Abstract
Much of our understanding of the genetic mechanisms that control planar cell polarity (PCP) in epithelia has derived from studies of the formation of polarized cell hairs during Drosophila wing development. The correct localization of an F-actin prehair to the distal vertex of the pupal wing cell has been shown to be dependent upon the polarized subcellular localization of Frizzled and other core PCP proteins. However, the core PCP proteins do not organize actin cytoskeletal polarity directly but require PCP effector proteins such as Fuzzy and Inturned to mediate this process. Here we describe the characterization of a new PCP effector gene, fritz, that encodes a novel but evolutionarily conserved coiled-coil WD40 protein. We show that the fritz gene product functions cell-autonomously downstream of the core PCP proteins to regulate both the location and the number of wing cell prehair initiation sites.
DURING animal development epithelial cells develop molecular and morphological asymmetries that give them a cellular polarity. Conventionally, epithelial cell polarity is defined along two orthogonal axes, one through the thickness of the epithelium (apical-basal cell polarity) and the other along the length of the cell layer (planar cell polarity or PCP). Apical-basal polarization occurs in a single dimension and defines the top and bottom of the cell and consequently the inside and outside of the epithelial layer. In contrast, planar polarization occurs within a two-dimensional field and epithelial cells frequently align their planar polarity with neighboring cells to give the whole epithelium a specific tissue polarity.
Evidence that genetic mechanisms exist specifically to organize PCP has come primarily from studies of the Drosophila epidermis (Adler 2002; Tree et al. 2002a). A group of Drosophila gene mutations that results in an altered patterning of polarized epidermal structures, such as the sensory bristles (macrochaetes and microchaetes) and cell hairs (trichomes), has been defined (Gubb and Garcia-Bellido 1982). These phenotypes are indicative of changes in the planar polarity of the epithelial cells that produce these structures. Significantly, these cell polarity changes occur largely without affecting cell fate decisions or tissue morphogenesis. Within this group a set of core PCP genes has been identified that not only controls the orientation of bristles and cell hairs but also controls ommatidial polarity in the eye and tarsal joint specification in the leg. The core PCP genes include frizzled (fz), dishevelled (dsh), prickle (pk), starry night (stan also called flamingo), van gogh (vang also called strabismus), and diego (dgo). There is also a group of planar polarity effector genes that includes fuzzy (fy), inturned (in), and multiple wing hairs (mwh), which are also required for the normal orientation of bristles and cell hairs but do not have substantial roles in ommatidial or tarsal joint development.
Much of our understanding of the genetic control of PCP in Drosophila has come from studies of wing cell hair development. Each wing cell produces a single hair that points toward the distal tip of the wing. The formation of this cell hair is initiated by the accumulation of F-actin at the distal vertex of the hexagonal pupal wing cell to form an actin-rich prehair (Wong and Adler 1993). Recent studies have shown that prior to prehair formation the core PCP proteins localize within the pupal wing cell to the distal edges (Frizzled, Dishevelled, and Diego) (Axelrod 2001; Strutt 2001; Das et al. 2004), the proximal edges (Prickle and Van Gogh) (Tree et al. 2002b; Bastock et al. 2003), or to both the distal and the proximal edges (Starry night) (Usui et al. 1999). In core PCP gene mutants the core PCP proteins fail to localize normally (McNeill 2002; Strutt 2002) and F-actin accumulation and prehair formation occurs at the apical center of the pupal wing cell rather than at the periphery, resulting in an abnormally oriented cell hair (Wong and Adler 1993). Therefore, it appears that the appropriate subcellular localization of the core PCP proteins is required to ensure the correct site of prehair initiation and cell hair polarity.
Several experiments have shown that the PCP effector genes act downstream of the core PCP genes in wing hair development. First, an analysis of epistatic interactions placed the PCP effector genes downstream of the core PCP genes in a regulatory pathway controlling the site of prehair initiation (Wong and Adler 1993). Second, temperature-shift experiments have shown that the PCP effector gene in is required later than the core PCP gene fz in wing hair development (Adler et al. 1994). Third, both fy and in gene mutations have been shown to block core PCP gene gain of function phenotypes (Lee and Adler 2002). It has also been found that the core PCP protein Stan localizes normally in a mwh mutant (Usui et al. 1999) and that Fz localizes normally in an in mutant background (Strutt 2001), implying that core PCP protein localization is independent of PCP effector gene function. However, despite the normal localization of the core PCP proteins, prehairs in PCP effector gene mutants form at aberrant sites around the apical periphery of the pupal wing cell and abnormal cell hair polarity results (Wong and Adler 1993). It appears, therefore, that the primary role of the PCP effector proteins in wing cell planar polarity is to link the site of F-actin accumulation and prehair formation with the polarized distribution of the core PCP proteins.
In PCP effector gene mutants, F-actin frequently accumulates at multiple sites on the pupal wing cell periphery, resulting in the production of multiple cell hairs (Wong and Adler 1993). This implies that the PCP effector proteins also have a role in restricting the number of sites of prehair initiation within the developing wing cell. This function is largely independent of the core PCP proteins as core PCP mutants display very weak multiple wing cell hair phenotypes. However, cytoskeletal regulators such as the small GTPases RhoA, Rac, and Dcdc-42, and Rho kinase also appear to play a role in restricting prehair initiation site number as loss-of-function or dominant-negative phenotypes include multiple wing cell hairs (Eaton et al. 1996; Strutt et al. 1997; Winter et al. 2001). Other genes implicated in the control of wing hair initiation sites include furry (Cong et al. 2001) and the Drosophila NDR kinase tricornered (Geng et al. 2000).
In this article we report the identification and characterization of fritz (frtz), a new PCP effector gene. frtz encodes a novel but evolutionarily conserved coiled-coil WD40 protein that functions cell-autonomously downstream of the core PCP proteins and is required for normal wing cell hair polarity and number.
MATERIALS AND METHODS
Phenotypic analysis:
All flies were raised at 25° unless indicated otherwise. Adult wings were washed with isopropanol and mounted in GMM (1:1 Canada balsam:methyl salicylate) or Euparol. Adult wing clones were produced by X-ray irradiating f36a/Y; f+30B ck13 pr1 pwn1/frtz33 b pr cn hv larvae with 1000 R at 48–72 hr old, and homozygous frtz clones were identified by the forked wing hair phenotype. Cuticle preparations of first instar larvae were made by mounting newly hatched larvae directly in Hoyer's medium and incubating the slides overnight at 60°.
Molecular characterization of mutant frtz alleles:
Homozygous or hemizygous frtz mutant genomic DNA was PCR amplified between frtz gene-specific oligonucleotide primers and the PCR products sequenced from oligonucleotides on the frtz sense strand. Nucleotide sequences were compared to wild type using the BDGP BLASTn server and sequence differences analyzed for potentially deleterious mutations (Table 1). Putative neutral polymorphisms occurring in two or more independent frtz alleles were Iso411Val (frtz1, frtz26, and frtz33), Asp542Asn (frtz1 and frtz33), Pro720Thr (frtz1, frtz26, frtz27, and frtz33), and Pro776Thr (frtz1, frtz26, frtz27, frtz30, and frtz33).
TABLE 1.
Molecular and phenotypic characteristics of extantfritz alleles
| Allele | Original name |
Mutagen | Source | Phenotype | Mutation | Protein product |
|---|---|---|---|---|---|---|
| fritz1 | EMS | Lancaster | Strong | TAT > TAA in exon 3 | Tyr506 > STOP | |
| fritz2 | Unknown | Cambridge | Strong | AAA > TAA in exon 3 | Lys558 > STOP | |
| fritz3 | GJ66/78 | X ray | Cambridge | Weak, temperature sensitive |
GAC > CAC in exon 3 | Asp375 > Ala (see Figure 7A) |
| fritz7 | GJ66/11 | X ray | Cambridge | Strong, reduced viability/fertility |
1329-nt deletion upstream from nt − 11 (see Figure 5A) |
Wild Type |
| fritz26 | fy-twin | EMS | Virginia | Weak | ND | ND |
| fritz27 | FK1172 | X ray | Virginia | Strong | AAG > ATG in exon 5 | Lys783 > Met |
| fritz28 | FK1521 | X ray | Virginia | Strong | 8 nt replaced by 9 nt in exon 3: TGCGGCTG CAGC > TGTCCGT CATTGC |
N-terminal 472 + 39 novel aa |
| fritz29 | FK4211 | X ray | Virginia | Strong | 96-nt deletion in exon 3 | Deletion Gln352 to Leu385 |
| fritz30 | FK3521 | X ray | Virginia | Strong | 7-nt deletion in exon 3: CATGTGCCTGA > CAGA (plus independent ∼1 kb deletion downstream) | N-terminal 493 + 7 novel aa |
| fritz33 | FK55a11 | X ray | Virginia | Strong | 1-nt insertion in exon 3: GATCTGCT > GATCCTGCT | N-terminal 309 + 37 novel aa |
EMS, ethyl methanesulfonate; nt, nucleotide; ND, not determined.
Analysis of pupal wing phenotypes:
We examined the frtz pupal wing phenotype in both completely mutant wings and somatic clones. Similar results were obtained by both approaches. Pupal frtz clones marked by a loss of GFP were generated by heat-shocking w hs-flp; + Ubi-GFP FRT40/frtz FRT40 larvae and collecting white prepupae, followed by dissection and fixation at desired times. To determine whether frtz was required for the asymmetric accumulation of Fz, we examined GFP in frtz; arm-fz-GFP pupal wings. To determine whether frtz was required for the asymmetric accumulation of Dsh or Stan, we stained frtz mutant wings using antibodies provided by T. Uemura. Pupal wings of the desired age were fixed in 4% paraformaldehyde PBS and then stained using standard procedures (Lee and Adler 2004). As cytoskeleton probes we used fluorescently labeled phalloidin (actin cytoskeleton) and antitubulin antibody (Sigma, St. Louis). Fluorescently labeled secondary antibodies were purchased from Molecular Probes (Eugene, OR). In most experiments we used direct visualization of GFP but on a few occasions we amplified the GFP signal using a rabbit polyclonal anti-GFP antibody (Molecular Probes). Samples were mounted using Prolong (Molecular Probes) and examined on either a Nikon laser scanning confocal microscope at the Keck Center for Cellular Imaging or a Nikon TE200 microscope equipped with an ATTO-CARV spinning disc confocal run by the Metamorph software package. Some images were deconvolved using Auto-DeBlur (AQI). Figures for the article were assembled using Adobe Photoshop.
Interaction of core PCP genes with frtz:
To determine whether frtz was required for the gain-of-function phenotypes that result from the directed expression of planar polarity genes, we generated flies that were mutant for frtz and that overexpressed one of the planar polarity genes using a Gal4 driver and the relevant UAS transgene. To determine whether frtz was required for cells to respond to the presence of a clone of cells that lacked fz function, we generated w hs-flp; frtz/frtz; fz trc FRT80/FRT80 flies and heat-shocked the larvae to induce clones. The clones could be identified by the strong multiple hair phenotype of tricornered (trc). As a control, we used a similar strategy to induce clones of cells mutant for trc in a frtz mutant background. In this experiment we used the null fz allele fzP21.
RESULTS
The frtz mutant phenotype is cold sensitive and cell autonomous:
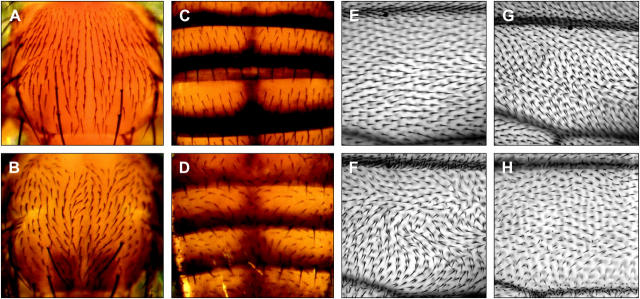
The frtz phenotype is strikingly similar to the phenotypes of the PCP effector genes fy and in (Wong and Adler 1993; Adler et al. 1994; Collier and Gubb 1997). For this reason we have also classified frtz as a PCP effector gene. The bristles, both macrochaetes and microchaetes, of the adult notum and abdomen of frtz mutants have an altered orientation and usually point toward the midline (Figure 1, B and D) rather then posteriorly as in wild type (Figure 1, A and C). The bristles of the triple row on the anterior wing margin of frtz mutants point more anteriorly than those of wild type and follow the local polarity of cell hairs (see Figure 2, A and B).
Figure 1.—
(A–D) Macrochaete and microchaete orientation is altered in frtz mutants. (A) Notum of wild type (Oregon-R) fly. (B) Notum of frtz33/Df fly raised at 18°. (C) Dorsal abdomen of wild type (Oregon-R) fly. (D) Dorsal abdomen of frtz33/Df fly raised at 18°. Anterior is uppermost. frtz mutant bristles point toward the midline rather than posteriorly. (E–H) Cell hair polarity and number are altered on a frtz mutant wing. Photomicrographs are of the “C” cell region of wing immediately anterior to the posterior cross-vein (PCV). The proximal-distal axis of the wings runs from left to right. Dorsal wing surface of (E) wild type (Oregon-R), (F) frtz33/Df raised at 18°, and (G) frtz33/Df raised at 29°. Note that the frtz phenotype is weaker at higher temperature. (H) Ventral surface of frtz33/Df wing at 18°. Most cells displaying reversed polarity have wild-type hair number.
Figure 2.—
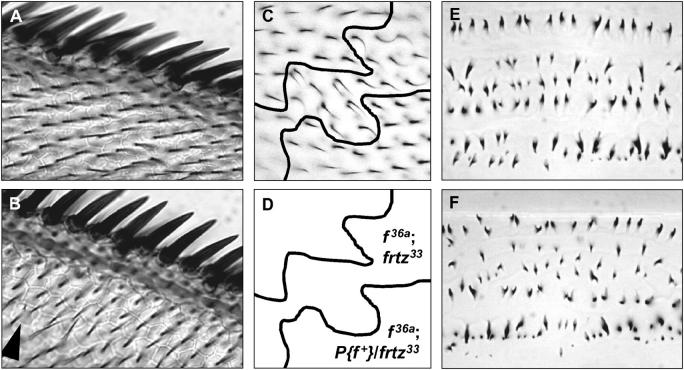
(A and B) Adult wing hairs emerge from the apical center of the cell in both wild-type and frtz mutants. (A) Anterior wing margin of m38c/Y mutant. (B) Anterior wing margin of m38c/Y; frtz1/ frtz1 mutant. The arrowhead indicates a frtz mutant cell producing two centrally located cell hairs. Note that margin bristle polarity follows local cell hair polarity. (C and D) The frtz wing cell hair phenotype is cell autonomous. (C) Small (∼60 cells) f36a; frtz33/frtz33 clone in the “D” region of a f36a; P{f+}30B/frtz33 wing. Proximal is to the left, distal to the right. Note the presence of frtz homozygous mutant cells carrying two cell hairs at the edge of the clone and the more posterior orientation of cell hairs within the clone. (E and F) Organization of larval denticles is disrupted in frtz mutants. Denticle belt A7 of cuticle preparations of LI larvae of (E) wild-type (Oregon-R) and (F) homozygous frtz33 mutants; anterior is at the top. Denticle rows are disrupted in frtz mutants and individual denticles often appear smaller.
The trichomes or cell hairs of the fritz mutant adult cuticle show altered, but reproducible, patterns of orientation. The majority of hairs on frtz mutant wings posterior to the L3 vein point more posteriorly than those of wild type and those anterior to the L3 vein point more anteriorly. A similar pattern has been described for the core PCP mutants and other PCP effector mutants (Gubb and Garcia-Bellido 1982; Wong and Adler 1993; Collier and Gubb 1997; Taylor et al. 1998; Chae et al. 1999) and can be regarded as the “default” PCP mutant pattern. All frtz alleles, with the exception of frtz3, have a cold-sensitive phenotype (Table 1) with mutant flies raised at 18° showing more dramatic alterations in wing hair polarity than those cultured at 25° or 29° (Figure 1, F and G). A similar conditional sensitivity has been described for strong alleles of the PCP effector genes fy and in (Adler et al. 1994; Collier and Gubb 1997) and is also true of mwh alleles. Changes in wing cell hair polarity in strong frtz mutants raised at 18° are more profound than those for loss-of-function core PCP gene mutations and display substantial regions of reversed (proximal pointing) hair polarity, especially on the ventral wing surface (Figure 1H).
frtz mutant wings are also characterized by a high proportion of cells producing two or more cell hairs (Figure 1, F and G). In the wild-type wing, a single F-actin rich prehair forms at the distal vertex of the developing wing cell between 31 and 34 hr after pupal formation (a.p.f.) but becomes localized to the apical center of the wing cell at ∼47–53 hr a.p.f. (Mitchell et al. 1983). We have found that frtz mutant prehairs form at the same time as wild type but initiate at multiple alternative sites at the apical periphery of the pupal wing cell (Figure 3A). To test whether this aberrant prehair initiation site leads to an altered final location of the mature cell hair, we made double mutants of a strong frtz allele (frtz1) with a miniature (m) mutation (In(1)m38c) in which wing cell boundaries remain visible in the adult wing (Newby et al. 1991). It is clear that, despite their aberrant initiation, wing cell hairs of m38c; frtz mutant adults arise from the apical center of the cell (Figure 2B) as they do in wild-type flies (Figure 2A). As a rule, the greater the deviation in hair polarity is from the proximal-distal axis of the wing, the more likely frtz mutant cells are to display multiple hairs. Cells that display either normal or reversed hair polarity usually retain wild-type cell hair number (Figure 1H), as has been observed on fy mutant wings (Collier and Gubb 1997).
Figure 3.—
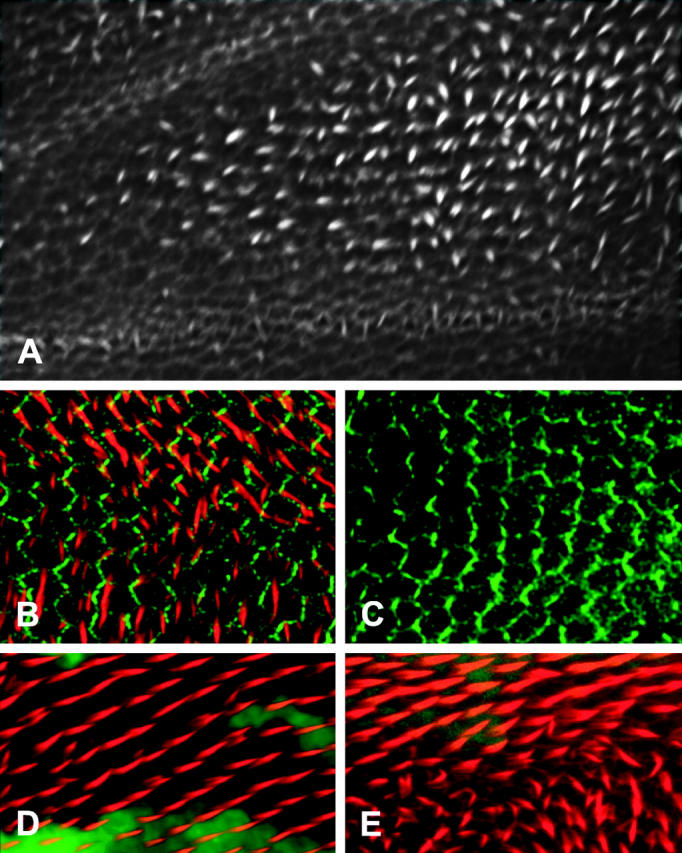
In all images distal is to the right and anterior at the top. (A) A 33-hr frtz2 pupal wing stained for F-actin by Alexa 568 phalloidin. Note the formation of multiple hairs located at the cell periphery. (B) A frtz mutant wing that expresses Fz-GFP (green) and is stained for actin (red-Alexa 568 phalloidin). Note the abnormal hair polarity and number due to the frtz mutation. Note also that the zig-zag pattern of Fz accumulation is present. (C) A frtz mutant wing stained with an anti-Dsh antibody. Once again note the asymmetric accumulation of Dsh in the stereotypic zig-zag pattern. (D and E) frtz clones marked by the loss of GFP. In D the clone was located in the mid-distal part of the “C” cell. This region is only weakly affected by frtz and the clone shows a correspondingly weak phenotype. The clone in E was located more proximally in a region that has a strong frtz phenotype. Note the correspondingly strong phenotype in the clone, that no wild-type cells differentiate in an abnormal way, and that mutant clone cells juxtaposed to wild-type cells can show a mutant phenotype.
The frtz mutant wing phenotype shows a high degree of cell autonomy with respect to both cell polarity and cell hair number. No substantive changes in hair polarity are seen outside of frtz homozygous mutant clones, but even small frtz clones can display hair polarity phenotypes (Figures 2C and 3E). The change of cell hair polarity seen within frtz clones is similar to that at the same position on a frtz homozygous mutant wing. Cells at the edges of homozygous frtz clones often produce additional hairs, whereas cells surrounding frtz homozygous clones never do (Figures 2C and 3, D and E). Similar cell autonomy is shown by the other mutations in other PCP effector genes (Gubb and Garcia-Bellido 1982; Adler et al. 1994; Collier and Gubb 1997) although weak nonautonomy has described for in clones (Park et al. 1996).
The legs of adult frtz mutants display multiple cell hairs of abnormal polarity, multiple bracts, and occasional partially formed ectopic joints in the third and fourth tarsal segments. Similar leg phenotypes are displayed by in mutants (Held et al. 1986; Coulson 1994; Lee and Adler 2002). However, preliminary analysis has not revealed defects in ommatidial rotation or chirality in sectioned frtz mutant eyes although a low level of these have been seen in in mutants (Lee and Adler 2002).
frtz is required for embryonic denticle organization:
Our RT-PCR and in situ hybridization experiments have shown that frtz is expressed during embryogenesis and that frtz transcripts are most abundant in the embryonic epidermis (data not shown). To investigate possible roles for frtz in patterning the embryonic epidermis, cuticle preparations of first instar (L1) larvae from homozygous stocks of the strong alleles frtz1 and frtz33 (Table 1) were made. We found that frtz mutant L1 larvae show abnormal patterning of ventral denticles. In wild-type larvae, rows of evenly spaced denticles of a common orientation (either anteriorly or posteriorly pointing) form the denticle belts (Figure 2E). In frtz mutant larvae, both the spacing and the alignment of denticles within the denticle rows is disrupted especially in the three anterior rows of the belts (Figure 2F). Significantly, frtz mutant denticles still point either anteriorly or posteriorly as in wild type. Individual frtz mutant denticles can also appear smaller or stunted compared to wild type. Similar denticle phenotypes have been reported in mwh mutant larva (Dickinson and Thatcher 1997). The PCP effector genes fy and in are also known to be expressed in the embryo (Park et al. 1996; Collier and Gubb 1997) and we have seen similar denticle phenotypes in L1 larvae of strong fy and in mutants (data not shown). In contrast, it has been reported that alleles of the core PCP genes, e.g., frizzled1 (Dickinson and Thatcher 1997) and pricklepk-sple13 (Gubb et al. 1999), do not affect embryonic denticle structure or organization.
frtz functions downstream of the core PCP genes:
The similarity of PCP mutant polarity patterns (see above) means that epistatic analysis of double mutants is not conclusive for polarity defects (Wong and Adler 1993). However, the directed expression of core PCP genes can produce polarity patterns that are distinctly different from the loss-of-function patterns. To determine whether frtz was required for the directed expression of the core PCP genes to alter hair polarity, we expressed different core PCP genes in a frtz mutant background using the Gal4 UAS system. When the expression of spiny-legs (sple) is driven relatively evenly across the wing using the actin-GAL4 driver, hair polarity is largely reversed. This dramatic gain-of-function phenotype was blocked in wings simultaneously mutant for frtz (Figure 4). We also used omb-GAL4 to drive the expression of UAS-stan. In the wing disc, omb-GAL4 drives expression in a band located centrally along the anterior/posterior axis. In the pupal wing, the expression pattern is more complex and in the distal region of the wing it consists of bands of expression and lack of expression. This leads to bands of polarity changes. This phenotype was also blocked in wings mutant for frtz (Figure 4). Similar results were obtained in analogous experiments using the expression of fz and pk (data not shown). These results are equivalent to those obtained previously for in and fy (Lee and Adler 2002).
Figure 4.—
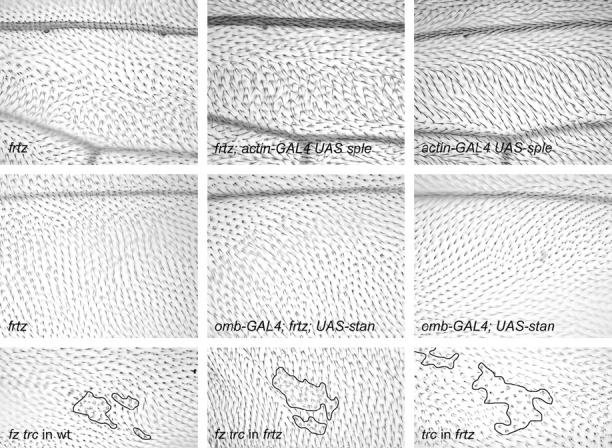
Distal is to the right in all photomicrographs. (Top) Micrographs of the dorsal surface of the wing just anterior to the posterior cross-vein. In the frtz mutant, many multiple hair cells tend to point posteriorly. The overexpression of sple driven by actin-gal4 leads to hairs in this region pointing proximally and anteriorly. This gain-of-function phenotype is blocked in wings simultaneously mutant for frtz. (Middle) Images from the posterior distal region of the wing (“D” cell). In this region of a frtz mutant, wing hairs point posteriorly and a substantial number of cells produce multiple hairs. When omb-GAL4 is used to drive expression of stan, this region of the wing hairs points anteriorly and multiple hair cells are rare. Once again the double mutant shows the frtz mutant phenotype. The requirement for the frtz function of cells to respond to a clone of cells lacking fz function is shown at the bottom. All images are from the posterior region of the wing (“E” cell). A fz trc mutant clone in an otherwise wild-type wing results in the typical fz domineering nonautonomy. In a frtz mutant wing this is not seen. A control trc clone in a frtz mutant wing is also shown. The trc multiple hair phenotype is not suppressed by a frtz mutant.
The presence of a clone of cells that lacks fz function results in neighboring cells responding and producing hairs that appear to be attracted to the clone (Vinson and Adler 1987). Previous experiments have shown that the ability of cells to respond to such a clone requires the function of both planar polarity genes, such as Vang and stan, and PCP effector genes, such as in and fy (Taylor et al. 1998; Chae et al. 1999; Lee and Adler 2002). To determine whether frtz was also required for cells to sense or to respond to a clone of fz mutant cells, we induced clones of fz cells marked with the multiple hair cell marker trc in flies mutant for frtz. As a control we first induced trc clones in a frtz mutant background and found that it was easy to identify the mutant cells on the basis of their hair phenotype (see Figure 4). In regions of frtz mutant wings where hair polarity is reproducible, albeit abnormal, we examined cells surrounding fz trc clones and saw no evidence for the clone acting nonautonomously. Hence, frtz, like in and fy, is required for cells to sense or to respond to a clone of cells lacking fz function (Lee and Adler 2002).
Mutations in other PCP effector genes are known to not block the assymetric accumulation of core PCP proteins such as Fz, Dsh, Stan, etc. (Usui et al. 1999; Strutt 2001). To determine if this was also the case for frtz, we examined the location of Fz, Dsh, and Stan in frtz mutant wings. All three of these proteins localized asymmetrically (Figure 3, B and C; data not shown for stan) in frtz mutant wing cells as is the case for in and fy mutant wings. This is consistent with frtz functioning downstream of the core PCP genes.
Mapping of the frtz locus:
There have been three independent isolations of frtz alleles: the frtz1 allele was recovered from an EMS screen by Allan Shiras at the University of Lancaster, Lancaster, United Kingdom; the frtz2 allele was identified during a screen for new pk mutants at the University of Cambridge, Cambridge, United Kingdom; and the frtz26 allele [originally fuzzy's-twin (fyt)] was recovered from an F1 FLP-FRT EMS screen for wing hair phenotypes at the University of Virginia, Charlottesville, Virginia. Seven additional frtz alleles have been recovered from F1 X-ray screens at the University of Cambridge and the University of Virginia (Table 1).
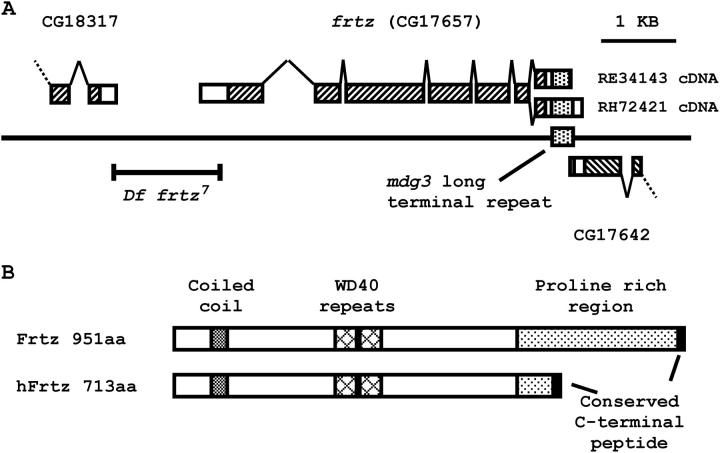
The frtz locus is uncovered by both Df(2L)S2 (21C8-D1;22A8-B1) and Df(2L)dp-79b (22A2-3;22D5-E1), placing it within the cytological region 22A2-3–22A8-B1. This localization is supported by the cytology of deficiencies recovered from an X-ray screen for new frtz mutants (Table 2). frtz is also uncovered by the small deletion Df(2L)F.1. Genetically, the distal breakpoint of Df(2L)F.1 is proximal to the capping protein beta (cpb) locus (M. Welte, unpublished data) and we have molecularly mapped the proximal endpoint of Df(2L)F.1 to between the P{lacW}k09624 insertion site and the distal break of a transposition event on the frtz22 chromosome within transcript CG17646 (position 1722500 on the GadFly annotated genome map). We screened frtz homozygous or hemizygous mutant genomic DNA by PCR to identify rearrangements or point mutations within seven of the eight candidate genes in this interval (CG17660, CG17642, CG17657, CG18317, CG17711, CG17652, and CG17646). The gene Eno (CG17654), which encodes a phosphopyruvate hydratase, was not considered a strong candidate for a PCP gene. We have identified putative deleterious mutations at the CG17657 locus on nine independent frtz mutant chromosomes (Table 1). We were able to confirm that these mutations were not present on the progenitor chromosome (or on an independent mutant chromosome from the same screen) for the seven alleles where these chromosomes were available, establishing that CG17657 is the frtz gene.
TABLE 2.
fritz deficiencies
| Deficiencies | Original name |
Mutagen | Source | Cytology |
|---|---|---|---|---|
| fritz11 | GJ66/41 | X ray | Cambridge | 22A1;B6-9 |
| fritz14 | GJ66/54 | X ray | Cambridge | 22A3;B3 |
| fritz19 | GJ66/69 | X ray | Cambridge | 22A2.3;B1 |
| fritz24 | GJ66/79 | X ray | Cambridge | 22A3.4;B1 |
| fritz25 | GJ66/83 | X ray | Cambridge | 22A3.4;C1.2 |
frtz encodes an evolutionarily conserved WD40 repeat protein:
The frtz transcript encodes a polypeptide of 951 amino acids with a predicted molecular weight of 106 kD. A single homologous protein is encoded by the human (Homo sapiens), mouse (Mus musculus), puffer fish (Fugu ruprides), and mosquito (Anopheles gambiae) genomes. The N-terminal 620 amino acids of fruit fly Frtz shares 30% amino acid identity with the mammalian Frtz proteins, which is evenly distributed over this region (Figure 5B). However, the next 320 amino acids of the fly Frtz protein are not conserved in the mammalian proteins. This unique region of the fly protein contains 14.5% proline and is predicted to fold as a random coil. The equivalent region of the mammalian Frtz proteins is shorter and not significantly proline rich but is remarkably diverged in sequence between mouse and human, showing just 45% identity compared with 82% for the rest of the protein. Despite this high degree of evolutionary variability, the nonconservative substitution of a lysine by a methionine in this region encoded by the frtz27 allele is associated with a strong frtz phenotype (Table 1). This suggests a functional constraint on this part of the Frtz protein that is either specific to planar polarity in Drosophila or not dependent on overall amino acid sequence conservation. The fly, mouse, and human Frtz proteins share a highly conserved hydrophobic 10-amino-acid peptide at the extreme C terminus of the protein (Figure 6C).
Figure 5.—
(A) Map of the frtz (CG17657) locus at chromosome band 22B1. Boxes represent exons; hatched regions within the boxes represent coding sequence. The presence of the mdg3 long terminal repeat sequence is indicated by a stippled box in both genomic DNA and transcripts. The RH72421 adult head cDNA and the RE34143 embryonic cDNA have different 3′-ends (Rubin et al. 2000). RH72421 extends beyond the mdg3 repeat and polyadenylates 113 nucleotides downstream within the 3′ untranslated region of the CG17642 gene. In contrast, RE34143 polyadenylates 17 nucleotides downstream of the native mdg3 polyA signal sequence (Arkhipova et al. 1986), suggesting that it has adopted the mdg3 signal sequence. (B) The comparative domain structure of the fruit fly Frtz and human Frtz (hFrtz) proteins. aa, amino acids.
Figure 6.—
Evolutionary conservation of Frtz protein domains. (A) Alignment by homology of the predicted N-terminal coiled-coil regions in Frtz proteins from fruit fly (Frtz), mosquito (aFrtz), mouse (mFrtz), and human (hFrtz). The phase of the heptad repeats is indicated below; the a and d (in boldface type) residues are conventionally hydrophobic. (B) Alignment by homology of the Frtz WD40a and WD40b repeats from fruit fly (Frtz), mosquito (aFrtz), puffer fish (fFrtz), mouse (mFrtz), and human (hFrtz) proteins. (C) Alignment by homology of the Frtz C terminus of fruit fly (Frtz), mouse (mFrtz), and human (hFrtz) proteins. All alignments were produced by the T-Coffee algorithm (Notredame et al. 2000). Residues identical to the Drosophila sequence are in boldface type. An asterisk (*) indicates absolute evolutionary conservation; (.) and (:) indicate increasing degrees of conservation as defined by the T-Coffee algorithm. The Frtz proteins cited are aFrtz, A. gambiae Frtz accession no. EAA03756; fFrtz, F. ruprides Frtz accession no. JGI 12262; mFrtz, M. musculus Frtz accession no. AAL24810; hFrtz, H. sapiens Frtz accession no. AAD20026.
The Frtz protein is predicted by the COILS program (Lupas et al. 1991) to have a short N-terminal coiled-coil region consisting of five heptad repeats. The phase of the Frtz coiled-coil region and its alignment with other Frtz proteins is shown is Figure 6A. Coiled-coil regions are known to mediate protein multimerization. The MultiCoil program (Wolf et al. 1997) predicts that the fly Frtz coiled coil forms a trimeric structure although the equivalent region in human Frtz is predicted to form a dimer.
A single WD40 repeat is strongly predicted in the fly Frtz protein by the SMART annotation tool (Schultz et al. 1998). SMART also predicts a WD40 repeat at an equivalent position in both human and mouse Frtz proteins with a second WD40 repeat immediately C-terminal to it. WD40 repeats fold together to form a β-propeller structure that provides surfaces for protein-protein interaction (Smith et al. 1999). The alignment of the Frtz WD40a and WD40b repeats from all of the Frtz sequences currently available is shown in Figure 6B. The sequences of the two Frtz WD40 repeats are unusual as both lack the highly conserved histidine in the loop between the propeller blades (between strands d and a) and WD40b also lacks the conserved aspartate normally present in the tight turn between strand b and strand c.
A frtz WD40b mutation interacts synergistically with hypomorphic fuzzy and inturned alleles:
The importance of the second Frtz WD40 repeat (WD40b) for the stability or function of the Frtz protein is demonstrated by the frtz29 allele, which encodes a protein that lacks the C-terminal 24 amino acids of WD40b and the adjacent 10 C-terminal amino acids and has a strong frtz phenotype (Table 1). In contrast, the frtz3 allele, which has a missense mutation resulting in the nonconservative substitution of aspartate by alanine at the C terminus of Frtz WD40b (Figure 7A), is associated with only a weak hypomorphic temperature-sensitive phenotype. At the permissive temperature of 18° the wing hair phenotype of homozygous or hemizygous frtz3 flies is close to wild type (Figure 7B). Such a weak phenotype is surprising as the terminal Frtz WD40b aspartate is absolutely conserved in both vertebrate and invertebrate Frtz proteins (Figure 6B). However, an alanine is present at the C terminus of ∼2% of WD40 repeats (Yu et al. 2000), suggesting that it does not preclude the formation of a standard WD40 β-propeller structure. At 29° homozygous or hemizygous frtz3 flies show moderate changes in hair polarity and cell hair number (Figure 7C), similar to strong cold-sensitive frtz mutants at this temperature (Figure 1G). It appears, therefore, that the frtz3 gene product is almost completely active at 18° and almost completely inactive at 29°.
Figure 7.—
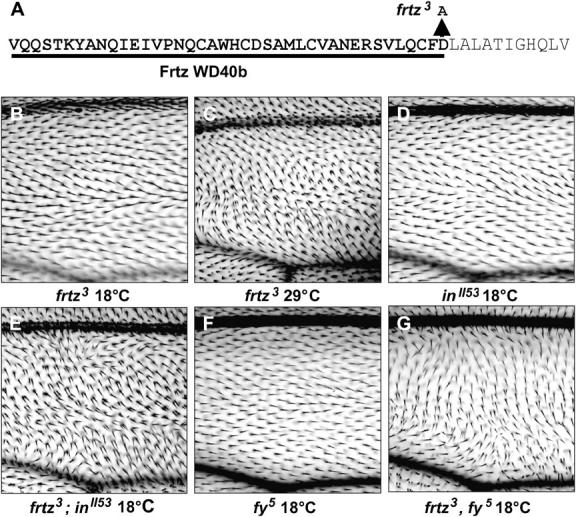
A frtz WD40 repeat mutation interacts synergistically with other hypomorphic PCP effector gene alleles. (A) The Asp-to-Ala substitution in the WD40b repeat of the frtz3 gene product. (B–G) Photomicrographs of the “C” region of PCP effector gene mutant wings immediately anterior to the PCV; the proximal-distal axis of the wings runs from left to right. Single mutants at 18° show an almost wild-type wing cell hair phenotype; double mutants show strong polarity and hair number phenotypes.
The frtz3allele shows strong synergistic interactions with the hypomorphic PCP effector gene alleles inII53 and fy5. The inII53 allele has a temperature-sensitive phenotype that is strikingly similar to frtz3 (Adler et al. 1994) (Figure 7D) and encodes a mutant protein with an additional nine C-terminal amino acids due to a point mutation in the normal termination codon. The fy5 allele is associated with a mild abdominal bristle phenotype but has virtually no effect on wing hair polarity (Figure 7F) and does not display temperature sensitivity. Molecularly, the fy5 allele has a nonsense mutation at codon 331 and so encodes a Fy protein with the C-terminal 85 amino acids deleted (Collier and Gubb 1997). Homozygous frtz3; inII53 mutant flies have a wing hair phenotype that closely resembles the cold-sensitivity phenotype of strong frtz or in alleles (Figure 7E). Therefore, although frtz3 and inII53 have little effect on cell polarity at 18°, the combination of the two alleles at the same temperature appears to completely block PCP effector gene function. The frtz3, fy5 allele combination also shows a strong synergistic interaction at 18° (Figure 7G) although the frtz3, fy5 phenotype is weaker than the frtz3; inII53 phenotype and shows temperature sensitivity.
DISCUSSION
The Frtz protein has dual functions in cytoskeletal regulation:
The frtz wing phenotype is characterized by altered cell hair polarity and an increase in cell hair number. This is a consequence of the formation of multiple F-actin-rich prehairs at aberrant sites around the apical periphery of the pupal wing cell. It is clear that the formation of multiple hairs is not an inevitable consequence of the loss of normal planar cell polarity since core PCP gene mutant wings display a low incidence of multiple hair cells. This implies that the Frtz protein has two distinct activities. The first is to link prehair localization to the subcellular localization of the core PCP proteins. The second is to restrict prehair initiation to a single site.
In wild-type wings, a prehair forms at the distal periphery of the cell but later translocates to the apical center where the mature hair is found in the adult wing. Prehairs in developing frtz mutant wings initiate at a different location along the apical periphery of the cell than in wild type but are found at the final same location in the adult wing. Therefore, the activity that translocates the developing wing hair from the cell periphery to the apical center must be independent of the position at which the prehair forms. One possibility is that this translocation process is microtubule driven as developing wing cells have webs of microtubules that connect the apical center of the cell to points on the periphery (Eaton et al. 1996). This mechanism would require that the microtubule network is still intact in frtz mutant epithelial cells and consistent with this hypothesis we have not found abnormalities in the microtubule cytoskeleton of frtz pupal wing cells. On a frtz mutant wing, cells that produce hairs with distal or proximal polarity almost invariably produce a single hair, implying that there is little requirement for frtz in restricting the number of sites of prehair initiation at the distal or proximal vertices of the cell. Perhaps the localization of the core PCP proteins at the distal and proximal ends of the pupal wing cell stabilizes hair formation and overcomes the requirement of the Frtz protein for restricting hair number.
The larval denticle phenotypes displayed by frtz and other PCP effector mutants do not appear to involve changes in epithelial cell planar polarity as individual denticles continue to point to either the posterior or the anterior of the embryo as in wild type. As this phenotype does not involve altered polarity and denticle organization does not require the core PCP genes, it is possible that the frtz denticle phenotype has the same origin as the multiple cell hair phenotype shown in frtz mutant wings. The reason that both frtz and mwh mutations (Dickinson and Thatcher 1997) preferentially disrupt denticles at the anterior end of the belts is not clear although this directionality is reminiscent of the way in which frtz and in mutants preferentially affect development of the distal over the proximal tarsal leg joints (Held et al. 1986; Coulson 1994; Lee and Adler 2002).
Frtz may be part of an evolutionarily conserved PCP effector protein complex:
The Frtz protein contains multiple domains that might mediate protein-protein interaction, which appears appropriate given its putative role in linking the actin cytoskeleton to the localized core PCP proteins. The Frtz WD40 repeats are most closely related to the third and fourth WD40 repeats in the p80 subunit of the microtubule-severing protein katanin, which target the protein to the centrosome, the organizer of the microtubule cytoskeleton (Hartman et al. 1998). It is possible that an association between Fritz and the microtubule cytoskeleton is required to regulate wing cell hair number as a microtubule antagonist mimics the frtz multiple wing hair phenotype (Turner and Adler 1998). The Frtz protein may homodimerize to bring together the four WD40 repeats required to form the archetypal β-propeller structure as has been proposed for proteins with less than four WD40 repeats (Smith et al. 1999). However, the homology with p80 katanin extends upstream of the Frtz WD40 repeats and into the second katanin WD40 repeat, suggesting that there is weak WD40 homology outside of Frtz WD40 repeats. Therefore, cryptic WD40-like sequences that can fold with the WD40a and b repeats to form a β-propeller-like structure may be present in the Frtz protein. Another potential protein interaction domain in Frtz is the evolutionarily conserved hydrophobic C terminus, which is a potential PDZ domain-binding motif (Harris and Lim 2001).This raises the possibility that Frtz physically interacts with PDZ PCP proteins such as the PCP effector protein Inturned or the core PCP protein Dishevelled.
The strong synergistic interactions among the hypomorphic frtz3, fy5, and inII53 alleles are not indicative of a functional redundancy among the PCP effector genes as double mutants of strong PCP effector alleles are not quantitatively stronger than single alleles (Wong and Adler 1993). Therefore it might reflect a threshold effect in which a combination of weak alleles decreases PCP signaling beyond a critical point where little or no signal is transduced. One possibility is that normal PCP signaling requires the PCP effector proteins to form a protein complex. PCP signaling would be lost if a combination of mutant protein components cause this complex to physically dissociate. Supportive evidence for this idea comes from the fact that the frtz3 mutation is within a putative protein-protein interaction domain and that the PCP effector proteins Fy and In have previously been shown to physically interact in Drosophila cell culture and transgenic flies (Lee 2002).
In both invertebrate and vertebrate genomes the frtz, fy, and in genes are present in single copy. The simplest assumption is that these genes arose prior to the branching of these evolutionary lines. Without gene duplication to introduce diversity into the function of these genes it is likely that they have maintained essentially the same functions in both invertebrates and vertebrates. If the PCP effector proteins function in a complex to control cytoskeletal integrity and polarity in invertebrates, then they most likely do in vertebrates and so observations made in Drosophila should have a wider evolutionary relevance.
Acknowledgments
Thanks go to Glynnis Johnson and John Roote in Cambridge and to Jeannette Charlton and Sreenatha Kirakodu in Virginia for help with mutant screens; to Michael Welte for sharing unpublished chromosomal deficiencies and mapping information; to Marian Wilkin for sectioning frtz mutant eyes; to Erica Anderson-Smith for discussion of denticle phenotypes; and to David Gubb and Maiyon Park for their valuable input. Work at the University of Virginia was supported by a grant from the National Institutes of Health to P.N.A., at the University of Manchester by a Royal Society grant to S.C., and at Marshall University by National Science Foundation award no. 0132740.
References
- Adler, P. N., 2002. Planar signaling and morphogenesis in Drosophila. Dev. Cell 2: 525–535. [DOI] [PubMed] [Google Scholar]
- Adler, P. N., J. Charlton and W. J. Park, 1994. The Drosophila tissue polarity gene inturned functions prior to wing hair morphogenesis in the regulation of hair polarity and number. Genetics 137: 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkhipova, I. R., A. M. Mazo, V. A. Cherkasova, T. V. Gorelova, N. G. Schuppe et al., 1986. The steps of reverse transcription of Drosophila mobile dispersed genetic elements and U3-R-U5 structure of their LTRs. Cell 44: 555–563. [DOI] [PubMed] [Google Scholar]
- Axelrod, J. D., 2001. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 15: 1182–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastock, R., H. Strutt and D. Strutt, 2003. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development 130: 3007–3014. [DOI] [PubMed] [Google Scholar]
- Chae, J., M. J. Kim, J. H. Goo, S. Collier, D. Gubb et al., 1999. The Drosophila tissue polarity gene starry night encodes a member of the protocadherin family. Development 126: 5421–5429. [DOI] [PubMed] [Google Scholar]
- Collier, S., and D. Gubb, 1997. Drosophila tissue polarity requires the cell-autonomous activity of the fuzzy gene, which encodes a novel transmembrane protein. Development 124: 4029–4037. [DOI] [PubMed] [Google Scholar]
- Cong, J., W. Geng, B. He, J. Liu, J. Charlton et al., 2001. The furry gene of Drosophila is important for maintaining the integrity of cellular extensions during morphogenesis. Development 128: 2793–2802. [DOI] [PubMed] [Google Scholar]
- Coulson, D., 1994 The genetic analysis of prickle and spiny-legs: two cuticular polarity mutants of Drosophila melanogaster. Ph.D. Thesis, University of Cambridge, Cambridge, UK.
- Das, G., A. Jenny, T. J. Klein, S. Eaton and M. Mlodzik, 2004. Diego interacts with Prickle and Strabismus/Van Gogh to localize planar cell polarity complexes. Development 131: 4467–4476. [DOI] [PubMed] [Google Scholar]
- Dickinson, W. J., and J. W. Thatcher, 1997. Morphogenesis of denticles and hairs in Drosophila embryos: involvement of actin-associated proteins that also affect adult structures. Cell Motil. Cytoskeleton 38: 9–21. [DOI] [PubMed] [Google Scholar]
- Eaton, S., R. Wepf and K. Simons, 1996. Roles for Rac1 and Cdc42 in planar polarization and hair outgrowth in the wing of Drosophila. J. Cell Biol. 135: 1277–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng, W., B. He, M. Wang and P. N. Adler, 2000. The tricornered gene, which is required for the integrity of epidermal cell extensions, encodes the Drosophila nuclear DBF2-related kinase. Genetics 156: 1817–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubb, D., and A. Garcia-Bellido, 1982. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J. Embryol. Exp. Morphol. 68: 37–57. [PubMed] [Google Scholar]
- Gubb, D., C. Green, D. Huen, D. Coulson, G. Johnson et al., 1999. The balance between isoforms of the prickle LIM domain protein is critical for planar polarity in Drosophila imaginal discs. Genes Dev. 13: 2315–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, B. Z., and W. A. Lim, 2001. Mechanism and role of PDZ domains in signaling complex assembly. J. Cell Sci. 114: 3219–3231. [DOI] [PubMed] [Google Scholar]
- Hartman, J. J., J. Mahr, K. McNally, K. Okawa, A. Iwamatsu et al., 1998. Katanin, a microtubule-severing protein, is a novel AAA ATPase that targets to the centrosome using a WD40-containing subunit. Cell 93: 277–287. [DOI] [PubMed] [Google Scholar]
- Held, L. I., C. M. Duarte and K. Derakhshanian, 1986. Extra tarsal joints and abnormal cuticular polarities in various mutants of Drosophila melanogaster. Roux's Arch. Dev. Biol. 195: 145–157. [DOI] [PubMed] [Google Scholar]
- Lee, H., 2002 Genetic and biochemical studies of Drosophila planar polarity. Ph.D. Thesis, University of Virginia, Charlottesville, VA.
- Lee, H., and P. N. Adler, 2002. The function of the frizzled pathway in the Drosophila wing is dependent on inturned and fuzzy. Genetics 160: 1535–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H., and P. N. Adler, 2004. The grainy head transcription factor is essential for the function of the frizzled pathway in the Drosophila wing. Mech. Dev. 121: 37–49. [DOI] [PubMed] [Google Scholar]
- Lupas, A., M. Van Dyke and J. Stock, 1991. Predicting coiled coils from protein sequences. Science 252: 1162–1164. [DOI] [PubMed] [Google Scholar]
- McNeill, H., 2002. Planar polarity: location, location, location. Curr. Biol. 12: R449–R451. [DOI] [PubMed] [Google Scholar]
- Mitchell, H. K., J. Roach and N. S. Petersen, 1983. The morphogenesis of cell hairs on Drosophila wings. Dev. Biol. 95: 387–398. [DOI] [PubMed] [Google Scholar]
- Newby, L. M., L. White, S. M. DiBartolomeis, B. J. Walker, H. B. Dowse et al., 1991. Mutational analysis of the Drosophila miniature-dusky (m-dy) locus: effects on cell size and circadian rhythms. Genetics 128: 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notredame, C., D. G. Higgins and J. Heringa, 2000. T-Coffee: a novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 302: 205–217. [DOI] [PubMed] [Google Scholar]
- Park, W. J., J. Liu, E. J. Sharp and P. N. Adler, 1996. The Drosophila tissue polarity gene inturned acts cell autonomously and encodes a novel protein. Development 122: 961–969. [DOI] [PubMed] [Google Scholar]
- Rubin, G. M., L. Hong, P. Brokstein, M. Evans-Holm, E. Frise et al., 2000. A Drosophila complementary DNA resource. Science 287: 2222–2224. [DOI] [PubMed] [Google Scholar]
- Schultz, J., F. Milpetz, P. Bork and C. P. Ponting, 1998. SMART, a simple modular architecture research tool: identification of signaling domains. Proc. Natl. Acad. Sci. USA 95: 5857–5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, T. F., C. Gaitatzes, K. Saxena and E. J. Neer, 1999. The WD repeat: a common architecture for diverse functions. Trends Biochem. Sci. 24: 181–185. [DOI] [PubMed] [Google Scholar]
- Strutt, D. I., 2001. Asymmetric localization of frizzled and the establishment of cell polarity in the Drosophila wing. Mol. Cell 7: 367–375. [DOI] [PubMed] [Google Scholar]
- Strutt, D. I., 2002. The asymmetric subcellular localisation of components of the planar polarity pathway. Semin. Cell Dev. Biol. 13: 225–231. [DOI] [PubMed] [Google Scholar]
- Strutt, D. I., U. Weber and M. Mlodzik, 1997. The role of RhoA in tissue polarity and Frizzled signalling. Nature 387: 292–295. [DOI] [PubMed] [Google Scholar]
- Taylor, J., N. Abramova, J. Charlton and P. N. Adler, 1998. Van Gogh: a new Drosophila tissue polarity gene. Genetics 150: 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tree, D. R., D. Ma and J. D. Axelrod, 2002. a A three-tiered mechanism for regulation of planar cell polarity. Semin. Cell Dev. Biol. 13: 217–224. [DOI] [PubMed] [Google Scholar]
- Tree, D. R., J. M. Shulman, R. Rousset, M. P. Scott, D. Gubb et al., 2002. b Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell 109: 371–381. [DOI] [PubMed] [Google Scholar]
- Turner, C. M., and P. N. Adler, 1998. Distinct roles for the actin and microtubule cytoskeletons in the morphogenesis of epidermal hairs during wing development in Drosophila. Mech. Dev. 70: 181–192. [DOI] [PubMed] [Google Scholar]
- Usui, T., Y. Shima, Y. Shimada, S. Hirano, R. W. Burgess et al., 1999. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell 98: 585–595. [DOI] [PubMed] [Google Scholar]
- Vinson, C. R., and P. N. Adler, 1987. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature 329: 549–551. [DOI] [PubMed] [Google Scholar]
- Winter, C. G., B. Wang, A. Ballew, A. Royou, R. Karess et al., 2001. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell 105: 81–91. [DOI] [PubMed] [Google Scholar]
- Wolf, E., P. S. Kim and B. Berger, 1997. MultiCoil: a program for predicting two- and three-stranded coiled coils. Protein Sci. 6: 1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, L. L., and P. N. Adler, 1993. Tissue polarity genes of Drosophila regulate the subcellular location for prehair initiation in pupal wing cells. J. Cell Biol. 123: 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, L., C. Gaitatzes, E. Neer and T. F. Smith, 2000. Thirty-plus functional families from a single motif. Protein Sci. 9: 2470–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]