Abstract
Linkage group identities and homologies were determined for metaphase chromosomes of Sorghum bicolor (2n = 20) by FISH of landed BACs. Relative lengths of chromosomes in FISH-karyotyped metaphase spreads of the elite inbred BTx623 were used to estimate the molecular size of each chromosome and to establish a size-based nomenclature for sorghum chromosomes (SBI-01–SBI-10) and linkage groups (LG-01 to LG-10). Lengths of arms were determined to orient linkage groups relative to a standard karyotypic layout (short arms at top). The size-based nomenclature for BTx623 represents a reasonable choice as the standard for a unified chromosome nomenclature for use by the sorghum research community.
LINKAGE mapping of Sorghum has progressed quickly, using diverse mapping populations and markers (Whitkus et al. 1992; Chittenden et al. 1994; Pereira et al. 1994; Xu et al. 1994; Dufour et al. 1997; Ming et al. 1998; Tao et al. 1998, 2000; Boivin et al. 1999; Crasta et al. 1999; Peng et al. 1999; Bhattramakki et al. 2000; Kong et al. 2000; Haussmann et al. 2002; Menz et al. 2002; Bowers et al. 2003). The lack of a common nomenclature system for sorghum linkage groups, however, has made it difficult and cumbersome to compare and use results obtained by different groups. For most well-studied genomes, linkage group nomenclature and chromosomal designations are integrated and are usually based on biological parameters, e.g., chromosome size, arm length, and arm orientation (Werner et al. 1992; Fransz et al. 1998; Künzel et al. 2000; Cheng et al. 2001; Kulikova et al. 2001; Howell et al. 2002; Anderson et al. 2003). Conventional and C-band karyotypes of Sorghum species were reported by Gu et al. (1984) and Yu et al. (1991), respectively, but means of evaluation were lacking and their relationship to molecular markers and genomic resources remains unknown. In contrast, identification of sorghum chromosomes by simultaneous fluorescence in situ hybridization (FISH) of a landed BAC cocktail was devised to establish a FISH-based karyotypic system for sorghum (Kim et al. 2002). It provides a cyto-genomic approach in which linkage group markers and cytological markers are integrated.
Here, we used FISH-based karyotyping in concert with analysis of chromosome lengths, arm lengths, and arm ratios to establish a size-based nomenclature for sorghum chromosomes. The ability to reliably identify contracted chromosomes facilitated development of a standardized karyotype (ideogram) for Sorghum bicolor (L.) Moench. The results enabled us to align and orientate the linkage maps relative to the 10 chromosome pairs, and to develop nomenclatures for chromosomes and linkage groups that are based on sorghum chromosome size.
MATERIALS AND METHODS
BACs used for FISH were derived from libraries prepared by Woo et al. (1994) and Tao and Zhang (1998). The BACs were located on the sorghum linkage map as described by Klein et al. (2000), and BAC DNA used for FISH was isolated as previously described (Islam-Faridi et al. 2002). Molecular cytogenetic methods were as described by Kim et al. (2002), except as follows. Root tips from glasshouse-grown sorghum [S. bicolor (L.) Moench] plants of the elite line BTx623 were treated with saturated aqueous α-monobromonaphthalene for 2 hr and then fixed and processed for slide making as described previously (Kim et al. 2002). Prior to FISH, chromosomal DNA on slides was denatured at 70° in 100 μl of 70% formamide in 2× SSC on a hot block for 1.5 min followed by dehydration in 70% ethanol at −20° and 85, 95, and 100% ethanol at room temperature, respectively. For single-probe FISH, the hybridization mixture (25 μl) contained 10 ng of labeled BAC probe DNA, 50% formamide, 10% dextran sulfate, and 2× SSC. The mixture was denatured at 90° for 10 min, chilled on ice, and added to the slide. For FISH of the multi-probe cocktail from 17 BAC clones, 50× Cot-1 DNA was added to the probe mixture, which was denatured at 90° for 10 min, chilled on ice, and then annealed for 30 min before application to the slide. Following overnight incubation at 37°, slides were rinsed at 40° for 5 min in a series of washes consisting of 2× SSC, 50% formamide in 2× SSC, 2× SSC, and 4× SSC plus 0.2% Tween 20, respectively.
Images were taken from an Olympus AX-70 epifluorescence microscope (Olympus America, Melville, NY) equipped with standard filter cubes, a Peltier-cooled monochrome 1.3 megapixel Sensys camera (Photometrics, Tucson, AZ), and MacProbe v.4.2.3 digital imaging system (Applied Imaging, San Jose, CA). Homologous chromosome pairs were identified with the aid of MacProbe v.4.2.3, according to the pattern of signal on each chromosome. For karyotyped images, DAPI-stained chromosomes were measured using Optimas v6.0 (Media Cybernetics, Silver Spring, MD). The centromere for each chromatid was identified by the primary constriction and also by FISH of the centromere-associated sequence pCEN38 (Zwick et al. 2000). FISH-identified chromatid arms were measured and averaged to determine the length for each arm of the genome. The arm ratio (average long arm/short arm ratio), total chromosome length (short arm + long arm), and relative chromosome length (length of the individual chromosome/total length of all chromosomes in the genome) were calculated for each chromosome in the complement. Data were exported to a spreadsheet (Microsoft Excel) and analyzed.
RESULTS AND DISCUSSION
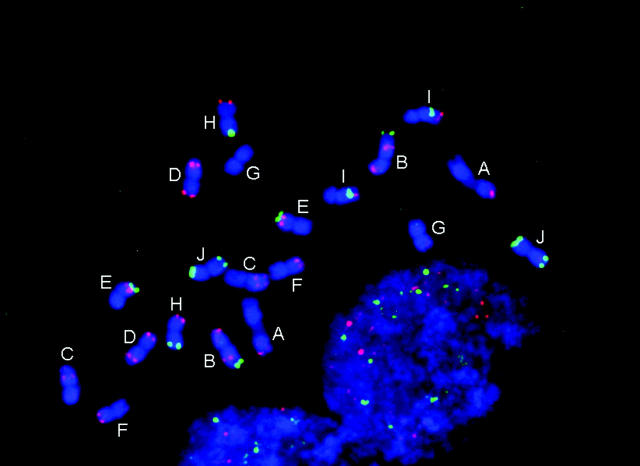
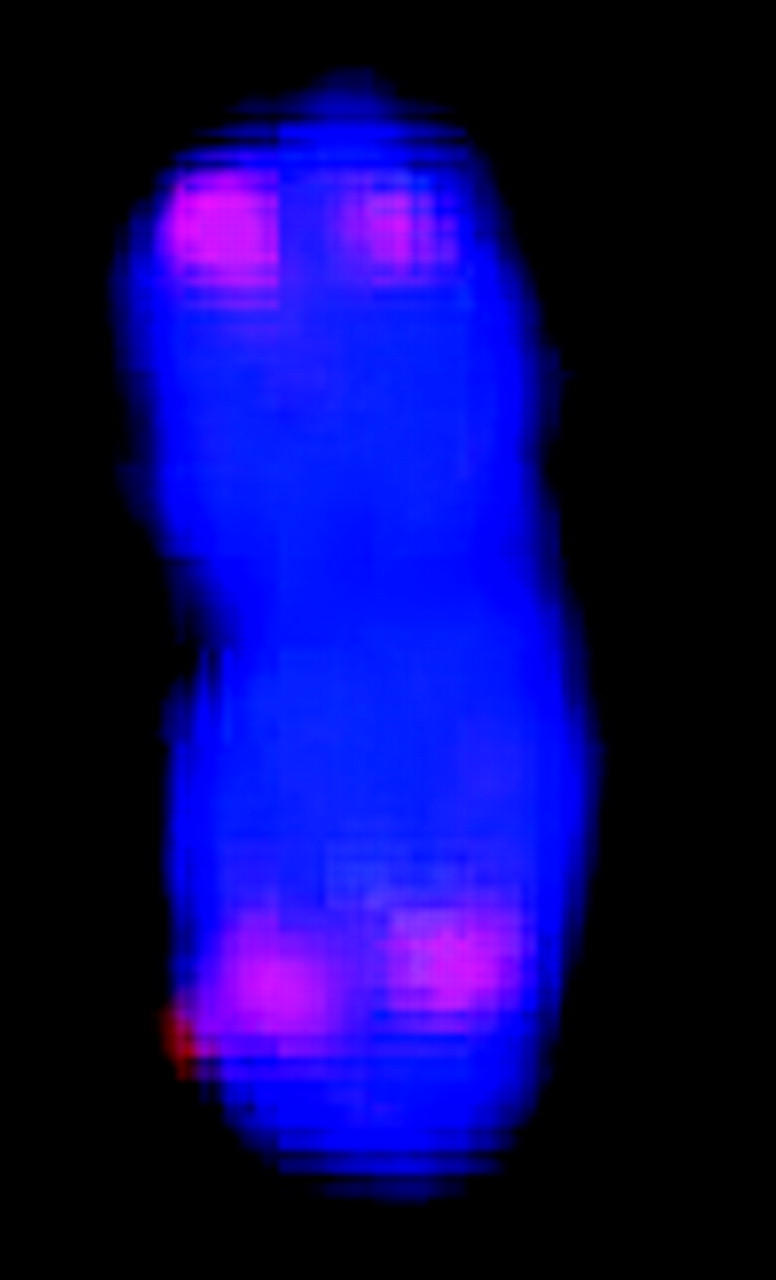
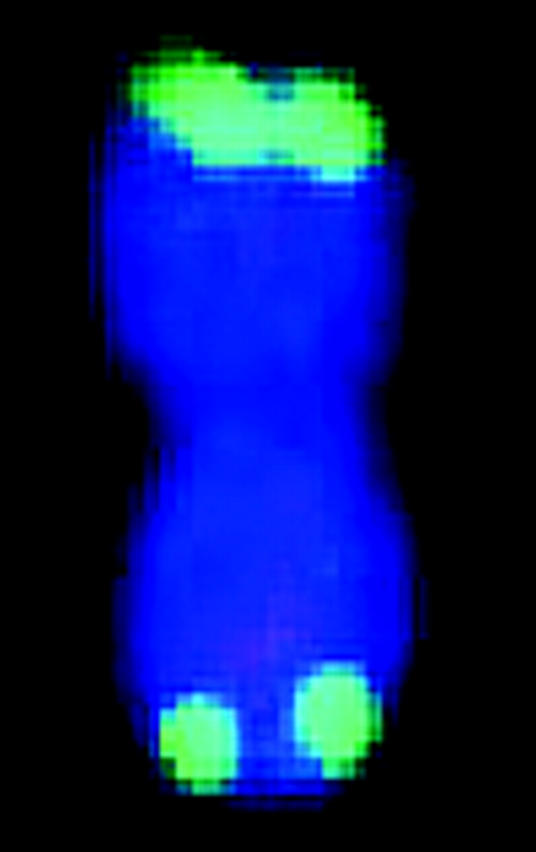
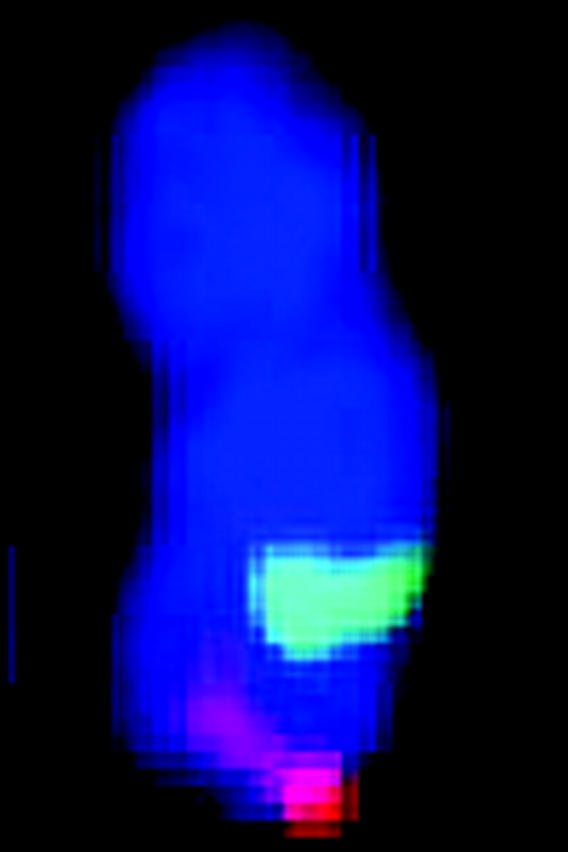
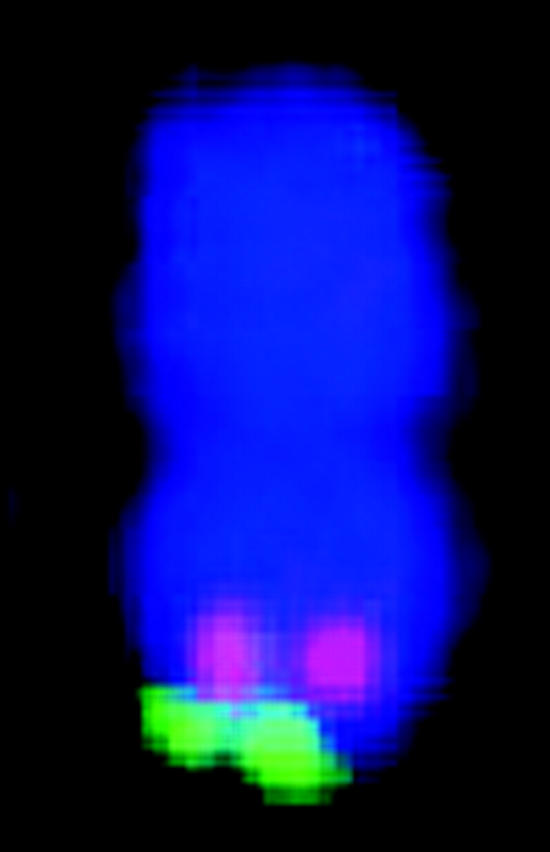
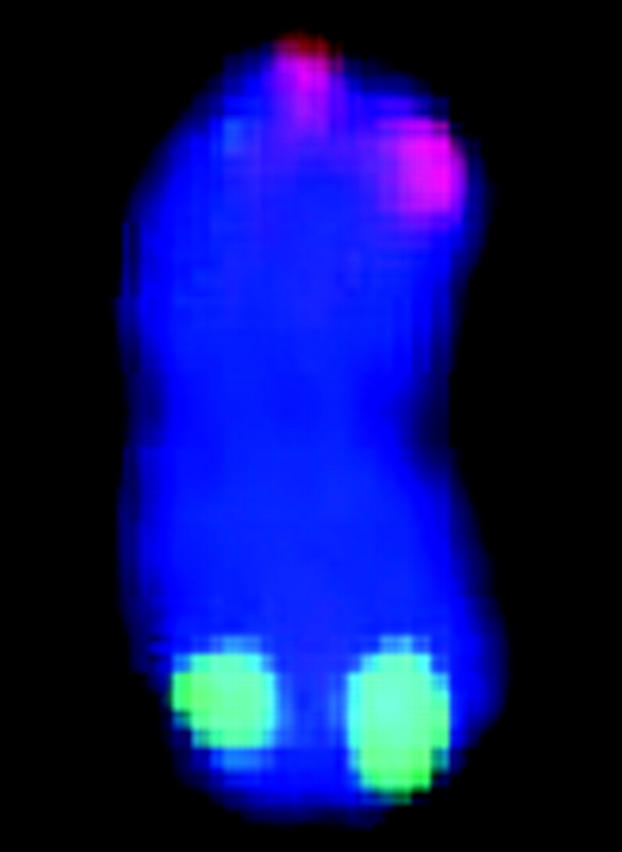
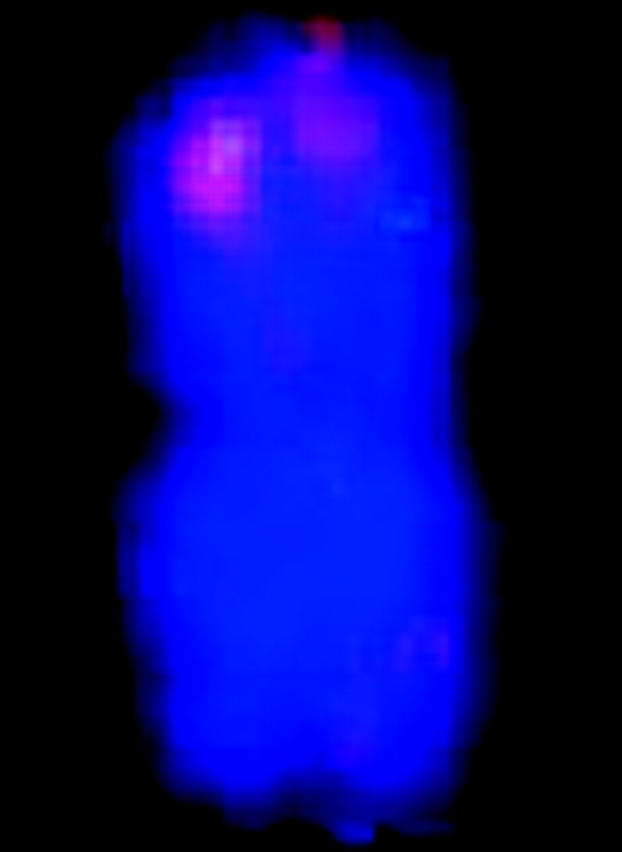
FISH markers enabled identification of all 20 mitotic metaphase chromosomes with respect to homology (within cells) and common identity (across cells) and relative to linkage groups (Figure 1, Table 1). Although C-banding can be used for identification of sorghum chromosomes that are not fully condensed (Yu et al. 1991), for the purpose of molecular size estimation, it is important to target metaphase, i.e., when molecular density is most uniform along the chromosome long axis and relative lengths most accurately reflect relative molecular size. Without FISH, reliable identification of all metaphase chromosomes would have been very difficult if not impossible, because distinctive features tend to vanish as chromatin becomes highly contracted.
Figure 1.—
Simultaneous FISH of a 17-BAC cocktail probe to sorghum mitotic metaphase chromosome spread. The patterns of signals enable FISH-based recognition of each chromosome pair and associate specific linkage groups with specific chromosomes. Each letter corresponds to a linkage group (Menz et al. 2002).
TABLE 1.
Relationship of the FISH-based karyotype of sorghum and the linkage groups composing the various linkage maps of the sorghum genome
| Chrom. no.a: | SBI-01 | SBI-02 | SBI-03 | SBI-04 | SBI-05 | SBI-06 | SBI-07 | SBI-08 | SBI-09 | SBI-10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Linkage group (LG): | LG-01 | LG-02 | LG-03 | LG-04 | LG-05 | LG-06 | LG-07 | LG-08 | LG-09 | LG-10 | |
| LG in Menz et al. (2002)b | A | B | C | D | J | I | E | H | F | G | |
| LG in Pereira et al. (1994) | C | F | G | D | J | B | A | I | E | H | |
| LG in Bowers et al. (2003)c | C | B | A | F | H | D | J | E | G | I | |
| LG in Crastraet al. (1999) | G, K | D | A | C | J | F | E | H | I | B | |
| LG in Boivin et al. (1999)d | C, K | F | G | D, L | J | B | A | I | E | H | |
| LG in Whitkus et al. (1992) | B, C | D | F, M | H | G | E | A | K, L | I | J | |
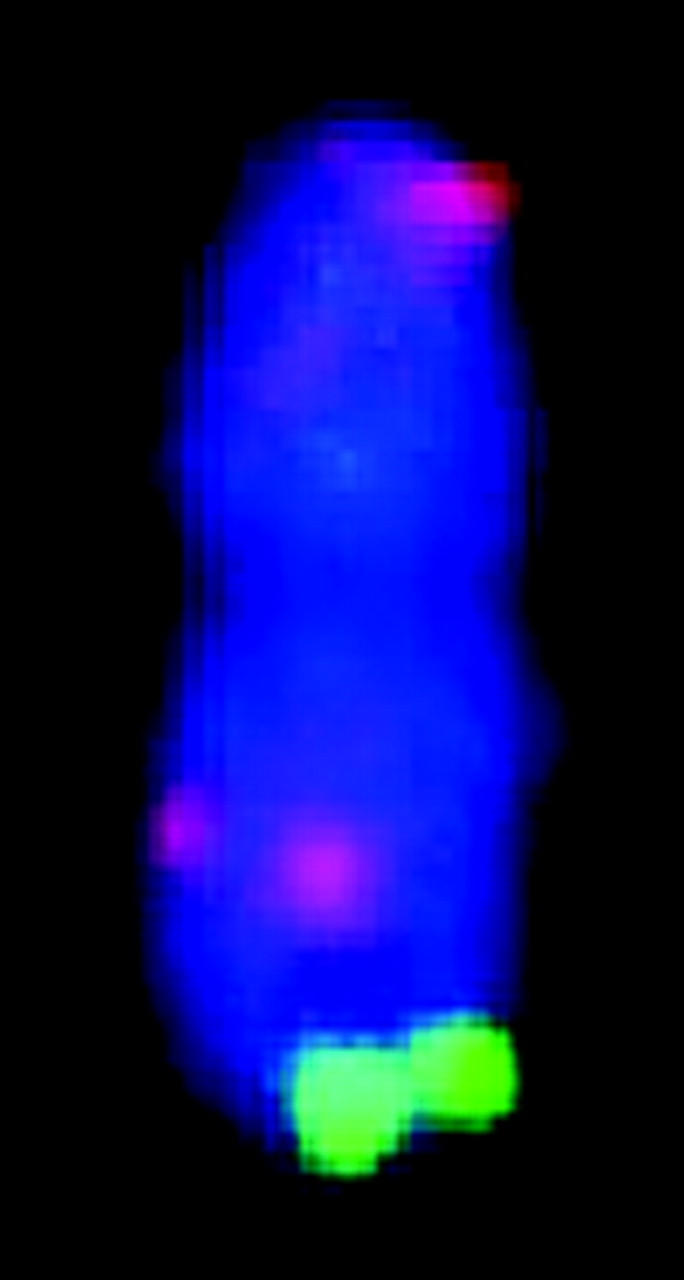
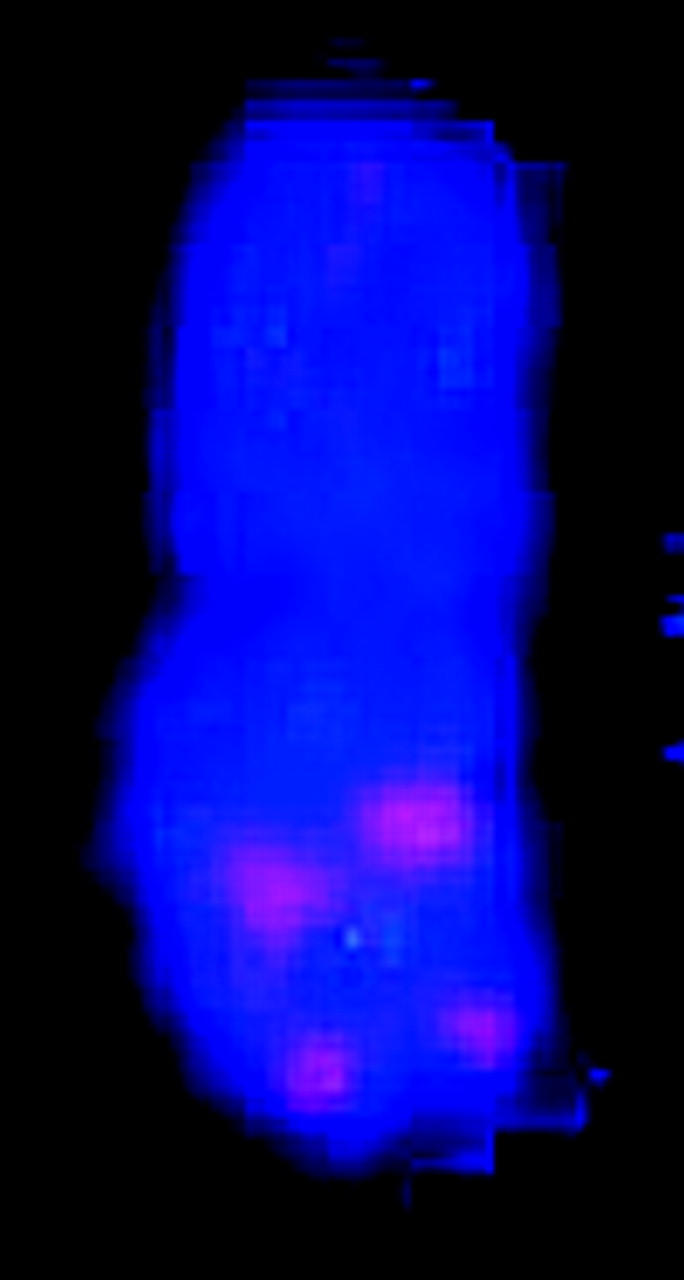
| FISH karyotypee | 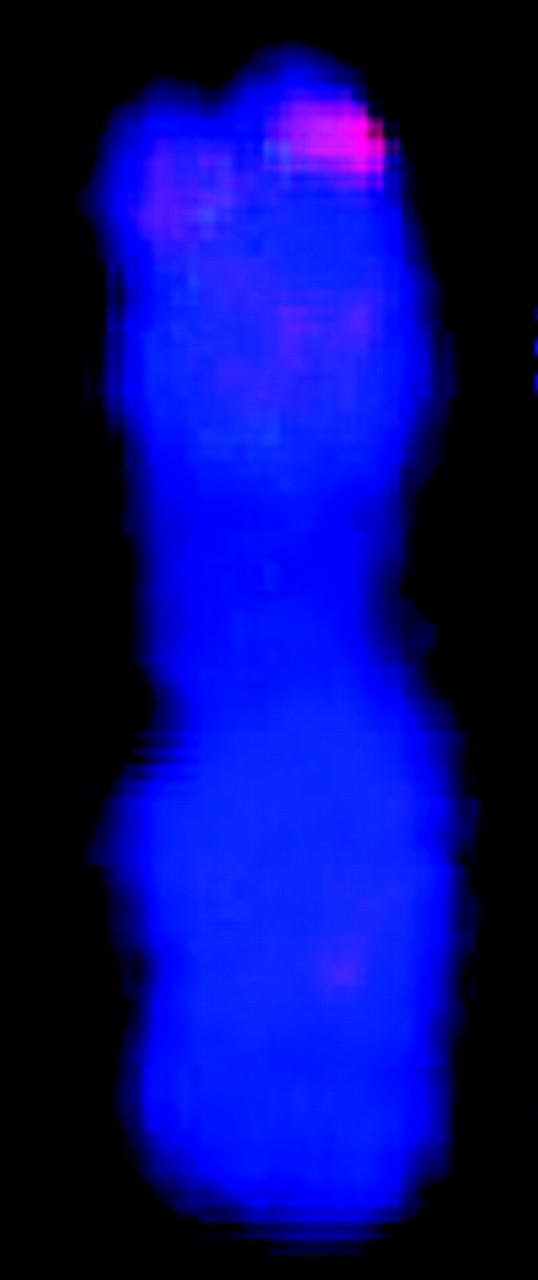 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
| Total length (μm) | 5.11 | 3.87 | 3.85 | 3.5 | 3.44 | 3.15 | 3.13 | 3.07 | 2.98 | 2.94 | |
| Standard errorf | 0.047 | 0.035 | 0.038 | 0.032 | 0.037 | 0.029 | 0.028 | 0.026 | 0.029 | 0.023 | |
| Relative lengthg | 14.59 | 11.06 | 10.98 | 9.99 | 9.82 | 9.00 | 8.92 | 8.75 | 8.51 | 8.39 | |
| Estimated DNA contenth | 119.3 | 90.5 | 89.8 | 81.7 | 80.3 | 73.6 | 73.0 | 71.6 | 69.6 | 68.6 | |
| Arm ratioi | 1.32 | 1.16 | 1.13 | 1.14 | 1.02 | 1.42 | 1.06 | 1.10 | 1.02 | 1.04 |
Chromosomes were ordered and numbered according to their rank of the total length at metaphase (full contraction).
Linkage group designations are identical to those described in Peng et al. (1999), Kong et al. (2000), Bhattramakki et al. (2000), and Haussmann et al. (2002).
Linkage group designations are identical to those described in Chittenden et al. (1994) and Tao et al. (2000).
Linkage group designations are identical to those described in Dufour et al. (1997).
The chromosomes are displayed according to cytogenetic convention with the short arm at the top of the vertical chromosome. The 17 BACs used for the karyotype are denoted in Figure 2 by an asterisk.
The sample size for measurements was 40.
Relative length = 100(chromosome length/genome length).
Estimated DNA content = relative length × estimated genome size, i.e., 818 Mbp (Price et al. 2005).
Arm ratio = length of the long arm/the length of the short arm.
Metaphase chromosome arms were measured and tabulated and later sorted by total chromosome length (Table 1). A FISH-based karyotype of S. bicolor inbred line BTx623 was developed, in which chromosomes were ordered and designated according to total length at metaphase, namely SBI-01 (longest) to SBI-10 (shortest). The three-letter acronym SBI designates the genus and species, and the two-digit numeric code denotes the chromosome number. The consistent use of two digits will facilitate data sorting by computers. For linkage groups that relate well to the structure of the BTx623 genome, we suggest that they be referred to analogously, as LG-01 to LG-10 and that arms be oriented as is customary in karyotypes: p (short) arm at the top and q (long) arm at the bottom (Figure 2). The relationship between sorghum chromosomes and many of the published sorghum linkage maps is also shown in Table 1. Adoption of a common nomenclature for sorghum linkage groups will facilitate the integration of data and genomic resources developed by independent research laboratories.
Figure 2.—
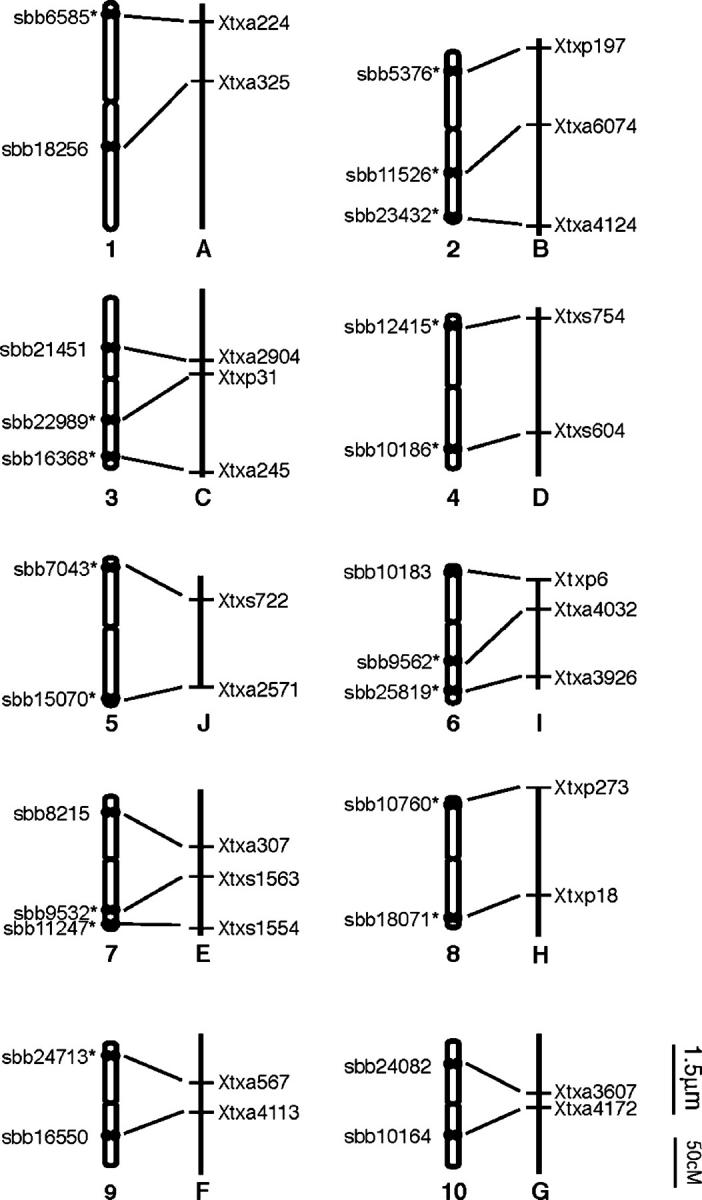
Correlation between mitotic metaphase chromosomes and linkage groups of sorghum using the map of Menz et al. (2002). Asterisks denote signals from the 17 BACs shown in Figure 1. Chromosomes are numbered according to size and linkage groups are labeled alphabetically. Chromosomes are depicted with the shorter arm in the top position. BAC clones are positioned on the ideogram according to their positions relative to the centromeres. Bar indicates 1.5 μm for metaphase chromosomes and 50 cM for linkage maps.
The karyotype of BTx623 is grossly similar to those of other sorghum accessions and cultivars (Magoon and Shambulingappa 1960; Magoon and Ramana 1961; Magoon et al. 1964; Bennett and Laurie 1995; Sang and Liang 2000). BTx623 contained an exceptionally long pair of chromosomes, SBI-01, eight pairs of metacentric chromosomes closely graded in size, SBI-02, -03, -04, -05, -07, -08, -09, and -10, and one pair of midsized submetacentric chromosomes, SBI-06. SBI-01 is morphologically the most distinct chromosome of the sorghum haploid complement. In addition to its distinctive length (5.11 μm), SBI-01 is one of only two submetacentric pairs and is the only “satellite” chromosome. Lengths of the remaining chromosomes followed a somewhat bimodal distribution, with SBI-02, -03, -04, and -05 constituting the group of longer chromosomes (3.87–3.44 μm) and SBI-06, -07, -08, -09, and -10 constituting the group of shorter ones (3.15–2.97 μm).
The only secondary constriction and nucleolus organizing region (NOR) observed in BTx623 was located near the centromere in the short arm of chromosome 1, SBI-01p. It should be noted, however, that the relative length of the two SBI-01 arms shifts during the mitotic chromosome contraction. Because NORs contract differentially late in the cell cycle and are otherwise very long, overall length of the NOR-bearing arm, SBI-01p, actually exceeds that of the long arm (SBI-01q) until the chromatin contraction process is nearly complete, i.e., at metaphase. Thus, the designation of relative arm sizes at metaphase should connote relative molecular size as well.
In most higher eukaryotes, NORs are situated in short arms of subacrocentric or submetacentric chromosomes. The medial position seen in BTx623 is of interest, but not unique. NORs in most S. bicolor genotypes (and a number of other Sorghum species) occur in medial locations of the largest chromosome of the genome (Magoon and Shambulingappa 1960; Magoon and Ramana 1961; Magoon et al. 1964; Bennett and Laurie 1995; Sang and Liang 2000). However, a temporary constriction occurs in the fifth largest chromosome of a variety of S. bicolor cultivated for silage, in addition to the major constriction in its largest chromosome (Gu et al. 1984). The NOR of S. bicolor Combine Kafir 60 is located in the middle of the fifth longest chromosome (Yu et al. 1991). The NOR of its close rhizomatous relative, S. propinquum, is located in the short arm of the smallest chromosome (Magoon and Shambulingappa 1961). Such structural differences between parents can complicate linkage analysis (e.g., see Bowers et al. 2003) and undermine the applicability of each linkage map beyond the respective parental combination.
We developed an integrated “cyto-genomic” map from FISH data on 24 BACs containing linkage markers from across the sorghum genome (Figure 2, Table 2). The centromere position of each chromosome was identified using the centromere-specific probe pCEN38, as previously described by Islam-Faridi et al. (2002; data not shown). Relative to the karyotyping convention (shorter arms at top), the orientations of linkage groups were concordant for SBI-01, -02, -04, -05, -06, -07, and -10, but inverted for SBI-03, -08, and -09 (Figure 2).
TABLE 2.
List of genetic markers and their anchored BACs used for integrating linkage and cytogenetic maps
| Chromosome map
|
Linkage map
|
|||||
|---|---|---|---|---|---|---|
| Chromosome no. | BAC | Arm location of signal |
Linkage group |
Marker | Map distance | Total map distance (cM) |
| SBI-01 | sbb6585 | Short | LG-01 | Xtxa224 | 16.2–19.1 | 232.2 |
| sbb18256 | Long | Xtxa325 | 75.6–80.5 | |||
| SBI-02 | sbb5376 | Short | LG-02 | Xtxp197 | 7.9–11.1 | 205.2 |
| sbb11526 | Long | Xtxa6074 | 89.7 | |||
| sbb23432 | Long | Xtxa4124 | 193.4–196.9 | |||
| SBI-03 | sbb21451 | Short | LG-03 | Xtxa2904 | 76.5–79.9 | 196.5 |
| sbb22989 | Long | Xtxp31 | 91.0–94.4 | |||
| sbb16368 | Long | Xtxa245 | 190.3–196.5 | |||
| SBI-04 | sbb12415 | Short | LG-04 | Xtxs754 | 9.7 | 174.6 |
| sbb10186 | Long | Xtxs604 | 130.1 | |||
| SBI-05 | sbb7043 | Short | LG-05 | Xtxs722 | 23 | 118 |
| sbb15070 | Long | Xtxa2571 | Off-end (118) | |||
| SBI-06 | sbb10183 | Short | LG-06 | Xtxp6 | 0 | 115.8 |
| sbb9562 | Long | Xtxa4032 | 26.0–29.3 | |||
| sbb25819 | Long | Xtxa3926 | 102.3 | |||
| SBI-07 | sbb8215 | Short | LG-07 | Xtxa307 | 73.9 | 155.9 |
| sbb9532 | Long | Xtxs1563 | 92.2-97.2 | |||
| sbb11247 | Long | Xtxs1554 | 151.2 | |||
| SBI-08 | sbb10760 | Short | LG-08 | Xtxp273 | 0 | 152.3 |
| sbb18071 | Long | Xtxp18 | 109.5-111.2 | |||
| SBI-09 | sbb24713 | Short | LG-09 | Xtxa567 | 54.4 | 153 |
| sbb16550 | Long | Xtxa4113 | 85 | |||
| SBI-10 | sbb24082 | Short | LG-10 | Xtxa3607 | 64.3 | 148 |
| sbb10164 | Long | Xtxa4172 | 77.9–80.3 | |||
The adoption of a common reference for nomenclature of sorghum chromosomes and a related nomenclature for linkage groups would facilitate development of gramineous genomics, e.g., by enhancing communication between research groups and data usage across genome maps. The unified nomenclature system for chromosomes and linkage groups of line BTx623 provide a reasonable basis for a genomic nomenclature for S. bicolor in that this line is readily available, highly inbred, and extensively used for genetic, breeding, and genomics research. However, caution must be exercised in applying the nomenclature to other mapping endeavors because the incidence of structural rearrangements in sorghum is inadequately studied, so it remains reasonably likely that genomes of mapping parents differ structurally. FISH-karyotypic analysis of parents and meiotic analysis of their F1 hybrids might alert researchers to perturbations that could otherwise cryptically distort linkage maps and predictions derived from them or preclude expected genetic gains.
Acknowledgments
We thank William L. Rooney, Department of Soil and Crop Sciences, for providing seed for mitotic preparations. We gratefully acknowledge support by the Texas Agricultural Experimental Station, Texas A&M University, the Perry Adkisson Chair (J.E.M.), and the National Science Foundation Plant Genome grants DBI-0077713 (J.E.M. and P.E.K.) and DBI-0321578 (P.E.K., J.E.M., and R.R.K.).
References
- Anderson, L. K., G. G. Doyle, B. Brigham, J. Carter, K. D. Hooker et al., 2003. High-resolution crossover maps for each bivalent of Zea mays using recombination nodules. Genetics 165: 849–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, M. D., and D. A. Laurie, 1995. Chromosome size in maize and sorghum using EM serial section reconstruction nuclei. Maydica 40: 199–204. [Google Scholar]
- Bhattramakki, D., J. Dong, K. Chhabra and G. E. Hart, 2000. An integrated SSR and RFLP linkage map of Sorghum bicolor (L.) Moench. Genome 43: 988–1002. [PubMed] [Google Scholar]
- Boivin, K., M. Deu, J-F. Rami, G. Trouche and P. Hamon, 1999. Towards a saturated sorghum map using RFLP and AFLP markers. Theor. Appl. Genet. 98: 320–328. [Google Scholar]
- Bowers, J. E., C. Abbey, S. Anderson, C. Chang, X. Draye et al., 2003. A high-density genetic recombination map of sequence-tagged sites for Sorghum, as a framework for comparative structural and evolutionary genomics of tropical grains and grasses. Genetics 165: 367–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Z., C. R. Buell, R. A. Wing, M. Gu and J. Jiang, 2001. Toward a cytological characterization of the rice genome. Genome Res. 11: 2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittenden, L. M., K. F. Schertz, Y.-R. Lin, R. A. Wing and A. H. Paterson, 1994. A detailed RFLP map of Sorghum bicolor × S. propinquum, suitable for high-density mapping, suggests ancestral duplication of Sorghum chromosomes or chromosomal segments. Theor. Appl. Genet. 87: 925–933. [DOI] [PubMed] [Google Scholar]
- Crasta, O. R., W. W. Xu, D. T. Rosenow, J. Mullet and H. T. Nguyen, 1999. Mapping of post-flowering drought resistance traits in grain sorghum: association between QTLs influencing premature senescence and maturity. Mol. Gen. Genet. 262: 579–588. [DOI] [PubMed] [Google Scholar]
- Dufour, P., M. Deu, L. Grivet, A. D'Hont, F. Paulet et al., 1997. Construction of a composite sorghum genome map and comparison with sugarcane, a related complex polyploid. Theor. Appl. Genet. 94: 409–418. [Google Scholar]
- Fransz, P. F., S. Armstrong, C. Alonso-Blanco, T. C. Fischer, R. A. Torres-Ruiz et al., 1998. Cytogenetics for the model system Arabidopsis thaliana. Plant J. 13: 867–876. [DOI] [PubMed] [Google Scholar]
- Gu, M. H., H. T. Ma and G. H. Liang, 1984. Karyotype analysis of seven species in the genus Sorghum. J. Hered. 75: 196–202. [Google Scholar]
- Haussmann, B. I. G., D. E. Hess, N. Seetharama, H. G. Welz and H. H. Geiger, 2002. Construction of a combined sorghum linkage map from two recombinant inbred populations using AFLP, SSR, RFLP, and RAPD markers, and comparison with other sorghum maps. Theor. Appl. Genet. 105: 629–637. [DOI] [PubMed] [Google Scholar]
- Howell, E. C., G. C. Barker, G. H. Jones, M. J. Kearsey, G. J. King et al., 2002. Integration of the cytogenetic and genetic linkage maps of Brassica oleracea. Genetics 161: 1225–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam-Faridi, M. N., K. L. Childs, P. E. Klein, G. Hodnett, M. A. Menz et al., 2002. A molecular cytogenetic map of sorghum chromosome 1: fluorescence in situ hybridization analysis with mapped bacterial artificial chromosomes. Genetics 161: 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.-S., K. L. Childs, M. N. Islam-Faridi, M. A. Menz, R. R. Klein et al., 2002. Integrated karyotyping of sorghum by in situ hybridization of landed BACs. Genome 45: 402–412. [DOI] [PubMed] [Google Scholar]
- Klein, P. E., R. R. Klein, S. W. Cartinhour, P. E. Ulanch, J. Dong et al., 2000. A high-throughput AFLP-based method for constructing integrated genetic and physical maps: progress toward a sorghum genome map. Genome Res. 10: 789–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, L., J. Dong and G. E. Hart, 2000. Characteristics, linkage-map positions, and allelic differentiation of Sorghum bicolor (L.) Moench DNA simple-sequence repeats (SSRs). Theor. Appl. Genet. 101: 438–448. [Google Scholar]
- Kulikova, O., G. Gualtieri, R. Geurts, D.-J. Kim, D. Cook et al., 2001. Integration of the FISH pachytene and genetic maps of Medicago truncatula. Plant J. 27: 49–58. [DOI] [PubMed] [Google Scholar]
- Künzel, G., L. Korzun and A. Meister, 2000. Cytologically integrated physical restriction fragment length polymorphism maps for the barley genome based on translocation breakpoints. Genetics 154: 397–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoon, M. L., and M. S. Ramana, 1961. Cytology of some “Eu-sorghums.” Genet. Iber. 13: 129–144. [Google Scholar]
- Magoon, M. L., and K. G. Shambulingappa, 1960. Karyomorphological studies in Sorghum ankolib var. annalib red, a Eu-Sorghum. Indian J. Genet. 20: 166–177. [Google Scholar]
- Magoon, M. L., and K. G. Shambulingappa, 1961. Karyomorphology of Sorghum propinquum and its bearing on the origin of 40-chromosome sorghum. Chromosoma 42: 460–465. [DOI] [PubMed] [Google Scholar]
- Magoon, M. L., P. L. Manchanda and M. S. Ramana, 1964. Cytological and morphological studies in the genus Sorghum. Cytologia 29: 42–60. [DOI] [PubMed] [Google Scholar]
- Menz, M. A., R. R. Klein, J. E. Mullet, J. A. Obert, N. C. Unruh et al., 2002. A high-density genetic map of Sorghum bicolor (L.) Moench based on 2926 AFLP, RFLP and SSR markers. Plant Mol. Biol. 48: 483–499. [DOI] [PubMed] [Google Scholar]
- Ming, R., S. C. Liu, J. da Silva, W. Wilson, D. Braga et al., 1998. Detailed alignment of Saccharum and Sorghum chromosomes: comparative organization of closely related diploid and polyploid genomes. Genetics 150: 1663–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Y., K. F. Schertz, S. Cartinhour and G. E. Hart, 1999. Comparative genome mapping of Sorghum bicolor (L.) Moench using an RFLP map constructed in a population of recombinant inbred lines. Plant Breed. 118: 225–235. [Google Scholar]
- Pereira, M. G., M. Lee, P. Bramel-Cox, W. Woodman, J. Doebley et al., 1994. Construction of an RFLP map in sorghum and comparative mapping in maize. Genome 37: 236–243. [DOI] [PubMed] [Google Scholar]
- Price, H. J., S. L. Dillon, G. Hodnett, W. Rooney, L. Ross et al., 2005. Genome evolution in the genus Sorghum (Poaceae). Ann. Bot. 95: 219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang, Y., and G. H. Liang, 2000. Comparative physical mapping of the 18S–5.8S- 26rDNA in three sorghum species. Genome 43: 918–922. [PubMed] [Google Scholar]
- Tao, Q., and H. B. Zhang, 1998. Cloning and stable maintenance of DNA fragments over 300 kb in Escherichia coli with conventional plasmid-based vectors. Nucleic Acids Res. 26: 4901–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, Y. Z., D. R. Jordan, R. G. Henzell and C. L. McIntyre, 1998. Construction of a genetic map in a sorghum RIL population using probes from different sources and its comparison with other sorghum maps. Aust. J. Agric. Res. 49: 729–736. [Google Scholar]
- Tao, Y. Z., R. G. Henzell, D. R. Jordan, D. G. Butler, A. M. Kelly et al., 2000. Identification of genomic regions associated with stay green in sorghum by testing RILs in multiple environments. Theor. Appl. Genet. 100: 1225–1232. [Google Scholar]
- Werner, J. E., T. R. Endo and B. S. Gill, 1992. Toward a cytogenetically based physical map of the wheat genome. Proc. Natl. Acad. Sci. USA 89: 11307–11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitkus, R., J. Doebley and M. Lee, 1992. Comparative genome mapping of sorghum and maize. Genetics 132: 1119–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, S. S., J. M. Jiang, B. S. Gill, A. H. Paterson and R. A. Wing, 1994. Construction and characterization of bacterial artificial chromosome library of Sorghum bicolor. Nucleic Acids Res. 22: 4922–4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, G.-W., C. W. Magill, K. F. Schertz and G. E. Hart, 1994. A RFLP linkage map of Sorghum bicolor (L) Moench. Theor. Appl. Genet. 89: 139–145. [DOI] [PubMed] [Google Scholar]
- Yu, H., G. H. Liang and K. D. Kofoid, 1991. Analysis of C-banding chromosome patterns of sorghum. Crop Sci. 31: 1524–1527. [Google Scholar]
- Zwick, M. S., M. N. Islam-Faridi, H. B. Zhang, G. L. Hodnett, M. Gomez et al., 2000. Distribution and sequence analysis of the centromere-associated repetitive element CEN38 of Sorghum bicolor (Poaceae). Am. J. Bot. 87: 1757–1764. [PubMed] [Google Scholar]