Abstract
People with rare, inherited chronic health conditions, such as hemophilia, face added physical, social, emotional, and fiscal challenges beyond those that are common to more prevalent chronic conditions. In 1975, a partnership among clinicians, consumers, and government agencies created a nationwide regional health delivery system that increased access to clinical care, prevention, and research, thereby improving health outcomes for people with hemophilia in the United States.
Today, more than 130 Comprehensive Hemophilia Diagnostic and Treatment Centers in 12 regions serve 70%–80% of the nation’s hemophilia patients. Health care leaders and advocates for other rare, expensive, chronic disorders may find that regionalization improves survival and reduces disability among affected populations. However, diverse and stable resources are needed to sustain such a model in our profit-oriented US health care arena.
SPECIAL CHALLENGES confront people with rare, chronic, genetic conditions, such as hemophilia. Specialists who provide clinical care are few and widely dispersed, and the disorders are familial. The benefits of specialist teams that direct care and coordination, teach self management strategies, refer to support services, and help patients navigate the health care system have been associated with improved health outcomes for people with prevalent conditions1,2 such as asthma3–5 and AIDS.6,7 Lasker outlined the value of these medical and public health partnerships.8
This article demonstrates how successful partnerships among health care professionals, consumers, and government agencies have created a nationwide regional health delivery system that has optimized access to care and prevention services and improved health outcomes for people with hemophilia in the United States. Elements of the chronic care model, described elsewhere,9–11 include community resources and policies, health care organizations, self-management support, delivery system design, and clinical information systems and are exemplified by our nationwide system of hemophilia care.
Hemophilia is an inherited bleeding disorder that primarily affects males and is characterized by bleeding into joints and muscles. Hemorrhages into the head, throat, and gastrointestinal areas can be fatal. Although not curable, hemophilia can be treated by replacing, via intravenous infusion, the missing proteins needed for clotting. Hemophilia is diagnosed at birth, and treatment lasts a lifetime. Hemophilia affects about 18 000 Americans of all racial and ethnic backgrounds.12 The article by Katon et al.1 on redefining practitioner roles in caring for people with complex, severe, and persistent disorders applies to people with hemophilia. The most successful treatment and care interventions require an organized multidisciplinary approach.
Thirty years ago, parents faced with the crippling and possible death of their hemophilic children formed a bond with their physicians that led to the development of an extremely effective health advocacy collaboration. That partnership created a nationwide hemophilia health delivery system that has grown beyond medical care to include research and the public health functions of needs assessment, capacity building, surveillance, prevention, and policy.13 Unique characteristics of the US hemophilia model are as follows: (1) its regional structure; (2) ongoing collaboration among patients, health care professionals, and government agencies; and (3) shared vision, goals and standards.14 Today there are more than 130 federally supported Comprehensive Hemophilia Diagnostic and Treatment Centers (HTC) throughout the United States organized into 12 regions.
History of the Regional System of Care and Outcomes
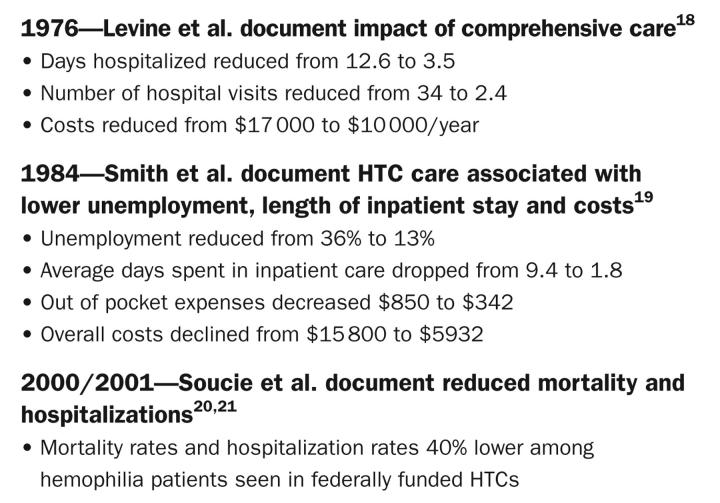
In 1975, through advocacy efforts led by those who treated hemophilia and parents affiliated with the consumer advocacy organization, the National Hemophilia Foundation (NHF), and based on the experience of early novel multidisciplinary approaches in Britain15 and California,16 Congress provided support to fund 26 comprehensive hemophilia specialty clinics through Health, Education, and Welfare, now the US Department of Health and Human Services Maternal and Child Health Bureau (HRSA).17 Early studies verified that HTC-based care drastically reduced many hemophilia-related physical and economic consequences, including days hospitalized by 72%, hospital visits by 93%, average length of stay by 7.6 days, out-of-pocket expenses by 60%, overall costs by 41% to 63%, and unemployment by 64%.18,19
A more recent study of 3000 hemophilic males funded by the Centers for Disease Control and Prevention (CDC) documents a 40% decrease in mortality20 and a 40% reduction in bleed-related hospitalizations21 among men who used an HTC at least once in the 3-year study period. These decreases occurred despite the HTC cohort having more severe symptoms and higher proportions of HIV and hepatitis than peers who obtain their care outside the HTC network (Figure 1 ▶).
FIGURE 1—
Outcomes of comprehensive care at US hemophilia treatment centers.
On the basis of early successes of the 26 original HTCs, in the early 1980s HRSA developed a regional framework for organizing the HTC network22 to encourage effective distribution of comprehensive services via organized linkages between HTCs in a defined geographic area. In each region, one HTC (or, in 3 cases, an NHF chapter) shouldered the added public health responsibilities of regional administration, including: (1) region-wide needs assessments, (2) grant writing and management, (3) staff training, (4) organizing an executive committee, (5) evaluation and fiscal over sight, and (6) liaising with related health agencies.23
The nascent HTC network was in place to respond to the HIV crisis. Most people with hemophilia were infected with HIV24 and hepatitis25 from contaminated blood-based treatment products. Advocacy in the 1980s succeeded in obtaining new Congressional support to the CDC to help HTCs develop expertise in HIV risk reduction counseling and testing, and in facilitating peer support. Thus began the new and lasting relationship between the HTCs, CDC, HRSA, and NHF. Advocacy with government regulators and manufacturers led to improved viral safety of the factor products that are used by this community. Blood products were free from HIV after 1984, from hepatitis C after 1992, and from hepatitis B after 1993.26
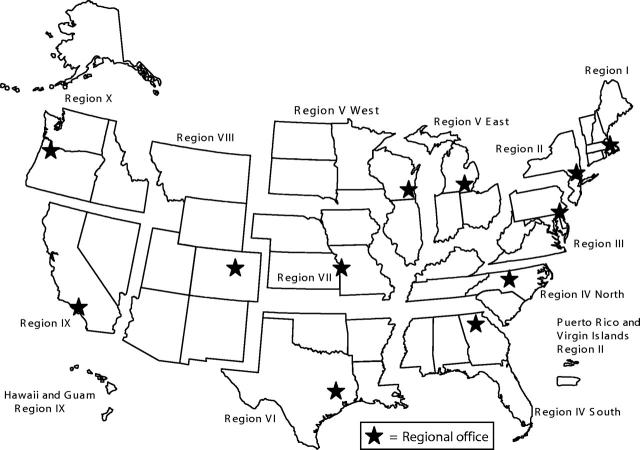
To reduce the new HIV grant-related administrative burdens on medical directors at the HTC regional centers, HTCs in California and New York each created a “regional coordinator” position. Because of their value, CDC and HRSA mandated the regional coordinator position for all regions in 1990.23 Currently, more than 130 HTCs in 12 regions serve patients in all US states, the District of Columbia, Puerto Rico, and Guam (Figure 2 ▶). HTCs care for 70%–80% of all US residents with hemophilia.12 The HTCs have proven effective in slowing disease progression and complications.
FIGURE 2—
US Hemophilia Treatment Center regions.
Comprehensive HTC Care: Philosophy, Goals, Team, and Services
The HTC model of care is multidisciplinary and integrates intensive patient and family education in its core philosophy. All of the HTCs provide diagnostic and treatment services in line with HRSA and CDC goals and NHF standards.14 HTCs are typically housed in university-based tertiary care hospitals, offering a full range of outpatient and inpatient services including case management and collaboration with primary care practitioners and other subspecialists. Because of the expertise of their medical directors, HTC care has expanded to other bleeding and, in two thirds of HTCs, clotting disorders.
In 2004,27 US HTCs provided care to 27 662 patients: 15 224 with the most common forms of hemophilia; 10 742 with von Willebrand disease (VWD), the most prevalent inherited bleeding disorder, affecting 1:100 males and females of all back-grounds28–30; 1553 with rarer hemophilias; and 143 with acquired symptomatic factor VIII inhibitors.27 Two thirds of HTCs optionally reported caring for an additional 2537 people with other bleeding disorders and 6444 with inherited thrombotic disorders.27 Twenty-nine percent of HTC patients were female, and 27% were non-white.27 More than 5000 HTC patients had hepatitis C,31 and 1600 had HIV.27 Only 4% had no insurance.27
The US HTCs conducted 14 151 annual evaluations, provided another 18 051 comprehensive examinations, gave 1903 consults, conducted 7759 diagnostic exams, and provided 7380 education seminars to groups separate from 1-on-1 teaching in clinic.27 HTCs monitor universal precautions with the 9119 people who they taught to self-infuse their medication.27 They reported that 21103 patients had a primary care practitioner; HTCs sent written summaries to 73% of them.27 HTCs enrolled over 16 000 of their patients (including 12 480 or 87% of patients with hemophilia) in the CDC’s nationwide prospective surveillance study to monitor hemophilia complications, logging more than 42 000 study visits since that study began 1998.31
Federal grants partially support salaries of HTC core staff to provide comprehensive care (HRSA) and to conduct research and provide prevention services (CDC) to monitor and prevent 2 main hemophilia complications: blood-borne viruses and joint disease. Both agencies share as their top goal enhanced access to the regional network of HTCs. HRSA-specific goals for HTCs are as follows: (1) to provide care that is family centered, culturally competent, community based, and easy to use; (2) to strengthen services to women, minorities, adolescents, the geographically distant, and the insurance restricted; (3) to encourage consumer partnership in decision making; (4) to conduct outreach and education; (5) to emphasize prevention; (6) to collaborate with health agencies; and (7) to link patients with primary care practitioners. The CDC-specific goals for HTCs are as follows: (1) to prevent complications through assessment, surveillance, outreach, education, consultation, and management; (2) to participate in blood safety monitoring; and (3) to collaborate with consumer organizations to deliver consistent prevention messages.
The core HTC team is comprised of experts from 4 disciplines: a board-certified hematologist who serves as HTC director, a nurse coordinator, a social worker, and a physical therapist. This team consults with subspecialists including those in dentistry, genetics, orthopedics, infectious disease, hepatology, and pharmacy. HTC staffs maintain long-term relationships with patients and families. HTC clinicians extensively educate the parents of newly diagnosed infants, teaching them how to recognize and effectively respond to bleeds. As children develop verbal skills, HTCs involve the children in their own treatment, teaching them self-infusion skills.32 This extensive family and patient education promotes normalcy: including the expectation of regular school attendance, physical activity, and employment.
HTC clinical services include identification of affected people and families at risk; diagnosis, treatment, self-infusion education, and monitoring; treatment plan development; counseling; case management; rehabilitation; genetic counseling and testing; dental and orthopedic care; nutritional and vocational counseling; and assessing and providing treatment and/or referrals for secondary conditions including hepatitis and HIV/AIDS. HTC community services include linkage with primary care practitioners, financial counselors, and insurance programs; referral to local NHF chapters; and educating and consulting with community providers, and school personnel via satellite clinics, telephone, telemedicine, and grand rounds. HTCs conduct research to develop new therapies, determine optimal treatment protocols, reduce complications, and determine the cost of care. HTC services reach isolated communities including the Amish; reservation-based American Indians; and residents of Alaska and Guam.33 HTC team members educate and advise policy makers. All of these services aim to improve the quality of life and lengthen the life span (Figure 3 ▶).
FIGURE 3—
Comprehensive hemophilia care components.
The achievement of HRSA and CDC goals is measured through site visits, review of quantified HTC goals and objectives, and analysis of 2 nationwide datasets: the Hemophilia Data Set27 and the CDC Universal Data Collection study (UDC).34 The Hemophilia Data Set annually tracks hemophilia patient demographics and a range of comprehensive care services. The UDC is a nationwide, prospective hemophilia surveillance system that monitors joint damage and blood-borne viruses. The largest hemophilia database in the world, the UDC measures clinical characteristics and outcomes, allows for multivariate analysis of orthopedic changes, and includes a plasma sample bank for rapid investigation of new threats to the blood supply. UDC data are collected annually for all consenting patients seen at HTCs.
Regional HTC Network Administration
The 12 regional HTC networks are administered through the leadership of a regional director and regional coordinator. The regional director is typically an HTC medical director, chosen by consensus for his or her leadership skills. Regional coordinator professional backgrounds include nursing, public health, social work, and law. Regional director/regional coordinator responsibilities were recently standardized in 2001 and embrace both HRSA and CDC duties. These leaders identify and respond to gaps in services via needs assessments, program planning, capacity building, technical assistance, data analysis, and site visits. They promote partnerships between HTCs, state health departments, and local chapters of the NHF. They preside over regional executive committees and foster intraregional communication and referrals. Regional coordinators write and monitor grants; provide fiscal management; oversee annual data collection, analysis, and evaluation; orient new HTC staff; and organize annual clinical education conferences. As regional points of contact and information, regional coordinators rapidly disseminate and replicate best practices, protocols, and new programs and efficiently mobilize HTCs and consumer leadership to respond to emerging health, economic, and social issues.
Because of the limited number of hemophilia experts, capacity building is critical. Regions use many cost-effective strategies to foster skill development and professional networking across institution and state boundaries. The strategies include regional conferences, discipline-specific work groups, listservers, shadowing HTC clinics, and linking mentor HTC clinicians with newly hired colleagues. The regional approach maximizes scarce resources, serving a key role in supporting the hemophilia health care and public health workforce. Regionalization reduces professional isolation and thereby enhances retention, improving the continuity of care. Shared goals, standards, philosophy, and a regional identity foster cooperation and referrals among HTCs, assisting patients who are traveling, going off to college, or relocating.
Multilevel Health Policy Collaboration
The regional infrastructure, HTC collaboration with the NHF and its local chapters and the strong relationships built over the past 30 years makes the hemophilia community effective health policy advocates. Leaders monitor and, together, take action at local, state, and federal levels to improve access to HTCs, strengthen insurance and reimbursement, and enhance coordination with state Title V and Medicaid programs. Hemophilia care is expensive, costing an average of $140 000 annually for a severely affected adult (1995 dollars).35 More than 90% of the cost of hemophilia care is in treatment products.35 Improved technology and an extremely limited market has caused a continued rise in costs,36 thereby requiring ongoing advocacy efforts.
The regional network of HTCs for hemophilia is the result of decades long collaboration between patients and their families, health care professionals, and federal public servants who all believe that an organized special care system is imperative to improve the health of affected people. The NHF, founded in 1948, was the first national hemophilia advocacy organization in the US. It was and continues to be instrumental in securing and sustaining federal funding for US HTCs. The NHF partners with HTC clinicians to develop national guidelines for treatment and health care policy. The NHF is the central point for information for people with hemophilia in the United States. Through its more than 45 chapters, the NHF provides the infrastructure for family support networks, advocacy, and education.
The NHF has a Medical and Scientific Advisory Council (MASAC) comprised of the leading bleeding disorder physicians and researchers in the United States. MASAC recommendations set guidelines for care for people with bleeding disorders. MASAC also has representation from HTC nurses, social workers, and physical therapists; patients; and government liaisons including the HRSA, CDC, National Institutes of Health (NIH), and US Food and Drug Administration.
The committee structure of NHF also supports the regional HTC model. Committees are organized to address national issues and develop strategies to meet constituent needs for high-quality medical and health care, information, and support services. NHF has working groups for HTC nurses, social workers, and physical therapists. Each consists of a representative from the 12 HTC regions. The working groups’ deliberations bolster the rapid dissemination of model programs and practices throughout the United States.
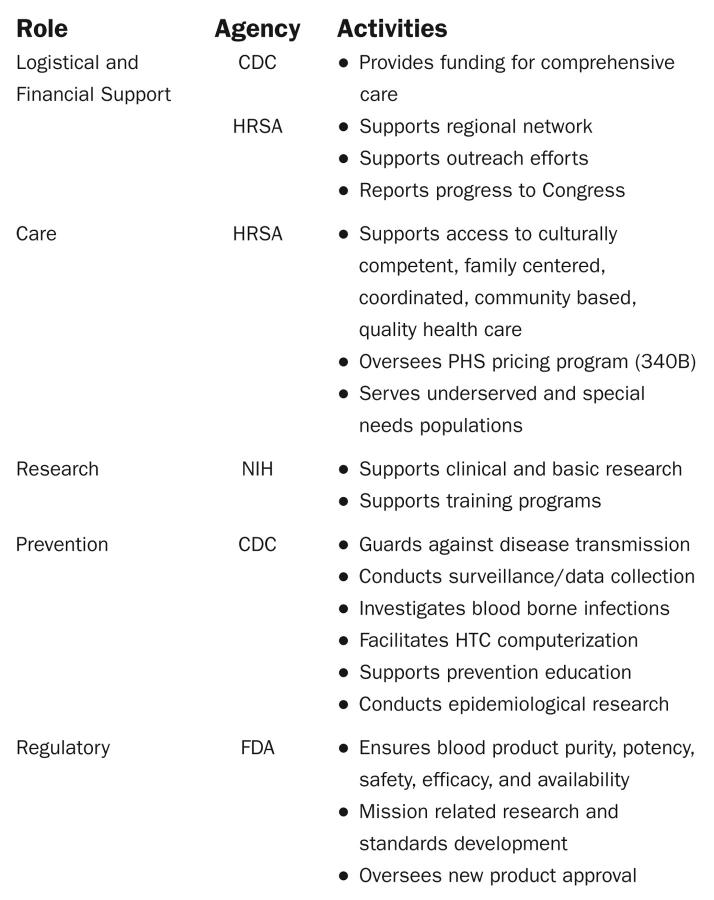
The NHF secures funding from private and public sources for basic science, gene therapy, clinical research, educational programs, and materials. These sources include the HRSA, CDC, and NIH National Heart, Lung and Blood Institute and other institutes, private foundations, and corporations. Consumers, HTC clinicians, and regional coordinators serve on blood safety advisory boards at the US Food and Drug Administration and US Department of Health and Human Service to represent constituent needs (Figure 4 ▶).
FIGURE 4—
US Department of Health and Human Services roles in hemophilia.
Other key nationwide agencies benefit the hemophilia population and health delivery system. The Hemophilia and Thrombosis Research Society is a professional organization consisting of HTC physicians who are interested in research to improve care and treatment. This group was born from the need to specifically develop research questions and protocols that advance our understanding of best treatments. The CDC supports clinical fellowships through the American Society of Hematology. The NIH provides fellowships and other training opportunities in nonmalignant hematology. Clinical fellowships are critical to recruit the next generation of physician leaders.
At the state level, HTC staff and regional leaders work with State Health Departments, focusing on policies and programs to ensure access to HTC care. NHF chapters and chapters of the Hemophilia Federation of America (HFA), another nationwide patient advocacy agency, assist HTC staff and regional leaders in statewide policy advocacy. At the local level, HTC clinicians staff infirmaries at summer hemophilia youth camps, serve as medical advisors to NHF and HFA local chapters, and present at patient education symposia. Regional coordinators sometimes provide local NHF and HFA chapters with linkage to mentors, and technical assistance in program planning and evaluation. The ongoing interaction between health care professionals and consumers at every level is what makes the partnership effective in furthering access to care.
Critical Challenges
The federally supported regional network of HTCs is a successful example of the type of health care delivery system called for in the 2001 Institute of Medicine study to improve the quality of US health care.37 Despite the success of this model, numerous challenges remain. They include inadequate reimbursement, managed care, static federal grant funding, lack of specialists choosing careers in non-malignant hematology,38 and diminished numbers of coagulation laboratories at US hospitals.39
Reimbursement and managed care problems abound. First, US health care reimbursement is on the basis of acute, not chronic, care.40 Third-party payers do not routinely reimburse care coordination.41 Inpatient reimbursement is inadequate: HTC host hospitals have documented several million dollars in debt for a single inhibitor patient.42 Managed care plans often limit access to HTCs outright or delay, deny, and modify HTC treatment recommendations. Many managed care laboratories lack the expertise and experience to accurately process and interpret hemophilia and VWD diagnostic tests. Pharmacy benefit management programs often limit treatment products and are unfamiliar with self-infusion supplies and factor concentrate, resulting in shipment inaccuracies and delays.
Federal support has faltered. Government subsidies for teaching hospitals and medical schools, where most HTCs reside, have been drastically reduced. HRSA and CDC HTC grant funding has been stagnant at $5.3 million43 and $6.8 million (S. Crudder, BSN, unpublished data, 2004), respectively, for the past 5 years, despite a 13% increase in hemophilia and a 68% increase in VWD patients from 199844 to 200427 HRSA/CDC grant dollars alone cannot support the HTC network, and recruit trained, but underpaid and institutionally under supported hemophilia specialists.
HTCs would not have survived without their inclusion in the 1992 legislation that created the federal Public Health Service (PHS) outpatient drug discount program.45 PHS programs reduce the cost of outpatient medication to third-party payers by about 50%.46 Nearly half of the US HTCs have developed PHS programs to generate desperately needed revenue to maintain and expand HTC services.47
The US regional hemophilia model is a highly beneficial, cost-effective, and clinically sound approach to health care for people with rare, genetic, chronic bleeding disorders.47 Its replication holds promise. HTCs care for nearly 8000 women with inherited bleeding disorders,27,48 and nearly 11000 people with VWD.27 Pilot projects were recently initiated to expand the model to people with thalassemia (CDC/HRSA), thrombophilia (CDC), and sickle cell disease (HRSA). Health care leaders and advocates for people who have other rare, expensive, chronic disorders may find a regional approach useful to improve health outcomes and reduce complications. However, they must be clear about the real economic impact: resources are needed from diverse and stable sources to sustain this model in our profit-oriented US health care arena.
Acknowledgments
The program is funded in part by US Department of Health and Human Services, HRSA Program Announcement 05-029 and Centers for Disease Control and Prevention Cooperative Agreement (Program Announcement 1049).
Human Participant Protection No protocol approval was required for this program.
Peer Reviewed
Contributors J. R. Baker originated the symposium which served as the basis of this article, organized the collaboration among authors, and oversaw the concept and article development, including literature review, writing, and editing. S. O. Crudder and B. Riske contributed to concept development, writing, literature review, and editing. V. Bias contributed to the concept development and writing. A. Forsberg contributed to concept development, writing, and editing.
References
- 1.Katon W, Von Korff M, Lin E, Simon G. Rethinking practitioner roles in chronic illness: the specialist, primary care physician, and the practice nurse. Gen Hosp Psychiatry. 2001;23: 138–144. [DOI] [PubMed] [Google Scholar]
- 2.Donoghue M. Comparing generalists, and specialists. Arch Intern Med. 1998;158:1596–1608. [DOI] [PubMed] [Google Scholar]
- 3.Mayo P, Richman J, Harris H. Results of a program to reduce admissions for adult asthma. Ann. Intern Med. 1990;112:864–871. [DOI] [PubMed] [Google Scholar]
- 4.Zeigler R, Heller S, Mellon M, et al. Facilitated referral to asthma specialist reduces relapse in emergency room visits. J Allergy Clin Immunol. 1991;87: 1160–1180. [DOI] [PubMed] [Google Scholar]
- 5.Gibson PG, Coughan J, Wilson A, et al. Self-management education and regular practitioner review of adults with asthma. Cochrane Database Syst Rev. 2000;2:CD001117. [DOI] [PubMed] [Google Scholar]
- 6.Kitahata M, Koepsell T, Deyo R, et al. Physicians’ experience with acquired immunodeficiency syndrome as a factor in patients’ survival. N Engl. J Med. 1996;334:701–706. [DOI] [PubMed] [Google Scholar]
- 7.Turner B, McKee L, Fanning T, Markson L. AIDS specialist versus general ambulatory care for advanced HIV infection, and impact on hospital use. Med Care. 1994;32:902–906. [DOI] [PubMed] [Google Scholar]
- 8.Lasker RD and the Committee on Medicine and Public Health. Medicine and Public Health: The Power of Collaboration. New York: New York Academy of Medicine; 1997.
- 9.Bodenheimer T, Wagner EH, Grumbach K. Development and use of the chronic care model improving primary care for patients with chronic illness. JAMA. 2002;288:1775–1779. [DOI] [PubMed] [Google Scholar]
- 10.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA. 2002;288:1909–1914. [DOI] [PubMed] [Google Scholar]
- 11.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood). 2001;20:64–78. [DOI] [PubMed] [Google Scholar]
- 12.Soucie JM, Evatt B, Jackson D. Occurrence of hemophilia in the United States. The Hemophilia Surveillance System Project Investigators. Am J Hemato1. 1998;59:288–294. [DOI] [PubMed] [Google Scholar]
- 13.Resnick Susan. Blood Saga: Hemophilia, AIDS, and the Survival of a Community. Berkeley, CA: University of California Press; 1999.
- 14.Standards and Criteria for the Care of People with Congenital Bleeding Disorders. MASAC Recommendation #132. New York: National Hemophilia Foundation; 2002.
- 15.Biggs R, Macfarlane RG. Treatment of Haemophilia and Other Coagulation Disorders. Oxford, UK: Blackwell Scientific; 1966.
- 16.Kasper CK. A total program for the patient with hemophilia. J Am Phys Ther Assoc. 1966;46:1268–1285. [DOI] [PubMed] [Google Scholar]
- 17.PL 9463: The Public Health Service Act Establishing the Hemophilia Diagnostic and Treatment Center Program. N0.1131 of Public Las 9463. Washington, DC: Government Printing Office; 1975.
- 18.Levine PH, McVerry BA, Segelman AE, Cranford CM, Zimbler S. Comprehensive health care clinic for hemophiliacs. Arch Intern Med. 1976;136: 792–794. [PubMed] [Google Scholar]
- 19.Smith PS, Levine PH. The benefits of comprehensive care of hemophilia: a five-year study of outcomes. Am J Public Health. 1984;74:616–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soucie JM, Nuss R, Evatt B, et al. and the Hemophilia Surveillance System Project Investigators. Mortality among males with hemophilia: relations with source of medical care. Blood. 2000; 96:437–442. [PubMed] [Google Scholar]
- 21.Soucie JM, Nuss R, Evatt B, et al., and the Hemophilia Surveillance System Project Investigators. Home based factor infusion therapy and hospitalization for bleeding complications among males with hemophilia. Haemophilia. 2001;7: 198–206. [DOI] [PubMed] [Google Scholar]
- 22.McPherson M. Hemophilia Newsnotes. National Hemophilia Foundation: New York, NY; May 1982.
- 23.Meredith K. The Maternal and Child Health Bureau. Hemophilia Regional Coordinators Assessment Project, Final Report. National Hemophilia Program, US Department of Health and Human Services: Rockville, MD; 1993.
- 24.Evatt BL, Gomperts ED, McDougal JS, Ramsey RB. Coincidental appearance of LAV/HTLV-III antibodies in hemophiliacs and the onset of the AIDS epidemic. N Engl J Med. 1985;312: 483–486. [DOI] [PubMed] [Google Scholar]
- 25.Soucie JM, Robertson BH, Bell BP, McCaustland KA, Evatt BL. Hepatitis A virus infections associated with clotting factor concentrate in the United States. Transfusion. 1998;38:573–579. [DOI] [PubMed] [Google Scholar]
- 26.Soucie JM, Richardson LC, Evatt B, et al., and the Hemophilia Surveillance System Project Investigators. Risk factors for infection with hepatitis B and hepatitis C viruses in a large cohort of hemophilic males. Transfusion. 2001; 41:338–343. [DOI] [PubMed] [Google Scholar]
- 27.Hemophilia Data Set, 2004. Unpublished data collected annually by 130+ US. federally supported Hemophilia Treatment Centers.
- 28.Dilley A, Drews C, Miller C, et al. Von Willebrand disease and other inherited bleeding disorders in women with diagnosed menorrhagia. Obstet Gynecol. 2001;97:630–636. [DOI] [PubMed] [Google Scholar]
- 29.Dilley A, Crudder SO. von Wille-brand disease in women: the need for recognition and understanding. J Women’s Health Gend Based Med. 1999;8: 443–445. [DOI] [PubMed] [Google Scholar]
- 30.Kadir RA, Economides DL, Sabin C, et al. Frequency of inherited bleeding disorders in women with menorrhagia. Lancet. 1998;351:485–489. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Division of Hereditary Blood Disorders. Bleeding and Clotting Disorder Surveillance. UDC Summary Reports. Available at: http://www2a.cdc.gov/ncbddd/htcweb/UDC_Report/UDC_Report.asp. Accessed August 19, 2005.
- 32.Butler RG, Crudder SO, Riske B, Toal S. Basic Concepts of Hemophilia. Atlanta, GA: Centers for Disease Control and Prevention; 2001.
- 33.Zabala RV, Baker JR. Guam’s Quest for improved hemophilia care. Pacific Health Dialog. 2002;9:317–320. [PubMed] [Google Scholar]
- 34.Report on the Universal Data Collection Program. Atlanta, GA: Centers for Disease Control and Prevention; 2002; 4:1–28.
- 35.Globe DR, Cunningham WE, Andersen R, et al. The Hemophilia Utilization Group Study: cost of outpatient, in-patient and pharmaceutical care. Int J Pediatr Hem/Onc. 2001;7:87–100. [Google Scholar]
- 36.Rogoff E.G., Guirguis HS, Lipton RA, et al. The upward spiral of drug costs: A time series analysis of drugs used in the treatment of hemophilia. Throb Haemost. 2002;88:545–553. [PubMed] [Google Scholar]
- 37.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the Twenty First Century. Washington, DC: National Academy Press; 2001.
- 38.Evatt BL, Black C, Batorova A, Street A, Srivastava A. Comprehensive care for haemophilia around the world. Haemophilia. 2004;10(Suppl 4): 9–13. [DOI] [PubMed] [Google Scholar]
- 39.Shahangian S, Stankovic AK, Lubin IM, et al. Survey of Hospital Coagulation Laboratory Practices, United States—2001. Atlanta, GA: Centers for Disease Control and Prevention; 2003.
- 40.Wagner EH. Chronic disease management: What will it take to improve care for chronic illness? Effect Clin Practice. 1998;1:2–4. [PubMed] [Google Scholar]
- 41.Anderson G, Knickman JR. Changing the chronic care system to meet people’s needs. Health Aff (Millwood). 2001;20:146–160. [DOI] [PubMed] [Google Scholar]
- 42.Winslow R. Intensive Care: One patient, 34 days in the hospital, $7,000 syringes, and a bill for $5.2 million. Wall Street Journal. August 2, 2001:A1.
- 43.US Department of Health and Human Services, Public Health Service, Health Resources and Services Administration, Maternal and Child Health Bureau. Understanding Title V of the Social Security Act. 2000; Rockville, MD.
- 44.Hemophilia Data Set, 1998. Unpublished data collected annually by 130+ US. federally supported hemophilia treatment centers.
- 45.PL 102–585, the Veteran’s Health Care Act (VHCA) of 1992, Sec. 602 subpart VII, Sec. 340B to Part D of Title III.
- 46.Prescription Drugs: Expanding Access to Federal Prices Could Cause Other Drug Price Changes. GAO/HEHS-00–118. Washington, DC: US General Accounting Office; 2000.
- 47.Hoots WK. Comprehensive care for hemophilia and related inherited bleeding disorders: Why it matters. Current Science. 2003;2:395–401. [PubMed] [Google Scholar]
- 48.Health Resources and Services Administration. Women’s Health 2003. Rockville, MD: US Department of Health and Human Services; 2003:37.