IT was with some self-consciousness that I accepted an invitation to reflect on my own work, a long Genetics article that appeared 20 years ago entitled, “Autoregulatory Functioning of a Drosophila Gene Product That Establishes and Maintains the Sexually Determined State” (Cline 1984). This article was a milestone in a line of work that began with an intriguing maternal effect and led ultimately to an understanding of the fly's sex-determination signal and the self-propagating response of that signal's target. The invitation to reflect on that 1984 article has given me an opportunity to make more explicit the lines of argument that I had not always made clear, writing at a time when authors stressed data presentation and expected readers to infer more of the logic and significance of the arguments. In relating the story of this article, I also emphasize two general points about experimental science. First, the shortest distance between two points is often not the straight line that one might have initially imagined. I certainly did not set out to “solve” fly sex determination. Second, although research stories often appear inevitable in retrospect, the humbling fact is that they almost always involve a great deal of irony and luck. While both of these points have been made by others, I hope an additional illustration might be useful, or at least entertaining.
The pleasure of the wild ride that the fruit fly took me on more than 2 decades ago addicted me to genetics for life. I was doubly fortunate to have had this genetic story unfold just as molecular tools were becoming available to confirm its validity and make the work more accessible to those who might not be genetically inclined. In that connection, I acknowledge a great debt to my former collaborator, the molecular biologist Paul Schedl.
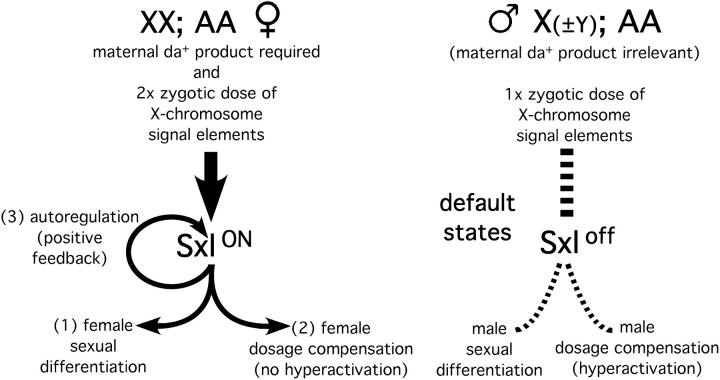
The 1984 article is best known for describing an epigenetic (determinative) aspect to the regulation of the X-linked gene that coordinately controls Drosophila sexual fate and X chromosome dosage compensation in response to X chromosome dose and for revealing a positive feedback activity of this gene's product that might explain how the active (female) state is maintained (see Figure 1). This gene was appropriately named Female-lethal by H. J. Muller in 1960 when he discovered a female-specific lethal allele. I felt compelled to violate protocol in 1978 by changing the gene's name to Sex-lethal (Sxl) when I isolated a male-specific lethal allele of Female-lethal and anticipated the confusion that might otherwise arise.
Figure 1.—
Figure adapted from Figure 5 in Cline (1984) with the legend taken verbatim: A summary of the functioning of Sxl+ in Drosophila development. Maternal da+ product and the proper balance (X/A = 1) of X-linked and autosomal signal elements in the zygote are required for the functioning of Sxl+. Sxl has three general classes of trans-acting functions: one that elicits female sex differentiation, probably through interactions with the genes tra and tra-2; one that suppresses dosage-compensated X-linked gene hyperactivation through interactions with the known autosomal male-specific lethal genes and other genes whose identity is not yet known; and one, demonstrated for the first time here, that can activate Sxl+ itself, even in the absence of maternal da+ activity. In the absence of Sxl+ activity, male development and an increased rate of dosage-compensated gene expression ensue.
The 1984 article seems like ancient history to me now, in part because it was necessarily devoid of molecular biology; nevertheless, its conclusions have survived subsequent molecular scrutiny. In the current genomics age with its emphasis on technology and massively parallel approaches, those who have never struggled through a highly focused “pure” genetics article like the 1984 article may not appreciate how much such a low-tech approach can achieve under the right circumstances, even for a subject as complex as metazoan development.
But any lesson that this article might provide regarding the enduring power of classical genetics pales in comparison with that of H. J. Muller's 1932 pure genetics article (Muller 1932). From mutant phenotypes alone, Muller deduced the various ways in which mutations can affect gene function and discovered X chromosome dosage compensation. One of the greatest ironies in genetics is that this brilliant man discovered Sxl, but died 7 years later with no inkling that he had found the master regulator of the dosage-compensation process that he had discovered so many years before. There was simply no place for sex-specific lethality in fly workers' thinking about dosage compensation or sex determination until the Sxl story unfolded (see Stewart and Merriam 1980; Lucchesi 1983).
I had the advantage of ignorance coming to the subject of fly sex-specific lethality. I had entered graduate school in 1968 with a biochemistry degree but no background in genetics, since in those days biochemistry mentors generally considered genetics irrelevant to their discipline. For my Ph.D., I studied how and when marine bacteria generate light, initially unconcerned that the system I had chosen lacked the prerequisites for genetic analysis. Nevertheless, I began to rely on mutations, especially temperature sensitives, and did genetics the hard way, for example, using in vitro complementation tests of enzyme extracts to determine allelism. I switched to Drosophila as a postdoc in part because I sought a bona fide genetic system and was stimulated by lectures on fly development by D. Suzuki, J. Postlethwaite, R. King, and W. Gehring. I joined the University of California at Irvine's Center for Pathobiology (not a germ warfare lab!) in 1973, having learned only shortly before how to distinguish male and female flies in a wonderful Cold Spring Harbor neurophysiology course taught by W. Pak and S. Benzer's talented postdocs.
The fondness for temperature-sensitive mutants that I had developed as a graduate student was shared by the Irvine labs led by H. Schneiderman and P. Bryant. Stimulated by Ernst Hadorn's work on transdetermination (Hadorn 1968), I planned to exploit temperature-sensitive mutants to address two general questions: (1) What are the signals that induce cells to adopt different fates? and (2) After fate decisions are made, how are they maintained as cells divide? With the goal of developing a biochemically tractable system in that prerecombinant-DNA dark age, I looked for mutations affecting maternally encoded signals in egg cytoplasm whose disruption would upset subsequent embryogenesis in enlightening ways.
At that time, pleiotropy was a somewhat dirty word in the world of maternal effects, but I embraced it, thinking it likely that molecules involved in developmental signaling would function at multiple stages. Thus with two others at the Center for Pathobiology, I screened for interesting temperature-sensitive maternal effects among a large collection of recently isolated temperature-sensitive zygotic lethals. We were surprised to discover the extent of pleiotropy: more than half of these zygotic lethals disrupted oogenesis when mutant adult females were shifted to the restrictive temperature. While pondering the difficult question of how to separate the wheat from the chaff among these mutants, I accidentally became distracted by sex.
I stumbled upon a description of the daughterless (da) gene while looking up the marker dp in the original “Red Book” of fly mutants (Lindsley and Grell 1968), the FlyBase of its day. I read that da1 mutant mothers produce sons (haplo-X progeny), but all their daughters (diplo-X progeny) die as embryos, even if they carry da+. Intrigued by such a striking maternal effect, I learned from the limited literature available that this gene was not really sex specific; da1 was a hypomorphic allele of a gene whose maternal and zygotic expression was essential for both sexes. In the first of several career-altering “ahah” moments in my new life as a developmental geneticist, I imagined that da might be just the kind of pleiotropic gene I had been anticipating, with a sex-specific aspect to its phenotype superimposed on other aspects unrelated to sex.
This reading got me thinking about sexual dimorphism in general, and sex-specific lethality in particular, not only as interesting developmental phenomena, but also as subjects whose study seemed to have tremendous practical advantages over studies of other processes such as early pattern formation, my original interest. Although I was unsure at that point just what sex-specific embryonic lethality would reveal about development, I felt certain it would be something important—although I never imagined how quickly it would lead to specific answers to the two questions that had launched my postdoctoral research.
Although da had been discovered in 1954, the gene had attracted remarkably little attention. Just a few years before my epiphany, the fly geneticist L. Sandler (see the Perspectives article by Lindsley 1999) had begun studying da, but with a very different motivation. Sandler believed that the maternal and zygotic effects of da were reflections of a single gene function acting on target genes in the heterochromatin, a function he believed was related to those of a group of genes near da that included abo (Sandler 1977).
Following my hunch that maternal and zygotic aspects of the da phenotype might instead reflect very different functions, one of which was specifically essential to females, I set out to determine whether sexually mosaic progeny called gynandromorphs would survive the female-lethal da maternal effect. Gynandromorphs have a nearly equal mix of haplo-X and diplo-X cells, but in different orientations in different individuals. They arise by X chromosome loss in diplo-X zygotes soon after fertilization. In what tissues would X chromosome dose matter for survival? How would female cells be affected? Would male cells be affected? Would male cells rescue female cells? In the course of setting up for this simple experiment, I was pleased to discover that all aspects of the da mutant phenotype are temperature sensitive, a fact that I exploited in teasing apart the complex phenotype.
My hunch that da is pleiotropic, with disruption of one of its multiple functions causing a truly diplo-X-specific-lethal maternal effect, proved correct. Together with temperature shifts, the gynander studies showed that this maternal activity functioned within the first few hours after fertilization to disrupt the subsequent growth and differentiation of diplo-X cells even days later, wherever those cells were located and regardless of their proximity to their normally developing haplo-X neighbors (Cline 1976). This cell-autonomous, diplo-X-specific effect on general cell growth and differentiation suggested a disruption in X chromosome dosage compensation. The fact that it also disrupted sex determination was obscured by the perverse fact that gyandromorphs never survived with abnormally differentiating diplo-X tissue in areas whose sexual phenotype could be determined unambiguously.
The greatest stroke of luck in my career helped me to identify the cause of this female-specific lethality. Since males survived this aspect of the da phenotype unscathed, I wondered whether there were any genetic perturbations that would allow females to survive as well. When a straightforward selection for single-hit suppressors at the restrictive temperature proved fruitless (in retrospect, only because the effort was too limited), I tried simply raising a homozygous da mutant stock at 18C where the viability of daughters was just high enough to maintain the line in the absence of suppression. I hoped for a slow improvement in the population sex ratio over time as weak modifiers accumulated. Far sooner than I expected, the sex ratio rose to nearly 1:1 even at higher temperatures, suggesting that all daughters as well as all sons were now surviving.
But when I tabulated the results of an outcross of this suppressed line, I discovered that the 1:1 sex ratio reflected instead the survival of exactly half the males and half the females. A spontaneous X-linked mutation that rescued heterozygous daughters had arisen, but remained heterozygous in da1 females because it killed hemizygous sons. Did those sons die simply because they lacked the wild-type allele of the suppressor gene? Answering this question required that the suppressor be mapped and its interactions with the appropriate deficiencies and duplications be determined.
That mapping effort led to the next eureka moment when it became clear that this mutation that rescued females and killed males colocalized with Muller's female-specific zygotic lethal, a mutation overlooked in the literature yet faithfully maintained by the Bowling Green Stock Center. Fortunately, chromosome rearrangements in this region that allowed me to discover that my da suppressor mutation was a dominant, male-specific-lethal, gain-of-function allele were available, while Muller's female-lethal mutation, which mapped just 0.007 cM away, was a loss-of-function allele that enhanced the da female-lethal maternal effect (Cline 1978). Although I introduced the SxlMale and Sxlfemale terminology for these opposite alleles, only in the 1984 article did I finally present the full description of the formal genetic proof of allelism, a proof that generated a number of useful SxlM,f double mutants.
The hypothesis that SxlM1 and Sxlf1 were lesions in the same gene led to a simple model: expression of Sxl+ is essential to females but lethal to males. Expression is induced in females by their double dose of X chromosomes in a process that requires maternal da+. SxlM1 is a constitutive allele that expresses this female-specific activity regardless of X chromosome dose and hence independently of da+. Such promiscuous expression kills males. Sex-specific lethality stems from the fact that Sxl+ product imposes an overall level of X-linked gene expression appropriate only for diplo-X cells, while the level appropriate for haplo-X cells occurs only in its absence.
Soon after I joined the Princeton faculty in 1976, I turned again to gynandromorphs to reveal the developmental basis for sex-specific lethality, this time asking how haplo-X SxlM1/0 mutant male tissue developed in association with diplo-X SxlM1/+ female cells. Again the effect of the mutation was severe, ubiquitous, and cell autonomous. As predicted by the dosage-compensation model, mutant haplo-X tissue displayed the strong Minute phenotype expected if X chromosome gene expression was abnormally low (Minutes are haplo-insufficient genes, several of which are X linked). Surprisingly, however, some of the surviving affected male tissue was feminized, the first indication that sex determination and dosage compensation might be coordinately controlled by Sxl.
Because incompletely penetrant, gain-of-function alleles can be misleading, my excitement over the possibility of a link between these two very different aspects of sexual dimorphism was tempered until I saw the opposite effect by the loss-of-function allele: Sxlf1/Sxlf1 clones generated by mitotic recombination in Sxlf1/+ females during the larval stage invariably differentiated as male. Wild-type Sxl product was both necessary and sufficient to impose the female pathway of sexual differentiation. Hence, at the level of the whole organism, sex-specific lethality rather than obvious sex transformations would be the hallmark of upsets in the primary events in sex determination—a major reason so little progress had been made on the sex-determination signal since 1916.
The fact that homozygous mutant clones were sex transformed even when the Sxl+ allele was removed as late as the third-instar larval stage showed that Sxl acted relatively late in development to direct sexual differentiation, yet the involvement of maternal da product in the regulation of Sxl by X chromosome dose seemed to be very early. If da could be shown to affect this sex-determination function of Sxl, one could infer that X chromosome dose must trigger a self-propagating change in Sxl gene expression. With that point in mind, I ended the article reporting these results with the statement, “Of particular interest is the question of how the expression of the Sxl locus may be controlled by the X-chromosome/autosome balance, and whether the control is determinative in nature” (Cline 1979, p. 274).
The first direct evidence that inappropriate Sxl expression upsets dosage compensation was published by Lucchesi and Skripsky (1981) in an article unfortunately plagued by confusing typos. They used the then-standard molecular assay for dosage compensation: autoradiography of labeled nascent transcripts on polytene chromosomes. I had eschewed this approach because the assay requires relatively healthy late-larval cells and is therefore biased against upsets. Moreover, the effects that one expects are of relatively low magnitude in an assay whose signal-to-noise ratio is low and for which statistical analysis is problematic. Nevertheless, an upset in dosage compensation was reported for one heteroallelic Sxl mutant female genotype (possibly animals who lost Sxl+ activity due to a failure in maintenance rather than activation). Although no reciprocal effect of the SxlM1 allele on male transcription was observed, a reciprocal effect on polytene X chromosome morphology was seen.
I opted to use the approach for assaying dosage compensation that Muller had used to discover the phenomenon: measurements of adult mutant phenotypes for dosage-compensated mutant alleles. I applied the assay to triploid intersexes, XX;AAA animals that I anticipated would tolerate Sxl-based upsets in dosage compensation far better than diploids because the genetic imbalances predicted would be lower. Indeed, I found that triploid intersexes survived, regardless of their Sxl expression state, at least to the pharate adult stage (fully differentiated pupae), allowing adult morphology to be assessed. This triploid intersex study (Cline 1983) complemented the autoradiography study in the sense that a strong effect of SxlM1 on dosage compensation was seen, but the reciprocal effect of Sxlf1 was far more modest, perhaps because Sxl+ might already have been off in most cells in the relevant tissues of the controls.
More significant for establishing an epigenetic aspect to the control of Sxl+ was the fact that both the da maternal genotype and the Sxl zygotic genotype affected the sexual phenotype of XX;AAA animals as predicted. With their ambiguous sex-determination signal, triploid intersexes normally develop as phenotypic mosaics of male and female cells. I found that any decrease in Sxl activity masculinized, whether by changes in Sxl itself or in maternal da alleles, while any increase feminized. These sex effects allowed me to infer that Sxl control must indeed be “determinative in nature,” although I suspected that more direct evidence of this point in diploids would be needed to convince others—evidence which I presented in the 1984 article.
It is easy to underestimate today how counterintuitive the argument once seemed for an epigenetic aspect to sex determination and how the overly broad use of the term “sex determination” had blurred important potential distinctions among events that initially set sexual fate, events that then maintain it in growing cells, and events that finally direct morphogenesis in terminally differentiating cells. Prior to the work with Sxl, the standard developmental sense of “determination” in Drosophila had always involved the paradigm of heritable developmental fate differences maintained epigenetically between genetically identical cells. Since male and female Drosophila cells were genetically different with respect to the key variable, what could sex determination possibly have to contribute regarding epigenetic processes of cell fate restriction?
Years earlier, Stern (1966) had shown that the mosaic sexual phenotype of triploid intersexes reflected genetically identical cells displaying opposite sexual phenotypes; however, without an understanding of Sxl and information on when in development such mosaicism developed, the implications of this intersexuality were unclear. Equally unclear were implications of another qualitatively different class of intersex: “true intersexes,” in which even individual cells displayed a sexually intermediate phenotype. The significance of the distinction between these two classes of intersexes, and of the fact that both classes can be generated by mutations in Sxl, were important elements in the 1984 article, although the points are discussed more explicitly in the reviews that soon followed (e.g., Maine et al. 1985).
It might seem more straightforward to deduce that the activity state of Sxl is maintained independently of the X chromosome dose signal that initiated it, from experiments in which the X chromosome dose was changed at various times in development, than from experiments leaving theX chromosome dose unchanged. This direct approach was in fact attempted by Sanchez and Nothiger (1983). But while their experiments were suggestive, their approach was fatally flawed, not only by an experimental design that did not include convincing evidence that the X chromosome dose had in fact been changed, but also by the fact that the cells whose X chromosome dose was thought to have changed simply died, disappearing before their sexual phenotype could be assessed. Hence, the possibility could not be excluded that the activity state of Sxl could be reversed, but simply not fast enough to avoid a transient growth handicap that would induce nonhandicapped neighbors to exclude them. Moreover, data (Sanchez and Nothiger 1983, Table 4) conflicting with their hypothesis regarding timing were ignored.
I had expected that an experiment that I began in 1979 would unambiguously demonstrate the epigenetic effect of da on Sxl's sex-determination function in diploids. This experiment failed, but its failure led to the hypothesis—which experiments in the 1984 article substantiated—that Sxl had a female-specific autoregulatory activity that could act in trans to bypass the normal Sxl activation process, an autoregulatory activity that might normally serve to maintain the active state triggered by the X chromosome dose. I had thought that the da maternal effect would block activation of the Sxl+ allele present in SxlM1/Sxl+ diploid daughters that had been rescued by SxlM1 and hence that Sxl+/Sxl+ clones induced in these daughters by mitotic recombination would survive and differentiate as male, just like the Sxlf/Sxlf clones induced in Sxlf/Sxl+ females. To my chagrin, such clones always differentiated as female. Clearly, the Sxl+ allele was active.
I guessed that Sxl+ activation in this case had been induced by wild-type Sxl product generated from the constitutive allele SxlM1 in trans. To test this hypothesis, I had to find a situation in diploid females in which activation of Sxl+ was blocked by the da maternal effect and transactivation did not occur, yet the cells still survived to display a masculinized phenotype that would unambiguously indicate that block. Moreover, if diplo-X cells survived with what I inferred to be a silent Sxl+ allele, I should be able to remove that allele without further effect. The 1984 article described such situations, thereby establishing the reality of Sxl autoregulation.
The successful approach was based on the expectation that the death of diplo-X cells lacking Sxl+ activity was due to inappropriate hyperactivation of their X-linked genes, and therefore that if there were some way to block that hyperactivation, diplo-X cells with epigenetically silenced Sxl+ alleles should survive to reveal their masculinization. Belote and Lucchesi (1980) reported the first evidence that a member of the set of genes known as male-specific lethals (msl's) was required for the transcriptional hyperactivation associated with male dosage compensation. Would mutations in these msl's suppress Sxl-based female lethality by blocking inappropriate hyperactivation? The confusing initial answer was “possibly yes and possibly no”: “yes” at the level of individual cells, but “no” at the level of the whole organism. Only when impairment of Sxl function was sufficiently mild to allow even msl+ females to survive would mutations in the msl's cause a mosaic intersex phenotype. But did this intersexuality really reflect cell rescue, or instead might the msl mutations simply reduce the effectiveness of Sxl+ product in directing female differentiation? Since mutations in the msl's by themselves did not affect sexual phenotype, any such reduction in Sxl effectiveness would have to be so mild that it was apparent only when Sxl+ levels were also reduced.
Three key points emphasized in the 1984 article supported the view that the msl mutations rescue functionally Sxl− cells and excluded the alternative: First, msl mutations suppressed developmental damage that had clearly indicated sporadic cell death among the surviving daughters of da mutant mothers. Sporadic masculinization was seen in its place. Second, at the cellular level, sporadic masculinization was always of the “all-or-none” type, never the incomplete (“true” intersex) type that one would expect from a simple reduction in Sxl+ effectiveness. Finally, the degree of interspersion of male and female cells showed that the critical events generating these mosaic intersexes occurred as soon as the imaginal disc primordia were formed, not later when the discs terminally differentiated.
The 1984 article dealt at some length with the question of why msl mutations rescued functionally Sxl− cells but not whole animals. The msl genes simply might not control as many aspects of dosage compensation as Sxl does, with regard to developmental time, tissue type, or gene target. One would expect rescue of imaginal disc cells to be easier than rescue of the whole organism if suppression of the dosage-compensation upset was incomplete, particularly if it were more incomplete earlier in development.
Gergen (1987) confirmed the key element in this hypothesis by showing that runt dosage compensation at the blastoderm stage is controlled by Sxl but not by the msl's. Subsequently, Franke et al. (1996) and Rastelli et al. (1995) showed that the msl's are unlikely to control any dosage compensation at the blastoderm stage [although one would not guess that from the Franke et al. (1996) title]. Kelley et al. (1995) presented a bioinformatic argument that X-linked genes are far more likely than autosomal genes to have multiple potential Sxl-binding sites in their 3′-UTRs (20 X-linked genes including runt vs. only two autosomal: msl-1 and msl-2!). They proposed that these sites might reflect an msl-independent post-transcriptional dosage-compensation mechanism directly mediated by Sxl in females. J. Kaminker, G. Rubin and T. Cline (unpublished results) extended this approach, using data on 2235 X-linked and 11,887 autosomal 3′-UTRs, respectively. Of the 84 potential Sxl target genes identified, 70 were on the X, including the dosage-compensation gene mof. Moreover, msl-3 was added to msl-1 and msl-2 among only 14 potential autosomal targets. A significant post-transcriptional contribution to dosage compensation would account for the often overlooked fact that transcriptional hyperactivation of X-linked genes in males relative to females is only 1.4 times overall, not 2 times (Holmquist 1972).
The argument that msl mutations rescue cells in which Sxl+ has been epigenetically silenced by da was clinched in the 1984 article by experiments exploiting Sxlf7,M1, a double-mutant allele recovered as a male-viable derivative of SxlM1. The article also provided the strongest evidence for Sxl positive autoregulation. The SxlM1 lesion allows Sxlf7,M1 to produce Sxlf7 mutant protein even in daughters of da mutant mothers under the most nonpermissive conditions. Sxlf7 protein cannot elicit female differentiation, but it does still have some dosage-compensation and autoregulatory activity. Since Sxlf7,M1 cannot feminize by itself, the sexual phenotype of Sxlf7,M1/Sxl+ cells reveals the activity state of Sxl+.
One to three percent of Sxlf7,M1/Sxl+ daughters survived the da maternal effect under conditions invariably lethal to their Sxl+/Sxl+ sisters. The sexual phenotype of these survivors showed they had activated Sxl+ in all nongonadal cells. With an additional copy of Sxlf7,M1, 49% of the daughters survived, again with Sxl+ activated in all nongonadal tissues. In contrast, doubling the dose of Sxl+ had relatively little effect, as expected if Sxl+ alleles were being silenced by the da maternal effect, and their activation by Sxlf7,M1 was rate limiting.
This dose effect of Sxlf7,M1 had to be due to an increase in the activation of Sxl+ by Sxlf7,M1, since when Sxlf7,M1/Sxl+ daughters were also mutant for msl's, 70% survived, but nearly all had a mosaic intersex phenotype signaling their failure to have activated Sxl+ in all nongonadal cells. For the first time, msl mutations rescued whole animals, not just cells. Most importantly, msl mutations did not increase the recovery or alter the phenotype of daughters who would have survived without msl mutations—those that had transactivated Sxl+ in all nongonadal cells.
Some Sxlf7,M1/Sxl+ daughters mutant for msl's were entirely male, indicating that they had survived the da maternal effect without activating Sxl+ in any adult precursor cells. This result suggested that msl mutations might be able to rescue Sxlf7,M1/Df(Sxl+) females, a genotype not otherwise viable. This prediction of the “epigenetically silenced Sxl+ allele” hypothesis was validated.
The degree of interspersion of male and female cells in the forelegs of msl-rescued females showed that if activation of Sxl+ by autoregulation was going to occur, it occurred only very early. Preliminary results of mitotic recombination experiments were mentioned, confirming that the Sxl+ activity state established so early was heritably maintained thereafter. But the question of whether continued Sxl autoregulation maintained female fate (with male fate being the default state) was answered definitively in the affirmative only years later with construction of a Sxl+ transgene whose expression could be manipulated (Bell et al. 1991).
The 1984 article described a variety of intriguing effects on the development of the ovary that we are still working to understand, including the inability of Sxlf7,M1 to transactivate Sxl+ alleles in the gonadal soma. The 1984 study came tantalizingly close to revealing the important role of the gonadal mesoderm in feminizing diplo-X germ cells, but it lacked the key Sxlf7,M1 pole-cell transplant experiment required for such a conclusion.
Seven years after the 1984 article demonstrated positive autoregulation as a mechanism by which Sxl might maintain the female fate decision in dividing cells, we described the molecular basis for this phenomenon (Bell et al. 1991): Transcripts generated from the maintenance promoter of Sxl, a promoter that is active in all cells of both sexes, can be processed into mRNA that encodes full-length Sxl protein only if full-length Sxl protein is already present. Hence, the active (female) state is maintained by a positive-feedback loop on Sxl pre-mRNA splicing, while the inactive (male) state is maintained by default. Of course, the question of the origin of the initial Sxl protein that engages this feedback loop in females remained.
Earlier genetic analysis had indicated that the mechanism by which the female state of Sxl expression was initiated by the X chromosome dose signal was likely to be very different from the mechanism by which it was subsequently maintained independently of that signal, since these two aspects of regulation could be disrupted separately by different mutations in Sxl (Maine et al. 1985). In 1992, we demonstrated that two different Sxl promoters were involved (Keyes et al. 1992). Transient activation of the establishment promoter in females in response to their double dose of X chromosomes is able to generate a burst of Sxl protein that subsequently will engage the autoregulatory loop for maintenance promoter transcripts because productive processing of transcripts from the establishment promoter does not require Sxl protein. The labels “establishment” and “maintenance” for the two Sxl promoters emphasize the striking formal parallels between initiation and maintenance of the female pathway choice in Drosophila and the lysogenic developmental pathway paradigm for phage λ, key elements of which are the repressor establishment and repressor maintenance promoters (see Ptashne 2004).
Undeniable analogies exist between the strategy that the fruit fly uses for sex determination and strategies used by a variety of organisms for initiating and maintaining other developmental fate decisions, many of which are known to involve binary switch genes like Sxl. Nevertheless, perhaps the greatest irony of the Sxl autoregulation story is that even now, so many years after the 1984 report, no other case of a developmental fate decision being maintained by positive feedback on pre-mRNA splicing seems to have emerged. Nature—or at least those who study Nature—would seem to be missing a good bet!
References
- Bell, L. R., J. I. Horabin, P. Schedl and T. W. Cline, 1991. Positive autoregulation of Sex-lethal by alternative splicing maintains the female determined state in Drosophila. Cell 65: 229–239. [DOI] [PubMed] [Google Scholar]
- Belote, J. M., and J. C. Lucchesi, 1980. Control of X chromosome transcription by the maleless gene in Drosophila. Nature 285: 573–575. [DOI] [PubMed] [Google Scholar]
- Cline, T. W., 1976. A sex-specific, temperature-sensitive maternal effect of the daughterless mutation of Drosophila melanogaster. Genetics 84: 723–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline, T. W., 1978. Two closely linked mutations in Drosophila melanogaster that are lethal to opposite sexes and interact with daughterless. Genetics 90: 683–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline, T. W., 1979. A male-specific lethal mutation in Drosophila melanogaster that transforms sex. Dev. Biol. 72: 266–275. [DOI] [PubMed] [Google Scholar]
- Cline, T. W., 1983. The interaction between daughterless and Sex-lethal in triploids: a lethal sex-transforming maternal effect linking sex determination and dosage compensation in Drosophila melanogaster. Dev. Biol. 95: 260–274. [DOI] [PubMed] [Google Scholar]
- Cline, T. W., 1984. Autoregulatory functioning of a Drosophila gene product that establishes and maintains the sexually determined state. Genetics 107: 231–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke, A., A. Dernburg, G. Bashaw and B. S. Baker, 1996. Evidence that MSL-mediated dosage compensation in Drosophila begins at blastoderm. Development 122: 2751–2760. [DOI] [PubMed] [Google Scholar]
- Gergen, J. P., 1987. Dosage compensation in Drosophila: evidence that daughterless and Sex-lethal control X chromosome activity at the blastoderm stage of embryogenesis. Genetics 117: 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadorn, E., 1968. Transdetermination in cells. Sci. Am. 219: 110–114. [DOI] [PubMed] [Google Scholar]
- Holmquist, G., 1972. Transcription rates of individual chromosome bands: effects of gene dose and sex in Drosophila. Chromosoma 36: 413–452. [DOI] [PubMed] [Google Scholar]
- Kelley, R. L., I. Solovyeva, L. M. Lyman, R. Richman, V. Solovyev et al., 1995. Expression of MSL-2 causes assembly of dosage compensation regulators on the X chromosomes and female lethality in Drosophila. Cell 81: 867–877. [DOI] [PubMed] [Google Scholar]
- Keyes, L. N., T. W. Cline and P. Schedl, 1992. The primary sex determination signal of Drosophila acts at the level of transcription. Cell 68: 933–943. [DOI] [PubMed] [Google Scholar]
- Lindsley, D., 1999. Larry Sandler: personal recollections. Genetics 151: 1233–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley, D. L., and E. H. Grell, 1968 Genetic Variations of Drosophila melanogaster. Pub. 627. Carnegie Institute, Washington, DC.
- Lucchesi, J. C., 1983. The relationship between gene dosage, gene expression, and sex in Drosophila. Dev. Genet. 3: 275–282. [Google Scholar]
- Lucchesi, J. C., and T. Skripsky, 1981. The link between dosage compensation and sex determination in Drosophila melanogaster. Chromosoma 82: 217–227. [DOI] [PubMed] [Google Scholar]
- Maine, E. M., H. K. Salz, P. Schedl and T. W. Cline, 1985. Sex-lethal, a link between sex determination and sexual differentiation in Drosophila melanogaster. Cold Spring Harbor Symp. Quant. Biol. 50: 595–604. [DOI] [PubMed] [Google Scholar]
- Muller, H. J., 1932. Further studies on the nature and causes of gene mutations. Proc. 6th Int. Congr. Genet. 1: 213–255. [Google Scholar]
- Ptashne, M., 2004 A Genetic Switch: Phage Lambda Revisited. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Rastelli, L., R. Richman and M. I. Kuroda, 1995. The dosage compensation regulators MLE, MSL-1, and MSL-2 are interdependent since early embryogenesis in Drosophila. Mech. Dev. 53: 223–233. [DOI] [PubMed] [Google Scholar]
- Sanchez, L., and R. Nothiger, 1983. Sex determination and dosage compensation in Drosophila melanogaster: production of male clones in XX females. EMBO J. 2: 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler, L., 1977. Evidence for a set of closely linked autosomal genes that interact with sex-chromosome heterochromatin in Drosophila melanogaster. Genetics 86: 567–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern, C., 1966. Pigment mosaicism in intersexes of Drosophila. Rev. Suisse Zool. 73: 339–355. [Google Scholar]
- Stewart, B., and J. Merriam, 1980 Dosage compensation, pp. 107–140 in The Genetics and Biology of Drosophila, edited by M. Ashburner and T. R. F. Wright. Academic Press, New York.