Abstract
National fish consumption advisories that are based solely on assessment of risk of exposure to contaminants without consideration of consumption benefits result in overly restrictive advice that discourages eating fish even in areas where such advice is unwarranted. In fact, generic fish advisories may have adverse public health consequences because of decreased fish consumption and substitution of foods that are less healthy.
Public health is on the threshold of a new era for determining actual exposures to environmental contaminants, owing to technological advances in analytical chemistry. It is now possible to target fish consumption advice to specific at-risk populations by evaluating individual contaminant exposures and health risk factors. Because of the current epidemic of nutritionally linked disease, such as obesity, diabetes, and cardiovascular disease, general recommendations for limiting fish consumption are ill conceived and potentially dangerous.
BOTH THE US ENVIRONMENTAL Protection Agency (EPA) and the US Food and Drug Administration (FDA) have issued national fish consumption advisories that recommend women of childbearing age restrict their consumption of fish to avoid excessive exposure to methylmercury. These advisories were issued on the basis of methylmercury levels found in fish in specific locations across the country and estimates of dietary consumption, and they were issued across the country regardless of actual levels of mercury found among human populations. Thus, we question the wisdom and the validity of the scientific basis of these advisories. First, by ignoring the benefits associated with consuming fish and the potentially decreasing fish consumption among women of childbearing age, these advisories may violate the ethical principles of beneficence: do not harm, and maximize possible benefits and minimize possible risks.1 Second, the advisories ignore the evolution of public health’s ability to measure actual exposure to environmental contaminants among specific populations.
Both advisories, which were issued in 2001 and reissued in 2004, restrict the fish consumption of women of childbearing age: the EPA’s advisory restricts consumption of recreationally caught fish to no more than 6 ounces a week; the FDA’s advisory restricts consumption of commercially caught fish to no more than 12 ounces a week.2–4 These consumption levels seem overly cautious after reviewing the 1999–2000 National Health and Nutrition Examination Survey (NHANES), which found that 92% of women of childbearing age (n=1709) had blood total mercury concentration levels that were below 5.8 μg/L, which is the blood level that corresponds with the EPA’s conservative reference dose. The EPA’s reference dose—the safe dose that can be consumed every day during a 70-year lifetime without any adverse health effects—was derived from the “benchmark dose” blood mercury concentration of 58 μg/L divided by a 10-fold uncertainty factor.5 The 95th percentile geometric mean from the NHANES study was 7.13 μg/L (95% confidence interval [CI] = 5.79, 8.48), which was well below the EPA’s “benchmark dose” level of 58 μg/L.5
Many Alaskans have no readily available alternative to fish. In fact, a large number of Alaskans rely on locally caught fish as their primary protein source.6 Thus, for Alaska public health officials, the EPA/FDA advisories have been particularly problematic. Even though available data show methylmercury concentrations in the most frequently consumed Alaska fish (e.g., chinook, coho, sockeye, chum, and pink salmon) are among the lowest of all fish species (average < 0.05 mg/kg),7 many Alaskans, particularly Alaska Natives, have begun questioning the safety of their traditional diets. The adverse effects on public health in communities that have moved away from traditional foods have been well documented.8,9 In Alaska, there is now evidence that Alaska Natives are replacing their traditional diets with foods that are far less healthy,10,11 and Alaska Natives are experiencing a significant increase in the prevalence of diabetes9 and overweight/obesity.12 Additionally, many Alaskans have serious problems with alcohol use and lack of physical exercise,13 conditions that may be partially attributed to the abandonment of a traditional diet and lifestyle.14
Alaska’s public health response to the EPA/FDA advisories has been to recommend unrestricted consumption of fish caught in Alaska waters.15 Furthermore, a biomonitoring program has been implemented to determine actual exposure levels of environmental contaminants among concerned populations. The program tests methylmercury exposure among pregnant women, with limited testing among women of childbearing age. It is expected to expand and eventually include all women of childbearing age; it also will test exposure to other chemicals of concern, such as polychlorinated biphenyls (PCBs), pesticides, and heavy metals. Exposure is determined by analyzing total mercury concentrations in hair, which is both noninvasive and relatively inexpensive. Hair mercury is a well-established biomarker for determining methylmercury exposure among fish-eating populations.16–20
The Alaska Division of Public Health (ADPH) began its Statewide Mercury Hair Biomonitoring Program during June 2002, when it offered free, confidential testing to all pregnant women in Alaska.21 The initial results of this program and the utility of targeted screening when recommending specific local-consumption advice are described in this article.
METHODS
All health care providers in Alaska received introductory materials during June 2002 and were encouraged to provide testing for their pregnant patients. Upon request, the ADPH provides health care providers with materials for collecting and submitting hair samples.21 The samples are about 1/8 inch in diameter and are collected from the back of the head close to the scalp. Initially, samples submitted to ADPH were analyzed by Frontier GeoSciences in Seattle, Wash, with cold vapor atomic fluorescence spectrometry.22 Today, unwashed samples are analyzed by the Alaska Public Health Laboratory with a direct mercury analyzer (DMA-80, Milestone, Inc). Samples that have total mercury levels higher than 10 mg/kg are sent to Frontier GeoSciences for methylmercury analysis.
Individual results and reference levels are sent to each woman’s health care provider. Women who have hair mercury levels higher than 5 mg/kg are interviewed to determine probable exposure sources, and their health care providers are consulted to determine optimal dietary advice.
The program also supports targeted testing of all women of childbearing age aged 15 to 45 years in areas of the state where relatively more fish, marine mammals, or both are consumed. In June 2003, ADPH began accepting hair samples from women of childbearing age who lived in 1 area of the state where there is heavy subsistence use.
RESULTS
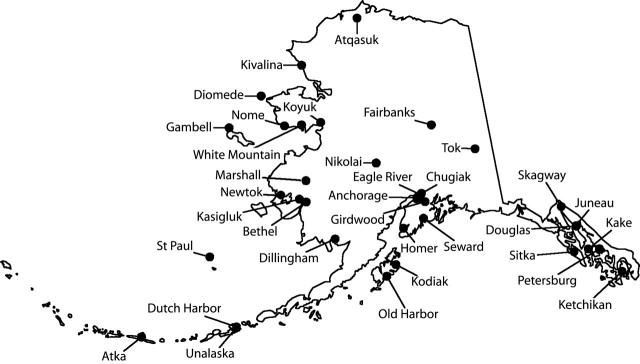
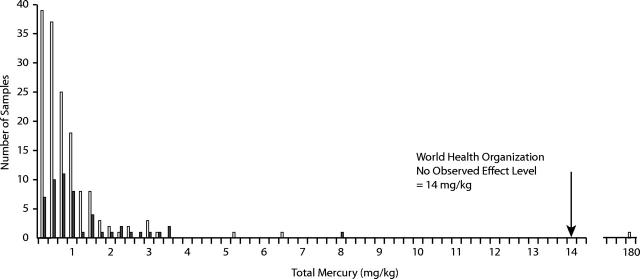
There are approximately 12000 births per year in Alaska. Between June 4, 2002, and December 31, 2003, the ADPH analyzed hair samples from 150 pregnant women and 52 women of childbearing age who lived in 34 Alaskan communities (Figure 1 ▶). The median and mean ages of the pregnant women were 28 and 29 years, respectively (range=15–47 years). The median and mean hair mercury levels among pregnant women were 0.47 mg/kg and 0.72 mg/kg, respectively (range = 0.02–6.35 mg/kg) (Figure 2 ▶). One pregnant woman’s hair mercury level was 180 mg/kg, but she was excluded from the statistical analysis because she was not representative of the population of interest and because her hair mercury level probably did not accurately reflect her internal exposure level (see next paragraph). The median and mean ages of women of child-bearing age were 32 and 31 years, respectively (range= 16–45 years). The median and mean hair mercury levels among women of childbearing age were 0.64 mg/kg and 1.12 mg/kg, respectively (range= 0.15–7.82 mg/kg). Overall, 77% and 91% of all women had levels of hair mercury below 1 and 2 mg/kg, respectively, and 81% had levels below 1.2 mg/kg, the level that corresponds with the EPA’s reference dose (Figure 2 ▶).
FIGURE 1—
The community residences (n = 34) of pregnant women (n = 150) and women of childbearing age (n = 52) who participated in the Alaska Statewide Maternal Hair Mercury Biomonitoring Program.
FIGURE 2—
The frequency distribution of hair total mercury levels among pregnant women (n = 150 [white bars]; median = 0.47 mg/kg) statewide and women of childbearing age (n = 52 [black bars]; median = 0.64 mg/kg) who participated in the Alaska Statewide Maternal Hair Mercury Biomonitoring Program, June 4, 2002–December 31, 2003. Note. The outlying value of approximately 180 mg/kg (on far right) was excluded from statistical analysis (see Results, first paragraph).
To date, 4 women have been found to have hair mercury levels higher than 5 mg/kg (Figure 2 ▶). The pregnant woman whose level of hair mercury was 180 mg/kg was visiting from Tanzania, Africa, and delivered her infant in Alaska. Although she had an extremely high level of hair mercury, she reported no clinical symptoms of mercury toxicity. She also reported that she had not consumed Alaska fish. An extensive case investigation—including testing the species of African fish she consumed—failed to identify a potential source of mercury exposure. Her blood mercury level was 23 μg/L, and her infant’s blood mercury level was 15 μg/L. Unfortunately, the small amount of hair initially collected precluded methylmercury analysis, and the patient would not provide another hair sample for additional testing.
Two pregnant women from western Alaska had hair mercury levels of 6.4 mg/kg and 5.2 mg/kg, respectively. They are enrolled in the Alaska Native Tribal Health Consortium’s maternal-infant cord blood study and have had medical follow-up as part of that project. One woman of childbearing age had a hair mercury level of 7.8 mg/kg. This level was confirmed during her follow-up investigation. Interviews with her family indicated the most likely food item that contributed to her mercury exposure was marine mammal muscle or organ meat. Testing was then offered to the entire community, and an additional 25 individuals provided hair samples. This community, which consists primarily of Alaska Natives, is very remote and has no readily available alternative to traditional subsistence foods. We are currently consulting with village-based community health aides and the regional Tribal Health Board to (1) analyze food items that may have high levels of methylmercury, and (2) evaluate the benefits of subsistence food consumption and the potential risks from exposure to methylmercury to determine optimum dietary advice.
DISCUSSION
To date, the hair mercury levels among pregnant women and women of childbearing age in Alaska are well below the World Health Organization’s No Observed Effect Level (NOEL) of 14 mg/kg18; however, our preliminary results are not representative of the entire Alaska population. Additionally, the small number of biomonitoring samples tested in Alaska to date precludes the development of a generalized estimate of mercury exposure statewide. However, our results are similar to the results (mean = 1.5 mg/kg; median=1.2 mg/kg) of a hair monitoring study conducted by Rothschild and Duffy23 in the western Alaska village of Napakiak (n=16). Similarly, low mercury levels have been observed in the blood of mothers who delivered babies from the Bethel (geometric mean=5.5 μg/L; n=23) and Barrow areas (arithmetic mean=1.3 μg/L; n = 23).24 The World Health Organization’s NOEL for blood is 56 μg/L.18
Recent advances in laboratory analytical methodology now allow public health officials to routinely measure exposure to mercury and other environmental contaminants.25,26 When high levels of contaminants are found in local foods, the ADPH suggests that human biomonitoring be used to identify at-risk sub-populations so that consumption advice can be tailored locally to balance benefits and risks. Biomonitoring can then determine if the advice needs to be changed.
We hold that dietary guidelines that are based on the EPA reference dose result in highly restrictive consumption advice that fails to consider the many benefits of fish consumption. Extensive scientific research has documented the numerous health, social and cultural, and economic benefits of eating fish.27 Proven health benefits include protection from cardiovascular disease28–30 and diabetes31 and improved maternal nutrition and neonatal and infant brain development.32–34 Additionally, fish consumption has been linked to the prevention of cancer, including cancers of the gastrointestinal tract and prostate gland.35–37 In the Seychelles Child Development Study, positive neurodevelopmental outcomes were shown among children exposed to methylmercury prenatally (mean maternal hair mercury level=6.8 mg/kg) and postnatally (mean child hair mercury level=6.5 mg/kg).38 It was hypothesized that nutrients derived from fish consumption provided the beneficial effect,38,39 and a new cohort has been established to evaluate this.39
CONCLUSION
When creating ethical public health recommendations for fish consumption, it is essential to weigh both benefits and risks. The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research identifies 3 “basic ethical principles” for protecting human subjects of research: respect for persons, beneficence, and justice.1 The principle of beneficence has been expressed as 2 complementary actions: (1) do not harm, and (2) maximize possible benefits and minimize possible risks. Although national fish advisories are not intended as research, they overemphasize risks and undervalue the benefits of fish consumption. Highly restrictive generic fish consumption advisories, such as the ones issued by the EPA and the FDA, can cause harm by unnecessarily warning people not to consume fish. Among Alaska Natives who rely heavily on these foods for their nutritional, spiritual, and cultural health, the results can be disastrous.40,41
The current EPA and FDA advisories should take into account recent advances in our ability to quantify actual exposure levels to environmental contaminants. These advances have made biomonitoring a cost-effective public health tool for helping federal, state, and local health agencies develop optimal dietary guidance.
Acknowledgments
The authors wish to thank Charles J. Utermohle, PhD, for database administration and programming and David A. Verbrugge and Frontier Geosciences, Inc, for analytical support. We also thank David O’Brien, PhD, who created Figure 1 ▶.
Human Participant Protection The Statewide Mercury Hair Biomonitoring Program was reviewed by the institutional review board of the Alaska Native Medical Center and was determined to be public health practice, not research.
Peer Reviewed
Contributors S. M. Arnold analyzed the data and led the writing. T. V. Lynn gathered the information on benefits, analyzed data, and contributed to the writing. L. A. Verbrugge led the development of the hair mercury biomonitoring program and contributed to the writing. J.P. Middaugh originated the idea for the paper and contributed to the writing. All the authors originated ideas, interpreted findings, and reviewed drafts of the article.
References
- 1.Department of Health, Education, and Welfare. The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research; 1979. Available at: http://ohsr.od.nih.gov/guidelines/belmont/html. Accessed December 17, 2004. [PubMed]
- 2.US Environmental Protection Agency. National Advice on Mercury in Fish Caught by Family and Friends: For Women Who Are Pregnant or May Become Pregnant, Nursing Mothers, and Young Children. Available at: http://www.epa.gov/ost/fishadvice/factsheet.html. Accessed January 29, 2004.
- 3.US Food and Drug Administration. Consumer Advisory. An Important Message for Pregnant Women and Women of Childbearing Age Who May Become Pregnant About the Risks of Mercury in Fish. Available at: http://vm.cfsan.fda.gov/~dms/admehg.html. Accessed January 29, 2004.
- 4.US Department of Health and Human Services and US Environmental Protection Agency. What You Need to Know About Mercury in Fish and Shellfish. 2004 EPA And FDA Advice for: Women Who Might Become Pregnant, Women Who Are Pregnant, Nursing Mothers, and Young Children. Available at http://www.cfsan.fda.gov/~dms/admehg3.html. Accessed May 18, 2004.
- 5.Schober SE, Sinks TH, Jones RL, et al. Blood mercury levels in US children and women of childbearing age, 1999–2000. JAMA. 2003;289: 1667–1674. [DOI] [PubMed] [Google Scholar]
- 6.Nobmann ED, Byers T, Lanier AP, Hankin JH, Jackson MY. The diet of Alaska Native adults: 1987–1988. Am J Clin Nutr. 1992;55:1024–1032. [DOI] [PubMed] [Google Scholar]
- 7.Alaska Department of Environmental Conservation. Alaska Fish Tested for Mercury Get Clean Bill of Health. Available at: http://www.state.ak.us/dec/eh/vet/HeavyMetals.htm. Accessed March 2004.
- 8.Kuhnlein HV, Receveur O, Chan HM. Traditional food systems research with Canadian Indigenous Peoples. Int J Circumpolar Health. 2001;60:112–122. [PubMed] [Google Scholar]
- 9.Ebbesson SO, Kennish J, Ebbesson L, Go O, Yeh J. Diabetes is related to fatty acid imbalance in Eskimos. Int J Circumpolar Health. 1999;58:108–119. [PubMed] [Google Scholar]
- 10.Nobmann ED, Ebbesson SO, White RG, Schraer CD, Lanier AP, Bulkow LR. Dietary intakes among Siberian Yupiks of Alaska and implications for cardiovascular disease. Int J Circumpolar Health. 1998;57:4–17. [PubMed] [Google Scholar]
- 11.Nobmann ED, Ebbesson SO, White RG, Bulkow LR, Schraer CD. Associations between dietary factors and plasma lipids related to cardiovascular disease among Siberian Yupiks of Alaska. Int J Circumpolar Health. 1999; 58:254–271. [PubMed] [Google Scholar]
- 12.Wertz-Stein L. The Burden of Overweight and Obesity in Alaska. Anchorage, Alaska: Alaska Division of Public Health, Health Promotion Unit; 2003.
- 13.Rarig A, Schumacher C, Crondahl J, et al. Health Status in Alaska, 2000 Edition. Available at: http://health.hss.state.ak.us/dph/targets/hs2000.htm. Accessed March 2004.
- 14.Risica PM, Ebbesson SO, Schraer CD, Nobmann ED, Caballero BH. Body fat distribution in Alaskan Eskimos of the Bering Straits region: the Alaskan Siberia Project. Int J Obes Relat Metab Disord. 2000;24:171–179. [DOI] [PubMed] [Google Scholar]
- 15.Alaska Division of Public Health. Mercury and National Fish Advisories. Statement From Alaska Division of Public Health. Recommendations for Fish Consumption in Alaska. State of Alaska Epidemiology Bulletin No. 6, June 15, 2001. Available at: http://www.epi.alaska.gov/bulletins/docs/b2001_06.htm. Accessed March 2004.
- 16.National Research Council. Toxicolgical Effects of Methymercury. Washington, DC: National Academy Press; 2000.
- 17.Myers GJ, Davidson PW, Cox C, et al. Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study. Lancet. 2003;361:1686–1692. [DOI] [PubMed] [Google Scholar]
- 18.Joint Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA). Summary and Conclusions, Sixty-First Meeting, Rome, 10–19, June 2003. Available at: http://www.chem.unep.ch/mercury/Report/JECFA-PTWI.htm. Accessed March 2004.
- 19.Harkins DK, Susten AS. Hair analysis: exploring the state of the science. Environ Health Perspect. 2003;111: 576–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Blood and hair mercury levels in young children and women of childbearing age—Unites States, 1999. MMWR Morb Mortal Wkly Rep. 2001; 50:140–143. [PubMed] [Google Scholar]
- 21.Alaska Division of Public Health. Statewide Mercury Hair Biomonitoring Program. State of Alaska Epidemiology Bulletin. No. 11, June 4, 2002. Available at: http://www.epi.alaska.gov/eh/hot0602/hairinfopacket.pdf. Accessed March 2004.
- 22.Bloom NS, Crecelius EA. Determination of mercury in seawater at sub-nanogram per liter levels. Marine Chem. 1983;14:49–59. [Google Scholar]
- 23.Rothschild RF, Duffy LK. Methylmercury in the hair of subsistence food users in a rural Alaskan village. Alaska Med. 2002;44:2–7. [PubMed] [Google Scholar]
- 24.Van Oostdam J, Tremblay N. Biological monitoring: human tissue levels of environmental contaminants. In: Hansen JC, Gilman A, Klopov V, Odland JØ, eds. AMAP Assessment 2002: Human Health in the Arctic. Oslo, Norway: Arctic Monitoring and Assessment Programme; 2003: 31–56.
- 25.Pirkle JL, Needham LL, Sexton K. Improving exposure assessment by monitoring human tissues for toxic chemicals. J Expo Anal Environ Epidemiol. 1995;5:405–424. [PubMed] [Google Scholar]
- 26.Needham LL, Sexton K. Assessing children’s exposure to hazardous environmental chemicals: an overview of selected research challenges and complexities. J Expo Anal Environ Epidemiol. 2000;10:611–29. [DOI] [PubMed] [Google Scholar]
- 27.Egeland GM, Middaugh JP. Balancing fish consumption benefits with mercury exposure. Science. 1997;278: 1904–1905. [DOI] [PubMed] [Google Scholar]
- 28.Albert CM, Campos H, Stampfer MJ, et al. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–1118. [DOI] [PubMed] [Google Scholar]
- 29.Dewailly E, Blanchet C, Lemieux S, et al. n-3 Fatty acids and cardiovascular disease risk factors among the Inuit of Nunavik. Am J Clin Nutr. 2001;74: 464–473. [DOI] [PubMed] [Google Scholar]
- 30.Harris WS, Isley WL. Clinical trial evidence for the cardioprotective effects of omega-3 fatty acids. Curr Atheroscler Rep. 2001;3:174–179. [DOI] [PubMed] [Google Scholar]
- 31.Adler AI, Boyko EJ, Schraer CD, Murphy NJ. Daily consumption of seal oil or salmon associated with lower risk of non-insulin dependent diabetes mellitus and impaired glucose tolerance in Yup’ik Eskimo and Athabaskan Indians of Alaska. Arctic Med Res. 1994;53: 271–275. [Google Scholar]
- 32.Allen KG, Harris MA. The role of n-3 fatty acids in gestation and parturition. Exp Biol Med. 2001;226: 498–506. [DOI] [PubMed] [Google Scholar]
- 33.Uauy-Dagach R, Mena P. Nutritional role of omega-3 fatty acids during the perinatal period. Clin Perinatol. 1995;22:157–175. [PubMed] [Google Scholar]
- 34.Uauy R, Peirano P, Hoffman D, Mena P, Birch D, Birch E. Role of essential fatty acids in the function of the developing nervous system. Lipids. 1996;31:S167–S176. [DOI] [PubMed] [Google Scholar]
- 35.Terry P, Lichtenstein P, Feychting M, Ahlbom A, Wolk A. Fatty fish consumption and risk of prostate cancer. Lancet. 2001;357:1764–1766. [DOI] [PubMed] [Google Scholar]
- 36.Augustsson K, Michaud DS, Rimm EB, et al. A prospective study of intake of fish and marine fatty acids and prostate cancer. Cancer Epidemol Biomarkers Prev. 2003;12:64–67. [PubMed] [Google Scholar]
- 37.Fernandez E, Chatenoud L, La Vecchia C, Negri E, Franceschi S. Fish consumption and cancer risk. Am J Clin Nutr. 1999;70:85–90. [DOI] [PubMed] [Google Scholar]
- 38.Davidson PW, Myers GJ, Cox C et al. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment. JAMA. 1998;280:701–707. [DOI] [PubMed] [Google Scholar]
- 39.Clarkson TW, Strain JJ. Nutritional factors may modify the toxic action of methyl mercury in fish-eating populations. J Nutr. 2003;133:1539S–1543S. [DOI] [PubMed] [Google Scholar]
- 40.Alaska Division of Public Health. Use of Traditional Foods in a Healthy Diet in Alaska: Risks in Perspective. State of Alaska Epidemiology Bulletin. 1998;2(1).
- 41.Deutch B. Recent dietary studies in the Arctic. In: Hansen JC, Gilman A, Klopov V, Odland JØ, eds. AMAP Assessment 2002: Human Health in the Arctic. Oslo, Norway: Arctic Monitoring and Assessment Programme;2003: 75–87.