Abstract
Objectives. We sought to estimate HIV incidence among injection drug users (IDUs) in New York City from 1990 to 2002 to assess the impact of an expansion of syringe exchange services. Syringe exchange increased greatly during this period, from 250000 to 3000000 syringes exchanged annually.
Methods. Serum samples were obtained from serial cross-sectional surveys of 3651 IDUs. HIV-positive samples were tested with the Serologic Test Algorithm for Recent HIV Seroconversion (STARHS) assay to identify recent HIV infections and to estimate HIV incidence. Consistency with other incidence studies was used to assess strengths and limitations of STARHS.
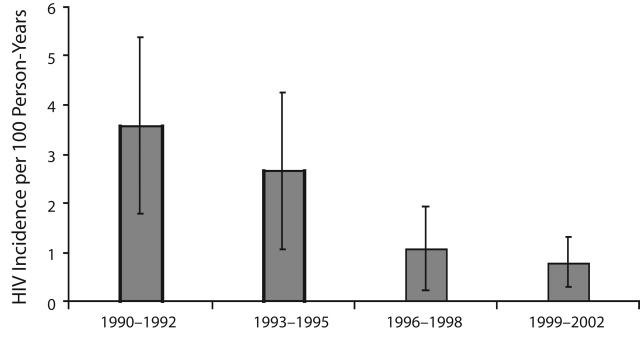
Results. HIV incidence declined from 3.55/100 person-years at risk (PYAR) from 1990–1992, to 2.63/100 PYAR from 1993–1995, to 1.05/100 PYAR from 1996–1998, and to 0.77/100 PYAR from 1999–2002 (P<.001). There was a very strong negative linear relationship (r= −.99, P<.005) between the annual numbers of syringes exchanged and estimated HIV incidence. These results were highly consistent with a large number of shorter incidence studies among IDUs conducted during the time period.
Conclusions. STARHS testing of samples from large serial cross-sectional surveys can provide important data for the assessment of community-level HIV prevention.
Measuring trends in HIV epidemics is critical for assessing the community-level effectiveness of HIV prevention programs and risk reduction and for eventually establishing control over HIV epidemics. Collecting valid HIV incidence data over time, however, is often quite difficult. Cohort studies, in which uninfected individuals are studied over time to observe the rate of new infections, are typically very expensive and logistically complex.
The recently developed Serologic Testing Algorithm for Recent HIV Seroconversion (STARHS) system1–3 provides a means for identifying likely incident HIV infections in cross-sectional HIV seroprevalence surveys. In this system (also known as the “sensitive/less sensitive” or “detuned” assay), 2 enzyme immunoassays (EIAs) are used to test each sample. One EIA is the standard highly sensitive test used to diagnose HIV infection; the other has been deliberately “detuned” to be less sensitive to antibody levels characteristic of early infection. Samples that test positive on both the diagnostic assay and the detuned assay are considered to have high levels of HIV antibody (both antibody titers and affinity) and to represent relatively long-term prevalent HIV infections. Samples that test positive on the diagnostic assay but negative on the less sensitive assay are considered to have lower antibody levels and to represent recent infections.
Multiple technical issues have delayed the widespread use of STARHS, but the few studies published to date suggest that the method may have substantial practical advantages over cohort studies as a method for estimating HIV incidence.4,5 We report data from STARHS testing on injection drug users (IDUs) entering the Beth Israel Medical Center drug abuse detoxification program in New York City from 1990 to 2002. This time period includes a “natural experiment” of substantial expansion of HIV prevention services for IDUs in New York City. We believe that this is the first use of STARHS to assess a large-scale expansion in HIV prevention for IDUs.
HIV was introduced into the IDU population in New York City in the middle 1970s and spread rapidly during the late 1970s and early 1980s.6 HIV prevalence then stabilized at approximately 50% from the early 1980s to the early 1990s.7 In the second half of 1992, syringe exchange programs received legal authorization, and funding from New York State subsequently underwent rapid expansion. The estimated numbers of syringes exchanged by the programs in New York City increased from approximately 250 000 in 1991 to 1 300 000 in 1994, to 2 400 000 in 1996, and stabilized at 3 000 000 in 2000 and 2002.8 In addition to exchanging needles and syringes, the programs provided free condoms, voluntary HIV counseling and testing, referrals to treatment for HIV infection, “prevention for positives” programs, and referrals to drug abuse treatment, among other services. This legalization, funding, and expansion of syringe exchange was undertaken with a clear expectation by public health authorities that it would be followed by a reduction in HIV transmission among IDUs in the city.9 The STAHRS testing reported here provides a direct assessment of whether that expectation was met.
METHODS
Subject Recruitment
The data reported here are part of an ongoing series of studies of IDUs entering the Beth Israel Medical Center drug detoxification program in New York City.6,7,10–12 The detoxification program serves the city as a whole; approximately half the patients live in Manhattan, one quarter in Brooklyn, one fifth in the Bronx, and the rest (5%) elsewhere. There were no substantive changes in program admissions criteria or study recruitment procedures over the period 1990 to 2002.
Patients were selected with the aim of producing an unbiased sample of IDUs in the detoxification program. Research staff visited the different general admission wards of the program in a preset order and examined the intake records to identify patients admitted within the past 3 days who had reported injecting illicit drugs within the previous 6 months. All of these newly admitted IDUs in the specific ward were then asked to participate in the study. The study was fully described to each potential subject, and a signed informed consent was obtained from those who agreed to participate. Twelve percent of eligible patients were not able to participate owing to appointments scheduled by hospital staff (for x-rays, appointments with social workers or physicians, etc.). Among patients approached by our interviewers, only 4% declined to participate. A structured interview was administered and a blood sample was obtained for HIV testing. After all of the patients admitted to a specific ward in the 3-day period had been asked to participate, the interviewer moved to the next ward in the preset order. Data collection was continuous throughout the study period.
HIV Testing
Blood specimens were sent to the New York City Department of Health and Mental Hygiene Public Health laboratory for HIV antibody analysis. Samples were screened in replicate for HIV antibody by commercially available EIA and confirmed by Western blot or indirect immunofluorescence. (The specific tests used over the time period are available from the first author.) Additional serum was divided into 0.5-mL aliquots and kept frozen at −70°C.
Serologic Test Algorithm for Recent HIV Seroconversion
STARHS was used to distinguish recent infections from long-term infections. All specimens testing positive by the highly sensitive EIA with confirmatory Western blot were retested with the less-sensitive EIA (bioMerieux Vironostika system, Durham, NC). This less-sensitive EIA detects HIV antibody after 170 days of infection (95% confidence interval [CI] = 162, 183) (Bernard Branson, Centers for Disease Control and Prevention; oral communication, November 2004). Specimens testing positive on the sensitive EIA and negative on the less-sensitive EIA were categorized as recent infections. The estimated sensitivity and specificity for STARHS are 95% and 98%, respectively.1 Persons with advanced HIV immunosuppression, those on highly active antiretroviral therapy (HAART) with durable viral suppression or on antiviral therapy for hepatitis B, long-term natural nonprogressors, and persons with non-clade B HIV-1 subtypes may also test positive in the STAHRS system, creating the possibility of false positive recent infections1,3,13 (B. Branson, Centers for Disease Control and Prevention, personal communication, November 2004); therefore, caution should be exercised in interpreting the results.
We decided not to conduct STARHS on the last remaining stored aliquot from any subject, so we did not test all confirmed HIV-positive subjects for recent infections. This required adjustment in calculating the estimated incidence rates for each year. We used the formula suggested by the Centers for Disease Control and Prevention (CDC) for making the adjustment: [(total detected) + (proportion detected) × (HIV positive not tested)]/[(total detected) + (proportion detected) × (HIV positive not tested) + (total HIV negative)]. This number was then multiplied by 365/170 × 100 to give the rate in incident infections per 100 person-years at risk. We compared the demographic characteristics of the HIV-positive persons whose samples were tested with STARHS and of those who were not. Even with the large sample sizes, there were no statistically significant differences in gender, racial/ethnic distribution, or age distribution. (Data not presented; available from the first author.)
The incidence data were grouped into 3-year periods in order to have approximately 200 HIV-positive samples for STARHS analysis for each period. This division yielded one 3-year period (1990–1992) prior to legalization and expansion of the syringe exchange programs and 3 periods (1993–1995, 1996–1998, and 1999–2002 [a 4-year period]) after expansion of the programs. To minimize possible problems of small numbers of incident HIV infections per time period, we also used a simple dichotomy of the first half of the study period (1990–1995) compared with the second half (1996–2002). This dichotomy corresponds to the period when the exchanges were modest but growing—from 250 000 to 2 000 000 syringes exchanged annually versus the period when they had reached large-scale operations—from 2 400 000 to 3 000 000 syringes exchanged annually.
RESULTS
Table 1 ▶ shows sociodemographic characteristics and the frequency of drug injection for all study participants. Most of the participants were male, and the mean age was 37 years. Almost half of the study subjects were Hispanic, 29% were Black, and 22% were White. Most subjects reported injecting on a daily or more frequent basis in the 6 months prior to study enrollment. Heroin was the most commonly injected drug.
TABLE 1—
Characteristics of Injection Drug Users Entering Beth Israel Medical Center Drug Detoxification Program: New York City, 1990–2002
| No. | Percentage | |
| Gender | ||
| Male | 2924 | 80 |
| Female | 727 | 20 |
| Race/ethnicity | ||
| White | 821 | 22 |
| Black | 1049 | 29 |
| Hispanic | 1737 | 48 |
| Other | 40 | 1 |
| Missing data | 4 | 0 |
| Frequency of injection | ||
| Less than daily | 955 | 26 |
| Daily | 2695 | 74 |
| Missing data | 1 | 0 |
| Drug injected | ||
| Heroin | 3006 | 82 |
| Speedball | 2191 | 60 |
| Cocaine | 1917 | 53 |
| Other | 11 | 0 |
Note. Average age of sample was 37 ±8.24 years.
A total of 959 of the 1161 HIV-positive samples (83%) were tested with STARHS. This testing initially identified 23 subjects as having recent HIV infections. Of these 23, however, 6 subjects reported that they had previously tested seropositive to HIV and were receiving treatment for HIV at the time of interview, and another 2 had CD4 cell counts of less than 200/mL3, the clinical definition of AIDS. We thus considered these 8 subjects as likely false positive recent infections. Four of these 8 were subjects who reported receiving treatment for HIV after 1998, which is consistent with the increasing trend in antiretroviral treatment among our HIV-positive subjects as a whole.
The first 4 rows of Table 2 ▶ present the total number of samples tested (combined HIV negative and HIV positive), the number of HIV-positive samples determined by STARHS, the number of HIV positives identified as probable incident infections, HIV incidence per 100 person-years at risk, and HIV seroprevalence for each 3-year period. There was a statistically significant decline in HIV incidence, from 3.55 per 100 person-years at risk from 1990 to 1992 to 0.77 per 100 person-years at risk from 1999 to 2002 (Cochran–Armitage test Z = 4.25, P< .0001). There was also a significant decline in HIV seroprevalence (Cochran–Armitage test Z = 18.08, P< .0001), with an average decline in seroprevalence of approximately 10% per 3-year period.
TABLE 2—
HIV Prevalence and Incidence Among Injection Drug Users Admitted to Beth Israel Medical Center Drug Detoxification Program: New York City, 1990–2002
| Period of Interview | Total No. of Injection Drug Users | No. HIV Negative | No. HIV Positive From EIA | No. HIV Positive From STARHS | Incident Cases | Annualized Incidence Rate, % (95% Confidence Interval) | HIV Prevalence, % |
| 4-time-period analysis | |||||||
| 1990–1992 | 791 | 392 | 399 | 303 | 5 | 3.55 (1.73, 5.37) | 50 |
| 1993–1995 | 686 | 383 | 303 | 255 | 4 | 2.63 (1.04, 4.23) | 44 |
| 1996–1998 | 705 | 489 | 216 | 180 | 2 | 1.05 (0.15, 1.95) | 31 |
| 1999–2002 | 1469 | 1226 | 243 | 221 | 4 | 0.77 (0.28, 1.26) | 17 |
| 2-time-period analysis | |||||||
| 1990–1995 | 1477 | 775 | 702 | 558 | 9 | 3.09 (1.88, 4.30) | 48 |
| 1996–2002 | 2174 | 1715 | 459 | 401 | 6 | 0.86 (0.42, 1.29) | 21 |
Note. EIA = enzyme immunoassay; STARHS = Serologic Test Algorithm for Recent HIV Seroconversion.
As shown in the last 2 rows of Table 2 ▶, dichotomizing the study period into 2 halves also showed a strong decline in incidence, from 3.09 per 100 person-years (95% CI = 1.88, 4.30) from 1990 to 1995 to 0.86 per 100 person-years (95% CI = 0.42, 1.29) from 1996 to 2002 (χ2 = 17.59, P< .0001). The modest number of recent HIV infections in this study does not permit distinguishing between a linear decline in HIV incidence associated with gradual expansion and a step decline associated with large-scale implementation, but both analyses show substantial and highly significant declines.
The modest number of probable recent infections14 did not provide sufficient power for statistical identification of risk factors for recent HIV infection. In logistic regression analyses, the only significant variable associated with probable recent HIV infection was the 3-year interview period variable, and the odds ratio for the 3-year period variable did not change with the addition of the demographic control variables to the model. Thus, the decline in HIV incidence from the period 1990 to 1992 to the period 1998 to 2002 was not the result of changes in the demographic composition of the sample.
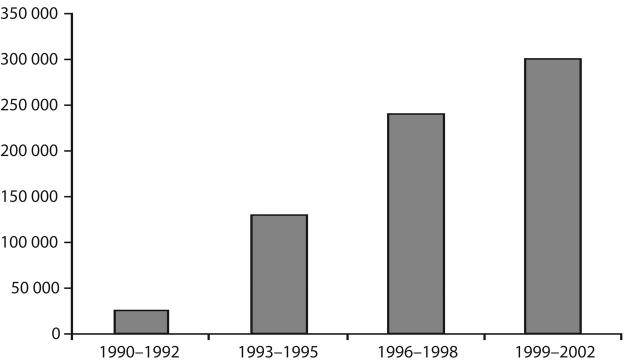
Figures 1 ▶ and 2 ▶ illustrate the decrease in HIV incidence among our subjects and the increase in the number of syringes exchanged by programs in New York City over the 3-year periods. (The numbers of syringes exchanged were obtained from biannual interviews with program directors.8) There appears to be a strong temporal relationship between the increase in syringe exchange services and the decrease in HIV incidence. The relationship was almost perfectly linear (r = − .99, P < .005). Also, it is important to remember that these publicly funded syringe exchange programs provided multiple services to their participants, and the other services provided also increased along with the numbers of syringes exchanged.
FIGURE 1—
HIV incidence among injection drug users entering Beth Israel Medical Center drug detoxification program, New York City, 1990–2002.
Note. T bars denote 95% confidence intervals.
FIGURE 2—
Annual mean number of syringes exchanged by programs in New York City, 1990–2002.
Note. Other services provided at syringe exchanges also expanded during this time period.
DISCUSSION
Limitations and Strengths of STARHS
One obvious limitation of STARHS is the need for large sample sizes. Note that we tested 3651 samples (including both HIV positives and HIV negatives) and identified only 15 probable recent HIV infections. This testing, however, was performed in a setting with declining HIV prevalence and incidence. A very large cohort study would similarly have been needed to assess whether HIV incidence declined over this time period.
A second important limitation is the potential for false negative results. Various factors, including advanced HIV infection, being on antiretroviral therapy, being on therapy for hepatitis B infection, and individual difference, may lead to the “less sensitive” EIA failing to detect antibody1,3,13 (B. Branson, Centers for Disease Control and Prevention; oral communication, November 2004) and providing a false positive recent infection result. In this study, we asked subjects about previous HIV testing and receiving treatment for HIV infection, and we had their CD4 cell counts; we could therefore exclude 8 instances where STARHS alone would have indicated a recent HIV infection. We did recalculate the incidence trend with these cases, and there still was a statistically significant decline over the period from 1990 to 2002, from 4.94 per 100 person-years at risk in the period from 1990 to 1992 to 2.6 per 100 person-years from 1993 to 1995, 1.6 per 100 person-years from 1996 to 1998, and 1.7 per 100 person-years from 1998 to 2002. Note, however, that the estimated incidence for the final period was more than twice as high with inclusion of the likely false positives (1.7 per 100 person-years vs 0.77 per 100 person-years).
It is clearly desirable to have clinical data to assess possible false positives from STARHS. Clinical data would be most helpful in “mature” epidemics in resource-rich areas, where there are many persons with long-time HIV infection, many persons receiving effective antiretroviral therapy, or both.
The most obvious strength of STARHS in this study is that the incidence data reported here could not have been generated without STARHS or some other similar test.
Perhaps the most important methodological finding of this study is the high consistency between these STARHS incidence results and the results of other, shorter-duration incidence studies conducted among IDUs in New York City. Studies conducted prior to the expansion of the syringe exchange programs show incidence rates of 3 to 7 per 100 person-years at risk.14–17 The STARHS incidence rate of 3.55 per 100 person-years at risk for IDUs entering the Beth Israel Medical Center detoxification program from 1990 to 1993 is consistent with the results of these studies.
HIV incidence studies of IDUs in New York City conducted after the expansion of the syringe exchange programs have shown rates of 0 to 3 per 100 person-years at risk.16,18,19 A meta-analysis of 10 incidence studies showed a weighted average incidence of 0.8 per 100 person-years at risk.10 As in the present study, this meta-analysis showed no significant association between HIV incidence and gender, race/ethnicity, or age. In 2003, the New York City Department of Health reported that STARHS testing of diagnostic specimens from IDUs screened at the city and state public health laboratories in 2001 showed an estimated incidence rate of 1 per 100 person-years at risk (95% CI = 0.9, 1.3),20 similar to the 0.77 per 100 person-years rate observed in the period from 1999 to 2002.
We also conducted a cohort study of persons entering the Beth Israel Medical Center detoxification program from 1995 to 1997. There were 757 participants in this study, of whom 72% provided at least 1 year of follow-up data. There were 10 HIV seroconversions in 744 person-years at risk, for an incidence rate of 1.35 per 100 person-years at risk (95% CI = 0.65, 2.47).10 The incidence rate seen in this cohort study of detoxification patients is quite similar to the STARHS incidence rate of 1.05 for 1996 to 1998.
The STARHS testing of our subjects gives incidence rates very similar to the rates in other HIV incidence studies among IDUs in New York City during the period from 1992 to 2002. This consistency creates some confidence that STARHS testing may be used in place of other types of HIV incidence studies, such as traditional cohort studies.
Effectiveness of Syringe Exchange in New York City, 1990–2002
A number of reviews have concluded that syringe exchange, in conjunction with other HIV prevention programming, can be effective in reducing risk behavior and HIV infection among IDUs, including reviews by the US National Commission on AIDS,21 a National Institutes of Health Consensus Development Conference,22 a National Academy of Sciences review,23 and, most recently, a Cochrane Collaboration review.24 The STARHS data reported here can be seen as additional evidence for the potential effectiveness of syringe exchange programs. This new evidence is of considerable importance for a large-scale test of syringe exchange in a high-prevalence HIV epidemic.
The behavioral and biological plausibility of expanded syringe exchange leading to reduced HIV incidence, and the strength of the temporal relationship between the expansion of the syringe exchange programs and the reduction in HIV incidence, suggest that it would be extremely unlikely for the expansion not to have contributed to the reduction in HIV incidence.
On the other hand, the existence of other HIV prevention programs for IDUs during this time period also suggests that the expansion of the syringe exchange programs was not the only causal factor in the reduction of HIV incidence. There are possible synergistic effects among different types of prevention programs; for example, the community outreach programs may educate drug users and motivate them to practice safer injection and safer sex, and the syringe exchange programs may then provide the means (clean needles and free condoms) to practice safer injection and safer sex. Or, the syringe exchange programs may successfully refer IDUs to both drug abuse treatment and treatment for HIV infection. While the expansion of the syringe exchange programs was clearly the most dramatic change in HIV prevention programming for IDUs in New York City during the period from 1990 to 2002, an attempt to assess the possible multiple causal relationships between this expansion, the other existing HIV prevention programs, and the observed reduction in HIV incidence is beyond the scope of this report. In our opinion, (1) the expansion of the syringe exchange programs was a critical component of the reduction in HIV incidence and (2) the existence of other prevention programs also contributed to the large reduction in incidence that was observed.
Implications for Future Research
The data reported here suggest that, in conjunction with standardized serial cross-sectional HIV seroprevalence surveys, STARHS testing may be particularly useful in assessing the effectiveness of HIV prevention programming at a community level. It will be important, however, to compare STARHS incidence estimates with incidence estimates from other studies whenever possible, and to conduct additional research on methods for minimizing and correcting for the problem of potential false positives.
Implications for Public Health Practice
The legalization, expansion, and public funding of syringe exchange programs in New York City was the result of a collaborative effort among public health officials, community-based organizations (which operated the exchanges), political leaders, researchers, and a private foundation (the American Foundation for AIDS Research), among others. This effort was undertaken in a city with a very large epidemic of high HIV seroprevalence, and where syringe exchange had been controversial.25 We suggest that the recent New York City experience can serve as a model for other areas with high HIV seroprevalence levels among IDUs who lack large-scale legal access to sterile injection equipment.
Acknowledgments
This study was supported primarily through a grant from the National Institute on Drug Abuse (DA 03574) and in part through grants from the National Institute of Allergy and Infectious Diseases (AI46370) and the New York State Department of Health AIDS Institute (AI4637).
Human Participant Protection The institutional review boards of Beth Israel Medical Center and the National Development and Research Institutes, Inc, approved the study. STARHS was performed only after the removal of all personal identifiers from the specimen and the subject’s case record.
Peer Reviewed
Contributors D. C. Des Jarlais originated the study, supervised all aspects of its implementation, wrote the original draft of the arricle, and supervised data analysis. T. Perlis, K. Arasteh, J. Milliken, and S. R. Friedman contributed to the conceptualization of the article and data analysis. L.V. Torian, S. Beatrice, D. Mildvan, and S. Yancovitz contributed to processing and testing of blood samples. All authors reviewed all drafts and contributed to revisions.
References
- 1.Janssen R, Statten G, Stramer S, et al. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA. 1998;280(1):42–48. [DOI] [PubMed] [Google Scholar]
- 2.Karon J, Kaplan EH, Brookmeyer R, Song R. Proposals for estimating HIV incidence in the United States from HIV reporting data and the detuned assay. Paper presented at: XIV International AIDS Conference; July 7–12, 2002; Barcelona, Spain.
- 3.Rawal B, Janssen R, Hecht F, Busch M. Development of less-sensitive anti-HIV-1 EIA to detect early HIV-1 infection using 96-well-formatted HIV-1 EIA kits. Paper presented at: 7th Conference on Retroviruses and Opportunistic Infections; January 30–February 2, 2000; San Francisco, Calif.
- 4.Kral AH, Lorvick J, Gee L, et al. Trends in human immunodeficiency virus seroincidence among street-recruited injection drug users in San Francisco, 1987–1998. Am J Epidemiol. 2003;157(10):915–922. [DOI] [PubMed] [Google Scholar]
- 5.McFarland W, Busch MP, Kellogg TA, Rawal BD, Satten GA, Katz MH. Detection of early HIV infection and estimation of HIV incidence using sensitive/less sensitive testing strategy at anonymous counseling and testing sites in San Francisco. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;22:484–489. [DOI] [PubMed] [Google Scholar]
- 6.Des Jarlais DC, Friedman SR, Novick D, et al. HIV-l infection among intravenous drug users in Manhattan, New York City, from 1977 through 1987. JAMA. 1989;261:1008–1012. [DOI] [PubMed] [Google Scholar]
- 7.Des Jarlais DC, Friedman SR, Sotheran JL, et al. Continuity and change within an HIV epidemic: injecting drug users in New York City, 1984 through 1992. JAMA. 1994;271(2):121–127. [PubMed] [Google Scholar]
- 8.Des Jarlais DC. The state of syringe exchange in the known universe. Paper presented at: 12th North American Syringe Exchange Convention; April 2002; Albuquerque, NM.
- 9.HIV Seroprevalence, Semiannual Report. Albany: New York State Dept of Health; 1992.
- 10.Des Jarlais DC, Marmor M, Friedmann P, et al. HIV incidence among injecting drug users in New York City, 1992–1997: evidence for a declining epidemic. Am J Public Health. 2000;90(3):352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Des Jarlais DC, Perlis T, Friedman SR, et al. Behavioral risk reduction in a declining HIV epidemic: injection drug users in New York City, 1990–1997. Am J Public Health. 2000;90(7):1112–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maslow CB, Friedman SR, Perlis TE, Rockwell R, Des Jarlais DC. Changes in HIV seroprevalence and related behaviors among male injection drug users who do and do not have sex with men: New York City, 1990–1999. Am J Public Health. 2002;92(3): 382–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parekh B, Dobbs T, Pau CP, et al. Quantitative detection of increasing HIV type 1 antibodies after seroconversion: a simple assay for detecting recent HIV infection and estimating incidence. AIDS Res Hum Retroviruses. 2002;18(4):295–307. [DOI] [PubMed] [Google Scholar]
- 14.Friedman SR, Jose B, Deren S, Des Jarlais DC, Neaigus A. Risk factors for HIV seroconversion among out-of-treatment drug injectors in high and low seroprevalence cities. Am J Epidemiol. 1995;142:864–874. [DOI] [PubMed] [Google Scholar]
- 15.Des Jarlais DC, Friedman SR, Marmor M, et al. Development of AIDS, HIV seroconversion, and potential co-factors for T4 cell loss in a cohort of intravenous drug users. AIDS. 1987;1:105–111. [PubMed] [Google Scholar]
- 16.Hartel DM, Schoenbaum EE. Methadone treatment protects against HIV infection: two decades of experience in the Bronx, New York City. Public Health Rep. 1998;113(suppl 1):107–115. [PMC free article] [PubMed] [Google Scholar]
- 17.Holmberg S. The estimated prevalence and incidence of HIV in 96 large US metropolitan areas. Am J Public Health. 1996;86(5):642–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Des Jarlais DC, Marmor M, Paone D, et al. HIV incidence among injecting drug users in New York City syringe-exchange programmes. Lancet. 1996;348: 987–991. [DOI] [PubMed] [Google Scholar]
- 19.Des Jarlais DC, Diaz R, Perlis T, et al. Variability in the incidence of HIV, HBV, and HCV infection among young injecting drug users in New York City. Am J Epidemiol. 2003;157(5):467–471. [DOI] [PubMed] [Google Scholar]
- 20.HIV Surveillance and Epidemiology Program, HIV Incidence in New York City, 2001: Estimates Using the Serologic Testing Algorithm for Recent HIV Seroconversion. New York City: Dept of Health and Mental Hygiene; 2003.
- 21.National Commission on AIDS. Full Report: The Twin Epidemics of Substance Use and HIV. Washington, DC: US National Commission on AIDS; July 1991.
- 22.Interventions to Prevent HIV Risk Behaviors, National Institutes of Health. Consensus Statement Online February 11–13, 1997; 15(2):1–41. Available at http://www.consensus.nih.gov/cons/104/104_statement.htm. Accessed May 27, 2005.
- 23.Normand J, Vlahov D, Moses LE, eds. Preventing HIV Transmission: The Role of Sterile Needles and Bleach. Washington, DC: National Academy Press/National Research Council/Institute of Medicine; 1995. [PubMed]
- 24.The Cochrane Collaborative Review Group on HIV Infection and AIDS. Evidence assessment: strategies for HIV/AIDS prevention, treatment and care. San Francisco, Calif: University of California, San Francisco, Institute for Global Health; 2004. Available at: http://www.igh.org/Cochrane/pdfs/EvidenceAssessment.pdf. Accessed May 27, 2005.
- 25.Des Jarlais DC, Perlis T, Arasteh K, et al. “Informed altruism” and “partner restriction” in the reduction of HIV infection in injecting drug users entering detoxification treatment in New York City, 1990–2001. J Acquir Immune Defic Syndr. 2004;35(2):158–166. [DOI] [PubMed] [Google Scholar]