Abstract
Objectives. We investigated the role of infant bedding items, as part of a composite bedding environment, in the development of childhood wheezing.
Methods. This prospective cohort investigation involved 863 children who participated in an infant survey in 1988 and an asthma study in Tasmania, Australia, in 1995. The derived 3 composite infant bedding categories corresponded to increasing numbers of house dust mite (HDM)–rich bedding items used. Outcomes measured included recent and frequent wheezing.
Results. Composite infant bedding used was associated with recent wheezing. Effects increased at increasing levels of HDM–rich bedding items used. Effects were further enhanced by home environmental factors of bedroom heating, recent bedroom painting, and absence of bedroom carpeting. When any 2 or more of these environmental factors were present, a strong dose–response relationship was evident.
Conclusions. Our results show that bedding exposures in infancy are prospectively associated with childhood wheezing and that home environmental conditions may modify this association.
The reasons for the changing incidence of childhood asthma over time are unclear. The indoor environment, in particular exposure to house dust mite (HDM) allergens, is an area of concern.1 High rates of exposure to HDM allergens are associated with increased risks of subsequent HDM sensitization, but the role of HDM exposure in asthma is less clear.2 Bedding is a significant source of HDM allergens, with infant bedding materials such as synthetic pillows and quilts3 and sheepskins4 harboring high HDM concentrations. One study involving adults showed that replacing old with new bed quilts led to a more than 10-fold reduction in airborne Dermatophagoides HDM allergen levels near the face during sleep.5
HDM allergen levels associated with bedding have been shown to be more important than bedroom floor HDM levels in determining airway responsiveness6 and asthma severity.7 This may explain why a prospective association between infant bedroom floor HDM levels and asthma has been found in only 1 of 3 studies of which we are aware.8–10 Furthermore, increased environmental exposures to endotoxins found in bedrooms have been postulated to influence risks of asthma and allergen sensitization.11,12
Difficulty in measuring indoor exposures during infancy has been 1 problem hampering research on early life influences on childhood asthma. Detailed information on the infant sleep environment has not previously been readily available, but a recent cohort of infants in Tasmania, Australia, provides such information. Earlier studies focusing on this cohort have demonstrated that infant exposures to synthetic bedding items such as pillows, quilts, and cocoons are associated with wheezing in childhood.13–15 Also, sheepskin use has been associated with HDM sensitization.16 In contrast, type of bedroom carpeting17 or infant mattress has appeared to be less important.16
Moreover, with regard to childhood asthma, the environmental model for infant HDM exposure may not be one of single, major independent risk factors but rather one of several environmental determinants synergistically increasing infant HDM exposures. For example, bedroom heating increases HDM levels in bedding.18 In addition to allergen exposure, environmental air quality may also be important, with substances such as volatile organic compounds (VOCs) possibly activating, enhancing, or otherwise potentiating allergen sensitization, whereas the presence of endotoxins may affect HDM sensitization.11
Another approach to investigating the role of infant bedding and bedroom environment in the development of asthma is one in which (1) bedding exposure measurements are further quantified by considering bedding not as a single item but as a composite of items and (2) bedding–environment interactions are assessed. We used this approach, on the basis of a theoretical model of HDM bedding loads, to investigate infant sleeping environment and subsequent wheezing by the age of 7 years in the Tasmanian infant cohort.
METHODS
Study Design
The Tasmanian Infant Health Survey (TIHS), conducted between 1988 and 1995, was a cohort study of infants from Tasmania, Australia; approximately 93% of births in the state occurred in hospitals participating in the cohort. Perinatal entry criteria were used to assess infants in regard to risk of sudden infant death syndrome.19 Infants exceeding a scoring cutoff were eligible for inclusion, together with all infants delivered in multiple births. Eligibility criteria and study methods have been discussed elsewhere.19 Data were obtained on 3 occasions, including a home interview conducted during the fifth postnatal week. Data were collected on parent and infant characteristics, home environment and child care factors, and the infant’s sleeping environment at the age of 1 month.
In 1995, all children aged 7 years of age in Tasmania took part in the cross-sectional Childhood Asthma Survey.17 We linked the 1995 asthma data of the 863 children whose parents had completed an at-home interview when the children were 1 month of age (in 1988) as part of the TIHS. Questions derived from the International Study of Asthma and Allergies in Childhood (ISAAC)20 were used in collecting data on sleeping environment and asthma symptoms. In addition, we obtained data on children’s age (in completed years) at onset of wheezing or asthma.
Definitions
Recent wheezing was defined as one or more wheezing episodes occurring over the past 12 months. Infants whose parents responded affirmatively to the ISAAC question “Has your child ever had asthma?” were categorized as having a history of asthma.20 (Bronchial hyperresponsiveness after exercise is related to both of the preceding outcomes.14) Night wheezing referred to the child’s sleep having been disturbed one or more nights per week during the past 12 months as a result of wheezing. Frequent wheezing referred to 12 episodes or more of wheezing over the past 12 months. Family history of asthma was defined as asthma among the infant’s siblings, parents, or grandparents at the time of the infant’s birth.
Classification of Bedding Items
Use of sheepskins referred to use of the animal hide with natural wool fibers attached. The infant cocoons manufactured and sold in southern Tasmania during the study period most commonly were made of outer coverings of cotton/polycotton with a synthetic filling.15 (A cocoon is similar to a padded sleeping bag, with a hood that comes close to the sides of the infant’s head, fully encasing the infant and leaving only the face exposed. While lying within the cocoon, the infant is then placed on a flat surface or in a cot or pram.) Pillows made of foam, sponge, tontine, polyester, or Dacron were classified as synthetic, and synthetic quilt use was defined as use of a synthetic quilt over the infant during cold weather. We recorded both use of sheepskins or cocoons at the time of the interview and intentions to use either of these items in cold weather, thereby avoiding seasonal effects.
We created 3 composite bedding groups on the basis of past work and the distribution of exposures to HDM-rich synthetic pillows, quilts and cocoons, and sheepskins.3,4 The reference group, bedding combination 0 (BC0), included infants with no exposure to synthetic items or sheepskin.16 Bedding combination 1 (BC1) comprised 1 synthetic item only (n = 279), 1 sheepskin only (n = 121), or 1 synthetic item and 1 sheepskin (n = 113). Bedding combination 2 (BC2) comprised 2 or more synthetic items without sheepskin (n = 57) or with sheepskin (n = 14). More emphasis was placed on synthetic bedding items than on sheepskin because of the associations with wheezing previously reported in this cohort.13–15 Here “composite bedding effect” refers to the increase from BC0 to BC1 and from BC1 to BC2.
Classification of Indoor Environmental Factors
We coded possible effect modifiers as binary. The heated bedroom variable referred to current use of heating at the time of the interview as well as intended use of heating in cold weather in the infant’s bedroom. Recent painting referred to the infant’s bedroom having been painted within the 12 months preceding the time of the interview. Absence of carpeting was defined as absence of wall-to-wall carpeting in the infant’s bedroom.
Statistical Analysis
We calculated pairwise correlations using Spearman’s rank order technique. Logit-based univariate odds ratios (ORs) and 95% confidence intervals (CIs) were calculated via logistic regression analyses. To assess type of bedding exposure in infancy as a risk factor for asthma at 7 years, we used multivariate logistic regression models controlling for multiple confounders simultaneously; these models provided adjusted odds ratios21 for the categorical “recent wheezing” and “history of asthma” outcome variables. Also, we examined “frequent wheezing” as a measure of asthma severity, and we assessed “night wheezing” because bedding was the exposure of interest. We explored as potential confounders factors shown in univariate analyses, or a priori, to be associated with wheezing.
Using change-in-estimate methods, we included in the final model factors altering the point estimate for the exposure–wheezing association by 10% or more. We examined the confounding effect of a number of factors, including number of siblings, in utero exposure to maternal smoking, air freshener use in infant’s bedroom, cat or dog as a family pet during infancy, visible indoor mold, use of a plastic mattress cover in infancy, low maternal education at infant’s birth, and paternal unemployment at infant’s birth. In addition, we used stratification to explore the contribution of family asthma history to the relationship between bedding and wheeze as either a confounder or an intervening variable. Factors that altered the exposure–wheeze association by 10% or more were family history of asthma, male gender, foam mattress use in infancy, and maternal cigarette smoking during infancy. The cohort entry criteria, either separately or as a set, did not alter any of the reported associations. We used the Wald linear trend test for the categorical bedding variables by replacing the binary predictors with a single predictor and determining category rank scores.
A model was built with terms for composite bedding and each confounder to assess whether associations between composite bedding classifications and given respiratory outcomes differed according to environmental factors after confounders had been controlled. The dependent variable was the respiratory outcome examined. A second model was built similar to the first but with an additional term for the environmental factor and an interaction term between composite bedding category and the environmental factor.
The significance value associated with the log-likelihood ratio test was then used in evaluating the resulting reduction in deviance produced by the second model relative to the first.21 Tests assessing interactions often involve a higher significance level than P = .05.22 Here interaction terms that improved the model by an increment of .1 were considered important. We used stratified multivariate logistic regression analyses to explore the potentiation of more than one environmental factor on the bedding–wheeze effect. In addition, we used discrete proportional hazard modeling21 to examine age at asthma onset or wheezing according to bedding category. Potentiation of age at onset by environmental factors was determined via a stratified log rank test.21 We conducted analyses using Stata 7.0 statistical software.23
RESULTS
The characteristics of the study sample are shown in Table 1 ▶. Infants in the BC2 category were at more than 2-fold risk of experiencing recent wheezing and night wheezing by the age of 7 years relative to infants who had not been exposed to any of the bedding items included in this category (Table 2 ▶). There was a dose–response relationship between exposure to increasing levels of composite bedding in infancy and risk of recent wheezing and history of childhood asthma. This trend was also observed for night wheezing and frequent wheezing. Parental history of asthma was not associated with selection of composite bedding combinations. Even among children without a family history of asthma, risk of wheezing increased with increasing numbers of bedding items (trend test P = .03).
TABLE 1—
Study Sample Characteristics (n = 863): Tasmania, Australia, 1995
| Sample | |
| Age, y, mean (SD) | 6.9 (0.3) |
| Male, no. (%) | 615 (71.3) |
| Family history of asthma, no. (%) | 301 (35.0) |
| No siblings in 1995, no. (%) | 48 (5.8) |
| Wheezing over past year, no. (%) | 222 (27.0) |
| Night wheezing over past year, no. (%) | 160 (19.2) |
| History of asthma, no. (%) | 280 (32.4) |
| Frequent wheezing over past year, no. (%) | 30 (4.8) |
| Bedroom factors in infancy, no. (%) | |
| Synthetic pillow use at age of 1 month | 127 (14.8) |
| Synthetic quilt use at age of 1 month | 396 (44.9) |
| Cocoon use at age of 1 month | 19 (2.4) |
| Sheepskin use at age of 1 month | 256 (29.8) |
| Foam mattress use in infancy | 531 (62.2) |
| Plastic mattress cover use in infancy | 352 (41.0) |
| Composite bedding category, no. (%) | |
| BC0 | 216 (27.0) |
| BC1 | 513 (64.1) |
| BC2 | 71 (8.9) |
| Environmental factors in infancy, no. (%) | |
| Heating in infant’s bedroom | 294 (34.3) |
| Carpet in infant’s bedroom | 767 (90.6) |
| Infant’s bedroom painted within previous 12 months | 287 (33.5) |
| Maternal cigarette smoking during infancy | 382 (44.7) |
| Use of home gas appliances (cooking or living room heating) in infancy | 28 (3.3) |
| Presence of mold in home (excluding bathroom) in infancy | 40 (4.7) |
| Cat as pet during infancy | 294 (34.3) |
| Dog as pet during infancy | 308 (35.9) |
| Parental factors, no. (%) | |
| Low maternal education level at child’s birth | 191 (22.2) |
| Paternal unemployment at child’s birth | 146 (17.4) |
Note. BC = bedding combination. The reference category, bedding combination 0 (BC0), represented no use of synthetic items or sheepskin. Bedding combination 1 (BC1) comprised 1 synthetic item only (n = 279), 1 sheepskin only (n = 121), or 1 synthetic item and 1 sheepskin (n = 113). Bedding combination 2 (BC2) comprised 2 or more synthetic items without sheepskin (n = 57) or with sheepskin (n = 14).
The sample size for the bedding item analysis was 800 respondents.
TABLE 2—
Associations Between Infant Composite Bedding Classifications and Childhood Wheezing
| Cases, No. (%) | Unadjusted OR (95% CI) | P | Adjusted ORa (95% CI) | P | |
| Recent wheezing | |||||
| BC0 | 49 (23.8) | Reference | Reference | ||
| BC1 | 126 (25.7) | 1.11 (0.76, 1.62) | .593 | 1.07 (0.72, 1.57) | .744 |
| BC2 | 29 (43.3) | 2.45 (1.37, 4.37) | .003 | 2.10 (1.15, 3.82) | .015 |
| Linear trend | .014 | .053 | |||
| History of asthma | |||||
| BC0 | 64 (29.6) | Reference | Reference | ||
| BC1 | 167 (32.6) | 1.15 (0.81, 1.62) | .439 | 1.15 (0.81, 1.64) | .437 |
| BC2 | 32 (45.1) | 1.95 (1.12, 3.38) | .018 | 1.80 (1.01, 3.18) | .045 |
| Linear trend | .039 | .072 | |||
Note. BC = bedding combination; OR = odds ratio; CI = confidence interval. The reference category, bedding combination 0 (BC0), represented no use of synthetic items or sheepskin. Bedding combination 1 (BC1) comprised 1 synthetic item only (n = 279), 1 sheepskin only (n = 121), or 1 synthetic item and 1 sheepskin (n = 113). Bedding combination 2 (BC2) comprised 2 or more synthetic items without sheepskin (n = 57) or with sheepskin (n = 14).
aAdjusted for male gender, family history of asthma, foam mattress use in infancy, and maternal cigarette smoking during infancy. Further adjustment for cohort entry criteria did not alter the results.
We examined the influence of bedroom heating, bedroom carpeting, and recent bedroom painting on wheezing and the interactions of these factors with use of composite bedding. No relationship was observed between bedroom heating and bedroom carpeting (Spearman r = 0.052), between bedroom heating and recent painting (Spearman r = 0.078), or between bedroom carpeting and recent painting (Spearman r = −0.029). Nor were these factors associated with composite bedding use (Spearman correlations were −0.015, −0.002, and −0.083 for bedroom heating, recent bedroom painting, and bedroom carpeting, respectively).
Bedroom heating, recent bedroom painting, and absence of bedroom carpeting did not significantly predict wheezing (ORs were 1.08, 0.82, and 1.12, respectively). Families with a history of asthma may remove carpeting in their attempts to reduce sources of allergens. In this cohort, there was no difference in the percentage of children who had a family history of asthma and had no carpeting in their bedrooms (9.1%) and the percentage of children who did not have such a family history of asthma and did not have carpeting (10.1%) (P = .576).
The 3 environmental factors just described altered the bedding–wheeze associations observed in this study. The dose–response relationship between composite bedding use and risk of subsequent recent wheezing was particularly evident when the infant slept in a heated room (Table 3 ▶). In particular, the BC2 bedding category was strongly associated with recent wheezing (adjusted OR = 7.87; 95% CI = 2.17, 28.51) when the infant’s bedroom had been heated. The difference in the potentiation of the composite bedding effect by room heating was significant (likelihood ratio test, P = .051). Similarly, children with a history of asthma demonstrated this potentiation of the composite bedding effect by room heating. No significant associations were found between each of the different types of heating used in the infant’s bedroom and recent wheezing. The bedding–wheeze interaction effect was potentiated by room heating irrespective of type of heating.
TABLE 3—
Potentiation of Infant Bedding Effects by Indoor Environmental Factors on Wheezing
| Recent Wheezing | History of Asthma | |||||
| Environmental Factors and Bedding Combination | Cases, No. (%) | Adjusted ORa (95% CI) | P | Cases, No. (%) | Adjusted ORa (95% CI) | P |
| No room heating | ||||||
| BC0 | 37 (27.0) | Reference | 49 (33.8) | Reference | ||
| BC1 | 74 (24.2) | 0.84 (0.2, 1.34) | .454 | 105 (32.6) | 0.91 (0.59, 1.40) | .673 |
| BC2 | 20 (38.5) | 1.39 (0.69, 2.79) | .354 | 23 (41.8) | 1.14 (0.59, 2.21) | .703 |
| Linear trend | .653 | .898 | ||||
| Room heating | ||||||
| BC0 | 12 (17.4) | Reference | 15 (21.1) | Reference | ||
| BC1 | 51 (28.0) | 1.77 (0.86, 3.65) | .120 | 61 (32.3) | 1.90 (0.97, 3.73) | .063 |
| BC2 | 9 (60.0) | 7.87 (2.17, 28.51) | .002 | 9 (56.3) | 7.07 (2.09, 23.85) | .002 |
| Linear trend | .004 | .003 | ||||
| Room heating difference in bedding effectb | .051 | .048 | ||||
| No recent painting | ||||||
| BC0 | 39 (27.2) | Reference | 48 (32.4) | Reference | ||
| BC1 | 82 (26.5) | 0.89 (0.57, 1.41) | .633 | 103 (31.5) | 0.98 (0.64, 1.51) | .944 |
| BC2 | 22 (43.1) | 1.65 (0.82, 3.31) | .162 | 20 (37.7) | 1.20 (0.60, 2.40) | .606 |
| Linear trend | .383 | .730 | ||||
| Recent painting | ||||||
| BC0 | 10 (15.4) | Reference | 16 (23.5) | Reference | ||
| BC1 | 44 (24.6) | 1.97 (0.89, 4.36) | .094 | 64 (34.6) | 1.81 (0.94, 3.49) | .075 |
| BC2 | 7 (43.8) | 5.39 (1.44, 20.25) | .013 | 12 (66.7) | 7.22 (2.25, 23.22) | .001 |
| Linear trend | .013 | .002 | ||||
| Room painting difference in bedding effectb | .095 | .039 | ||||
| No carpeting | ||||||
| BC0 | 2 (13.3) | Reference | 5 (33.3) | Reference | ||
| BC1 | 14 (30.4) | 2.51 (0.47, 13.55) | .284 | 20 (41.7) | 1.61 (0.42, 6.19) | .487 |
| BC2 | 5 (58.3) | 8.13 (1.14, 58.20) | .037 | 7 (53.9) | 2.40 (0.46, 12.48) | .298 |
| Linear trend | .032 | .298 | ||||
| Carpeting | ||||||
| BC0 | 46 (24.7) | Reference | 58 (29.6) | Reference | ||
| BC1 | 112 (25.5) | 1.01 (0.67, 1.51) | .966 | 146 (31.7) | 1.10 (0.76, 1.60) | .621 |
| BC2 | 22 (40.7) | 1.82 (0.94, 3.53) | .075 | 25 (43.9) | 1.72 (0.92, 3.23) | .090 |
| Linear trend | .200 | .157 | ||||
| Room carpeting difference in bedding effectb | .003 | .001 | ||||
Note. BC = bedding combination; OR = odds ration; CI = confidence interval. The reference category, bedding combination 0 (BC0), represented no use of synthetic items or sheepskin. Bedding combination 1 (BC1) comprised 1 synthetic item only (n = 279), 1 sheepskin only (n = 121), or 1 synthetic item and 1 sheepskin (n = 113). Bedding combination 2 (BC2) comprised 2 or more synthetic items without sheepskin (n = 57) or with sheepskin (n = 14).
aAdjusted for male gender, family history of asthma, foam mattress use in infancy, and maternal cigarette smoking during infancy. Further adjustment for cohort entry criteria did not alter the results.
bLikelihood ratio test.
A greater increase in risk of subsequent wheezing according to type of bedding used was observed in the case of infants sleeping in recently painted bedrooms (Table 3 ▶). Among infants in recently painted rooms, those in the BC2 group had a more than 5-fold increased risk of recent wheezing by the age of 7 years relative to infants in the BC0 group. We next examined whether the relative importance of type of bedding used in regard to subsequent wheezing would be greater in uncarpeted or in carpeted rooms. Stronger composite bedding effects on wheezing were found in uncarpeted than carpeted bedrooms (Table 3 ▶). The potentiation of the composite bedding effect by these 3 environmental factors was also evident for night wheezing and frequent wheezing.
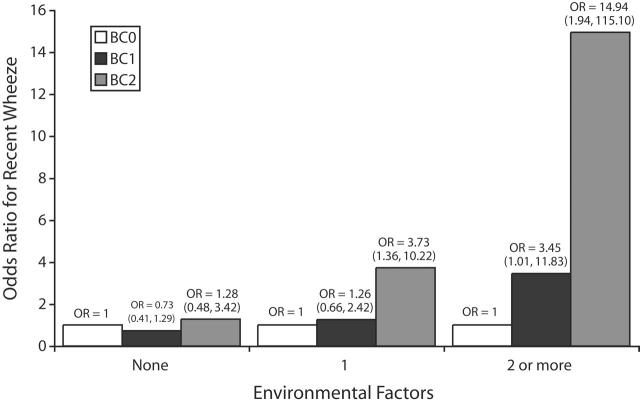
The effect modification patterns reported for bedroom heating, recent bedroom painting, and absence of carpeting remained after adjustment for the other 2 environmental factors, either individually or together, along with the confounders listed in Table 2 ▶. These 3 environmental factors appeared to independently potentiate the risk of composite bedding categories on wheeze. Moreover, Figure 1 ▶ shows that when any 2 or more of these environmental factors were present, odds ratios were 3.45 (95% CI = 1.01, 11.83) for the association between membership in the BC1 group and wheezing and 14.94 (95% CI = 1.94, 115.1) for the association between membership in the BC2 group and wheezing. The difference for the potentiation of the BC2–wheezing effect in the presence of between 0 and 2 or more environmental factors was significant (P = .012).
FIGURE 1—
Relationship between number of environmental factors (bedroom heating, recent bedroom painting, absence of bedroom carpeting) and potentiation of composite bedding–wheezing effect by bedding combinations BC0, BC1, and BC2.
Note. The reference category, bedding combination 0 (BC0), represented no use of synthetic items or sheepskin. Bedding combination 1 (BC1) comprised 1 synthetic item only (n = 279), 1 sheepskin only (n = 121), or 1 synthetic item and 1 sheepskin (n = 113). Bedding combination 2 (BC2) comprised 2 or more synthetic items without sheepskin (n = 57) or with sheepskin (n = 14). The difference in effect for potentiation of the BC1–wheezing effect from 0 to 2 or more of the environmental factors was P = .017. The difference in effect for potentiation of the BC2–wheezing effect from 0 to 2 or more of the environmental factors was P = .012. OR = odds ratio.
Among infants with childhood asthma, increasing levels of composite bedding were associated with increasing risks of earlier asthma onset. Mean ages of onset were 3.7, 3.2, and 2.1 years, respectively, for infants in the BC0, BC1, and BC2 groups (log-rank test for equality of survivor functions, P = .002). The presence of the 3 environmental factors described earlier also potentiated the shift to an earlier age at onset among children with a history of asthma.
DISCUSSION
To our knowledge, ours is the first study investigating infant bedding use and subsequent wheezing to consider composite bedding and modification in regard to the home environment. Risk of childhood wheezing increased linearly when infants were exposed to increasing numbers of potentially HDM-rich bedding items at 1 month of age. This composite bedding effect was further modified by indoor environment factors: bedroom heating, recent bedroom painting, and absence of bedroom carpeting. When 2 or more of these environmental factors were present, the association between type of bedding and wheezing was markedly exacerbated.
The strengths of this study include the prospective sleeping environment data, minimizing recall bias, and the use of a composite bedding classification. In addition, at the time of assessment of wheezing, the research nurse was unaware of infants’ bedding status. Moreover, our results were not likely to have been influenced by differences in rates of follow-up, given that the proportion of TIHS children followed up from infancy did not differ according to use of particular bedding items. The study sample was not representative of all live births occurring in the geographical area covered, in that it consisted of infants born at higher risk of sudden infant death syndrome; however, the TIHS cohort entry criteria were unlikely to have affected the generalizability of our results. These eligibility criteria were well defined,24 and we considered the potential effects of the entry scoring system components in our multivariate analyses. Thus, the study children should not differ from other children to any large extent in regard to biological development of asthma.
Importantly, the associations observed between type of bedding used and wheezing were evident even among children without a family history of asthma. A potential weakness of this study is the absence of a biological or physician assessment of asthma. The ISAAC questionnaire, however, is considered a valid instrument for determining current asthma symptoms; reports of wheezing over the past 12 months had a sensitivity of 0.85 and a specificity of 0.81 when related to physician diagnosis of asthma in childhood.25
Our findings emphasize the important role of infant sleeping environment in the development of asthma. Moreover, this study demonstrates features suggestive of a causal relationship26 between type of bedding used and wheezing: a high strength of association, a marked dose–response effect, consistency in the bedding–wheezing effect, and the significant earlier shift in age at asthma onset according to type of bedding used. Furthermore, environmental enhancers not only increased the magnitudes of bedding–wheeze associations but led to infants developing asthma at an earlier age. Our results are also consistent with those of past research. In studies of the Tasmanian cohort, increases in sensitization to HDM have been shown among children exposed to sheepskin16 or to synthetic bedding relative to those exposed to feather bedding.13 Moreover, associations between synthetic bedding materials and childhood wheezing have been reported in several cross-sectional27,28 and prospective14,15 studies.
A number of mechanisms provide biologically plausible explanations for the increased risk of wheezing observed here. Increased exposure to HDM allergen levels, the model on which this study was based, has been reported with use of synthetic or sheepskin bedding items3,4 in early childhood. Thus, HDM-rich bedding items may increase the risk of HDM sensitization and airway inflammation because the allergen load near the infant’s airway is higher.14 This possibility is further reinforced by work showing that the adverse effect of synthetic quilts on wheezing varies according to sleeping position.29
Atopic individuals are overrepresented at the severe end of the asthma spectrum, with earlier studies suggesting that frequent wheezing may be a better marker than a history of asthma or milder symptoms of airway disease related to HDM allergens.13,28,30,31 Exposure to HDM allergens is associated with increasing frequencies of wheezing.32 Here the risk of frequent wheezing in childhood also increased linearly with increasing numbers of potentially high HDM-rich bedding items at the age of 1 month. This finding is consistent with a bedding effect on airway disease related to HDM allergens but is not definitive, because data on child HDM–atopy status were not available.
Conflicting results regarding associations between type of mattress used and respiratory symptoms or HDM sensitization have been reported.32,33 Mattress HDM allergen levels may be a proxy for measurement of other allergen sources closer in proximity to the affected individual.34 A recent meta-analysis showed that bedding changes aimed at reducing exposures to HDM allergens appeared to be ineffective in improving symptoms in HDM-sensitive asthmatics.35 Overall, no statistically significant difference in regard to improvements in asthma symptoms was found, even among the subgroup for whom effective allergen reductions were documented.35 The findings reported indicated that the lack of effect may relate to the fact that allergen reduction was effective in regard to type of mattress but not other allergen sources. It may be that multiple components, and the relationship of these components to proximal sleeping environments, play a role in childhood asthma.
We did not determine HDM allergen levels. However, consider a single allergen measure of, for example, bedroom carpeting,9 which may not exhibit consistent patterns of allergen distribution.36 Such a measure would not have encompassed the bedding and indoor environmental determinants leading to personal allergen exposures studied here. Thus, exposure models that take into account the multiple determinants of personal allergen exposures are now required to identify the mechanisms involved.
Various environmental factors influence the development of asthma symptoms.37 In the case of atopic infants, the presence of environmental exposures in addition to aeroallergens has been hypothesized to increase the risk of asthma.38 Here the potentiation of the composite bedding–wheezing effect by indoor environmental factors could reflect various synergistic mechanisms, including increased HDM exposure, increased allergen sensitization, increased VOC-induced inflammation in vulnerable infant airways, and reduced exposure to endotoxins. The potentiation of the composite bedding effect on wheezing by room heating may have been because of an enrichment of the allergen content of bedding, consistent with previous studies showing that room heating increases HDM loads in bedding.18
Our finding that bedroom heating potentiation did not vary according to heating type is consistent with the effect being that of increased heat in the bedroom environment as opposed to characteristics associated with type of heating used. The potentiation of the composite bedding effect on wheezing by recent painting may reflect that recently painted walls release VOCs,39 and their presence in the infant’s bedroom may enhance HDM sensitization by airway inflammation; this situation can result in increased permeability and enhanced allergen presentation to the immune system40 or through VOC-induced Th2 deviation.41 Several birth cohort studies have also revealed an exposure–response relationship between recent home redecoration, including wall painting, and increased risk of wheezing39 or asthma.42
The potentiating effect of absence of bedroom carpeting is intriguing. The promoting effect of lack of carpeting did not operate independently but was seen in association with composite bedding exposures. Possibly, absence of carpeting reduces an HDM habitat,36 potentially increasing the relative importance of bedding as an HDM allergen reservoir. Another possibility is that bedroom carpets, which contain significantly more endotoxins than bedding,43 are a significant source of infantile endotoxin exposures.44,45 The fact that synthetic bedding may contain fewer endotoxins than other types of bedding46 could be counterbalanced by increased levels in bedroom carpeting. Although the role of environmental endotoxin exposures in the development of asthma is unclear, several researchers have postulated that such exposures in infancy may be protective.12,47
Infant exposures to composite HDM-rich bedding items at the age of 1 month predict subsequent childhood wheezing, particularly if exacerbating home environmental factors are in place. Infants spend a significant proportion of their time in bed, with consequent opportunities for prolonged exposures to adverse environmental influences. Our findings indicate the need for a greater public health effort to ensure optimal infant sleeping environments that will assist in asthma prevention.
Acknowledgments
The Tasmanian Infant Health Survey was supported by the US National Institutes of Health (grant 001HD28979–01A1), the Tasmanian State Government, the Australian Rotary Health Research Fund, the National Health and Medical Research Council of Australia, the National Sudden Infant Death Syndrome Council of Australia, and the Sudden Infant Death Research Foundation of Victoria, along with the Community Organisations’ Support Program of the Department of Human Services and Health, Zonta International, Wyeth Pharmaceuticals, and the Tasmanian Sanatoria After-Care Association. The Public Health Research and Development Committee of the Australian National Health and Medical Research Council funded the asthma study. Portions of the project analysis were supported by a grant from Blundstone’s Pty Ltd, by a Coles Supermarket grant to the Canberra Region Medical Foundation, and by the Australian Commonwealth Government Department of Health and Aged Care, Australian National Priority Areas Initiative (Asthma).
We thank the parents, infants, and children who participated in these studies, the research staff for data collection and collation, and the hospitals participating in the infant cohort study. We thank the participating schools; the Tasmanian State Department of Education, Cultural, and Community Development; and the Catholic Education Office.
Peer Reviewed
Contributors L. F. Trevillian was the principal contributor to the analysis and the preparation of the article. A. Ponsonby, T. Dwyer, and A. Kemp also contributed. L. L. Lim provided input on the analytical approach. All of the authors contributed to interpretation of the data and reviewed the final version.
Human Participant Protection This research was approved by the ethics committee of the University of Tasmania. Parents provided informed written consent, including consent for data linkage between the Tasmanian Infant Health Survey and the 1995 Childhood Asthma Survey.
References
- 1.Simpson A, Woodcock A, Custovic A. Housing characteristics and mite allergen levels: to humidity and beyond. Clin Exp Allergy. 2001;31:803–805. [DOI] [PubMed] [Google Scholar]
- 2.Grad R. Risk of asthma in children with exposure to mite and cat allergens. Lancet. 2000;356: 1369–1370. [DOI] [PubMed] [Google Scholar]
- 3.Mills S, Siebers R, Wickens K, Crane J, Purdie G, Fitzharris P. House dust mite allergen levels in individual bedding components in New Zealand. N Z Med J. 2002;115:151–153. [PubMed] [Google Scholar]
- 4.Tovey ER, Guinan JE, Vandenberg RA. Mite populations in Sydney household bedding with particular reference to nursery sheepskins. Med J Aust. 1975;2: 770–772. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi M, Inouye S, Yasueda H, Shida T. Concentration of airborne mite allergens (Der I and Der II) during sleep. Allergy. 1992;47:55–57. [DOI] [PubMed] [Google Scholar]
- 6.Jalaludin B, Xuan W, Mahmic A, Peat J, Tovey E, Leeder S. Association between Der p 1 concentration and peak expiratory flow rate in children with wheeze: a longitudinal analysis. J Allergy Clin Immunol. 1998; 102:382–386. [DOI] [PubMed] [Google Scholar]
- 7.Chan-Yeung M, Manfreda J, Dimich-Ward H, et al. Mite and cat allergen levels in homes and severity of asthma. Am J Respir Crit Care Med. 1995;152: 1805–1811. [DOI] [PubMed] [Google Scholar]
- 8.Hide DW, Matthews S, Tariq S, Arshad SH. Allergen avoidance in infancy and allergy at 4 years of age. Allergy. 1996;51:89–93. [PubMed] [Google Scholar]
- 9.Lau S, Illi S, Sommerfeld C, et al. Early exposure to house-dust mite and cat allergens and development of childhood asthma: a cohort study. Lancet. 2000; 356:1392–1397. [DOI] [PubMed] [Google Scholar]
- 10.Sporik R, Holgate ST, Platts-Mills TA, Cogswell JJ. Exposure to house-dust mite allergen (Der p I) and the development of asthma in childhood: a prospective study. N Engl J Med. 1990;323:502–507. [DOI] [PubMed] [Google Scholar]
- 11.Braun-Fahrlander C, Riedler J, Herz U, et al. Environmental exposure to endotoxin and its relation to asthma in school-age children. N Engl J Med. 2002; 347:869–877. [DOI] [PubMed] [Google Scholar]
- 12.von Mutius E. Environmental factors influencing the development and progression of pediatric asthma. J Allergy Clin Immunol. 2002;109:S525–S532. [DOI] [PubMed] [Google Scholar]
- 13.Ponsonby AL, Kemp A, Dwyer T, Carmichael A, Couper D, Cochrane J. Feather bedding and house dust mite sensitization and airway disease in childhood. J Clin Epidemiol. 2002;55:556–562. [DOI] [PubMed] [Google Scholar]
- 14.Ponsonby AL, Dwyer T, Kemp A, Cochrane J, Couper D, Carmichael A. Synthetic bedding and wheeze in childhood. Epidemiology. 2003;14:37–44. [DOI] [PubMed] [Google Scholar]
- 15.Trevillian LF, Ponsonby AL, Dwyer T, et al. A prospective association between cocoon use in infancy and childhood asthma. Paediatr Perinat Epidemiol. 2004;18:281–289. [DOI] [PubMed] [Google Scholar]
- 16.Trevillian LF, Ponsonby AL, Dwyer T, et al. An association between plastic mattress covers and sheepskin underbedding use in infancy and house dust mite sensitization in childhood: a prospective study. Clin Exp Allergy. 2003;33:483–489. [DOI] [PubMed] [Google Scholar]
- 17.Ponsonby AL, Couper D, Dwyer T, Carmichael A, Kemp A, Cochrane J. The relation between infant indoor environment and subsequent asthma. Epidemiology. 2000;11:128–135. [DOI] [PubMed] [Google Scholar]
- 18.Mihrshahi S, Marks G, Vanlaar C, Tovey E, Peat J. Predictors of high house dust mite allergen concentrations in residential homes in Sydney. Allergy. 2002;57: 137–142. [DOI] [PubMed] [Google Scholar]
- 19.Dwyer T, Ponsonby AL, Newman NM, Gibbons LE. Prospective cohort study of prone sleeping position and sudden infant death syndrome. Lancet. 1991;337: 1244–1247. [DOI] [PubMed] [Google Scholar]
- 20.International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema. Lancet. 1998; 351:1225–1232. [PubMed] [Google Scholar]
- 21.Armitage P, Berry G. Statistical Methods in Medical Research. Oxford, England: Blackwell Scientific Publications; 1994.
- 22.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York, NY: John Wiley & Sons Inc; 2000.
- 23.Stata, Version 7.0. College Station, Tex: Stata Corp; 1999.
- 24.Miettinen O. Design of the Study Base. New York, NY: John Wiley & Sons Inc; 1985:46–68.
- 25.Jenkins MA, Clarke JR, Carlin JB, et al. Validation of questionnaire and bronchial hyperresponsiveness against respiratory physician assessment in the diagnosis of asthma. Int J Epidemiol. 1996;25:609–616. [DOI] [PubMed] [Google Scholar]
- 26.Rothman KJ, Greenland S. Modern Epidemiology. Philadelphia, Pa: Lippincott-Raven Publishers; 1998.
- 27.Butl andand BK, Strachan DP, Lewis S, Bynner J, Butler N, Britton J. Investigation into the increase in hay fever and eczema at age 16 observed between the 1958 and 1970 British birth cohorts. BMJ. 1997;315: 717–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strachan DP, Carey IM. Home environment and severe asthma in adolescence: a population-based case-control study. BMJ. 1995;311:1053–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ponsonby AL, Dwyer T, Trevillian L, et al. The bedding environment, sleep position, and frequent wheeze in childhood. Pediatrics. 2004;113: 1216–1222. [DOI] [PubMed] [Google Scholar]
- 30.Peat JK, Tovey E, Gray EJ, Mellis CM, Woolcock AJ. Asthma severity and morbidity in a population sample of Sydney schoolchildren: Part II—importance of house dust mite allergens. Aust N Z J Med. 1994;24: 270–276. [DOI] [PubMed] [Google Scholar]
- 31.Ponsonby AL, Gatenby P, Glasgow N, Mullins R, McDonald T, Hurwitz M. Which clinical subgroups within the spectrum of child asthma are attributable to atopy? Chest. 2002;121:135–142. [DOI] [PubMed] [Google Scholar]
- 32.Peat JK, Tovey E, Toelle BG, et al. House dust mite allergens: a major risk factor for childhood asthma in Australia. Am J Respir Crit Care Med. 1996; 153:141–146. [DOI] [PubMed] [Google Scholar]
- 33.Frischer TH, Kuehr J, Meinert R, Karmaus W, Urbanek R. Relationship between exposure to dust mite allergen and bronchial response to exercise in schoolchildren sensitized to dust mites. Pediatr Pulmonol. 1993;16:13–18. [DOI] [PubMed] [Google Scholar]
- 34.Tovey ER, Peat JK. Bedding and childhood asthma. Aust N Z J Med. 1994;24:679–681. [DOI] [PubMed] [Google Scholar]
- 35.Gotzsche PC, Johansen HK, Hammarquist C, Burr ML. House dust mite control measures for asthma. Cochrane Database of Systematic Reviews. 2004, Issue 4. Article No. CD001187. DOI: 10.1002/14651858.CD001187.pub2. [DOI] [PubMed]
- 36.Colloff MJ. Distribution and abundance of dust mites within homes. Allergy. 1998;53:24–27. [DOI] [PubMed] [Google Scholar]
- 37.Halken S, Host A, Husby S, Hansen LG, Osterballe O, Nyboe J. Recurrent wheezing in relation to environmental risk factors in infancy: a prospective study of 276 infants. Allergy. 1991;46:507–514. [DOI] [PubMed] [Google Scholar]
- 38.Lindfors A, van Hage-Hamsten M, Rietz H, Wickman M, Nordvall SL. Influence of interaction of environmental risk factors and sensitization in young asthmatic children. J Allergy Clin Immunol. 1999;104: 755–762. [DOI] [PubMed] [Google Scholar]
- 39.Diez U, Kroessner T, Rehwagen M, et al. Effects of indoor painting and smoking on airway symptoms in atopy risk children in the first year of life: results of the LARS study. Int J Hyg Environ Health. 2000;203: 23–28. [DOI] [PubMed] [Google Scholar]
- 40.Koren HS, Graham DE, Devlin RB. Exposure of humans to a volatile organic mixture: III. Inflammatory response. Arch Environ Health. 1992;47:39–44. [DOI] [PubMed] [Google Scholar]
- 41.Lehmann I, Rehwagen M, Diez U, et al. Enhanced in vivo IgE production and T cell polarization toward the type 2 phenotype in association with indoor exposure to VOC: results of the LARS study. Int J Hyg Environ Health. 2001;204:211–221. [DOI] [PubMed] [Google Scholar]
- 42.Emenius G, Nordvall EHO, Pershagen G, Wickman M. Indoor environment and asthma in children up to two years of age: a case control study within the BAMSE birth cohort. Allergy. 2001;56(suppl 68):175.11167380 [Google Scholar]
- 43.Douwes J, Zuidhof A, Doekes G, et al. (1→3)-beta-D-glucan and endotoxin in house dust and peak flow variability in children. Am J Respir Crit Care Med. 2000;162:1348–1354. [DOI] [PubMed] [Google Scholar]
- 44.Wickens K, Douwes J, Siebers R, et al. Determinants of endotoxin levels in carpets in New Zealand homes. Indoor Air. 2003;13:128–135. [DOI] [PubMed] [Google Scholar]
- 45.Gereda JE, Klinnert MD, Price MR, Leung DY, Liu AH. Metropolitan home living conditions associated with indoor endotoxin levels. J Allergy Clin Immunol. 2001;107:790–796. [DOI] [PubMed] [Google Scholar]
- 46.Weernink A, Severin WP, Tjernberg I, Dijkshoorn L. Pillows, an unexpected source of Acinetobacter. J Hosp Infect. 1995;29:189–199. [DOI] [PubMed] [Google Scholar]
- 47.Douwes J, Pearce N, Heederik D. Does environmental endotoxin exposure prevent asthma? Thorax. 2002;57:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]