Abstract
Flagellar length is tightly regulated in the biflagellate alga Chlamydomonas reinhardtii. Several genes required for control of flagellar length have been identified, including LF1, a gene required to assemble normal-length flagella. The lf1 mutation causes cells to assemble extra-long flagella and to regenerate flagella very slowly after amputation. Here we describe the positional cloning and molecular characterization of the LF1 gene using a bacterial artificial chromosome (BAC) library. LF1 encodes a protein of 804 amino acids with no obvious sequence homologs in other organisms. The single LF1 mutant allele is caused by a transversion that produces an amber stop at codon 87. Rescue of the lf1 phenotype upon transformation was obtained with clones containing the complete LF1 gene as well as clones that lack the last two exons of the gene, indicating that only the amino-terminal portion of the LF1 gene product (LF1p) is required for function. Although LF1 helps regulate flagellar length, the LF1p localizes almost exclusively in the cell body, with <1% of total cellular LF1p localizing to the flagella.
CILIA and flagella are found on a variety of cell types such as sperm and respiratory epithelial cells, where they function to propel cells through fluid or to move fluid over the cell surface. Recently, an unexpected role for cilia and flagella in left/right axis determination during embryonic development was found by examining mice with mutations in KIF3, a gene required for maintenance and assembly of the embryonic nodal cilia (Nonaka et al. 1998; reviewed by Hirokawa 2000; Wagner and Yost 2000). Other human diseases, such as primary ciliary dyskinesia (or immotile cilia syndrome) and polycystic kidney disease have been associated with defects in cilia or flagella (Pazour and Rosenbaum 2002; reviewed by Zein et al. 2003). Assembly of cilia or flagella to a defined length is critical for normal functioning of these structures. How cells monitor and maintain the length of their cilia or flagella is unknown.
Flagellar length in the unicellular, biflagellate, green alga Chlamydomonas reinhardtii is tightly regulated. Wild-type cells display a narrow distribution of flagellar lengths between 10 and 15 μm, never exceeding 16 μm. Mutants with abnormal flagellar length include both long-flagella (lf) mutants, with flagella up to three times the length of wild-type cells, and short-flagella (shf) mutants, with flagella approximately one-half the length of wild-type cells (Mcvittie 1972a; Jarvik et al. 1984; Kuchka and Jarvik 1987; Barsel et al. 1988; Asleson and Lefebvre 1998).
Chlamydomonas cells actively maintain two flagella of equal length. Within 2 hr after amputation, wild-type cells regrow their flagella to predeflagellation lengths. If one of the two flagella is severed, the remaining flagellum immediately begins to shorten while the amputated flagellum begins to regrow (Rosenbaum et al. 1969). This simultaneous shortening and lengthening of the flagella continues until the two flagella reach the same length; the two flagella then grow out together to the wild-type length. The control of flagellar equality is further demonstrated in the null mutants of LF3 that have an unequal-length-flagella (Ulf) phenotype; these mutants are mostly flagella-less, but under certain growth conditions, two flagella of unequal length are produced (Tam and Lefebvre 1993; Tam et al. 2003). The null lf3 mutants have lost the ability to enforce the equality of length between the two flagella.
The most striking demonstration of the dynamic nature of flagellar length control is the behavior of lf or shf mutants during mating. Chlamydomonas gametes fuse to form a temporary dikaryon cell with four flagella. When a wild-type cell is mated to an shf mutant, the two short flagella rapidly elongate to wild-type length (Kuchka and Jarvik 1987). When a wild-type cell is mated to an lf mutant, the two long flagella shorten to wild-type length within 15 min (Starling and Randall 1971). Even though 20–40 μm of flagellar material is resorbed into the dikaryon cell in this experiment, the wild-type flagella do not lengthen, implying that the growth of flagella is actively limited rather than being a passive response to the pool size of flagellar proteins. These observations demonstrate that a mechanism contributed by the cytoplasm of the wild-type gametic cell recognizes abnormal-length flagella and restores length control.
In Chlamydomonas, four long-flagella genes (LF1, LF2, LF3, and LF4) have been identified that can be mutated to produce cells with abnormally long flagella (Mcvittie 1972a; Barsel et al. 1988; Asleson and Lefebvre 1998). Within a population of lf cells, the flagellar length distribution is broad, and the flagella can reach lengths three times that of wild-type cells. Some lf mutants, including lf1 and three of the five alleles of lf2, also demonstrate a severe defect in regenerating flagella. These strains do not fully regenerate their flagella up to 24 hr after amputation (Mcvittie 1972a; Barsel et al. 1988). Genetic analysis of double and triple mutants of LF1, LF2, and LF3 suggests that these genes lie in the same regulatory pathway. The double mutants, lf1 lf2, lf2 lf3, and lf1 lf3, exhibit a synthetic, flagella-less phenotype (Barsel et al. 1988). This phenotype is dependent on the action of the wild-type LF4 gene product as demonstrated by the observation that triple mutants of lf4 lf1 and lf2 have long flagella (Asleson and Lefebvre 1998).
The mechanisms that regulate flagellar length are being identified by cloning and characterizing each of the genes that can be mutated to produce a long-flagella phenotype. The LF4 gene product is a MAP kinase with high sequence similarity to a mammalian MAP kinase of unknown function called MOK (Berman et al. 2003). The LF3 gene product is a large (133 kD) protein with no sequence homologs in other organisms. LF3p localizes to small foci scattered throughout the cytoplasm, with little or no LF3p localizing to the flagella (Tam et al. 2003). LF2 encodes a cytoplasmically localized serine-threonine protein kinase (L. W. Tam, N. W. Wilson and P. A. Lefebvre, unpublished observations).
In this report we describe the cloning and characterization of the first length control gene to be identified genetically, LF1. The only LF1 mutant allele, lf1, was identified in a motility screen following chemical mutagenesis (Mcvittie 1972a). We used a positional cloning approach employing an indexed bacterial artificial chromosome (BAC) library to clone the LF1 gene. LF1 encodes a novel protein found predominately in the cytoplasm in discrete foci similar to those containing LF3p. A very small amount of LF1p (<1%) localizes to the flagella.
MATERIAL AND METHODS
Strains and culture conditions:
C. reinhardtii wild-type strain 137c (CC125) and lf1 strain (CC802) are available through the Chlamydomonas Genetics Center (Duke University, Durham, NC). Strain A-A6 (lf1 arg7), was constructed using parental strains lf1 (CC802) and arg7 and used for transformation rescue experiments. In strains 1-F10 and F7 the lf1 phenotype was rescued by transformation with BAC clones. Strain 1-F10 was used for RNA analysis and strain F7 was used for flagellar regeneration and flagellar length measurements. Phenotypically rescued strains E1, G9, G12, and G2 express an LF1 protein tagged with a triple hemagglutinin (HA) epitope. Strains were grown in liquid media with aeration on a 14-hr light:10-hr dark cycle at 24° in either minimal M media (Sager and Granick 1953) or Tris acetate/phosphate media (TAP; Harris 1989) supplemented with 0.02% arginine when needed. Cultures were maintained on solid agar media (1.2%) in constant light.
Flagellar length measurements:
Cells fixed with 0.5% glutaraldehyde were observed using differential interference contrast (DIC) microscopy. Images were captured with a video camera and Scion Image 1.59 software, and flagellar lengths were measured using PhotoShop 5.5 and Scion Image 1.59 software. For flagellar regeneration experiments, cells were deflagellated using homogenization with a hand-held blender for 3 min. Deflagellation was confirmed by phase contrast microscopy. Cell samples taken before deflagellation, immediately following deflagellation, and at time points after deflagellation (30, 60, 90, 120, and 180 min) were fixed in 0.5% glutaraldehyde, and the lengths of 100 random flagella were measured per time point.
Cell swimming velocity measurements:
Swimming velocities from wild-type (137c) and lf1 mutant cells were measured as described by Sakakibara et al. (1993). Average swimming velocity measurements were obtained for 25–30 samples per strain.
Chlamydomonas transformation:
An lf1 arg7 (A-A6) double-mutant strain was cotransformed with plasmid pARG7.8 DNA containing the complete argininosuccinate lyase gene (Debuchy et al. 1989) and BAC or plasmid DNA using the glass bead method (Kindle 1990). Cells (5 × 107) were treated with Chlamydomonas autolysin for 45 min at room temperature under bright light. Cells were lightly pelleted and resuspended in 0.3 ml TAP media. Two to five micrograms of pARG7.8 DNA, 5–10 μg of BAC DNA (or 2–5 μg plasmid DNA), 5% PEG 8000, and 0.3 ml acid-treated glass beads were added to cells and vortexed together at top speed for 45 sec. TAP media (1 ml) was added to cells, and the entire volume of cells was plated on 1.2% TAP agar plates and placed in bright, constant light for 1 week. Transformed Arg+ colonies were picked individually into liquid M media and screened for rescue of the long-flagella phenotype using a Zeiss stereomicroscope to screen for normal swimming motility. The cotransformation efficiency rate was ∼1–5% using BAC clones.
Cloning and sequencing of LF1:
DNA probes Dhc4 and Dhc5 (kindly provided by Mary Porter, University of Minnesota, Minneapolis), GSP1, and GP225 were labeled with [32P]dCTP by random primer labeling to use as hybridization probes for screening a BAC library of Chlamydomonas genomic DNA (available from the Clemson University Genomics Institute, Clemson, SC). Comparing restriction patterns of BAC clones digested with HindIII and BamHI, we identified BAC end fragments; fragments that were not common between BAC clones were assumed to be at the end of the BAC clone. BAC end fragments were purified from 0.8% TAE agarose gels using the GeneCleanII kit (Bio101 Systems, Vista, CA) and prepared for hybridization by random primer labeling. The smallest fragment found to be unique to a BAC clone was used to screen the library. Hybridization of the BAC library filters was performed at 42°, overnight, in 50% formamide, 5× SSPE, 10× Denhardt's, 1% SDS, and 300 μg/ml salmon sperm DNA followed by one wash at 42° in 2× SSPE, 1% SDS buffer, and three washes at 65° in 0.2× SSPE, 0.2% SDS buffer (Sambrook et al. 1989). DNA from BAC clones hybridizing to the labeled probes was isolated by alkaline lysis. BAC DNA was transformed into the A-A6 lf1 arg7 strain using the glass bead method described above, and rescue of the lf1 phenotype was determined by visual screens for normal swimming motility.
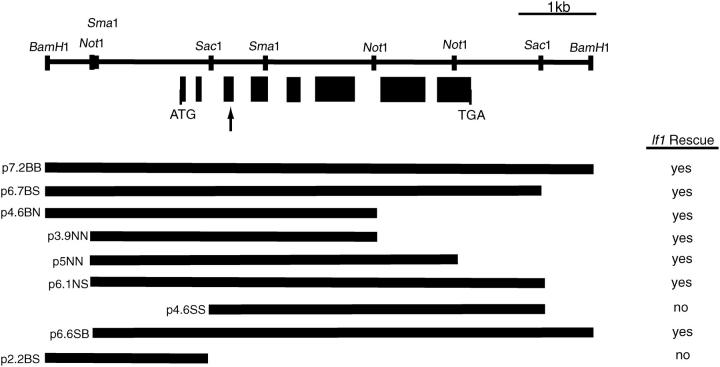
A 7.2-kb subclone (p7.2BB) containing the entire LF1 gene was obtained by digesting BAC 13m9 with BamHI and ligating the resulting fragment into pBluescript KS+. The subclone was sequenced on both DNA strands (Advanced Genetics Analysis Center, University of Minnesota, St. Paul) using gene-specific primers, and the DNA sequence was assembled using the Genetics Computer Group software (Madison, WI). Subsequent subclones were cloned into pBluescript KS+ (Figure 4) and tested for their ability to rescue the lf1 phenotype upon transformation. The sequence of the lf1 mutant allele was obtained by sequencing overlapping PCR amplification products that covered the entire LF1 coding region using gene-specific primers and the Epicentre (Madison, WI) Failsafe PCR kit, following the manufacturer's protocol. Eight independent PCR reactions from three independent DNA preparations confirmed the single-base-pair mutation in DNA from the lf1 mutant.
Figure 4.—
The LF1 gene contains eight exons and requires only the amino-terminal half of the protein for rescue of the lf1 phenotype. Shown is a restriction map of the 7.2-kb genomic plasmid (p7.2BB) containing the LF1 gene with the exons indicated as solid bars. Various subclones were able to rescue the lf1 phenotype upon transformation including three (p4.6BN, p3.9NN, and p5NN) that lack the 3′ end of the LF1 gene. Arrow indicates location of lf1 mutation.
cDNA analysis:
The exon/intron structure of the LF1 gene was predicted using the GeneMark (http://opal.biology.gatech.edu/GeneMark/) and GenScan (http://genes.mit.edu/GENSCAN.html) gene prediction programs. The cDNA sequence was obtained by amplifying fragments from a lambda-ZAP gametic cDNA library (kindly provided by Bill Snell, University of Texas Southwestern Medical Center, Dallas), by PCR using gene-specific primers and the Epicentre Failsafe PCR kit, and by RT-PCR using cDNAs prepared from poly(A) RNA by reverse transcription using Superscript II reverse transcriptase (GIBCO Life Technologies, Carlsbad, CA).
RNA analysis:
Total RNA was isolated using the LiCl precipitation method of Wilkerson et al. (1994) except that 100 μg/ml of proteinase K was used in the lysis buffer. To obtain RNA from deflagellated cells, wild-type cells were deflagellated by pH shock (Witman et al. 1972) and allowed to regrow their flagella for 15, 30, 45, or 60 min before RNA isolation. Poly(A) RNA was isolated using Magnetight oligo(dT) particles (Novagen, Madison, WI) starting with ∼1 mg of total RNA. Poly(A) RNA (4–5 μg/lane) was size fractionated in a 1% MOPS-formaldehyde agarose gel (Sambrook et al. 1989) and transferred to Brightstar Plus membrane (Ambion, Austin, TX). cDNA probes were labeled by random priming with [32P]dCTP, and the membrane was hybridized in ULTRAHyb solution (Ambion) overnight at 42°, washed in 2× SSC and 0.2× SSC solutions containing 0.2% SDS at 65°, and exposed to X-ray film. Hybridization with two cDNA fragment probes, one from the 5′ end and the other from the 3′ end, confirmed that a single transcript was detected for LF1. Membranes were rehybridized using labeled DNA from the CRY1 gene [encoding the ribosomal protein S14 (Nelson et al. 1994)] as a loading control.
HA epitope tagging, immunoblot, and immunofluorescence analysis:
A triple HA tag [described by Silflow et al. (2001)] was inserted at the second SmaI site of the SmaI-BamHI(p6.6SB) subclone. Rescue of the lf1 phenotype by transformation with a plasmid having the HA tag in the forward orientation was observed. A plasmid with the HA tag in the reverse orientation was unable to rescue the lf1 phenotype, presumably due to the introduction of premature stop codons. To observe expression of the HA-tagged LF1 protein (HA-LF1p), immunoblot analysis was performed using the method of Wilson et al. (1999) with modifications. To analyze HA-LF1p expression in whole cells, 2 × 106 cells per sample were pelleted and resuspended in 1× SDS sample buffer (10% glycerol, 2% SDS, 0.1% bromphenol blue, 0.1 m dithiothreitol, and 0.0625 m Tris, pH 6.8), boiled for 5 min, and separated on an 8% acrylamide gel (20 mA, 1.5 hr). Protein was transferred to Immobilon P membranes (Millipore, Bedford, MA) in a buffer containing 192 mm glycine, 20% methyl alcohol, and 25 mm Tris, pH 8.3, at 102 V for 1 hr at room temperature using the Mini Transblot electrophoresis transfer cell (Bio-Rad, Hercules, CA). The membrane was fixed in 0.2% glutaraldehyde in 1× TBS (137 mm NaCl, 20 mm Tris, pH 7.6) for 45 min at room temperature, washed two times in 1× TBS, and blocked overnight at 4° in TBST (137 mm NaCl, 20 mm Tris, pH 7.6, and 0.05% Tween 20) containing 5% Carnation dry milk. After blocking, the membrane was rinsed twice with TBST and incubated with anti-HA antibody 3F10 (Roche, Indianapolis) at 1:1000 dilution in TBST containing 3% dry milk for 1 hr at room temperature. After the primary antibody incubation, the membrane was washed three times for 5 min each in TBST, incubated with anti-rat POD (Roche) antibody at 1:1000 dilution in TBST containing 3% dry milk for 1 hr at room temperature, and washed three times for 7 min each in TBST. The protein was detected using the ECL detection system (Amersham Pharmacia Biotech, Piscataway, NJ). To analyze HA-LF1 protein expression in both cell bodies and flagella, cells were deflagellated by pH shock and flagella were isolated as described by Witman (1986) except flagella and cell bodies were resuspended in 10 mm HEPES, pH 7.0. As a control for contamination of isolated flagella by cell body proteins, the membrane was incubated with an antibody to OEE1, a chloroplast protein, (kindly provided by Bridgette Barry, University of Minnesota, St. Paul; Mayfield et al. 1987) at a 1:400 dilution.
Immunofluorescence was performed using the method of Sanders and Salisbury (1995) with cells fixed in 4% paraformaldehyde, 10 mm HEPES, or methanol (−20°). We used anti-HA antibodies (Roche) at a 1:200 dilution and anti-rat antibodies conjugated to the Alexa 488 fluorochrome (Molecular Probes, Eugene, OR) at a 1:200 dilution or anti-α tubulin (kindly provided by Carolyn Silflow, University of Minnesota, St. Paul) at a 1:1000 dilution and anti-rabbit antibodies conjugated to Texas red (ICN, Aurora, OH) at a 1:200 dilution. Cells were mounted as described in the Slowfade Light Antifade kit (Molecular Probes) and observed with a Nikon800 microscope. Digital images were collected using a CoolCam liquid-cooled, three-chip color CCD camera and captured using Image Pro Plus v4.0 software.
RESULTS
Characterization of the lf1 phenotype:
lf1 cells had a wide flagellar length distribution, with some flagella longer than 30 μm (Figure 1, A and B; Barsel et al. 1988); wild-type cells had a narrow distribution of flagellar lengths never exceeding 16 μm (Figure 1, A and B). lf1 mutant cells also lacked the ability to regenerate their flagella rapidly after deflagellation. If wild-type cells were deflagellated by either mechanical shearing or pH shock, they regenerated flagella to the predeflagellation length within 2 hr (Figure 1C). By contrast, after deflagellation by mechanical shearing or pH shock, >50% of the lf1 cells remained flagella-less after 2 hr (Figure 1D; Barsel et al. 1988). Even by 14 hr after deflagellation, >35% of the lf1 cells remained aflagellate (data not shown) indicating that in addition to the flagellar length defect, these cells have either a flagellar assembly defect or a defect in the response to flagellar amputation.
Figure 1.—
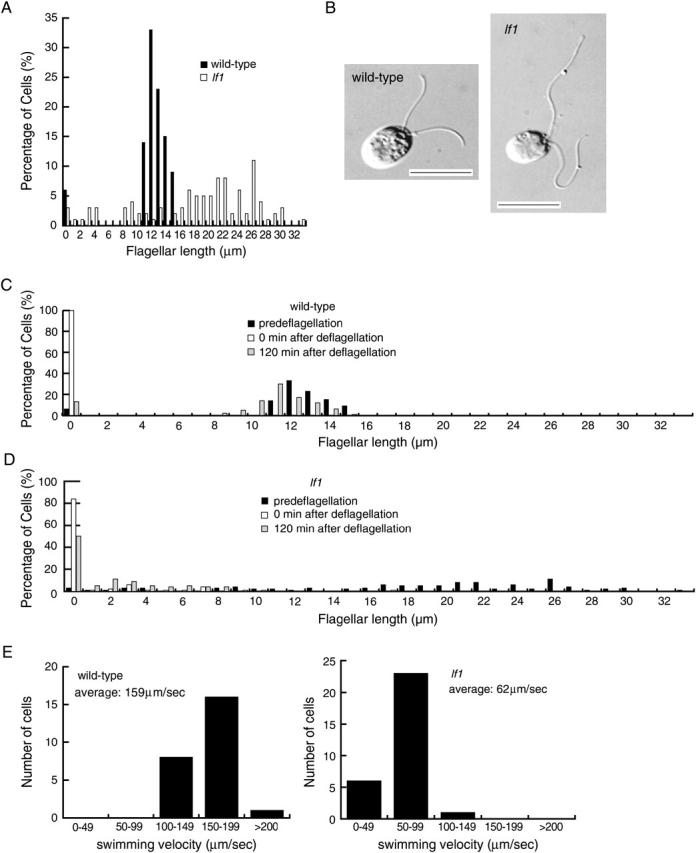
lf1 cells have defects in flagellar length control, assembly, and motility. (A) Histogram of flagellar lengths of wild-type (solid bars) and lf1 (open bars) cells. (B) DIC images of wild-type and lf1 cells. Bars, 10 μm. (C) Histogram of flagellar lengths of wild-type cells before deflagellation (solid bars). (D) Histogram of flagellar length of lf1 cells before deflagellation (solid bars), immediately following deflagellation (open bars), and 120 min after deflagellation (shaded bars). (E) Histogram of wild-type and lf1 cell swimming velocity.
The lf1 mutation also resulted in abnormal cell motility. The swimming velocity of lf1 cells (62 μm/sec) was <40% that of wild-type cells (159 μm/sec; Figure 1E). The lf1 cells had a jerky swimming motility compared to the smooth swimming of wild-type cells. This motility defect allowed us to distinguish lf1 cells from wild-type cells by examining swimming in liquid culture using a dissecting stereomicroscope.
LF1 cloning and characterization:
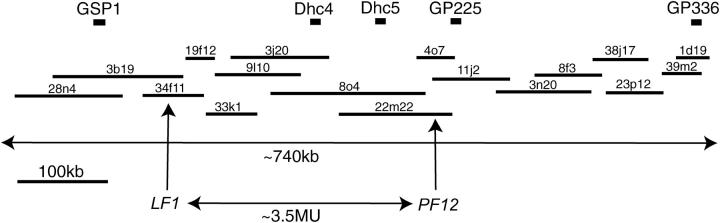
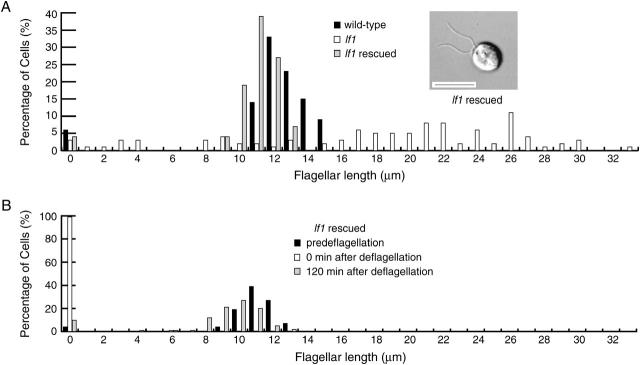
Although we have generated many different motility mutants using insertional mutagenesis, including >10 insertional alleles of lf4 (Tam and Lefebvre 1993; Smith and Lefebvre 1996; Asleson and Lefebvre 1998), no insertional alleles of lf1 have been isolated. We therefore obtained the LF1 gene by performing a chromosome walk using molecular markers near LF1 as probes to screen an indexed BAC library of Chlamydomonas DNA. The lf1 mutation maps to the right arm of linkage group II between ac12 and pf12 (Mcvittie 1972b). Beginning with dynein heavy chain clones Dhc4 and Dhc5 (Porter et al. 1996) as hybridization probes, we performed a chromosome walk that spanned an ∼740-kb region of linkage group II (Figure 2). Using molecular markers GSP1, GP225, and GP336 (Kathir et al. 2003) and numerous BAC end fragments as probes, the BAC library was screened 20 times. A total of 101 BAC clones were identified and assembled into a contig of >740 kb of genome sequence. Seventeen clones spanned the entire length of the contig. Each of the 17 clones was introduced into lf1 cells by cotransformation with a selectable marker gene and transformed lines were screened for rescue of the lf1 phenotype. Both the flagellar length defect and the flagellar regeneration defect of lf1 cells were rescued by transformation with BAC clones 3b19 and 34f11. Mutant cells rescued by transformation had flagella of wild-type length (Figure 3A) and regenerated their flagella within 2 hr after deflagellation, as seen for wild-type cells (Figure 3B).
Figure 2.—
Approximately 740-kb chromosome walk of linkage group II identifies location of LF1 gene. Contig of chromosome walk of linkage group II containing 17 of 101 BACs found in the region is shown. BAC names indicate plate number and well location. Molecular marker probes used to facilitate the walk are indicated on the top line. The walk spanned ∼740 kb and covered at least two genes identified by mutation, LF1 and PF12, separated by 3.5 MU. The entire contig is available at the Chlamydomonas Genetics Center web site: http://www.biology.duke.edu/chlamy_genome/BAC/GP366ext.html.
Figure 3.—
lf1 rescued cells rescue both the length defect and the regeneration defect of lf1 cells. (A) Histogram of flagellar lengths of wild-type cells (solid bars), lf1 cells (open bars), and lf1 cells rescued by transformation with the wild-type LF1 gene (shaded bars). DIC image of lf1 rescued cell is also shown. Bar, 10 μm. (B) Histogram of flagellar lengths of lf rescued cells before deflagellation (solid bars), immediately after deflagellation (open bars), and 120 min after deflagellation (shaded bars).
Included in the walk was a second mutation, pf12, a paralyzed-flagella mutation that maps near lf1 (Mcvittie 1972b). The pf12 mutation causes cells to swim in a jerky fashion in liquid media while remaining at the bottom of a well. The pf12 phenotype was rescued by transformation with BAC 4o7, allowing us to correlate genetic distance with physical distance using these two genes. The lf1 and pf12 mutations are separated by ∼3.5 map units (Mcvittie 1972b). The corresponding physical distance is ∼300 kb, indicating that in this genomic region one genetic map unit (1 cM) corresponds to ∼85 kb.
A 7.2-kb BamHI genomic fragment (p7.2BB) was subcloned and shown to rescue the lf1 defect upon transformation. Examination of the DNA sequence of this clone using the GenScan and GeneMark gene prediction programs revealed a single open reading frame (ORF) of 804 amino acids. cDNA library screening and RT-PCR confirmed the exon/intron boundaries (Figure 4). LF1 encodes a glycine-rich (21%), novel protein with a predicted molecular weight of ∼80 kD and a pI of 7.9 (Figure 5A). Searches of databases showed no homology to any known protein and no conserved functional motifs. The lf1 allele was sequenced to reveal a single transversion mutation (G to T) resulting in an amber stop codon at amino acid 87 (Figure 5B). The resulting truncated protein would be ∼10% the length of the wild-type protein.
Figure 5.—

LF1 encodes a novel protein of 804 amino acids. (A) Amino acid sequence of LF1. Underlined amino acid indicates location of lf1 amber stop codon. Sequence in boldface type is sufficient to rescue lf1 phenotype by transformation with p3.9NN plasmid (Figure 4). (B) Genomic sequence of the lf1 allele indicates a single-base-pair change from a G to a T leading to a premature stop codon at amino acid 87. (C) RNA blot analysis shows the 3.1-kb LF1 transcript is present in the lf1 mutant and (D) is not upregulated significantly after deflagellation. A total of 4–5 μg of poly(A) RNA was loaded per lane. P, predeflagellation. A cDNA fragment from the 3′ end of the LF1 gene was used as a probe. The same result was obtained with a probe of a cDNA fragment from the 5′ end of the LF1 gene (data not shown). A fragment of the CRY1 gene (encoding the ribosomal protein S14) was radiolabeled and used as a hybridization control for equal loading.
Interestingly, only a portion of the predicted coding region was required to rescue the lf1 phenotype. Using eight subclones of p7.2BB, we determined that all clones containing the entire ORF as well as three clones (p4.6BN, p3.9NN, and p5NN) that lacked the 3′ end of the gene were able to rescue the lf1 phenotype upon transformation (Figure 4). The first 404 amino acids, therefore, are sufficient to rescue the lf1 phenotype. Clone p4.6SS, which lacks the first two exons of the gene, was unable to rescue the lf1 phenotype, indicating that the 5′ end of the gene is necessary for rescue.
Using probes from the 5′ and the 3′ ends of the LF1 gene, we measured transcript levels in wild-type, lf1, and lf1 rescued cells (Figure 5C). The ∼3.1-kb transcript was present in all strains, but the LF1 expression level was ∼60% lower in the lf1 mutant than in wild-type cells. This result would be expected for the lf1 amber mutation if Chlamydomonas uses the nonsense-mediated decay mechanism for degrading untranslatable mRNAs seen in many eukaryotic systems (reviewed by Culbertson and Leeds 2003). The transcript was restored to wild-type levels in the lf1 rescued strain. Chlamydomonas cells upregulate the transcripts of many flagellar proteins after the cells are deflagellated. Using poly(A) RNA isolated from wild-type cells both before (lane P, Figure 5D) and at 15, 30, 45, and 60 min after deflagellation (Figure 5D), we found that the ∼3.1-kb LF1 transcript was not upregulated after flagellar amputation. A probe for the CRY1 gene encoding the ribosomal protein S14, whose transcript level is unaffected by deflagellation, was used as a loading control.
LF1p localization:
Attempts to generate antibodies to the LF1 protein have not been successful. As an alternative approach, we tagged the LF1 protein with a triple HA epitope for immunolocalization experiments (Figure 6A). Transformation of HA-LF1 gene constructs into lf1 cells rescued the lf1 phenotype. When the epitope tag was inserted in the reverse orientation, rescue of the lf1 phenotype was not obtained, presumably due to the introduction of multiple stop codons. To observe HA-LF1p expression, we performed an immunoblot of proteins from whole cells using four different transformed strains that had been rescued with the HA-tagged construct. A protein of ∼120 kD was observed in transformed strains that was not expressed in strains lacking the HA tag (Figure 6B). We compared levels of HA-LF1p in whole cells, cell bodies, and isolated flagella by loading equal amounts of protein (Figure 6C, lanes 1, 2, and 3). HA-LF1p was detected in each fraction. Incubation of the blot with an antibody to a chloroplast protein (OEE1p; Mayfield et al. 1987) that should not be present in the flagella showed that the isolated flagella were not contaminated by cytosolic proteins. To determine the relative amounts of HA-LF1p in the flagella vs. the cell bodies, dilutions of flagellar protein samples were examined. When protein samples from whole cells, cell bodies, and flagella from equivalent numbers of cells (2 × 106) were loaded on the gel, no band corresponding to HA-LF1p was detected in the lane containing isolated flagella (Figure 6C, lanes 4, 5, and 6). If a flagellar protein sample from 2 × 109 cells (Figure 6C, lane 10) was compared with a whole-cell or cell-body protein sample from 2 × 106 cells (Figure 6C, lanes 4 and 5), equivalent amounts of HA-LF1p were detected, indicating that ∼1000 times more LF1 protein is present in the cell body than in the flagella.
Figure 6.—
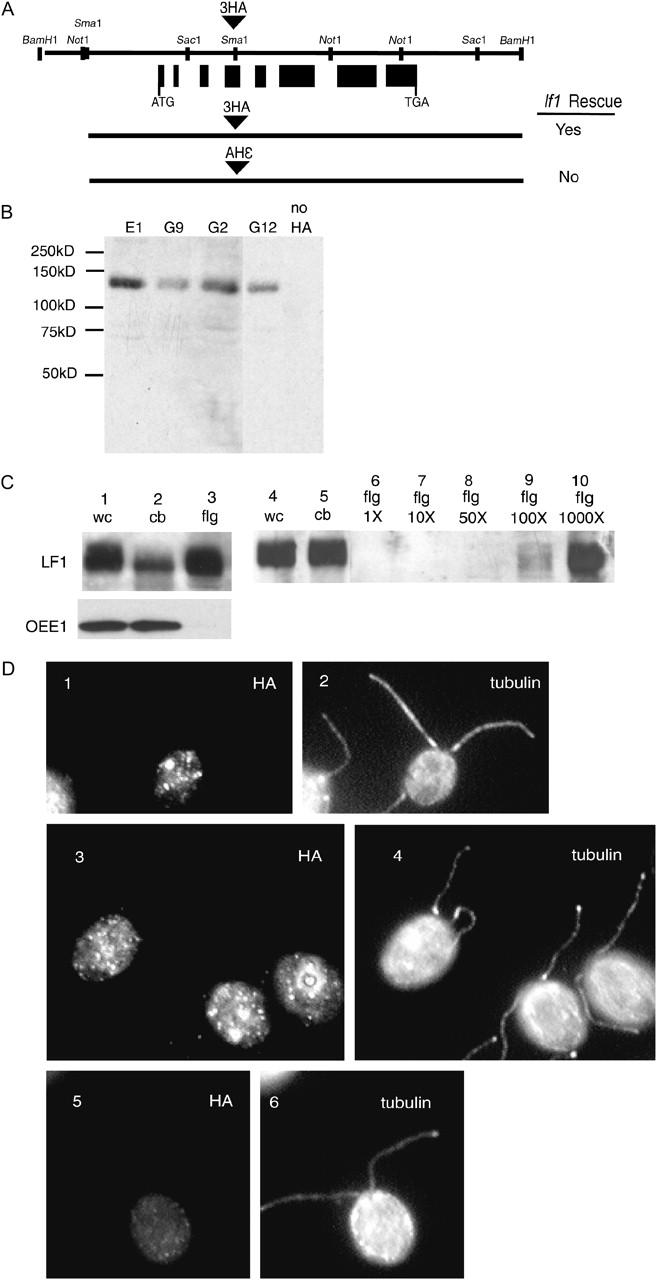
HA-tagged LF1 is present in the cell body and flagella. (A) Restriction map of 7.2-kb genomic plasmid showing the placement of the triple HA tag within the LF1 gene. (B) Immunoblot using an HA antibody shows four HA-tagged lf1 rescued strains (E1, G9, G12, and G12) express the HA-LF1 tagged protein, whereas an lf1 rescued strain transformed with a plasmid lacking the HA tag is not reactive to the HA antibody. (C) Immunoblot using an HA antibody indicates HA-LF1p is present in whole cells, cell bodies, and flagella. Lanes 1, 2, and 3 show protein from whole cell, cell body, and flagella (30 μg). Lanes 4 and 5 show protein from whole cells and cell bodies (2 × 106). Lanes 6, 7, 8, 9, and 10 show protein from flagella isolated from 2 × 106 (1×), 2 × 107 (10×), 1 × 108 (50×), 2 × 108 (100×), and 2 × 109 (1000×) cells. (D) Immunofluorescence of HA-LF1 tagged cells. Shown are phenotypically rescued strains, G2 and E1, stained with anti-HA antibody (1 and 3) and anti-tubulin antibody (2 and 4) and wild-type cells lacking the HA tag stained with anti-HA (5) and anti-tubulin (6) antibodies.
Using the anti-HA antibody to perform immunofluorescence on cells transformed with the HA-LF1 construct, we found that HA-LF1p localizes as punctate spots within the cell body (Figure 6D, 1 and 3), with no detectable localization within the flagella. The same punctate staining was observed with all HA-LF1 transformed strains examined. No staining was observed with strains lacking the HA tag (e.g., wild type; Figure 6D5). Various fixation methods (e.g., methanol alone, acetone alone, paraformaldehyde and methanol together, paraformaldehyde alone, and paraformaldehyde and acetone together) all gave the same punctate staining pattern, with varying degrees of background staining (data not shown). The punctate spots do not localize specifically with any known organelle (e.g., basal bodies or nucleus), although the localization is similar to that observed for two other proteins involved in flagellar length control, LF3 (Tam et al. 2003) and LF2 (L. W. Tam, N. W. Wilson and P. A. Lefebvre, unpublished observations).
DISCUSSION
We show in this report that the LF1 gene in Chlamydomonas encodes a novel protein of 804 amino acids localized primarily in the cytoplasm. The protein must be involved in both flagellar assembly and function, as lf1 mutants have defects in flagellar beating and flagellar growth, in addition to defects in flagellar length control. The localization of the protein in discrete spots throughout the cytoplasm does not suggest a mechanism as yet for how LF1 exerts its effects on flagella.
Potential interactions of LF1p with other LF gene products:
Several lines of evidence suggest that the LF1 gene product interacts with the products of the LF2 and LF3 genes in the regulation of flagellar assembly and length (Mcvittie 1972a,b; Barsel et al. 1988). Not only do double mutants of lf1 with lf2 or lf3 show a synthetic flagella-less phenotype, but also all three gene products are similarly localized in discrete spots in the cytoplasm with very little in the flagella (Figure 6D; Tam et al. 2003). LF1p and LF3p also cofractionate at 11s upon sucrose gradient centrifugation of cytoplasmic extracts (Tam et al. 2003). Triple mutants of lf4, lf1, and lf2-3 had long flagella, suggesting that LF4 may act downstream of LF1, possibly to induce flagellar shortening (Asleson and Lefebvre 1998). LF4 also acts downstream of LF1 in the control of flagellar regeneration. lf1 lf4 double mutants regenerate flagella after amputation with the rapid kinetics of wild-type cells, whereas lf1 mutants alone regenerate flagella very slowly. This result suggests that the wild-type LF4 gene product is required to produce the flagellar regeneration defects in lf1 mutants. LF4 has recently been shown to encode a novel MAP kinase (Berman et al. 2003), suggesting the possible involvement of LF1 in the upstream portion of a signal transduction cascade involved in flagellar length control.
It is interesting that only 50% of the protein, from the amino terminus, is needed to rescue the lf1 phenotype upon transformation; the carboxyl end of the protein seems dispensable. The IC140 protein of Chlamydomonas axoneme acts in a similar manner (Perrone et al. 1998) with the first 283 amino acids of the protein being dispensable for function. Perrone et al. concluded that the carboxyl terminus is sufficient to assemble a dynein protein complex. The carboxyl terminus of LF1p is not essential for function, but perhaps it helps to stabilize the protein. The protein is glycine rich (∼21%) especially within the carboxyl terminus. Glycine-rich regions are thought to be sites of protein:protein interactions that help with stabilization and flexibility of protein complexes (Sachetto-Martins et al. 2000). The C terminus of LF1p may interact with other length control proteins, most likely LF2 and/or LF3, to form a cytoplasmic complex, visualized as discrete foci by immunofluorescence.
It seems likely that the single available mutant allele of lf1 is a null allele. The genetic lesion in lf1 generates a stop codon at amino acid 87, and there are no methionine codons for potential downstream reinitiation within the following 380 codons. If lf1 is a null allele, it is somewhat surprising that no new lf1 alleles were found among >10 long-flagella mutants identified in insertional mutagenesis screens (Asleson and Lefebvre 1998). All insertional mutants with the long-flagella phenotype to date have proven to be lf4 alleles. Insertional null alleles of lf3 (Tam et al. 2003) and lf2 (L. W. Tam and P. A. Lefebvre, unpublished observations) have an “unequal-length flagella” or Ulf phenotype. Populations of Ulf mutants have many flagella-less cells; those cells with flagella often have flagella of unequal length, and a few cells have long flagella. This phenotype is clearly different from the long-flagella phenotype of the lf1 mutant, as well as putative hypomorphic alleles of lf2 and lf3. It is possible that LF4 is a hotspot for plasmid insertion during transformation or that LF1 is in an unfavorable genomic environment for plasmid insertion.
Control of flagellar length:
No detailed molecular models have yet been advanced to explain how Chlamydomonas cells can recognize the length of their flagella and maintain them at an equal and fixed length. Recently a proposal has been advanced that length regulation is a consequence of a balance between rates of assembly and disassembly of the doublet microtubules of the axoneme and that this balance is a consequence of a limiting supply of IFT proteins in the flagella (Marshall and Rosenbaum 2001). Two different long-flagella mutants, lf3 (Tam et al. 2003) and lf1 (Perrone et al. 2003), have now been shown to accumulate IFT proteins into their flagella above the levels seen in wild-type flagella. These proteins appear to accumulate in bulges at the tips and to a lesser extent along the length of the flagella. These bulges contain accumulations of IFT protein particles. Perhaps one role for the proteins in the cytoplasmic complex containing LF1p, LF2p, and LF3p is to control the partitioning of IFT protein components into and out of the flagella.
Positional cloning using BAC clones is feasible in Chlamydomonas: We determined, by obtaining rescue with BAC clones of both the pf12 and the lf1 mutations, that one map unit corresponds to ∼85 kb of genomic DNA. Dutcher et al. (2002) performed an ∼720-kb BAC walk in their cloning of the Chlamydomonas bld2 gene, which encodes epsilon tubulin, and determined that in the relevant portion of linkage group III one map unit corresponds to ∼160 kb.
Our results highlight the ease with which BAC clones can be used for positional cloning of mapped genes. The transformation efficiency with BAC clones in Chlamydomonas cells is 1–5%, and transformation and phenotypic screening of transformed strains can be completed in 7–10 days. A molecular map containing >280 markers with an average spacing of 3–4 MU has been aligned with the genetic map (Kathir et al. 2003). BAC contigs have been constructed around many of these markers. By using a nearby molecular marker as a starting point, positional cloning of genes identified by mapped mutations should become routine using the BAC library. The Chlamydomonas genome has been sequenced, and the draft sequence is available at the Chlamydomonas web site of the Joint Genome Institute (JGI) of the Department of Energy (http://genome.jgi-psf.org/chlre2/chlre2.home.html). The sequence of the ends of all 15,000 BAC clones in the indexed library is also available at the JGI site. With the availability of the genomic sequence and BAC clones, as well as the ease and efficiency of cotransformation, positional cloning of any gene on the basis of its position on the genetic map becomes possible.
Acknowledgments
We thank Carolyn Silflow and Nedra Wilson for critically reading this manuscript. We also thank Ritsu Kamiya for assistance with swimming velocity measurements. We thank Nancy Haas for construction of the BAC library. We also thank members of the Lefebvre, Silflow, Porter, and Linck laboratories at the University of Minnesota for helpful comments and technical advice. This work was supported by National Institutes of Health grant GM-34437. R.N. was supported in part by the Plant Molecular Genetics Institute of the University of Minnesota.
Sequence data from this article have been deposited with the EMBL/GenBank Data Libraries under accession no. AY298951.
References
- Asleson, C. M., and P. A. Lefebvre, 1998. Genetic analysis of flagellar length control in Chlamydomonas reinhardtii: a new long-flagella locus and extragenic suppressor mutations. Genetics 148: 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsel, S.-E., D. E. Wexler and P. A. Lefebvre, 1988. Genetic analysis of long-flagella mutants of Chlamydomonas reinhardtii. Genetics 118: 637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman, S. A., N. F. Wilson, N. A. Haas and P. A. Lefebvre, 2003. A novel MAP kinase regulates flagellar length in Chlamydomonas. Curr. Biol. 13: 1145–1149. [DOI] [PubMed] [Google Scholar]
- Culbertson, M. R., and P. F. Leeds, 2003. Looking at mRNA decay pathways through the window of molecular evolution. Curr. Opin. Genet. Dev. 13: 207–214. [DOI] [PubMed] [Google Scholar]
- Debuchy, R., S. Purton and J. D. Rochaix, 1989. The argininosuccinate lyase gene of Chlamydomonas reinhardtii: an important tool for nuclear transformation and for correlating the genetic and molecular maps of the ARG7 locus. EMBO J. 8: 2803–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutcher, S. K., N. S. Morrissette, A. M. Preble, C. Rackley and J. Stanga, 2002. ε-Tubulin is an essential component of the centriole. Mol. Biol. Cell 13: 3859–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, E. H., 1989 The Chlamydomonas Sourcebook. Academic Press, San Diego.
- Hirokawa, N., 2000. Stirring up development with the heterotrimeric kinesin KIF3. Traffic 1: 29–34. [DOI] [PubMed] [Google Scholar]
- Jarvik, J. W., F. D. Reinhart, M. R. Kuchka and S. A. Adler, 1984. Altered flagellar size-control in shf-1 short-flagella mutants of Chlamydomonas reinhardtii. J. Protozool. 31: 199–204. [Google Scholar]
- Kathir, P., M. Lavoie, W. J. Brazelton, N. A. Haas, P. A. Lefebvre et al., 2003. Molecular map of the Chlamydomonas reinhardtii nuclear genome. Eukaryotic Cell 2: 362–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindle, K. L., 1990. High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 87: 1228–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchka, M. R., and J. W. Jarvik, 1987. Short-flagella mutants of Chlamydomonas reinhardtii. Genetics 115: 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall, W. F., and J. L. Rosenbaum, 2001. Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J. Cell Biol. 155: 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield, S. P., P. Bennoun and J. D. Rochaix, 1987. Expression of the nuclear encoded OEE1 protein is required for oxygen evolution and stability of photosystem II particles in Chlamydomonas reinhardtii. EMBO J. 6: 313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcvittie, A., 1972. a Flagellum mutants of Chlamydomonas reinhardii. J. Gen. Microbiol. 71: 525–540. [DOI] [PubMed] [Google Scholar]
- Mcvittie, A., 1972. b Genetic studies on flagellum mutant of Chlamydomonas reinhardii. Genet. Res. 9: 157–164. [Google Scholar]
- Nelson, J. A., P. B. Savereide and P. A. Lefebvre, 1994. The CRY1 gene in Chlamydomonas reinhardtii: structure and use as a dominant selectable marker for nuclear transformation. Mol. Cell Biol. 14: 4011–4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka, S., Y. Tanaka, Y. Okada, S. Takeda, A. Harada et al., 1998. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95: 829–837. [DOI] [PubMed] [Google Scholar]
- Pazour, G. J., and J. L. Rosenbaum, 2002. Intraflagellar transport and cilia-dependent diseases. Trends Cell Biol. 12: 551–555. [DOI] [PubMed] [Google Scholar]
- Perrone, C. A., P. Yang, E. O'Toole, W. S. Sale and M. E. Porter, 1998. The Chlamydomonas IDA7 locus encodes a 140-kDa dynein intermediate chain required to assemble the I1 inner arm complex. Mol. Biol. Cell 9: 3351–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone, C. A., D. Tritschler, P. Taulman, R. Bower, B. K. Yoder et al., 2003. A novel dynein light intermediate chain colocalizes with the retrograde motor for intraflagellar transport at sites of axoneme assembly in Chlamydomonas and mammalian cells. Mol. Biol. Cell 14: 2041–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter, M. E., J. A. Knott, S. H. Myster and S. J. Farlow, 1996. The dynein gene family in Chlamydomonas reinhardtii. Genetics 144: 569–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum, J. L., J. E. Moulder and D. L. Ringo, 1969. Flagellar elongation and shortening in Chlamydomonas: the use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J. Cell Biol. 41: 600–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachetto-Martins, G., L. O. Franco and D. E. De Oliveira, 2000. Plant glycine-rich proteins: A family or just proteins with a common motif? Biochim. Biophys. Acta 1492: 1–14. [DOI] [PubMed] [Google Scholar]
- Sager, R., and S. Granick, 1953. Nutritional studies in Chlamydomonas reinhardi. Ann. NY Acad. Sci. 56: 831–838. [DOI] [PubMed] [Google Scholar]
- Sakakibara, H., S. Takada, S. M. King, G. B. Witman and R. Kamiya, 1993. A Chlamydomonas outer arm dynein mutant with a truncated β heavy chain. J. Cell Biol. 122: 653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., E. F. Fritsch and T. Maniatis, 1989 Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Sanders, M. A., and J. L. Salisbury, 1995. Immunofluorescence microscopy of cilia and flagella. Methods Cell Biol. 47: 163–169. [DOI] [PubMed] [Google Scholar]
- Silflow, C. D., M. Lavoie, L.-W. Tam, S. Tousey, M. Sanders et al., 2001. The Vfl1 protein in Chlamydomonas localizes in a rotationally asymmetric pattern at the distal ends of the basal bodies. J. Cell Biol. 153: 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, E. F., and P. A. Lefebvre, 1996. PF16 encodes a protein with armadillo repeats and localizes to a single microtubule of the central apparatus in Chlamydomonas flagella. J. Cell Biol. 132: 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starling, D., and J. Randall, 1971. The flagella of temporary dikaryons of Chlamydomonas reinhardii. Genet. Res. 18: 107–113. [Google Scholar]
- Tam, L.-W., and P. A. Lefebvre, 1993. Cloning of flagellar genes in Chlamydomonas reinhardtii by DNA insertional mutagenesis. Genetics 135: 375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam, L.-W., W. L. Dentler and P. A. Lefebvre, 2003. Defective flagellar assembly and length regulation in LF3 null mutants in Chlamydomonas. J. Cell Biol. 163: 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, M. K., and H. J. Yost, 2000. Left-right development: the roles of nodal cilia. Curr. Biol. 10: R149–R151. [DOI] [PubMed] [Google Scholar]
- Wilkerson, C. G., S. M. King and G. B. Witman, 1994. Molecular analysis of the gamma heavy chain of Chlamydomonas flagellar outer-arm dynein. J. Cell Sci. 107: 497–506. [DOI] [PubMed] [Google Scholar]
- Wilson, N. F., J. S. O'Connell, M. Lu and W. J. Snell, 1999. Flagellar adhesion between mt+ and mt− Chlamydomonas gametes regulates phosphorylation of the mt+ specific homeodomain protein GSP1. J. Biol. Chem. 26: 34383–34388. [DOI] [PubMed] [Google Scholar]
- Witman, G. B., 1986. Isolation of Chlamydomonas flagella and flagellar axonemes. Methods Enzymol. 134: 280–290. [DOI] [PubMed] [Google Scholar]
- Witman, G. B., K. Carlson, J. Berliner and J. L. Rosenbaum, 1972. Chlamydomonas flagella. I. Isolation and electrophoretic analysis of microtubules, matrix, membranes, and mastigonemes. J. Cell Biol. 54: 507–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zein, L. E., H. Omran and P. Bouvagnet, 2003. Lateralization defects and ciliary dyskinesia: lessons from algae. Trends Genet. 19: 162–167. [DOI] [PubMed] [Google Scholar]