Abstract
We analyzed gestational diabetes mellitus trends in New York City between 1990 and 2001 by using information obtained from birth certificates. Gestational diabetes diagnoses among women who delivered babies increased 46%, from 2.6% (95% confidence interval [CI]=2.5, 2.7) to 3.8% (95% CI=3.7, 3.9) of births. Prevalence was highest among South and Central Asian women (11%). Given risks for adverse fetal outcomes and maternal chronic diabetes, prompt screening is critical. Metabolic control should be maintained during pregnancy and assessed postpartum for women with gestational diabetes.
Maternal glucose tolerance can deteriorate during pregnancy, usually without adverse maternal or fetal health effects. For a small proportion of women, glucose tolerance declines below a healthy range, and gestational diabetes mellitus develops. Gestational diabetes mellitus is a well-established risk factor for adverse infant health outcomes, including fetal macrosomia, birth trauma, neonatal hypoglycemia, and fetal death.1,2 Gestational diabetes mellitus also has been shown to predict later maternal development of type 2 diabetes.3,4
Existing evidence indicates that women at greatest risk for development of gestational diabetes mellitus include those who are obese, are older, have family members with diabetes, or are a member of an ethnic group with high prevalence of diabetes.5 The current confluence of the obesity epidemic in the United States,6,7 and the trend toward older maternal age,8 may place women at increased risk for development of gestational diabetes mellitus and its potential sequelae. Few population-based studies have examined trends in gestational diabetes mellitus prevalence, especially across racial/ethnic groups.9
We describe findings from an analysis of New York City birth records registered between 1990 and 2001. We examined gestational diabetes mellitus trends by race/ethnicity, which included Hispanic and Asian subgroups.
METHODS
In New York City, universal screening of pregnant women for gestational diabetes mellitus has been widely practiced since the 1980s. The recommended initial screening test is a 1-hour 50-g oral glucose load, typically administered between 24 and 28 weeks’ gestation; women exceeding glucose thresholds then receive a diagnostic oral glucose tolerance test.10–12 A first-time detection of glucose intolerance during pregnancy can reflect either new onset of diabetes resulting from the pregnancy or new detection of a preexisting diabetic condition. The gestational diabetes mellitus definition includes any first recognition of diabetes during pregnancy.
Data for this study were obtained from birth certificate records maintained by New York City Department of Health and Mental Hygiene. New York City birth certificates include 2 check boxes to indicate the presence of either gestational diabetes mellitus or previously diagnosed chronic diabetes. We examined trends in both conditions among women who were residents of New York City who completed a live singleton delivery between 1990 and 2001.
In addition to diabetes status, maternal demographic characteristics extracted from birth certificates included age at delivery, country of origin, and racial and ethnic ancestry (self-identified by the mother). Racial/ethnic categories are listed in Table 1 ▶. Hispanic and Asian categories were broken down into subgroups based on country of origin or ancestry.
TABLE 1—
Gestational Diabetes Among Pregnant Residents of New York City Who Delivered in 1990 and 2001, by Age Group and Race/Ethnicity
| 1990 | 2001 | ||||||
| Population | % | % GDM (95% CI) | Population | % | % GDM (95% CI) | % Change | |
| Total deliveries | 125 663 | 100 | 2.6 (2.5, 2.7) | 110 340 | 100 | 3.8 (3.7, 3.9) | 46 |
| Age, y | |||||||
| < 25 | 43 860 | 35 | 1.1 (1.0, 1.2) | 35 565 | 32 | 1.5 (1.4, 1.7) | 36 |
| 25–34 | 66 169 | 53 | 2.9 (2.7, 3.0) | 55 543 | 50 | 4.3 (4.1, 4.5) | 48 |
| ≥35 | 15 395 | 12 | 5.8 (5.4, 6.2) | 19 235 | 17 | 6.7 (6.3, 7.0) | 16 |
| Race/ethnicitya | |||||||
| Non-Hispanic White | 32 875 | 26 | 2.2 (2.1, 2.4) | 28 084 | 26 | 2.4 (2.2, 2.5) | 9 |
| Non-Hispanic Blackb | 27 777 | 22 | 1.7 (1.6, 1.9) | 20 583 | 19 | 3.1 (2.9, 3.3) | 82 |
| Asian | 9878 | 8 | 3.9 (3.5, 4.2) | 13 978 | 13 | 7.4 (7.1, 7.9) | 90 |
| South and Central Asianc | 2263 | 2 | 5.7 (4.8, 6.7) | 4685 | 4 | 11.1 (10.2, 12.0) | 95 |
| Other Asian | 7615 | 6 | 3.3 (2.9, 3.7) | 9293 | 8 | 5.6 (5.1, 6.1) | 70 |
| Hispanic or Caribbean | 53 909 | 43 | 3.1 (2.9, 3.2) | 47 233 | 43 | 3.9 (3.8, 4.1) | 26 |
| Puerto Rican | 18 325 | 15 | 2.8 (2.6, 3.0) | 10 149 | 9 | 3.1 (2.7, 3.4) | 11 |
| Mexican | 2916 | 2 | 2.5 (1.9, 3.1) | 6780 | 6 | 4.9 (4.4, 5.5) | 96 |
| Dominican Republic | 11 128 | 9 | 3.3 (2.9, 3.6) | 9531 | 9 | 3.4 (3.1, 3.8) | 3 |
| Other Central or South Americand | 7212 | 6 | 3.3 (2.9, 3.7) | 8048 | 7 | 3.6 (3.2, 4.0) | 3 |
| Other Caribbeane | 12 357 | 10 | 3.5 (3.2, 3.8) | 9481 | 9 | 5.2 (4.8, 5.7) | 49 |
| Unknown Hispanic origin | 1971 | 2 | 1.9 (1.3, 2.5) | 3244 | 3 | 3.0 (2.4, 3.5) | 58 |
Note. GDM = gestational diabetes mellitus; CI = confidence interval.
aRace/ethnicity was unknown or listed as “other” for 2.5% of birth certificates.
bDoes not include women of Caribbean descent; these are listed separately.
cIncludes women from Afghanistan, Bangladesh, Bhutan, Ceylon, India, Kashmir, Kazakhstan, Maldives, Nepal, Pakistan, Sri Lanka, Tajikistan, Turkmenistan, and Uzbekistan or women who reported being of Hindu or Sikh ancestry.
dIncludes women from Argentina, Belize, Bolivia, Chile, Colombia, Costa Rica, Dominican Republic, Ecuador, El Salvador, Guatemala, Honduras, Nicaragua, Panama, Peru, Uruguay, and Venezuela.
eIncludes women from Aruba, Barbados, Cuba, Grenada, Guyana, Haiti, Jamaica, Martinique, and West Indies.
Annual prevalence rates of gestational diabetes mellitus and chronic diabetes were calculated per every 100 births for the years 1990 through 2001, as well as by racial/ethnic subgroup. Confidence intervals (CIs) for prevalence rates were calculated with Fleiss quadratic methods.13 Data were analyzed with SAS 8.0 software (SAS Institute Inc, Cary, NC).
RESULTS
Birth Trends
In New York City, more than 1.5 million births by resident women were recorded between 1990 and 2001. During that time, the annual number of births declined 12%, from 125663 in 1990 to 110340 in 2001 (Table 1 ▶). Although the total number of deliveries decreased, the proportion of births to women aged 35 and older increased from 12% to 17%. Births to older women were most common among non-Hispanic White residents, but increases were observed in all racial/ethnic groups. The overall racial/ethnic distribution of births in New York City did not change substantially between 1990 and 2001, with modest exception. Deliveries to Asian women increased; among Hispanic women, deliveries to women of Mexican ethnicity increased while deliveries to women of Puerto Rican ethnicity decreased.
Gestational Diabetes
In 2001, the prevalence of any diabetes among delivering mothers in New York City was 4.2% (95% CI=4.1, 4.3). Most diabetes identified during pregnancy (91%) were determined to be gestational diabetes mellitus, as opposed to chronic diabetes. The specific prevalence of gestational diabetes mellitus was 3.8% of births (95% CI=3.7, 3.9) in 2001. This represented a 46% increase since 1990, when prevalence of gestational diabetes mellitus was 2.6% (95% CI=2.5, 2.7). The temporal increase observed for chronic diabetes was similar to that found for gestational diabetes mellitus (42%).
Gestational diabetes mellitus increased significantly among all major racial/ethnic groups except non-Hispanic White women. Gestational diabetes mellitus prevalence was highest and increased dramatically among Asian women (90% increase to 7.4% by 2001). Rates were lower but rose most rapidly among non-Hispanic Black women (124% increase to 3.8% by 2001). Gestational diabetes mellitus prevalence among Hispanic women was high in 1990, but the rate increased less rapidly (26% increase to 3.9% by 2001). The lowest rates were observed among non-Hispanic White women.
Stratifications of Asian and Hispanic mothers into ethnic subgroups showed stark differences in the prevalence and trends of gestational diabetes mellitus. The highest prevalence in the city, and one of the highest demonstrated increases, was observed in South and Central Asian mothers (95% increase to 11.1% by 2001). Among Hispanic women, the most rapid increase in gestational diabetes mellitus in the past decade occurred in Mexican mothers (96% increase to 4.9% by 2001); the highest prevalence among Hispanic women was observed in mothers from Caribbean countries or of Caribbean descent (49% increase to 5.2% by 2001).
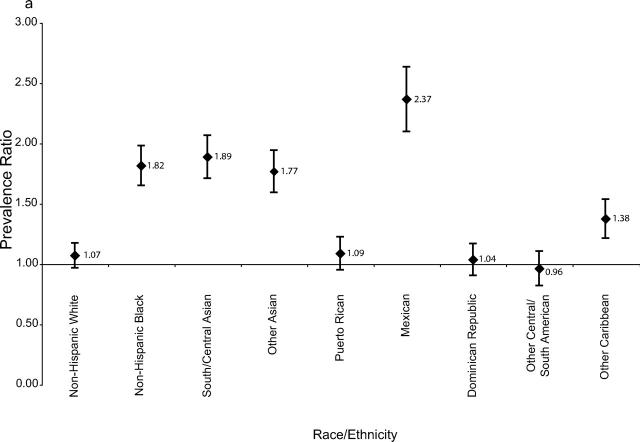
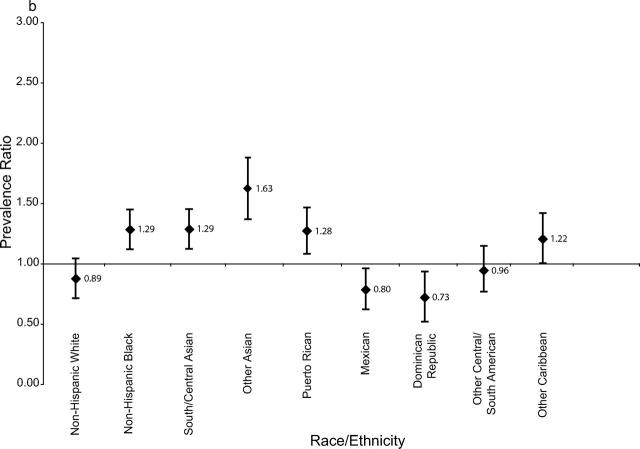
The effect of increasing maternal age on the prevalence of gestational diabetes mellitus was examined by stratifying results into younger and older age groups (Figure 1 ▶). The relative increase in gestational diabetes mellitus was greatest among women delivering before age 35—more than 75%. This was most apparent for younger non-Hispanic Black, Asian, and Mexican women. Increases among older women were generally more attenuated.
FIGURE 1—
Ratio with error bars of gestational diabetes mellitus prevalence in 1990 to 2001 among pregnant women whose age at delivery was (a) younger than 35 years and (b) 35 years and older, by race/ethnicity.
DISCUSSION
Findings from this study showed a rapid increase in gestational diabetes mellitus prevalence among most racial/ethnic groups between 1990 and 2001, according to population-based birth certificate data. The increase was particularly evident among younger women and among South and Central Asian, Mexican, and non-Hispanic Black women. Levels observed among New York City women of South and Central Asian ethnicity are among the highest documented nationwide, comparable to levels found in some Native American populations (up to 14%).14
The increase in gestational diabetes mellitus prevalence observed in New York City has been found in other settings, although at lower magnitudes of increase.9,15 Although age stratification showed that increasing maternal age plays some role in the observed trend, gestational diabetes mellitus has increased more dramatically in younger age groups. These findings suggest that the observed increase is likely influenced more by the obesity epidemic than by increasing maternal age, particularly because increases in diabetes mellitus were greatest among racial/ethnic minority groups that have been most affected by increasing obesity. Unfortunately, we were not able to examine the role of prepregnancy weight in gestational diabetes mellitus trends directly because this information was recorded poorly on birth certificates.
This analysis had several limitations. First, birth certificates may underreport diabetes during pregnancy.16 However, validation studies elsewhere have shown that gestational diabetes mellitus prevalence estimates obtained from birth certificates are comparable to those obtained from hospital discharge summaries.17 Also, the New York City birth certificate uses a check box system, which has been shown to result in better ascertainment of pregnancy complications than open-ended queries.18
Second, increased gestational diabetes mellitus diagnoses could result from increases in gestational diabetes mellitus screening, leading to the inclusion of milder cases of gestational diabetes mellitus. However, a large study of health plan members in California from 1991 to 2000 showed a 35% increase in gestational diabetes mellitus prevalence with no significant changes in screening practices, and plasma glucose screening values in the 25th percentile range did not decline.9 In our study, we noted a concomitant increase in chronic diabetes, suggesting that gestational diabetes mellitus increases were not solely the product of increases in screening for gestational diabetes mellitus. We also observed increases in the prevalence of chronic diabetes among delivering mothers from all major racial/ethnic groups (data not shown), suggesting that our findings were not a result of differential diabetes screening practices.
Despite controversy over the optimal management of gestational diabetes mellitus,10–12 the risk of maternal hyperglycemia on fetal development is well documented,19 as is maternal risk for later development of type 2 diabetes.20 The rapid increases in and higher prevalence of gestational diabetes mellitus observed among South and Central Asian, Mexican, and non-Hispanic Black pregnant women in New York City may be key influences on the rising disparities in both development of maternal type 2 diabetes and childhood obesity.21,22 Whether this may affect prevalence of and disparity in type 2 diabetes in youths is less clear. The findings underscore the importance of prompt screening and the maintenance of metabolic control during and after pregnancy, particularly among women with gestational diabetes mellitus. Women at high risk, especially women of South Asian descent, should be screened before pregnancy, and blood sugar control should be optimized before becoming pregnant.
Acknowledgments
The authors would like to thank the staff at the Bureau of Vital Statistics for providing access to birth certificate records and for their advice on interpreting the information collected. Additional thanks go to Dr Steven Helgerson for his early encouragement of our conducting this study.
Human Participant Protection No protocol approval was needed for this study.
Peer Reviewed
Contributors L.E. Thorpe and D. Berger originated the study, and L.E. Thorpe, with J.A. Ellis, V.R. Bettegowda, and G. Brown, analyzed the data and wrote the first draft of the brief. T. Matte, M. Bassett, D. Berger, and T.R. Frieden revised and edited the final draft.
References
- 1.Cousins L. Pregnancy complications among diabetic women: review 1965–1985. Obstet Gynecol Surv. 1987;42:140–149. [PubMed] [Google Scholar]
- 2.Xiong X, Saunders LD, Wang FL, Demianczuk NN. Gestational diabetes mellitus: prevalence, risk factors, maternal and infant outcomes. Int J Gynaecol Obstet. 2001;75:221–228. [DOI] [PubMed] [Google Scholar]
- 3.Catalano P, Vargo KM, Bernstein IM, et al. Incidence and risk factors associated with abnormal postpartum glucose tolerance in women with gestational diabetes. Am J Obstet Gynecol. 1991;165:914–919. [DOI] [PubMed] [Google Scholar]
- 4.Kim C, Newton KM, Knopp RH: Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862–1868. [DOI] [PubMed] [Google Scholar]
- 5.Solomon C, Willet W, Carey V, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997;278:1078–1083. [PubMed] [Google Scholar]
- 6.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related risk factors, 2001. JAMA. 2003;289:76–79. [DOI] [PubMed] [Google Scholar]
- 7.Okusun IS, Dinesh Chandra KM, Boev A, et al. Abdominal adiposity in US adults: prevalence and trends, 1960–2000. Prev Med. 2004;39:197–206. [DOI] [PubMed] [Google Scholar]
- 8.Births: Final Data for 2002. Atlanta, Ga: Centers for Disease Control and Prevention; December 2003. DHHS publication PHS 2004-1120. [PubMed]
- 9.Ferrara A, Kahn HS, Quesenberry CP, Riley C, Hedderson MM. An increase in the incidence of gestational diabetes mellitus: Northern California, 1991–2000 [published erratum appears in Obstet Gynecol. 2004; 103:799]. Obstet Gynecol. 2004;103: 526–533. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Public health guidelines for enhancing diabetes control through maternal- and child-health programs. MMWR Morb Mortal Wkly Rep. 1986;35:201–208, 213. [PubMed] [Google Scholar]
- 11.US Preventive Services Task Force. Screening for Gestational Diabetes Mellitus: Recommendations and Rationale. Rockville, Md: Agency for Healthcare Research and Quality; February 2003. Available at: http://www.ahrq.gov/clinic/3rduspstf/gdm/gdmrr.htm. Accessed March 1, 2005.
- 12.O’Sullivan JB, Mahan CM, Charles D, et al. Screening criteria for high-risk gestational diabetic patients. Am J Obstet Gynecol. 1973;116:895–900. [DOI] [PubMed] [Google Scholar]
- 13.Fleiss JL. Statistical Methods for Rates and Proportions. 2nd ed. New York, NY: John Wiley & Sons; 1981.
- 14.Benjamin E, Winters D, Mayfield J, Gohdes D. Diabetes in pregnancy in Zuni Indian women: prevalence and subsequent development of clinical diabetes after gestational diabetes. Diabetes Care. 1993;16: 1231–1235. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Pregnancy complications and perinatal outcomes among women with diabetes—North Carolina, 1989–1990. MMWR Morb Mortal Wkly Rep. 1993;42(43): 847–851. [PubMed] [Google Scholar]
- 16.Piper JM, Mitchel EF, Snowden M, Hall C, Adams M, Taylor P. Validation of 1989 Tennessee birth certificates using maternal and newborn hospital records. Am J Epidemiol. 1993;137:758–768. [DOI] [PubMed] [Google Scholar]
- 17.Meyer RE, Buescher PA, Sullivan LA. Diabetes in Pregnancy in North Carolina. Raleigh, NC: State Center for Health and Environmental Studies; May 1992; CHES Studies Report No. 64.
- 18.Frost F, Starzyk P, George S, et al. Birth complication reporting: the effect of birth certificate design. Am J Public Health. 1984;74:505–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjos SL, Buchanan TA. Gestational diabetes mellitus. N Engl J Med. 1999;341:1749–1756. [DOI] [PubMed] [Google Scholar]
- 20.Kim C, Newtown KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862–1868. [DOI] [PubMed] [Google Scholar]
- 21.Thorpe LE, Mostashari F, Berger DK, Cobb LK, Helgerson SD, Frieden TR. Diabetes is epidemic. NYC Vital Signs. 2003;2(1):1–4. Available at: http://www.nyc.gov/html/doh/downloads/pdf/survey/survey-2003diabetes.pdf. Accessed February 28, 2005. [Google Scholar]
- 22.Thorpe LE, List DG, Marx T, May L, Helgerson SD, Frieden TR. Childhood obesity in New York City elementary school students. Am J Public Health. 2004;94: 1496–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]