica 99: 15–29. [DOI] [PubMed] [Google Scholar]
- Sarkar, A., R. Sengupta, J. Krzywinski, X. Wang, C. Roth et al., 2003. a P elements are found in the genomes of nematoceran insects of the genus Anopheles. Insect Biochem. Mol. Biol. 33: 381–387. [DOI] [PubMed] [Google Scholar]
- Sarkar, A., C. Sim, Y. S. Hong, J. R. Hogan, M. J. Fraser et al., 2003. b Molecular evolutionary analysis of the widespread piggyBac transposon family and related “domesticated” sequences. Mol. Genet. Genomics 270: 173–180. [DOI] [PubMed] [Google Scholar]
- Schmidt, H. A., K. Strimmer, M. Vingron and A. von Haeseler, 2002. TREE-PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics 18: 502–504. [DOI] [PubMed] [Google Scholar]
- Schneider, I., 1972. Cell lines derived from late embryonic stages of Drosophila melanogaster. J. Embryol. Exp. Morphol. 27: 363–365. [PubMed] [Google Scholar]
- Swofford, D. L., 1998 PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods), Version 4. Sinauer Associates, Sunderland, MA.
- Taylor, C. E., and N. C. Manoukis, 2003 Effective population size in relation to genetic modification of Anopheles gambiae sensu stricto, pp. 133–146 in Ecological Aspects for Application of Genetically Modified Mosquitoes, edited by W. Takken and T. W. Scott. Kluver Academic, Dordrecht, The Netherlands.
- Taylor, C. E., Y. T. Toure, M. Coluzzi and V. Petrarca, 1993. Effective population size and persitance of Anopheles arabiensis during the dry season in west Africa. Med. Vet. Entomol. 7: 351–357. [DOI] [PubMed] [Google Scholar]
- Thompson, J. D., D. G. Higgins and T. J. Gibson, 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, W. D., P. W. Atkinson and D. A. O'Brochta, 1995. The Australian bushfly Musca vetustissima contains a sequence related to transposons of the hobo, Ac and Tam3 family. Gene 154: 133–134. [DOI] [PubMed] [Google Scholar]
- Whelan, S., and N. Goldman, 2001. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol. Biol. Evol. 18: 691–699. [DOI] [PubMed] [Google Scholar]
- Wilson, R., J. Orsetti, A. K. Klocko, C. Aluvihare, E. Peckham et al., 2003. Post-integration behavior of a Mos1 mariner gene vector in Aedes aegypti. Insect Biochem. Mol. Biol. 33: 853–863. [DOI] [PubMed] [Google Scholar]
- Zhou, L., R. Mitra, P. W. Atkinson, A. B. Hickman, F. Dyda et al., 2004. Transposition of hAT elements links transposable elements and V(D)J recombination. Nature 432: 995–1001. [DOI] [PubMed] [Google Scholar]