Abstract
Drugs that target the serotonergic system are the most commonly prescribed therapeutic agents and are used for treatment of a wide range of behavioral and neurological disorders. However, the mechanism of the drug action remain a conjecture. Here, we dissect the genetic targets of serotonin (5HT), the selective 5HT reuptake inhibitor (SSRI) fluoxetine (Prozac), the tricyclic antidepressant imipramine, and dopamine. Using the well-established serotonergic response in C. elegans egg-laying behavior as a paradigm, we show that action of fluoxetine and imipramine at the 5HT reuptake transporter (SERT) and at 5HT receptors are separable mechanisms. Even mutants completely lacking 5HT or SERT can partially respond to fluoxetine and imipramine. Furthermore, distinct mechanisms for each drug can be recognized to mediate these responses. Deletion of SER-1, a 5HT1 receptor, abolishes the response to 5HT but has only a minor effect on the response to imipramine and no effect on the response to fluoxetine. In contrast, deletion of SER-4, a 5HT2 receptor, confers significant resistance to imipramine while leaving the responses to 5HT or fluoxetine intact. Further, fluoxetine can stimulate egg laying via the Gq protein EGL-30, independent of SER-1, SER-4, or 5HT. We also show that dopamine antagonizes the 5HT action via the 5HT-gated ion channel MOD-1 signaling, suggesting that this channel activity couples 5HT and dopamine signaling. These results suggest that the actions of these drugs at specific receptor subtypes could determine their therapeutic efficacy. SSRIs and tricyclic antidepressants may regulate 5HT outputs independently of synaptic levels of 5HT.
THE selective serotonin reuptake inhibitor (SSRI) fluoxetine and the tricyclic antidepressant imipramine represent two major classes of therapeutics used for treatment of a wide range of behavioral and neurological disorders from depression to autism and schizophrenia. The classically recognized action of these drugs is to block uptake of serotonin (5HT) by the membrane 5HT transporter (SERT), which results in an increase in synaptic 5HT availability (Baldessarini 1996). In addition, it has been shown in vitro that these drugs, independently and selectively, act as agonists and antagonists at receptors for a variety of neurotransmitters and neurohormones (Baldessarini 1996; Blier and de Montigny 1998; Kroeze and Roth 1998). For example, tricyclic antidepressants have moderate to high affinity for 5HT1a (Blier and de Montigny 1998), muscarinic, cholinergic, and histaminergic receptors (Baldessarini 1996). SSRIs have high affinity for at least four 5HT receptor subtypes (Kroeze and Roth 1998). Both fluoxetine and imipramine can antagonize 5HT2 in cultured smooth muscle cells (Pitt et al. 1994). Furthermore, it has been reported that drugs that antagonize 5HT2 receptors may induce dopamine neurotransmission (Di Matteo et al. 2001). On the other hand, antagonists to the ionotropic receptor 5HT3 inhibit dopamine function in the rat mesocorticolimbic system (Gillies et al. 1996). Therefore, the action of antidepressants at specific 5HT receptor subtypes could underlie the unique spectrum of the therapeutic efficacy of the drugs. However, distinction between the action of a drug on SERT and on 5HT receptors in vivo is difficult, and the role of 5HT receptor subtypes in the efficacy of the drugs may be significantly underestimated.
Caenorhabditis elegans egg laying has been used as a simple genetic system for identification and characterization of the action of drugs on neurotransmitter pathways that generate and modulate a specific behavior (Trent et al. 1983; Desai and Horvitz 1988; Weinshenker et al. 1995; Waggoner et al. 1998, 2000; Bany et al. 2003). In C. elegans, fertilized eggs are propelled from the uterus through contractions of the vulval muscles, and the rate of egg laying is controlled by the availability of food. On a lawn of bacteria (food) young adult hermaphrodites periodically release fertilized eggs at the stage of gastrulation ∼3 hr after fertilization (Sulston et al. 1983). When deprived of food, egg laying ceases and fertilized eggs are retained in the uterus where they continue to develop. This modulation of egg-laying behavior by food involves an antagonistic action between 5HT and dopamine: application of 5HT to worms immediately elicits egg laying in the absence of food (Horvitz et al. 1982; Trent et al. 1983), and dopamine inhibits egg laying induced by either 5HT or food (Schafer and Kenyon 1995).
One major neuronal input to the vulval muscles is from a pair of serotonergic motor neurons, HSN (Desai et al. 1988; Desai and Horvitz 1988). The cell bodies of the HSN neurons reside bilaterally symmetrical at the subventral region posterior to the vulva, and each neuron extends a single axon. The axon branches at the region of the vulva, the short branches form extensive synapses onto the vulval muscles, and the main process runs anteriorly, eventually entering the nerve ring where it receives synaptic inputs from sensory neurons and interneurons that detect and integrate external environmental signals (White et al. 1986; Bargmann and Mori 1997). Ablation of the HSN neurons in wild-type animals by a laser beam (Desai et al. 1988) or deletion of the gene encoding the key 5HT biosynthetic enzyme, tryptophan hydroxylase, tph-1 (Sze et al. 2000), causes accumulation of late-stage embryos in the uterus even under optimal growth conditions. In addition to 5HT, the HSN neurons also release acetylcholine and several neuropeptides (Schinkmann and Li 1992; Weinshenker et al. 1995). Disruption of acetylcholine or neuropeptide signaling diminishes the egg-laying response following 5HT stimulation, whereas nicotinic and muscarinic acetylcholine agonists stimulate egg laying (Weinshenker et al. 1995; Waggoner et al. 1998, 2000). Another neuronal input to the egg-laying circuit is from the ventral-type C (VC) neurons, which synapse to the vulval muscles as well as with the HSN neurons (White et al. 1986). Intriguingly, acetylcholine released from the VC neurons negatively regulates the activity of the vulval muscles and the HSN neurons (Schafer et al. 1996; Bany et al. 2003). Thus, endogenous 5HT controls egg-laying behavior, and the HSN neurons integrate multiple neurotransmitter signals to optimize the rate of egg laying.
This study identifies receptors that couple 5HT signaling to egg-laying behavior. We test the effect of mutations in individual receptor subtypes on actions of 5HT. By identification of mutants that cannot respond to 5HT, we test whether fluoxetine, imipramine, and dopamine can regulate egg-laying behavior via alternative signaling pathways. The construction of double mutants that cannot synthesize and cannot respond to 5HT allows us to test whether fluoxetine and imipramine have the potential to activate egg laying independently of 5HT. Here we report that pharmacological actions at 5HT receptors and at SERT are two separable components of the in vivo response to both fluoxetine and imipramine. Consistent with a previous report (Ranganathan et al. 2001), we find that SERT is a minor target of fluoxetine and imipramine in the egg-laying circuit. Furthermore, we demonstrate that fluoxetine and imipramine each stimulate egg laying via distinct G-protein-coupled 5HT receptor signaling pathways independently of 5HT or SERT. We also show that dopamine antagonizes 5HT stimulatory action via MOD-1, a 5HT-gated channel (Ranganathan et al. 2000). Thus, this channel activity may couple 5HT and dopamine signaling in the egg-laying circuit. The results from this study provide genetic evidence that the actions of antidepressant drugs at specific receptor subtypes may score their unique therapeutic efficacy and suggest that SSRIs and tricyclic antidepressants may regulate 5HT outputs independently of the synaptic level of 5HT.
MATERIALS AND METHODS
Worm strains:
Maintenance and manipulation of C. elegans have been previously described (Brenner 1974). Animals were cultivated at 20° and fed with Escherichia coli OP50 as food. Wild type is the C. elegans variety Bristol strain (N2). The following genes and alleles were used in this work: ser-1(ok345) (C. elegans Gene Knockout Consortium), ser-2(pk1357) (Tsalik et al. 2003), ser-4(ok512) (C. elegans Gene Knockout Consortium), mod-1(ok103) (Ranganathan et al. 2000), mod-5(n3314) (Ranganathan et al. 2001), egl-30(ad806) (Brundage et al. 1996), egl-8(ok934) (C. elegans Gene Knockout Consortium), tph-1(mg280) (Sze et al. 2000), tph-1(mg280);ser-1(ok345), tph-1(mg280);ser-4(ok512), mod-5(n3314);ser-1(ok345), mod-5(n3314); ser-4(ok512), egl-1(n487) (Trent et al. 1983), itr-1(sa73) (Iwasaki et al. 1995), tpa-1(k530) (Tabuse et al. 1995), and dgk-1(nu62) (Nurrish et al. 1999).
Behavioral assays and statistical analyses:
Egg-laying behavior was evaluated by two methods, following well-established drug concentrations and assay protocols (Trent et al. 1983; Desai and Horvitz 1988; Schafer and Kenyon 1995; Weinshenker et al. 1995; Sawin 1996). The animals used for both assays were prepared as follows: Well-fed larval stage 4 animals (L4) were picked onto fresh plates seeded with bacteria and allowed to develop ∼20 hr at 20°, and the resultant young adults were assayed. One assay determines the number of eggs laid. To test the response to drugs, individual young adults were each transferred into a well of a 96-well microtiter plate containing 100 μl of a solution of a particular drug, and the number of eggs released at room temperature scored after 60 min. Unless specified, the concentrations of drugs in M9 buffer are: 5HT, 5 mg/ml; dopamine, 3 mg/ml; imipramine, 0.75 mg/ml; fluoxetine, 0.5 mg/ml; mianserinm 20 μm; and methiothepin, 50 μm (fluoxetine is a gift from William Miles of Lafayette University, Easton, PA; the other drugs were obtained from Sigma, St. Louis) Controls in M9 buffer alone were performed on each strain every time. Assays on all the strains were replicated by two to three people in the lab; each assay tested eight animals/strain/treatment.
To test the egg-laying response of animals preexposed to ligands for 5HT receptor subtypes, animals were preincubated for 6 hr on a cultivation plate containing the indicated drug and seeded with bacteria, and their egg-laying behavior was assayed immediately afterward and compared with sham-treated siblings. To test the effect of dopamine on food-dependent egg-laying behavior, young adult animals were transferred onto standard worm cultivation plates containing 3 or 6 mg/ml dopamine and seeded with bacteria for 60 min and then these animals were transferred to another plate containing the same concentration of dopamine also seeded with food; the number of eggs released on the second plate was scored after 60 min. The dopamine-containing plates were freshly prepared on the day of the assay. Controls are siblings of each strain assayed on plates without dopamine.
The second assay scored the number of eggs carried in the uterus. In this assay, young adult animals prepared as described above were individually transferred to a drop of solution containing commercial bleach and 1 n NaOH on a glass slide covered with an agar pad. The bleach solution dissolves the body of the adult animal, and eggs, which are protected by their egg shells, were scored immediately under DIC optics with a Zeiss axioscope.
Routine statistical analyses were performed using Minitab 12.1 (Minitab 1998). Tests used were ANOVA (one-way), followed by a Tukey's pairwise multi-comparison procedure. Where applicable, chi-square tests and Student's t-tests were carried out. In a few cases where the number of eggs released was very low and the data were found not to be normally distributed, the data were analyzed using an arcsine transformation or the nonparametric Mann-Whitney U-test and Kruskal-Wallis tests.
Construction and observation of a GFP reporter of the ser-1 gene:
ser-1::gfp is a transcriptional fusion of the ser-1 genomic sequence containing 4.9 kb 5′-upstream sequence to the first methionine of the second isoform of ser-1 (Hamdan et al. 1999) with the green fluorescent protein (GFP) sequence and unc-54 3′-untranslated sequence in the vector pPD95.75 (A. Fire). The ser-1 sequence was amplified from wild-type genomic DNA and ligated to the GFP and unc-54 sequence by PCR. Note that the 5′-upstream sequence included in this construct is 3.2 kb longer than that in the ser-1 GFP reporter described by Tsalik et al. (2003). This could account for the extra cells and tissues expressing our ser-1::gfp reporter (Figure 2).
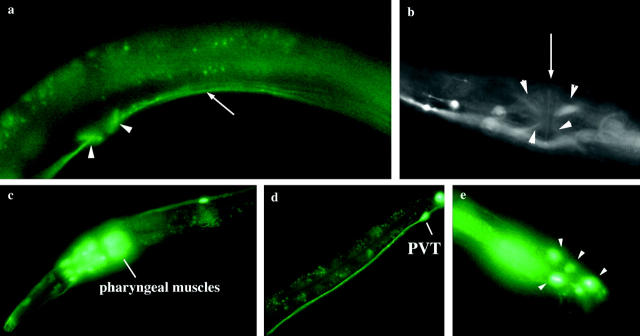
Figure 2.—
Expression pattern of ser-1 observed by a GFP fusion reporter. (a) Lateral view of the vulval region, showing vulval muscles (arrowheads) and the ventral nerve cord (arrow). (b) Ventral view of the vulval muscles (arrowheads). Arrow indicates the vulval opening. (c) Expression in the pharyngeal muscles as reported (Tsalik et al. 2003), and in many amphid sensory neurons and interneurons in the head region. (d) PVT neuron in the tail. (e) Expression in ray sensory neurons (arrowheads) in the male sex organ. Adult animals are shown, and the anterior is to the left.
The PCR-amplified fusion products were micro-injected into wild-type animals to generate transgenic animals, with the plasmid pRF4 that contains the dominant Rol-6 gene coinjected as a genetic marker for the transgene. The GFP expression pattern in two transgenic lines was observed under a Zeiss Axioplane 2 microscope equipped with a fluorescence light source.
5HT receptor rescue constructs:
We generated by PCR two constructs driving ser-1 expression by tissue-specific promoters to determine the site of action of ser-1. A genomic fragment encompassing the sequence from the start codon of the second isoform of ser-1 to the stop codon was amplified from wild-type genomic DNA. To express ser-1 in neuronal cells, this ser-1 genomic fragment was fused in frame to a promoter fragment of unc-119 (containing 3.3 kb of the upstream sequence, the first 25 amino acid codons and the first intron). This promoter directs a strong expression in all the neuronal cells (Maduro and Pilgrim 1995). To express ser-1 in muscles, the ser-1 genomic fragment was fused in frame to a promoter fragment of unc-45 (containing 4.4 kb of the upstream sequence and the first 6 amino acid codons). This promoter directs a strong expression in the pharyngeal muscles, body-wall muscles, vulval muscles, and anal depressor muscles (Venolia et al. 1999).
A ser-4 rescue construct was generated by PCR fusion of the full-length ser-4 cDNA to a ser-4 promoter fragment (containing 4.1 kb upstream sequence from the predicted start codon). A GFP reporter containing this promoter sequence is expressed in interneurons and motor neurons located in the head and tail regions (Tsalik et al. 2003). The ser-4 cDNA was isolated by RT-PCR from mixed stage of total RNA prepared from wild-type worms, using the SuperScript III first-strand system (Invitrogen, San Diego) and gene-specific primers. This ser-4 cDNA can confer 5HT receptor activities when it is expressed in culture cells (Olde and McCombie 1997).
The PCR-amplified fusion products were micro-injected into respective mutant animals to generate transgenic animals, with the plasmid pRF4 that contains the dominant Rol-6 gene coinjected as a genetic marker for the transgene. The Rol animals were scored for their egg-laying responses to 5HT and imipramine. In each assay, transgenic animals, wild type, and corresponding nontransgenic mutant animals were raised at the same time and assayed side by side as described above.
RESULTS
The G-protein-coupled 5HT receptor SER-1 is essential for egg-laying response to 5HT:
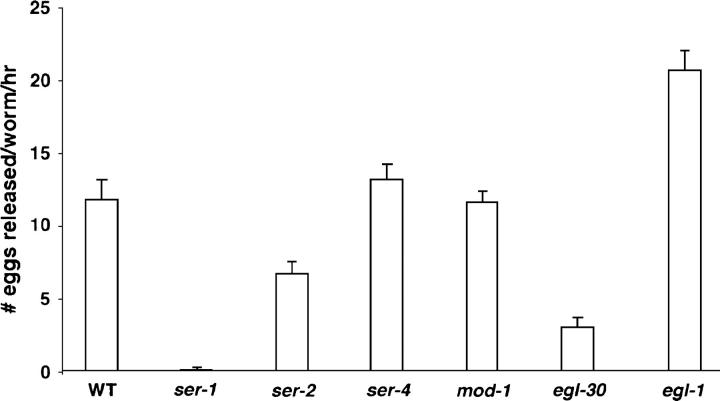
We first sought to identify the receptors that mediate the egg-laying response to 5HT. Similar to mammals, two major classes of 5HT receptors exist in C. elegans: mod-1 encodes an ionotropic 5HT receptor (Ranganathan et al. 2000), and ser-1 and ser-4 are two members of the metabotropic G-protein-coupled 5HT receptor superfamily and have pharmacological profiles similar to mammalian 5HT2 and 5HT1a subtype receptors (Olde and McCombie 1997; Hamdan et al. 1999). In addition, ser-2 encodes a G-protein-coupled receptor with pharmacological properties of a tyramine receptor (Rex and Komuniecki 2002). Using a well-established protocol (Trent et al. 1983; Desai and Horvitz 1988; Weinshenker et al. 1995; Bastiani et al. 2003), we characterized the egg-laying behavior of mod-1, ser-1, ser-4, and ser-2 mutants in response to exogenous 5HT (Figure 1). When bathed for 1 hr in a solution containing 5 mg/ml of 5HT, a wild-type young adult hermaphrodite releases ∼12 eggs. ser-4 knockout and mod-1 mutant animals respond to 5HT equivalently to wild type (Figure 1). Mutants bearing the ser-2(pk1357) deletion, which spans the first four transmembrane segments including the predicted ligand binding site (Tsalik et al. 2003), release a reduced number of eggs compared to wild type following 5HT treatment (Figure 1). Under the same growth condition, ser-2(pk1357) mutant animals carry at least as many eggs as wild type in the uterus (Table 1); thus the ser-2 mutation does not affect the ability to fertilize or to retain the eggs. ser-2 is not expressed in muscles or in neurons directly involved in egg laying, but it is expressed in the AIZ and BDU interneurons (Tsalik et al. 2003), which are known to synapse onto the HSN neurons. AIZ and BDU are the postsynaptic targets of many sensory neurons that detect environmental signals (White et al. 1986). Thus, SER-2 signaling is likely to occur via these interneurons and to enhance the egg-laying response to 5HT by modulation of HSN activity. MOD-1 and SER-4 are not required for egg-laying response to 5HT.
Figure 1.—
Egg-laying response to exogenous 5HT in 5HT receptor mutants. A deletion mutation in the metabotropic receptor gene ser-1 eliminates 5HT stimulation of egg laying. Mutants bearing a deletion in the metabotropic receptor SER-4 or the ionotropic receptor MOD-1 respond as well as wild type to 5HT stimulation of egg laying (one-way ANOVA, P > 0.05, comparing the response of the mutants with that of wild type assayed on the same day). Egg-laying assays were performed on individual young adult animals. All the strains have been tested many times. Each bar represents the mean of two to three representative assays, eight animals tested/strain/drug/assay ±SEM. Except egl-1 mutants, all the strains laid, on average, less than one egg in the control buffer M9.
TABLE 1.
Accumulation of fertilized eggs in 5HT signaling mutants
| Strains | No. of eggs in uterus |
SD | N |
|---|---|---|---|
| Wild type | 10.3 | 3.3 | 16 |
| tph-1(mg280) | 15.8 | 3.9 | 21 |
| ser-1(ok345) | 15.5 | 2.3 | 22 |
| ser-1(ok345); tph-1(mg280) | 17.2 | 1.6 | 51 |
| ser-2(pk1357) | 13.2 | 3.1 | 30 |
| ser-4(ok512) | 10.4 | 3.8 | 29 |
| ser-4(ok512); tph-1(mg280) | 15.0 | 2.2 | 50 |
| mod-1(ok103) | 11.7 | 3.3 | 29 |
| mod-5(n3314) | 11.3 | 3.5 | 20 |
| mod-5(n3314); ser-1(ok345) | 14.8 | 2.1 | 50 |
| mod-5(n3314); ser-4(ok512) | 10.3 | 2.8 | 54 |
| egl-30(ad806) | 25.3 | 4.8 | 15 |
The assays were performed with first-day adults as described in materials and methods. Although tph-1(mg280) and ser-1 (ok345) mutant animals retained a similar amount of eggs in the uterus at the age of the assay, tph-1 mutants tended to accumulate more eggs in older adults. Both tph-1 and ser-1 mutants are less defective than egl-30/Gq mutants (Bastiani et al. 2003) or egl-1 mutants that lack the HSN neurons (Trent et al. 1983). The data are the summary of at least two trials. SD, standard deviation; N, number of animals assayed.
In contrast, we found that the deletion allele ser-1(ok345), which is an in-frame truncation that removes the last two transmembrane segments of the receptor (C. elegans Gene Knockout Consortium), completely abolishes egg-laying response following 5HT treatment (Figure 1). This result suggests that the metabotropic 5HT receptor SER-1 is the major determinant in coupling 5HT to egg-laying behavior.
SER-1 is expressed in the vulval muscles to control egg-laying behavior:
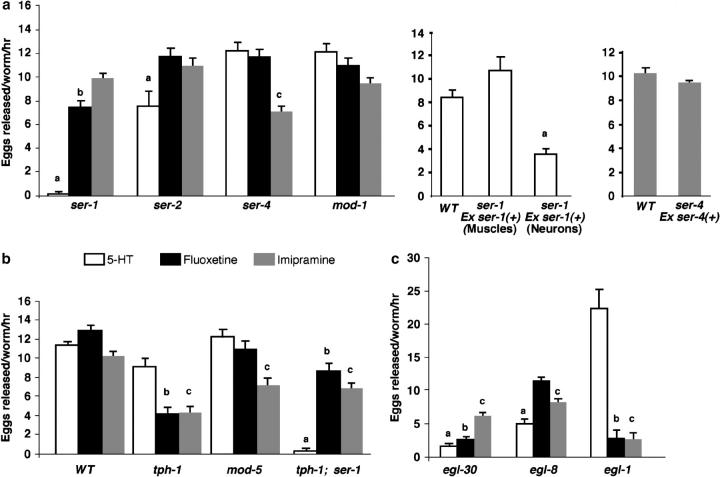
To identify the site of ser-1 action in the egg-laying circuit, we used GFP as a reporter to examine the ser-1 expression pattern. As previously reported, we observed ser-1::gfp expression in the pharyngeal muscles (Tsalik et al. 2003). In addition, we observed ser-1::gfp expression in the vulval muscles, as well as in many neurons (Figure 2). The difference in the expression pattern that we observed to that previously reported apparently arises from the additional 5′-upstream sequence of ser-1 included in our construct (see materials and methods). In addition to HSN, the VC4/5 motor neurons synapse onto the vulval muscles (White et al. 1986); ser-1::gfp is not detectable in HSN or VC. To determine if indeed SER-1 acts in the muscles to regulate egg laying, we expressed the wild-type ser-1 coding sequence, including introns and exons, under the control of an unc-45 promoter, which is specifically expressed in muscles, including the pharyngeal, body-wall, and vulval muscles (Venolia et al. 1999), and introduced this fusion construct into ser-1(ok345) mutant animals. This fusion construct fully rescues the ability of the ser-1 mutants to induce egg laying following 5HT treatment (Figure 3a). This indicates that it is the mutation in ser-1 that causes 5HT resistance of the mutants and that expression of SER-1 in the muscles is sufficient to confer the 5HT sensitivity. Together, these observations suggest that 5HT acts on the vulval muscles and that the SER-1 receptor-coupled signaling pathway in the vulval muscles regulates vulval contraction. However, expression of the same wild-type ser-1 coding sequence under the control of the unc-119 promoter that is expressed in all the neurons, but not in muscles, can partially restore the egg-laying response of ser-1(ok345) mutants to 5HT (Figure 3a). This result suggests that some yet-unidentified neurons expressing ser-1 may trigger neurotransmitter release in response to 5HT to activate egg laying. It is not known whether these neurons normally express SER-1 or if they ectopically express SER-1 due to the unc-119 promoter.
Figure 3.—
Stimulation of egg laying by 5HT, fluoxetine, and imipramine is mediated by distinct signaling pathways. (a) Differential effect of drugs on 5HT and tyramine receptor mutants. ser-1(ok345) mutants fail to respond to exogenous 5HT but respond vigorously to fluoxetine and imipramine. The ser-4(ok512) mutation specifically affects the sensitivity to imipramine. mod-1(ok103) mutants respond well to all the drugs. Expression of wild-type ser-1 coding regions under the control of an unc-119 promoter, which is expressed in all the neurons (Maduro and Pilgrim 1995), partially rescues the 5HT sensitivity of ser-1(ok345) mutants, whereas expression of the same ser-1 coding sequence under the control of a unc-45 promoter, which is expressed in muscles, including the vulval muscles (Venolia et al. 1999), confers a full rescue. Expression of a wild-type ser-4 cDNA under the control of a ser-4 promoter (Tsalik et al. 2003) restores the imipramine sensitivity in ser-4(ok512) mutants. (b) A part of the efficacy of fluoxetine and imipramine is dependent on endogenous 5HT and correlates to the activity of SERT. Note that tph-1(mg280) mutants, lacking detectable 5HT, are equally resistant to fluoxetine and imipramine and that mod-5/SERT n3314 null mutants exhibit resistance only to imipramine. (c) Resistance to fluoxetine and imipramine is mediated by distinct Gq signaling pathways. egl-30/Gq mutants are strongly resistant to 5HT and fluoxetine but retain a substantial sensitivity to imipramine. However, animals bearing a null mutation in egl-8/PLCβ show a partial resistance to 5HT and imipramine and have no significant resistance to fluoxetine. We have also tested the drug responses of mutants lacking other predicted components in Gq signaling pathways. We found that loss-of-function mutations in C. elegans homologs of inositol trisphosphate receptor itr-3 (Iwasaki et al. 1995), protein kincase C tpa-1 (Tabuse et al. 1995), and diacylglycerol kinase dgk-1 (Nurrish et al. 1999) have relatively mild and variable effects on egg-laying response to the drugs (data not shown). Because some of the mutations also affect brood size, their egg-laying behavior is difficult to evaluate. Individual young adults were assayed. Except for egl-8, the number of eggs carried in the uterus of individual strains at the stage of the assays was scored. None of the strains carried fewer eggs than wild type (Table 1). Each bar represents the mean of two or more assays and eight animals tested/strain/drug/assay ±SEM. Letters denote a significant reduction in the response of a mutant strain relative to that of wild type to a specific drug (one-way ANOVA, P > 0.05): a, 5HT; b, fluoxetine; c, imipramine. M9 buffer was used as the control each time for each strain, and the average of eggs released in M9 is between 0 and 1.7.
We have reported that animals bearing a deletion of the 5HT biosynthetic enzyme tryptophan hydroxylase, tph-1, lack detectable 5HT and accumulate excess numbers of fertilized eggs (Sze et al. 2000). If SER-1 is the receptor through which endogenous 5HT controls egg-laying behavior, ser-1 mutants should show a similar egg-laying defect. We addressed this question by comparing the number of fertilized eggs held in the uterus of wild-type, tph-1, and ser-1 mutant animals (Table 1). A wild-type young adult accumulates ∼10 eggs in the uterus, but age-matched tph-1(mg280) and ser-1(ok345) animals accumulate ∼150% that of wild type (Table 1), showing that SER-1 is required for normal egg-laying behavior. This result also indicates that the reason ser-1 mutant animals fail to lay eggs in response to exogenous 5HT is not because they cannot fertilize eggs, but rather because vulval muscles lack the required receptor to 5HT. The tph-1(mg280);ser-1(ok345) double mutants do not retain significantly more eggs than either of the single mutants, reconfirming that 5HT and ser-1 act in the same pathway and that loss of either receptor or transmitter disables the signaling. tph-1(mg280), ser-1(ok345), and the double-mutant animals are less defective in egg laying than are egl-1 mutants that lack the HSN neurons (Trent et al. 1983; Table 1). The HSN neurons release multiple neurotransmitters and neuropeptides controlling egg-laying behavior (Weinshenker et al. 1995; Waggoner et al. 2000); the relatively mild egg-laying deficit of ser-1 and tph-1 mutants suggests that the ser-1 mutation eliminates vulval muscle response to 5HT but may not significantly affect the response to the other neuronal signals mediated by HSN.
Fluoxetine and imipramine can stimulate egg laying independently of SER-1, SERT, or 5HT:
The finding that ser-1 mutants are unresponsive to 5HT provides us a unique opportunity to explore the genetic targets of SSRIs and tricyclic antidepressants in the egg-laying circuit. It has been established that applying the SSRI fluoxetine or the tricyclic antidepressant imipramine to worms promptly induces egg laying much like 5HT (Trent et al. 1983; Desai and Horvitz 1988; Weinshenker et al. 1995). mod-5 is the only gene in the C. elegans genome encoding a SERT (Ranganathan et al. 2001). Fluoxetine and imipramine have been shown to block endogenous 5HT reuptake by serotonergic neurons in worms (Sze et al. 2002), and this blockade requires MOD-5 (Ranganathan et al. 2001). The null allele mod-5(n3314) diminishes egg-laying responses to imipramine (Figure 3b), indicating that the blockade of MOD-5 is at least a part of the drug's action for inducing egg laying. Furthermore, tph-1(mg280) mutants respond as well as wild type to 5HT, but their responses to fluoxetine and imipramine are ∼50% those of wild type (Figure 3b). Thus, endogenous 5HT must be present for a complete effect of the drugs.
However, if SERT were the only target of the drugs and enhancement of 5HT synaptic levels was their only action, mod-5, tph-1, and ser-1 mutants should not respond to fluoxetine or imipramine. We find, to the contrary, that these mutants retain significant responses to fluoxetine and imipramine, and in particular, that ser-1(ok345) animals that cannot respond to 5HT exhibit a substantial response to fluoxetine and a nearly full response to imipramine (Figure 3). Further, if the drug responses of ser-1 mutants were due to the potentiation of endogenous 5HT acting at residual SER-1(ok345) receptors, we should expect the ser-1 mutants, in the absence of endogenous 5HT, to exhibit a complete drug resistance. To test this possibility, we constructed a double mutant of ser-1(ok345) and tph-1(mg280). We find that the double-mutant animals respond to fluoxetine comparably to ser-1(ok345) or tph-1(mg280) alone (Figure 3c). These observations suggest that fluoxetine can stimulate egg laying via at least two alternative target pathways. One pathway is dependent on endogenous 5HT and SER-1; mutation in either tph-1 or ser-1 would cause an equivalent drug resistance. The other pathway (or pathways) is independent of 5HT or SER-1.
Interestingly, the tph-1;ser-1 double-mutant animals exhibit a response to imipramine similar to the tph-1 mutants. This observation suggests that, like fluoxetine, imipramine can partially induce egg laying independently of endogenous 5HT or SER-1. In addition, imipramine can induce a full egg-laying response in ser-1(ok345) mutants in the presence of endogenous 5HT. Because ser-1(ok345) animals can lay eggs in response to fluoxetine and imipramine, the muscles and other components of the egg-laying apparatus must be functioning in the mutant.
5HT, fluoxetine, and imipramine stimulate egg laying via distinct 5HT receptor pathways:
It has been shown that animals lacking the HSN neurons are resistant to fluoxetine and imipramine (Trent et al. 1983; Weinshenker et al. 1995). This suggests that the drugs function through these major motor neurons connected to the vulval muscles. Because application of 5HT cannot elicit egg laying in ser-1 mutants, fluoxetine and imipramine must activate HSN to release inducing signals distinct from 5HT to stimulate egg laying in ser-1 mutants. To assess if any other 5HT receptors play a role in these actions of fluoxetine and imipramine, we tested the egg-laying responses of ser-4 and mod-1 mutants (Figure 3a). mod-1(ok103) mutants show no detectable defects in responding to any of the drugs (Figure 3a).
ser-4(ok512) mutants show significant resistance to imipramine while responding normally to fluoxetine and 5HT (Figure 3a), indicating that SER-4 is a critical component for imipramine to induce egg laying. ser-4(ok512) is a 1.3-kb in-frame deletion that removes transmembrane segment 5 and a part of the intracellular loop connecting to transmembrane segment 6 (C. elegans Gene Knockout Consortium). Tsalik et al. (2003) have constructed a ser-4::gfp reporter and shown that the GFP is expressed in a number of interneurons in the head region but not in the neurons or muscles directly involved in egg laying. This suggests that SER-4 may mediate a 5HT input distinctly from SER-1 and regulate egg-laying behavior indirectly. To assess whether SER-4 acts in these neurons to mediate the response to imipramine, we expressed the ser-4 cDNA under the control of the ser-4 promoter sequence used in the GFP reporter and introduced the construct into ser-4(ok512) mutants. This transgene can rescue ser-4 (ok512) imipramine sensitivity and mediate an egg-laying response equivalent to that of wild type (Figure 3a). These observations suggest that SER-4 acts upstream in the neuronal circuit to modulate egg-laying behavior. One plausible mechanism could be that imipramine activates the SER-4 signaling pathway, which promotes HSN to release, for example, acetylcholine or neuropeptides that induce egg laying (Weinshenker et al. 1995; Waggoner et al. 2000).
ser-4(ok512) mutants exhibit a significant deficit in response to imipramine but respond normally to 5HT and fluoxetine (Figure 3a). To the contrary, ser-1 mutants cannot respond to 5HT and are defective in response to fluoxetine, but respond normally to imipramine (Figure 3a). Together, these observations suggest that the action of imipramine is distinct from fluoxetine or 5HT. One probable explanation for the discrete effects could be that imipramine and fluoxetine regulate the activity of distinct 5HT receptor pathways independently of 5HT itself. If this is the case, mutations in the signaling components downstream of a specific 5HT receptor subtype should disrupt the response to one drug but not to the other. It has been reported that the Gq protein EGL-30 acts downstream of 5HT to regulate egg laying and the egl-30(ad806) loss-of-function mutants do not lay eggs in response to exogenous 5HT (Bastiani et al. 2003). As the first step in elucidating the signaling pathway acted on by fluoxetine and imipramine in vivo, we compared the egg-laying response of ser-1(ok345) and egl-30(ad806) mutants to 5HT, fluoxetine, and imipramine. Supporting the view that SER-1 is coupled to EGL-30, mutants lacking either ser-1 or egl-30 could not respond to 5HT (Figure 3). However, fluoxetine, which potently stimulates egg laying in ser-1 mutants, fails to induce egg laying in egl-30(ad806) mutants (Figure 3c). This observation is consistent with the report that the EGL-30 acts in both the vulval muscles and the presynaptic neurons to regulate egg laying (Bastiani et al. 2003). The result suggests that EGL-30 acts downstream of SER-1 to mediate the response to 5HT, but that fluoxetine can activate EGL-30 via other receptors independently of SER-1. In contrast, egl-30(ad806) mutants remain substantially responsive to imipramine (Figure 3c). Therefore, egl-30 is not essential for imipramine to stimulate egg laying. Together, these results suggest that EGL-30 is an essential component in two distinct receptor pathways that independently mediate the response to 5HT and fluoxetine and that imipramine can activate a pathway that is independent of EGL-30.
Although phospholipase Cβ (PLCβ) has been the only identified effector of Gq family members in mammals, the only C. elegans PLCβ homolog, EGL-8 (Lackner et al. 1999), is a minor factor downstream of EGL-30 for egg laying (Bastiani et al. 2003). Consistent with this notion, we found that egl-8 mutants exhibit pharmacological profiles distinct from egl-30 mutants: the egl-8 (ok934) deletion mutants are only partially resistant to 5HT and imipramine and have a nearly wild-type response to fluoxetine (Figure 3c). Together, these data indicate discrete mechanisms mediating the action of 5HT, fluoxetine, and imipramine in the egg-laying circuit (Figure 4): The vulval response to 5HT is mediated by the pathway from SER-1 to EGL-30; imipramine can activate a pathway that involves SER-4, EGL-30, and EGL-8; and fluoxetine can activate an EGL-30-mediated signaling cascade independently of SER-1, SER-4, or EGL-8. Because administration of fluoxetine and imipramine, but not 5HT, stimulates egg laying in ser-1 mutants, fluoxetine and imipramine must be capable of activating these pathways more efficiently than 5HT.
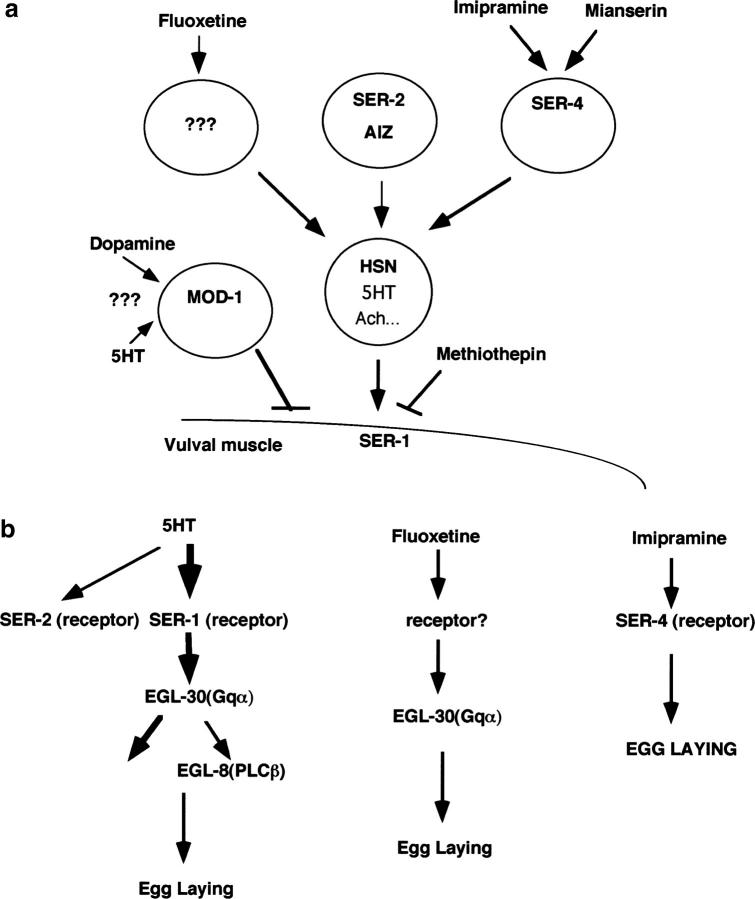
Figure 4.—
Model for 5HT receptor inputs to egg-laying behavior. (a) The role of the 5HT receptor subtypes in the egg-laying circuit. SER-1 acts postsynaptically and couples 5HT signals from HSN into vulval muscle contraction. SER-2 is a putative tyramine receptor and is expressed in the AIZ and BDU interneurons (Tsalik et al. 2003) that receive synaptic inputs from many sensory neurons (White et al. 1986). SER-2 might transduce sensory inputs about the environment and modulate the release of acetylcholine and neuropeptides from HSN to influence the efficacy of 5HT. SER-4 is expressed in the neurons adjacent to the serotonergic neurons ADF, AIM, and RIH in the head region (Tsalik et al. 2003) and is required for a full egg-laying response to imipramine. Because egl-1 mutants, which lack the HSN neurons, are strongly resistant to fluoxetine and imipramine (Trent et al. 1983; Figure 3c), fluoxetine and imipramine act via HSN. mod-1 is required for dopamine to antagonize 5HT action and this mod-1-mediated inhibitory action is independent of that of HSN and SER-1 (Figure 6). (b) Molecular pathways of the 5HT receptors. EGL-30 must be activated to produce an egg-laying response to 5HT, implying that SER-1 signaling is coupled to Gq. Fluoxetine can stimulate egg laying in ser-1 mutants but not in egl-30 mutants, indicatingt hat fluoxetine can activate EGL-30 via non-SER-1 receptors. Because the egl-8 deletion mutation affects the response to 5HT but not to fluoxetine, EGL-8 is an effector of 5HT but is not required for the action of fluoxetine. ser-4 deletion mutation confers resistance to imipramine but does not affect the response to fluoxetine; SER-4 may be a target of imipramine.
Antagonists to mammalian 5HT1 and 5HT2 receptor subtypes modulate egg laying via SER-1 and SER-4:
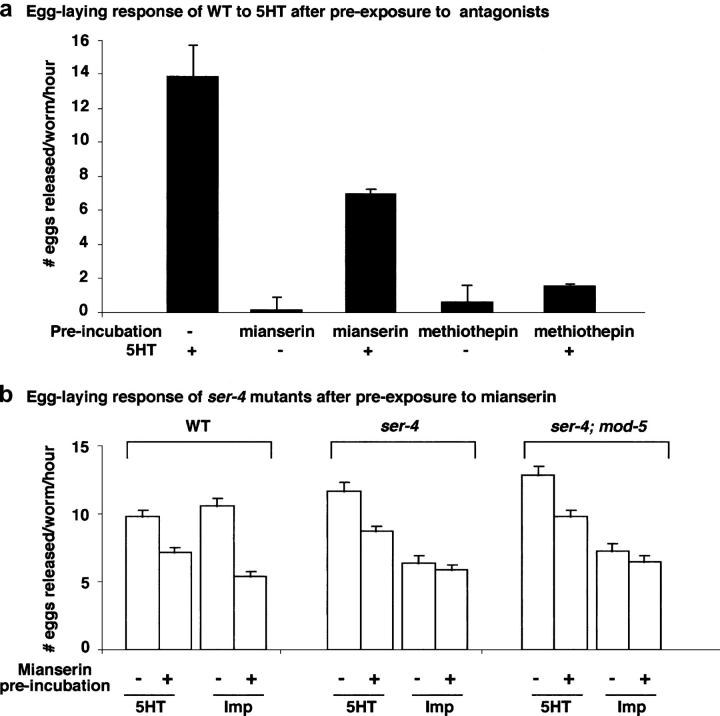
In cultured mammalian cells, cloned SER-1 and SER-4 exhibit characteristic pharmacological profiles of mammalian 5HT1 and 5HT2 (Olde and McCombie 1997; Hamdan et al. 1999). As one step toward understanding the genetics underlying pharmacological action and interaction, we tested the effect of ligands to mammalian 5HT1 and 5HT2 on egg-laying behavior. Preincubation of wild-type worms with the mammalian 5HT1/2 antagonist methiothepin completely blocks egg-laying response to 5HT (Figure 5a). Because SER-1 activity is required for 5HT to stimulate egg laying, methiothepin most likely competes with 5HT action at SER-1.
Figure 5.—
Egg-laying response of ser-1 and ser-2 mutants to ligands of mammalian 5HT1 and 5HT2a subtype receptors. (a) The 5HT1-like receptor antagonist methiothepin competes with 5HT in wild type. Applying 5 mg/ml to worms stimulates egg laying, but this 5HT stimulation is dramatically diminished when worms are preincubated on a plate containing 50 μm methiothepin for 6 hr. However, preincubation of worms with 5HT2a ligand mianserin has much less of an effect on 5HT-stimulated egg-laying behavior. (b) SER-4 is a common target of mianserin and imipramine. Wild-type animals that are preincubated on a plate containing 20 μm mianserin for 6 hr exhibit a reduced egg-laying response to 5HT and imipramine, indicating that mianserin competes with the action of both 5HT and imipramine in the egg-laying circuit. The ser-4(ok512) mutants have a normal response to 5HT but exhibit a reduced response to imipramine-induced egg laying. Preexposure of ser-4(ok512) mutants with mianserin causes a reduction in the 5HT response but does not cause a further reduction in the response to imipramine. Note that the wild-type animals preexposed to mianserin responded to imipramine comparably to ser-4(ok512) mutants or ser-4; mod-5 double-mutant animals without the mianserin treatment. Thus, mianserin can compete with 5HT and imipramine independently, and SER-4 is required for mianserin to compete with imipramine. Each bar represents the mean of three assays, eight animals tested/strain/drug/assay ±SEM. M9 buffer was used as the control each time for each strain, and the average of eggs released in M9 is between 0.6 and 1.8.
Preincubation of wild-type animals with the mammalian 5HT2 ligand mianserin partially blocks the egg-laying response to 5HT as well as to imipramine (Figure 5b). Interestingly, wild-type animals preexposed to mianserin for 6 hr exhibit an egg-laying response to imipramine comparable to ser-4(ok512) mutants without mianserin treatment (Figure 5b). A parsimonious interpretation would be that mianserin inhibits the action of imipramine at SER-4. If this should be the case, then ser-4 mutants should not respond to mianserin. To test this possibility, we preexposed ser-4(ok512) mutants with mianserin for 6 hr and tested their egg-laying response to imipramine. The mianserin treatment does not further reduce imipramine response in ser-4(ok512) mutants (Figure 5b). However, the ser-4(ok512) mutation does not significantly affect the ability of mianserin to block 5HT action (Figure 5b). These results further indicate that signaling from SER-4 and SER-1 represents two different mechanisms in the control of egg-laying behavior and that imipramine can regulate the egg-laying circuit via the SER-1 signaling pathway.
A mutation in the ionotropic 5HT receptor MOD-1 blocks dopamine action:
Extensive evidence from neuroanatomical, pharmacological, and behavioral studies in rats indicates that dopamine function may be either enhanced or blocked by a host of drugs that target specific 5HT receptor subtypes (Gillies et al. 1996; Lejeune and Millan 1998; Miner et al. 2000; Di Matteo et al. 2001). To explore the genetic basis of the interaction between 5HT and dopamine signaling, we tested the effect of mutations in individual 5HT receptor subtypes on dopamine action in egg-laying behavior.
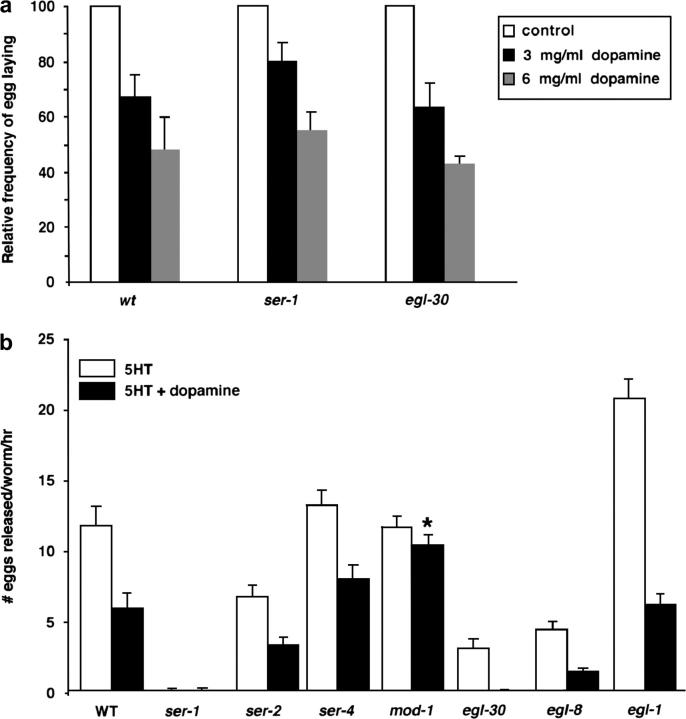
Activation of egg laying by food and 5HT can be inhibited by exogenous dopamine (Schafer and Kenyon 1995). One possible mechanism would be that dopamine signaling interdicts the response to 5HT by inhibiting SER-1 activity. In this model ser-1 and egl-30 mutants should no longer be sensitive to dopamine. Because ser-1 and egl-30 mutants are resistant to 5HT, we examined the effect of dopamine on the egg-laying behavior stimulated by food. Wild-type, ser-1(ok345), and egl-30(ad806) animals were incubated on culture plates containing dopamine at 3 and 6 mg/ml for 2 hr, and the rate of egg laying was compared to that of their age-matched untreated siblings. Dopamine caused an equivalent reduction in the rate of egg laying of wild-type and ser-1 or egl-30 mutants (Figure 6a). Thus, dopamine inhibits egg laying via a pathway independent of SER-1.
Figure 6.—
Egg-laying response of 5HT-signaling mutants to dopamine. (a) Dose response to dopamine inhibition of egg laying is not affected by loss-of-function alleles of ser-1 and egl-30. Young adult animals were placed on plates containing 3 or 6 mg/ml dopamine and seeded with food for 2 hr, and the number of eggs laid during the second hour was scored as described in materials and methods. The data represent the average of the number of eggs released per worm as a percentage of the eggs laid by their untreated siblings assayed at the same time. No difference was found between the egg-laying patterns of strains assayed (chi-square test, P > 0.05). Each bar represents the mean of two or more assays ±SEM. (b) The mod-1(ok103) deletion mutation blocks dopamine inhibition of egg laying. Mutants that are resistant to 5HT and egl-1 mutants that lack the egg-laying serotonergic neurons HSN are fully sensitive to dopamine. The asterisk indicates no significant difference in the egg-laying response of the mod-1(ok103) mutants to 5HT alone and to 5HT plus dopamine (Student's t-test, P > 0.05). Dopamine does not induce egg laying in any of the strains. Each bar represents the mean of three assays ±SEM. M9 buffer and dopamine alone were used as the controls each time; all the strains laid, on average, less than one egg.
To assess the role of 5HT receptors in functional interaction between 5HT and dopamine signaling, we compared egg-laying responses to 5HT alone with that to 5HT plus dopamine in mutants with loss-of-function alleles of 5HT receptors or of components of Gq signaling pathways. Dopamine inhibits the 5HT action in all of the mutants except the mod-1(ok103) deletion mutants (Figure 6b), indicating that MOD-1 must be active for dopamine to inhibit egg laying. To determine if MOD-1 activity normally couples food signals and egg-laying behavior, we compared egg-laying behavior of wild-type and mod-1(ok103) mutants in response to starvation. We transferred well-fed animals onto a fresh culture plate with or without bacterial food and counted the number of eggs released 1 hr later. On average, wild-type animals on the plate without food laid 68% fewer eggs than their fed sibling (N = 45/condition), whereas food-deprived mod-1(ok103) animals laid only 33% fewer eggs than their fed sibling (N = 40/condition). Prolonged starvation causes both wild-type and mod-1 mutant animals to cease egg laying. These observations are consistent with the reports that MOD-1 is involved in a pathway that modulates locomotory behavior in response to brief food deprivation (Sawin et al. 2000; Ranganathan et al. 2001) and suggest that MOD-1 also modulates the egg-laying circuit. mod-1 encodes a 5HT-gated ion channel and is expressed in many neurons but not in muscles (Ranganathan et al. 2000). Modulation by MOD-1 of dopamine function in C. elegans egg laying may be analogous to the role played by the mammalian 5HT-gated channel 5HT3 in the mesolimbic system, where activation of 5HT3 receptors results in an increase in dopaminergic activity and 5HT3 antagonists block dopamine function (Carboni et al. 1989; Costall et al. 1990; Pei et al. 1993; Gillies et al. 1996).
DISCUSSION
The egg-laying circuit in C. elegans represents a part of the modulatory system by which the animal adjusts its behavior in response to environmental signals for self-preservation. Our previous study indicated that endogenous 5HT controls the rate of egg laying (Sze et al. 2000). In this study, we have extended that work by identification of three 5HT receptors, which each play a unique role in modulating egg-laying behavior (Figure 4). The metabotropic receptor SER-1 acts predominantly in the vulval muscles and is essential for activation of egg laying following 5HT stimulation. The metabotropic receptor SER-4 is expressed in neurons near the nerve ring, but not in the vulval muscle (Tsalik et al. 2003), and therefore is likely to couple 5HT signals from the nerve ring to those mediated by other neurotransmitters and neuromodulators, ultimately acting through the HSN neurons, the motor neurons to the vulva. The ionotropic receptor MOD-1 is required for the inhibitory response to dopamine. The specificity of the function and distinct pharmacological properties of each 5HT receptor subtype identified through this study should provide an experimentally tractable system in which to define the molecular mechanisms of individual 5HT receptor subtypes, to explore the interaction between 5HT and other neuronal signaling pathways, and to integrate a role of the 5HT receptor subtypes into the overall therapeutic efficacy of antidepressants in vivo.
The role of 5HT receptors in the action of antidepressants:
Using C. elegans egg-laying behavior as a model, our data provide genetic evidence that pharmacological actions at 5HT receptors and at SERT are two separable components of the in vivo response to both fluoxetine and imipramine. We and others have shown that fluoxetine and imipramine can block 5HT uptake by serotonergic neurons and that this blockage requires the SERT protein MOD-5 (Ranganathan et al. 2001; Sze et al. 2002). We show in this study that a complete action of fluoxetine and imipramine is dependent on endogenous 5HT and that a full action of imipramine requires MOD-5 (Figure 3b). Thus, one mechanism by which fluoxetine and imipramine induce egg laying is to block MOD-5 from the reuptake of endogenous 5HT. This leads to an increase of synaptic 5HT, much as if the worms were treated with exogenous 5HT. This mechanism is very similar to the classic and well-established role of SSRIs and tricyclic antidepressants as inhibitors of SERT in mammals (Baldessarini 1996).
While C. elegans shows a major site of antidepressant action similar to that well established in mammals, stimulation of egg laying by fluoxetine and imipramine in tph-1, mod-5, and ser-1 mutants (Figure 3) argues persuasively for a second mechanism of drug action, which is independent of synaptic 5HT. The distinct drug resistance profiles displayed by ser-1, ser-2, ser-4, egl-30, and egl-8 mutants (Figure 3) suggest that the alternative targets for these drugs are distinct G-protein-coupled receptors. Because of the extensive structural and functional conservation of the 5HT receptors across phyla, the 5HT-independent actions of these drugs at 5HT receptors are also likely to be conserved. Indeed, extensive pharmacological evidence indicates that SSRIs and tricyclic antidepressants can bind to distinct mammalian 5HT receptor subtypes and modulate receptor activity in cultured cells (Pitt et al. 1994; Baldessarini 1996; Blier and de Montigny 1998; Kroeze and Roth 1998). Our data show that such actions occur in vivo, can influence a 5HT-modulated behavior, and require specific signaling components for individual antidepressants.
How may the identification of the action of antidepressants at 5HT receptor subtypes add to our understanding of their therapeutic efficacy? The most striking result is that the ser-1(ok345) mutants completely fail to respond to exogenous 5HT but lay eggs if fluoxetine or imipramine is provided even in the absence of endogenous 5HT (Figure 3b). This observation shows that these drugs do not simply increase 5HT synaptic levels, but rather that the drugs can activate the targets even more potently than 5HT itself and can enhance signals other than 5HT to stimulate egg laying. Therefore, the receptor, instead of SERT, could be the major determinant of antidepressant action. This perspective provides a novel insight into the therapeutic efficacy of these drugs and suggests that SSRIs and tricyclic antidepressants may correct a 5HT-regulated behavior in mutants that cannot produce or respond to 5HT.
The ionotropic 5HT receptor MOD-1 is required for dopamine action:
The dopamine-resistance observed in mod-1(ok103) mutants has two implications. First, it provides genetic evidence that a 5HT receptor is an intermediary of dopamine signaling. Second, this result suggests that 5HT exerts both stimulatory and inhibitory roles in controlling a behavior by acting through different receptor subtypes: the pathways from SER-1 and SER-4 act in concert to activate vulval muscle contraction, and the pathway from MOD-1 inhibits it.
The inhibitory signaling from dopamine MOD-1 and stimulatory signaling from 5HT, fluoxetine, and imipramine are mediated by different cellular pathways. The action of both fluoxetine and imipramine requires the HSN neurons, and egg-laying response to 5HT requires SER-1. However, egl-1(n487) mutants (Trent et al. 1983; Schafer and Kenyon 1995; Weinshenker et al. 1995), which lack the HSN neurons, and ser-1 mutants respond strongly to dopamine inhibition (Figure 6a). Thus, HSN specifically mediates stimulatory 5HT signaling; inhibitory signaling is transduced by MOD-1 in a different cellular pathway. It has been proposed that the VC neurons may have a role in food-deprivation-induced locomotion behavioral change (Sawin et al. 2000). The VC neurons are connected to the vulval muscles as well as to the HSN neurons (White et al. 1986), and VC neurons release acetylcholine to inhibit egg laying (Bany et al. 2003). It is possible that MOD-1 acts through the VC neurons to modulate the inhibitory signaling. Further experiments are necessary to determine the role of MOD-1 in the dopamine signaling pathway. Interaction between the serotonergic and dopaminergic systems is believed to be a basic mechanism for synaptic plasticity underlying behavior, emotion, and learning. Disruption of the balance between dopamine and 5HT systems has been implicated in cocaine addiction, cognitive dysfunction, and in both the positive and negative symptoms of schizophrenia (Di Matteo et al. 1999; Blows 2000; Czoty et al. 2002; Mateo et al. 2003). It is therefore possible that a human MOD-1 homolog is dysregulated, perhaps mutated, in some of those patients.
Acknowledgments
We thank J. Gargus for critical reading of the manuscript; R. Horvitz, T. Stiernagle, and the Caenorhabditis Genetics Center for worm strains; and the C. elegans Knockout Consortium for deletion mutants. We are particularly grateful to W. Miles for providing us with fluoxetine. This work is supported by grants from the Whitehall Foundation and the National Institutes of Health (NIH) to J.Y.S; G.B. was supported by the NIH minority biomedical research support program.
References
- Baldessarini, R. J., 1996 Drugs and the treatment of psychiatric disorders, pp. 249–263 in Goodman & Gilman's Pharmacological Basis of Therapeutics, Ed. 9, edited by Hardman et al. McGraw-Hill, New York.
- Bany, I. A., M. Q. Dong and M. R. Koelle, 2003. Genetic and cellular basis for acetylcholine inhibition of Caenorhabditis elegans egg-laying behavior. J. Neurosci. 23: 8060–8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann, C., and I. Mori, 1997 Chemotaxis and thermotaxis, pp. 717–737 in C. elegans II, edited by D. L. Riddle et al. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- Bastiani, C. A., S. Gharib, M. I. Simon and P. W. Sternberg, 2003. Caenorhabditis elegans Galphaq regulates egg-laying behavior via a PLCβ-independent and 5HT-dependent signaling pathway and likely functions both in the nervous system and in muscle. Genetics 165: 1805–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier, P., and C. De Montigny, 1998. Possible serotonergic mechanisms underlying the antidepressant and anti-obsessive-compulsive disorder responses. Biol. Psychiatry 44: 313–323. [DOI] [PubMed] [Google Scholar]
- Blows, W. T., 2000. The neurobiology of antidepressants. J. Neurosci. Nurs. 32: 177–180. [DOI] [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundage, L., L. Avery, A. Katz, U. J. Kim, J. E. Mendel et al., 1996. Mutations in a C. elegans Gqalpha gene disrupt movement, egg laying and viability. Neuron 16: 999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni, E., E. Acquas, R. Frau and G. Di Chiara, 1989. Differential inhibitory effects of a 5–HT3 antagonist on drug-induced stimulation of dopamine release. Eur. J. Pharmacol. 164: 515–519. [DOI] [PubMed] [Google Scholar]
- Costall, B., A. M. Domeney and R. J. Naylor, 1990. 5–HT3 receptor antagonists attenuate dopamine-induced hyperactivity in the rat. Neuroreport 1: 77–80. [DOI] [PubMed] [Google Scholar]
- Czoty, P. W., B. C. Ginsburg and L. L. Howell, 2002. Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J. Pharmacol. Exp. Ther. 300: 831–837. [DOI] [PubMed] [Google Scholar]
- Desai, C., and H. R. Horvitz, 1988. Caenorhabditis elegans mutants defective in the functioning of the motor neurons responsible for egg laying. Genetics 121: 703–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai, C., G. Garriga, S. L. McIntire and H. R. Horvitz, 1988. A genetic pathway for the development of the Caenorhabditis elegans HSN motor neurons. Nature 336: 638–646. [DOI] [PubMed] [Google Scholar]
- Di Matteo, V., G. Di Giovanni, M. Di Mascio and E. Esposito, 1999. SB 242 084, a selective serotonin(2C) receptor antagonist, increases dopaminergic transmission in the mesolimbic system. Neuropharmacology 38: 1195–1205. [DOI] [PubMed] [Google Scholar]
- Di Matteo, V., A. De Blasi, C. Di Giulio and E. Esposito, 2001. Role of 5-HT(2C) receptors in the control of central dopamine function. Trends Pharmacol. Sci. 22: 229–232. [DOI] [PubMed] [Google Scholar]
- Gillies, D. M., E. J. Mylecharane and D. M. Jackson, 1996. Effects of 5–HT3 receptor-selective agents on locomotor activity in rats following injection into the nucleus accumbens and the ventral tegmental area. Eur. J. Pharmacol. 303: 1–12. [DOI] [PubMed] [Google Scholar]
- Goodman, L. S., A. Gilman, J. G. Hardman, A. G. Gilman and L. E. Limbird, 1996 Goodman & Gilman's the Pharmacological Basis of Therapeutics. McGraw-Hill, New York.
- Hamdan, F. F., M. D. Ungrin, M. Abramovitz and P. Ribeiro, 1999. Characterization of a novel 5HT receptor from Caenorhabditis elegans: cloning and expression of two splice variants. J. Neurochem. 72: 1372–1383. [DOI] [PubMed] [Google Scholar]
- Horvitz, H. R., M. Chalfie, C. Trent, J. E. Sulston and P. D. Evans, 1982. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science 216: 1012–1014. [DOI] [PubMed] [Google Scholar]
- Iwasaki, K., D. W. Liu and J. H. Thomas, 1995. Genes that control a temperature-compensated ultradian clock in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 92: 10317–10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeze, W. K., and R. L. Roth, 1998. The molecular biology of 5HT receptors: therapeutic implications for the interface of mood and psychosis. Biol. Psychiatry 44: 1128–1142. [DOI] [PubMed] [Google Scholar]
- Lackner, M. R., S. J. Nurrish and J. M. Kaplan, 1999. Facilitation of synaptic transmission by EGL-30 Gqalpha and EGL-8 PLCbeta: DAG binding to UNC-13 is required to stimulate acetylcholine release. Neuron 24: 335–346. [DOI] [PubMed] [Google Scholar]
- Lejeune, F., and M. J. Millan, 1998. Induction of burst firing in ventral tegmental area dopaminergic neurons by activation of 5HT (5-HT)1A receptors: WAY 100,635-reversible actions of the highly selective ligands, flesinoxan and S 15535. Synapse 30: 172–180. [DOI] [PubMed] [Google Scholar]
- Maduro, M., and D. Pilgrim, 1995. Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics 141: 977–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo, Y., E. A. Budygin, C. E. John and S. R. Jones, 2004. Role of serotonin in cocaine effects in mice with reduced dopamine transporter function. Proc. Natl. Acad. Sci. USA 101: 372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner, L. H., S. Schroeter, R. D. Blakely and S. R. Sesack, 2000. Ultrastructural localization of the 5HT transporter in superficial and deep layers of the rat prelimbic prefrontal cortex and its spatial relationship to dopamine terminals. J. Comp. Neurol. 427: 220–234. [DOI] [PubMed] [Google Scholar]
- Nurrish, S., L. Segalat and J. M. Kaplan, 1999. 5HT inhibition of synaptic transmission: Galpha(0) decreases the abundance of UNC-13 at release sites. Neuron 24: 231–242. [DOI] [PubMed] [Google Scholar]
- Olde, B., and W. R. McCombie, 1997. Molecular cloning and functional expression of a 5HT receptor from Caenorhabditis elegans. J. Mol. Neurosci. 8: 53–62. [DOI] [PubMed] [Google Scholar]
- Pei, Q., T. Zetterstrom, R. A. Leslie and D. G. Grahame-Smith, 1993. 5–HT3 receptor antagonists inhibit morphine-induced stimulation of mesolimbic dopamine release and function in the rat. Eur. J. Pharmacol. 230: 63–68. [DOI] [PubMed] [Google Scholar]
- Pitt, B. R., S. A. R. Weng, R. D. Blakely, I. Reynolds and P. Davies, 1994. 5HT increases DNA synthesis in rat proximal and distal pulmonary vascular smooth muscle cells in culture. Am. J. Physiol. 266: L178–L186. [DOI] [PubMed] [Google Scholar]
- Ranganathan, R., S. C. Cannon and H. R. Horvitz, 2000. MOD-1 is a 5HT-gated chloride channel that modulates locomotory behaviour in C. elegans. Nature 408: 470–475. [DOI] [PubMed] [Google Scholar]
- Ranganathan, R., E. R. Sawin, C. Trent and H. R. Horvitz, 2001. Mutations in the Caenorhabditis elegans 5HT reuptake transporter MOD-5 reveal 5HT-dependent and -independent activities of fluoxetine. J Neurosci. 21: 5871–5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex, E., and R. W. Komuniecki, 2002. Characterization of a tyramine receptor from Caenorhabditis elegans. J. Neurochem. 82: 1352–1359. [DOI] [PubMed] [Google Scholar]
- Riddle, D. L., 1997 C. elegans II. Cold Spring Harbor Laboratory Press, Plainview, NY. [PubMed]
- Sawin, E. R., 1996 Genetic and cellular analysis of modulated behaviors in Caenorhabditis elegans. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA.
- Sawin, E. R., R. Ranganathan and H. R. Horvitz, 2000. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26: 619–631. [DOI] [PubMed] [Google Scholar]
- Schafer, W. R., and C. J. A. Kenyon, 1995. Calcium-channel homologue required for adaptation to dopamine and 5HT in Caenorhabditis elegans. Nature 375: 73–78. [DOI] [PubMed] [Google Scholar]
- Schafer, W. R., B. M. Sanchez and C. J. Kenyon, 1996. Genes affecting sensitivity to serotonin in Caenorhabditis elegans. Genetics 143: 1219–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkmann, K., and C. Li, 1992. Localization of FMRFamide-like peptides in Caenorhabditis elegans. J. Comp. Neurol. 316: 251–260. [DOI] [PubMed] [Google Scholar]
- Sulston, J. E., E. Schierenberg, J. G. White and J. N. Thomson, 1983. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100: 64–119. [DOI] [PubMed] [Google Scholar]
- Sze, J. Y., M. Victor, C. Loer, Y. Shi and G. Ruvkun, 2000. Food and metabolic signalling defects in a Caenorhabditis elegans 5HT-synthesis mutant. Nature 403: 560–564. [DOI] [PubMed] [Google Scholar]
- Sze, J. Y., S. Zhang, J. Li and G. Ruvkun, 2002. The C. elegans POU-domain transcription factor UNC-86 regulates the tph-1 tryptophan hydroxylase gene and neurite outgrowth in specific serotonergic neurons. Development 129: 3901–3911. [DOI] [PubMed] [Google Scholar]
- Tabuse, Y., T. Sano, K. Nishiwaki and J. Miwa, 1995. Molecular evidence for the direct involvement of a protein kinase C in developmental and behavioural susceptibility to tumour-promoting phorbol esters in Caenorhabditis elegans. Biochem. J. 312: 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent, C., N. Tsuing and H. R. Horvitz, 1983. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics 104: 619–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsalik, E. L., T. Niacaris, A. S. Wenick, K. Pau, L. Avery et al., 2003. LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev. Biol. 263: 81–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venolia, L., W. Ao, S. Kim, C. Kim and D. Pilgrim, 1999. unc-45 gene of Caenorhabditis elegans encodes a muscle-specific tetratricopeptide repeat-containing protein. Cell Motil. Cytoskeleton 42: 163–177. [DOI] [PubMed] [Google Scholar]
- Waggoner, L. E., G. T. Zhou and W. R. Schafer, 1998. Control of alternative behavioral states by 5HT in Caenorhabditis elegans. Neuron 21: 203–214. [DOI] [PubMed] [Google Scholar]
- Waggoner, L. E., L. A. Hardaker, S. Golik and W. R. Schafer, 2000. Effect of a neuropeptide gene on behavioral states in Caenorhabditis elegans egg-laying. Genetics 154: 1181–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker, D., G. Garriga and J. H. Thomas, 1995. Genetic and pharmacological analysis of neurotransmitters controlling egg laying in C. elegans. J. Neurosci.. 15: 6975–6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, J. G., E. Southgate, J. N. Thomson and S. Brenner, 1986. The structure of the nervous system of the nematode C. elegans. Phil. Trans. R. Soc. Lond. B Biol. Sci. 314: 1–340. [DOI] [PubMed] [Google Scholar]