Abstract
Dosage compensation refers to the equal expression of X-linked genes despite the difference in copy number between the two sexes. The male-specific lethal (MSL) complex is concentrated on the X chromosome in males. A gene expression assay for embryos was developed to examine the function of this complex. In mutant male embryos without either the MSL complex or MOF histone acetylase, dosage compensation is retained but autosomal expression is increased. Dosage compensation is lost in the double-mutant embryos. In embryos in which the MSL complex and MOF are targeted to the X chromosomes in females, the results are consistent with previous surveys showing that in general the X expression remains unchanged, but autosomal expression is reduced. Mutations in the ISWI chromatin-remodeling component cause increases specifically of X-linked genes in males. Thus, the function of the MSL complex in conjunction with ISWI is postulated to override the effect on gene expression of high histone acetylation on the male X. The basic determinant of dosage compensation is suggested to be an inverse dosage effect produced by an imbalance of transcription factors on the X vs. the autosomes. The sequestration of the MSL complex to the male X may have evolved to counteract a similar effect on the autosomes and to prevent an overexpression of the X chromosome in males that would otherwise occur due to the high levels of histone acetylation.
DOSAGE compensation mechanisms equalize the expression of genes between the sexes despite the difference in sex chromosomal dosage. In Drosophila, the transcription of most X-linked genes in males with only one X chromosome is doubled to achieve an activity equal to that of the two X's in females (Muller 1932; Arkhipova et al. 1997).
Changes in X chromosome chromatin organization between the sexes have occurred in Drosophila as evidenced by the high accumulation of the male-specific lethal (MSL) chromatin remodeling complex on the X chromosome in males. The protein products of at least six genes (mle, msl1, msl2, msl3, mof, and JIL-1) and two noncoding RNAs (roX1 and roX2) have been identified in this complex (Kelley and Kuroda 2000). At least two histone modifiers (MOF, JIL-1 kinase) are sequestered from the autosomes (Hilfiker et al. 1997; Bhadra et al. 1999; Jin et al. 2000; this study) by the complex. Because the MSL complex binds the male X at high concentration, the argument has been put forth that the MSL complex and the associated histone modifications are directly responsible for X chromosomal dosage compensation (Belote and Lucchesi 1980; Kuroda et al. 1991; Bone et al. 1994; Jin et al. 1999).
However, several gene expression studies have now indicated that the mutational effect of the msl mutants, in which the MSL complex is destroyed, is that the majority of X-linked genes remain compensated and many autosomal genes are increased in expression, as the most common effect (Hiebert and Birchler 1994; Bhadra et al. 1999, 2000). A similar result was found in microarray analysis of germline expression (Parisi et al. 2003), where the MSL complex is not present (Rastelli and Kuroda 1998), but dosage compensation of the X still occurs and many autosomal genes exhibit male-biased expression. These gene expression results suggest a function for the MSL complex different from mediating dosage compensation directly.
The MSL proteins (minus MSL-2) are associated with all female chromosomes at a low uniform level but are sequestered to the X chromosome in males (plus MSL-2) (Bhadra et al. 1999). The histone modifiers follow a similar pattern and therefore are higher on the X and lower on the autosomes in males compared to females. In msl mutant males, the MSL binding to the X chromosome is disrupted, resulting in MOF and JIL-1 becoming distributed on all chromosomes (Bhadra et al. 1999, 2000; this study), which results in an increase of acetylation level on the autosomes. The increased autosomal gene expression in the msl males parallels the increase of histone acetylation.
In contrast, most X-linked genes do not respond to the high levels of histone acetylation present on the normal male X (Hiebert and Birchler 1994; Bhadra et al. 1999, 2000). Thus, the co-localization of the MSL complex and the histone modifiers under normal circumstances suggests that some components of the MSL complex have the unique activity of overriding the response of genes to histone acetylation. Indeed, when the MSL complex is targeted to the X chromosomes in females, there is no overall increase of X chromosomal gene expression, but there is a reduction in expression of autosomal genes (Bhadra et al. 1999, 2000).
Given these initial studies of gene expression in msl mutants, we conducted experiments to understand the function of the MSL complex. In this study, we report evidence that the MSL complex insulates X-linked genes from the effects of histone acetylation. This property prevents an overexpression of X-linked genes in normal males but still allows a twofold upregulation to achieve the proper level of dosage compensation. We postulate that this upregulation results from a twofold imbalance of transcription factors on the X relative to the autosomes, which causes a twofold negative dosage effect on target gene expression. Such trans-acting dosage effects have been observed in a wide range of aneuploids in different species (Birchler 1979; Devlin et al. 1988; Guo and Birchler 1994; Birchler et al. 2001; Phillips et al. 2001; Bahn et al. 2002; FitzPatrick et al. 2002; Veitia 2002; Kaushal et al. 2003; Matzke et al. 2003; Saran et al. 2003; Lyle et al. 2004) and specifically with the Drosophila X chromosome (Birchler 1992).
Our results suggest that at least one required function for the override of histone acetylation is provided by ISWI, an ATP-dependent chromatin remodeling component (Corona et al. 2002). Mutations in this gene result in overexpression of the male X, indicating a novel involvement in the override of histone acetylation in conjunction with the MSL complex. This overexpression lends further support to the concept that the MSL complex counteracts the impact of histone acetylation on gene expression. The data suggest that MSL sequestration and action equalizes both X and autosomal gene expression between the sexes despite the chromosomal imbalance in Drosophila males.
MATERIALS AND METHODS
Drosophila strains and crosses:
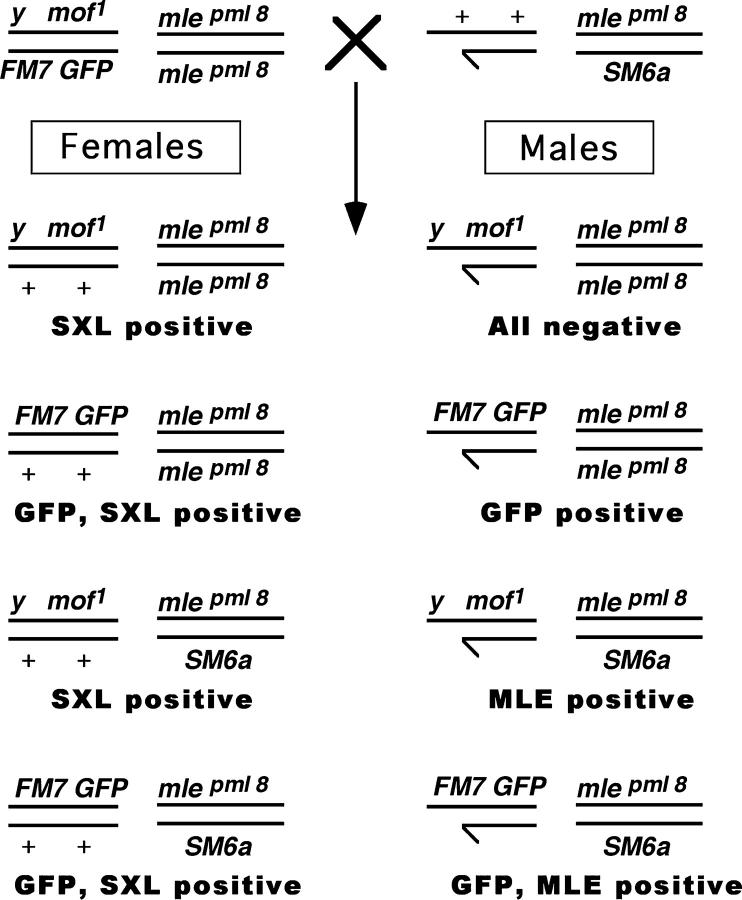
All stocks mentioned in the text are described in FlyBase (http://flybase.bio.indiana.edu). To determine the effect of the males absent on the first (mof1) and maleless (mlepml8) mutations on gene expression, we generated a double-mutant stock using multiple balancer chromosomes. The stock contains balancer chromosomes with dominant and green fluorescent protein (GFP) markers to distinguish each genotype in a segregating population at different developmental stages. The GFP marker facilitates genotyping of progeny at all developmental stages. Females from this stock (y1 mof1/FM7 GFP; mlepml8/mlepml8) were mated with mle heterozygous males (+/Y; mlepml8/CyO). Eight different F1 classes of embryos can be identified (Figure 1). The sex and genotype were determined by immunostaining for Sex lethal (SXL), MLE, and the presence of GFP.
Figure 1.—
Genetic cross and resulting progeny to generate a segregating population for mle and mof. The cross +/Y; mle[pml8]/SM6a × y[1] mof[1]/FM7(GFP); mle[pml8]/mle[pml8] produces male progeny that are segregating for both mle and mof. The four types of males are normal [FM7(GFP)], mutant for mle alone (mle[pml8]/mle[pml8]), mutant for mof alone (mof[1]), and mutant for both mof and mle (mof[1]; mle[pml8]/mle[pml8]). The presence or absence of GFP, SXL, and MLE can be used to distinguish all four classes of males.
The cross to examine the effect of ISWI on X and autosomal gene expression was w−/Y; CyO GFP/ISWI1 × w−/w−; CyO GFP/ISWI2. Each individual allele was also examined separately. This cross produces a segregating progeny of males and females of normal and mutant ISWI. The white gene was not examined in this case because the X chromosome carries a mutant allele.
The cross to examine the effect of the Sxl mutation on X and autosomal gene expression was Sxlfhv/Y × Sxlf1/FM7 GFP. This cross produces four types of progeny: Sxlf1/Sxlfhv, Sxlfhv/FM7 GFP, Sxlf1/Y, and FM7 GFP/Y. The genes examined in this case could not include yellow and white because mutant alleles were present in some genotypes.
A quantitative fluorescent RNA in situ assay:
The gene expression assay in embryos is based on fluorescent RNA in situ hybridization. The MSL complex is sequestered to the X chromosome at the blastoderm stage (Franke et al. 1996). We examined gene expression of X and autosomal genes shortly thereafter, but well before the msl mutant lethal phase at the larval/pupal transition. Embryos at approximately stage 13 (Campos-Ortega and Hartenstein 1985) were selected for analysis. In a confocal microscopic field, embryos can be identified at a particular developmental stage on the basis of their morphology. By using a combination of balancer chromosomes carrying GFP, SXL antibody staining to determine sex, and MLE antibody labeling to classify mutants, one can distinguish the different genotypes. These determinations were accomplished using UV and multiple channels of laser excitation in confocal microscopy as described below. SXL and MSL antibodies are detected with fluorescent-tagged secondary antibodies. With digoxygenin-tagged UTP incorporated into antisense RNA probes followed by fluorescent-tagged antibody detection, one can identify the messenger RNA of specific genes using otherwise standard techniques for RNA in situ hybridization (Tautz and Pfeifle 1989).
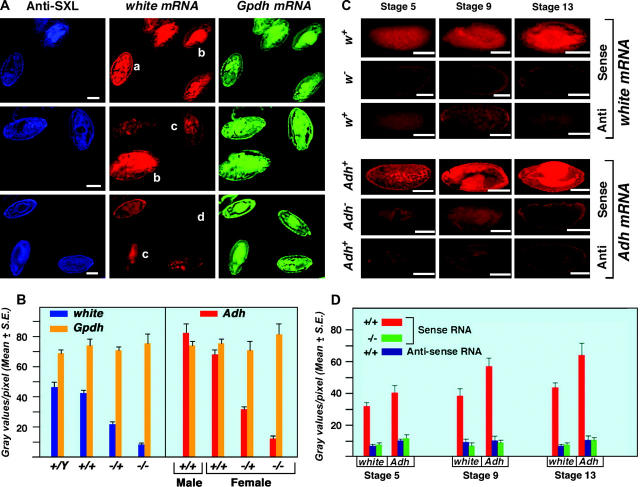
The ability of this assay to detect twofold differences in gene expression was determined by producing segregating populations of embryos heterozygous and homozygous for white− and Adh− (Alcohol dehydrogenase) (Figure 2). The white gene is present on the X chromosome and has been used extensively in studies of dosage compensation. Adh resides on chromosome 2. After adjustment of the raw values for background in the white deficiency embryos, females heterozygous for a deficiency of white (w67c23) have 47% of the measured expression as do homozygous normal females. An equal dosage of autosomal α-Glycerophosphate dehydrogenase (α-Gpdh) in each genotype acts as an internal control. Heterozygotes for Adh (Adhfn6/+) contribute 54% of the homozygous normal level (Figure 2). Background levels were determined by probing the null for white and Adhfn6, which contributes only 5–10% of the normal transcript level. The measurement of transcripts from 21 embryos at approximately stage 13 for each genotype exhibits standard errors that are typically only a small percentage of the mean. These data indicate that a twofold difference can be robustly detected by this technique. We also note that while the expressions of white and Adh in adults are at opposite ends of the spectrum for Drosophila genes, they are more similar in embryos and span the range of expression of the genes examined.
Figure 2.—
Development of a quantitative fluorescent gene expression assay. (A) Embryos stained with SXL antibodies and probed with antisense white and α-Gpdh RNAs. The anti-SXL labeling (blue) distinguishes the sex of the embryos. Females express SXL while males do not. The w mRNA hybridization was visualized using Cy3-conjugated UTP incorporated into the antisense probe. α-Gpdh mRNA was hybridized with an antisense probe labeled with Cy5-conjugated UTP. The pattern of Canton-S males and females is shown at the top. A segregating progeny of normal males and w67c23/+ heterozygous females, generated by crossing Canton-S females by males of a white deficiency strain (w67c23), are in the middle. A population of w−/+ female and w−/Y male embryos, which result from the reciprocal cross, are shown at the bottom. The genotype of each embryo is (a) Canton-S female, (b) Canton-S male, (c) w−/+ female, and (d) w− male. Bars, 50 μm. (B) A quantitative measurement of w, Adh, and α-Gpdh mRNA in embryos segregating for normal or null alleles of white or Adh, respectively. The white genotypes are described above. The heterozygous Adh normal/null genotype was produced by crossing Adhfn6 by Canton-S wild type, whereas the null homozygotes were obtained from an Adhfn6/ Adhfn6 stock. Normal males and females were obtained from the Canton-S stock. The Adh mRNA was detected with a digoxygenin-labeled antisense probe followed by incubation with Cy5-conjugated anti-dioxygenin antibodies. The same specific probe preparation for each of the three genes analyzed was used on all genotypes. Twenty-one embryos at approximately stage 13 were analyzed for each genotype. The amount of white, Adh, and α-Gpdh mRNA in each respective embryo was estimated by measuring the gray values per pixel using Metamorph software. The results indicate that the amount of mRNA detected by this technique is proportional to functional allele dosage and that a twofold difference in expression can be robustly distinguished. The results also illustrate that the quantitation of different genes (w vs. α-Gpdh) is independent of each other. (C) Comparison of null and antisense determinations of background. Normal embryos were probed for sense and antisense RNAs of white and Adh. In parallel, embryos homozygous for the respective null alleles were probed for mRNA. Embryos from three developmental stages are shown. The results illustrate that probing for antisense RNA is an effective background measurement. (D) Histograms of the comparison of background determinations via antisense probing or a null allele. The same digoxygenin-labeled probe preparation was used for analysis of normal and null genotypes for the respective genes. Twenty-one embryos were used to determine each mean. The results illustrate that the background determination by the two methods is comparable at the three developmental stages examined.
Because an RNA null allele is not available as a background control for most genes, we tested whether probing for antisense RNA could serve this purpose. The white and Adh genes were chosen to calibrate the method because RNA level loss-of-function alleles were available for these loci. The antisense background level of hybridization was similar to the white and Adh null level in the sense probings (Figure 2). Thus a probe for antisense can be used to determine background in the absence of a genetic null. This technique appears to be useful for any gene whose expression is present in most tissues and for which there is minimal or no maternal RNA contribution. Such was the case for the genes analyzed in this study.
Probe preparation:
One to three micrograms of linearized plasmid DNA of a selected gene was incubated with 20 μl of digoxygenin labeling mixture (containing 10 mm ATP, 10 mm GTP, 10 mm CTP, 6.5 mm UTP, and 3.5 mm digoxygenin-conjugated UTP), including10× transcription buffer and 2 μl of promoter-specific enzyme (T7, SP6, or T3 RNA polymerase; Roche, Indianapolis) at 37° for at least 4 hr (Bhadra et al. 1999). After incubation, the reaction was terminated with 0.2 m EDTA, pH 8.0. The labeled RNA was precipitated with 2.5 μl 4 m LiCl and 75 μl prechilled ethanol at −20°. The RNA was pelleted via centrifugation at 12,000 rpm, washed in 70% ethanol, and dissolved in 25 μl double-distilled water. Probes prepared in this way could be stored for several months before use. In the case of the white dosage series analysis, Cy3-conjugated UTP and Cy5-conjugated UTP were used to generate directly labeled fluorescent probes for w and α-Gpdh, respectively.
Immunostaining of embryos:
Embryos were collected on agar plates at 25° (Wieschaus and Nusslein-Volhard 1986). The digoxygenin-labeled antisense RNA probes for the selected X and autosomal genes were prepared as noted above. The subsequent steps for detection of labeled probe were performed according to the Genius kit (Roche). Gene-specific antisense and sense RNA probes were prepared using template plasmids as previously described (Bhadra et al. 1999, 2000).
The embryos were processed for hybridization with RNA probes as noted (Pal Bhadra et al. 1999). For fluorescence color detection, we used AMCA-conjugated goat anti-mouse antibodies for SXL and rhodamine-conjugated goat anti-rabbit antibodies for MLE. To measure the gene-specific transcripts, the hybridized embryos were probed with Cy-5-conjugated anti-digoxygenin antibodies at a dilution of 1:200. The embryos were examined under epifluorescence excitation channels using a Bio-Rad (Richmond, CA) 600 confocal microscope with three different laser excitation filters together with a UV filter. The detection of SXL antibodies was performed with the UV filter and the images were captured for later sexing of the embryos. The HQ 515/30 confocal channel was used for the detection of GFP, MLE was detected in the HQ600/40 channel, and individual transcripts were detected in the HQ 660 long pass channel. Cy5-conjugated antibodies for quantification of specific transcripts were used because Cy5 excitation exhibits negligible bleed through with the other channels. Approximately 15 Z sections were taken in each field and stacked into a single image. The various images were obtained using a ×5, ×10, or ×20 lens. The figures were prepared using Adobe Photoshop 7.0 software.
Quantification of transcript amounts in embryos:
Once sexing and genotyping were completed, the embryo images from the Cy5 excitation channel were analyzed for estimation of the quantity of each transcript. The amount of gene-specific transcripts per embryo was estimated by determining the gray scale level using Metamorph version 4.6 (Universal Imaging, Westchester, PA) software. Gray scale, which is defined as the brightness of pixels in a digitized image, is an eight-bit digital signal with 256 possible values ranging from 255 (white) to 0 (black). The mean gray scale values equal the ∑ gray scale values per number of pixels. For estimating the mean gray scale value, we calculated the total gray scale values of whole embryos divided by the total pixel area for each embryo image.
The probe for antisense RNA served as a background determination for each gene examined. The mean value of the background images was calculated for each genotype and then individually subtracted from each embryo sense RNA determination before calculating the mean sense value for a particular genotype. The use of segregating populations of embryos with comparison of genotypes from the same progeny allows a determination of gene expression in each case using a single probe preparation applied under identical conditions for all genotypes.
Immunostaining of chromosomes:
Third instar salivary glands were dissected and fixed temporarily. The polytene chromosomes were immunostained with antibodies as described by Pal Bhadra et al. (1997). Chromosomes were probed with anti-JIL-1 antibodies at a dilution of 1:50. Cy5-conjugated goat anti-chicken secondary antibodies were used for JIL-1 at a standard 1:200 dilution. In other experiments, the chromosomes were probed with anti-MSL1 antibodies at a dilution of 1:100 and subsequently with Cy5-conjugated anti-rabbit antibodies at a 1:200 dilution. The chromosomes were mounted in a mixture of Vectashield mounting media with propidium iodide and examined with a Bio-Rad 600 confocal microscope using a ×60 water immersion lens.
RESULTS
To address the question of whether the MSL complex overrides the effect of histone acetylation, we examined gene expression in a population of individuals with or without the MSL complex as well as the MOF histone acetylase. This analysis allowed a test of (1) whether reduction of the histone acetylation level on the X chromosome would be without consequence in the presence of the MSL complex and (2) whether gene expression would change in parallel to the acetylation level in the absence of the MSL complex. If the MSL complex does affect the action of histone acetylation, there should be no overall change in dosage compensation in the former case, but a change should occur in the latter. This comparison was accomplished by producing a segregating population for mle and mof. No detectable MSL complex is bound to the X chromosome in the mle mutant males (Bhadra et al. 1999, 2000) and the mof1 mutation is depleted for histone acetylase activity (Hilfiker et al. 1997). The two genes are on different chromosomes; therefore, the double-mutant stock will generate a population of progeny with the four genotypes needed to perform the tests. The double-mutant individuals were not viable to the third larval instar, so it was necessary to develop a gene expression assay for embryos.
A fluorescent in situ hybridization expression assay was formulated by modifying standard protocols for RNA detection in situ (see materials and methods). The method relies on the quantification of fluorescently labeled antisense RNA probes using confocal microscopy and the Metamorph software. When this procedure is applied to a segregating population of genotypes in the same hybridization reaction, the randomization of genotypes minimizes any skewed variation in hybridization efficiency on any particular genotype. The procedure was demonstrated to detect twofold differences with very low variance and can be applied to the expression of any gene provided there is little or no maternal RNA contribution (Figure 2).
Analysis of gene expression in msl mutant and normal embryos:
Using this assay, embryos from the cross +/Y; mle[pml8]/CyO × y[1] mof[1]/FM7(GFP); mle[pml8]/mle[pml8] were analyzed. The four types of males are normal, mutant for mle alone, mutant for mof alone, and mutant for both mof and mle (Figure 1). The presence or absence of GFP, SXL, and MLE proteins was used to identify all four classes of male embryos and then individual genes were assayed for their level of expression. The different genotypes in a field of embryos of various developmental stages are shown in Figure 3. The X-linked white and the second chromosomal α-Gpdh genes are shown.
Figure 3.—
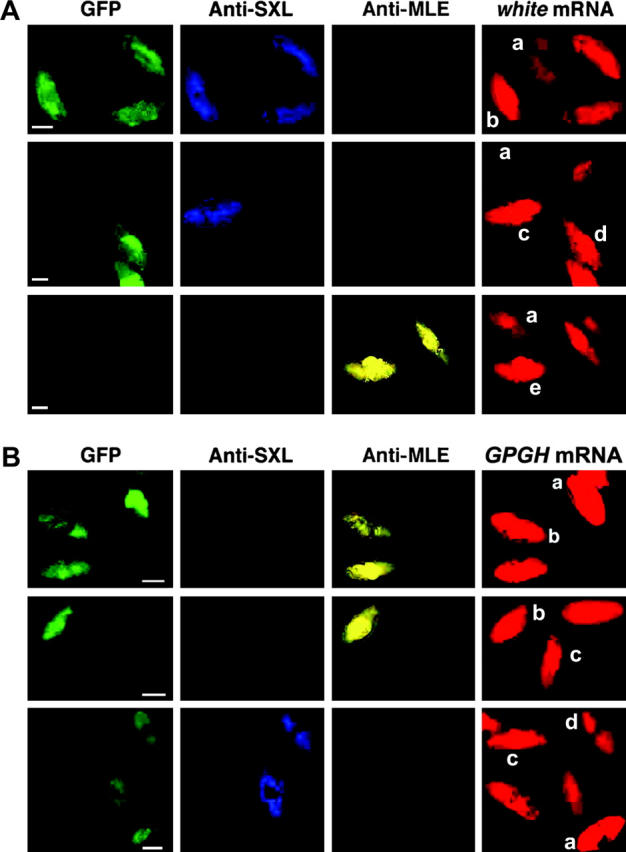
Gene expression analysis of embryos in the same microscopic field. (A) Effect of the mle and/or mof mutation on X-linked white transcripts in developing embryos. A collection of embryos segregating for the mle and mof mutations was probed with anti-SXL and anti-MLE antibodies followed by hybridization with an antisense white RNA probe. Each embryo was classified on the basis of GFP, SXL, and MLE. The genotypes are (a) mof/Y; mle/mle male, (b) GFP/+; mle/mle female, (c) y mof/+; mle/mle female, (d) GFP/Y; mle/mle male, and (e) y mof/Y; mle/+ male. Bars, 50 μm. (B) Effect of the mle and/or mof mutation on autosomal Gpdh RNA levels. Embryos were probed with two antibodies, SXL for sexing and MLE for genotyping, and also were hybridized with an antisense Gpdh RNA probe. The genotypes of each type of embryo are (a) GFP/Y; mle/mle male, (b) GFP/Y; mle/SM6a male, (c) mof/Y; mle/mle male, and (d) GFP/+; mle/mle female. Bars, 50 μm.
For comparisons of genotypes at the same developmental stage, the experimental time-point chosen was approximately stage 13 (Campos-Ortega and Hartenstein 1985). This developmental time-point is sufficiently late that substantial zygotic expression has occurred; yet, it is beyond the time of the sequestration of the MSL complex to the X chromosome at blastoderm (Franke et al. 1996). Finally, it is well before the lethal phase of the mof; mle double mutants.
Images of representative embryos of each male genotype for the six genes studied are assembled in Figure 4. The genes analyzed were yellow, white, vermilion, and 6Pgdh on the X chromosome vs. Adh and α-Gpdh on chromosome 2. This group was selected on the basis of their representative behavior from a larger collection of genes examined in previous studies (Hiebert and Birchler 1994; Bhadra et al. 1999, 2000). Note that there is no substantial RNA from these six genes in early embryos. The average expression of each gene in embryos of the four genotypes is given in Figure 4B. In the mof+; mle/mle male embryos, the X-linked genes retain dosage compensation while the autosomal genes are increased in expression in parallel to previous Northern results of many genes in larvae (Hiebert and Birchler 1994; Bhadra et al. 1999, 2000). [Note: the yellow[1] mutation produces normal amounts of RNA (Bhadra et al. 1999).] Thus, the two methods provide consistent results for the generalized profile of expression of various genes in the mutant individuals. The mof[1]; mle/+ males, which lack acetylase activity but retain substantial MSL complex on the X (Hilfiker et al. 1997; see below), also exhibit dosage compensation of the X-linked genes, but Adh is not elevated in expression to the same degree as in the mle mutant males. The level of RNA from α-Gpdh is reduced in the mof mutants. In the double-mutant mof[1]; mle/mle males, the X-linked genes are reduced in expression to about half the normal level, but the autosomal genes are only slightly changed in expression, falling slightly below the normal male values. A separate comparison of the six genes in males and females of a Canton-S wild strain showed similar expression between the sexes for all genes (Figure 4B), illustrating the compensation of the X and equal autosomal expression under normal conditions.
Figure 4.—
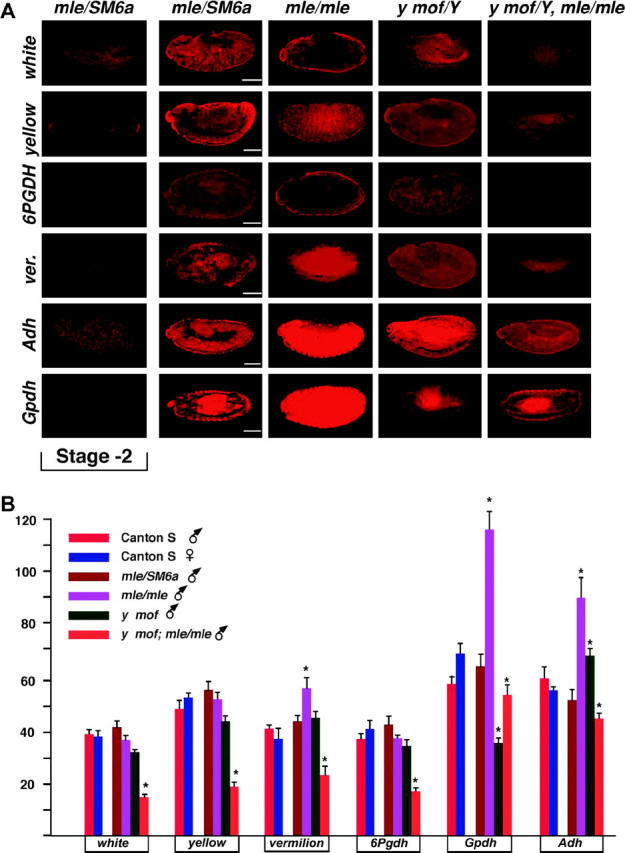
Embryo expression of the six selected genes analyzed. (A) A representative embryo at approximately stage 13 from each of the four male genotypes for each gene analyzed is shown. In addition, an early stage embryo is presented to illustrate the absence of maternal RNA. The sense mRNA determination represents the relative expression in each genotype. Probings for antisense RNA established the background hybridization. Bars, 50 μm. (B) Histogram of the gene expression in a population of embryos from Canton-S wild-type males and females as well as the mof/mle segregating population. Fifteen to 23 embryos were analyzed per genotype. Probing for antisense RNA served as the background for each gene, which was subtracted from the determination of each embryo. Those average values that differ from the normal male genotype at the 95% confidence level are marked by an asterisk.
These results suggest that the acetylation level on the X can influence gene expression only in the absence of the MSL complex. In the presence of the MSL complex, the reduction of acetylation in the mof mutant has little effect on X-linked gene expression. Because compensation is present in the mof mutants, but not in the double-mutant mof; mle genotype, these results suggest that histone acetylation or the MSL complex alone allows transcription factors to produce dosage effects on their target loci. If this is the case, it is possible that some X-linked target genes might increase in expression in mle mutant males because they change from being unresponsive to histone acetylation to being capable of responding to it. Such a response in fact occurs for vermilion (Figure 4) and for other genes assayed in a previous survey (e.g., vermilion, rudimentary, Bar, and Notch) (Hiebert and Birchler 1994). It is also possible that some few X-linked genes may have increased H4Lys16Ac, as is more generally true of autosomal loci, because they may have low acetylation in normal males compared to the uniform redistribution in the mle/mle mutant males.
Binding of the MSL complex in the mof mutants:
Previous studies have found no detectable MSL complex remaining on the chromosomes in mle/mle mutant males, although the MOF acetylase and H4 Lys16 acetylation become distributed throughout the genome (Bhadra et al. 1999). In female genotypes that allow expression of MSL2 and thus sequestration of the complex to the two X chromosomes, the further introduction of homozygous mle to the genotype results in a partial complex remaining at high-affinity sites along the X (Palmer et al. 1994; Lyman et al. 1997; Bhadra et al. 2000; Gu et al. 2000; Kageyama et al. 2001). It was previously determined that such binding is not detectable in the mle/mle male genotype (Bhadra et al. 2000).
To examine the binding of the MSL complex in the mof mutant in order to understand the changes in gene expression in this mutant, two sets of experiments were performed. In the first, a mixture of salivary gland chromosomes from mof males and mle/mle females was prepared and probed with antibodies against MSL1. Previous work has shown that MSL1 is present on all female chromosomes, but was removed in the mle mutant (Bhadra et al. 1999). The mle females serve as a negative control on the same slides with the mof males. The second comparison was of mof and normal males, again in mixed preparations on the same slides.
The results (Figure 5) illustrate that the MSL complex on the X chromosome is destabilized to some degree in the mof mutant males (see also Hilfiker et al. 1997). While the presence of MSL1 remains high on the X chromosome, some degree of labeling is also detected on the autosomes compared to the mutant female chromosomes or the normal male autosomes. The mixed analysis with a null control is critical to establish that this labeling is above background. The low level of binding on the autosomes indicates the presence of at least a partial MSL complex, given that autosomal binding is removed in females when any one member of the complex is mutant (Bhadra et al. 1999; Figure 5). A higher magnification of a confocal image of the X chromosome from normal males, normal females, mof males, and mle/mle females illustrates the changes in X binding of the MSL complex under the various circumstances (Figure 5C). The normal male X has the highest concentration compared to the level of MSL1 present in females. The mof males have strong labeling of the typical set of high-affinity sites along the X chromosome as previously noted (Gu et al. 2000), but substantial labeling remains throughout the length of the X.
Figure 5.—
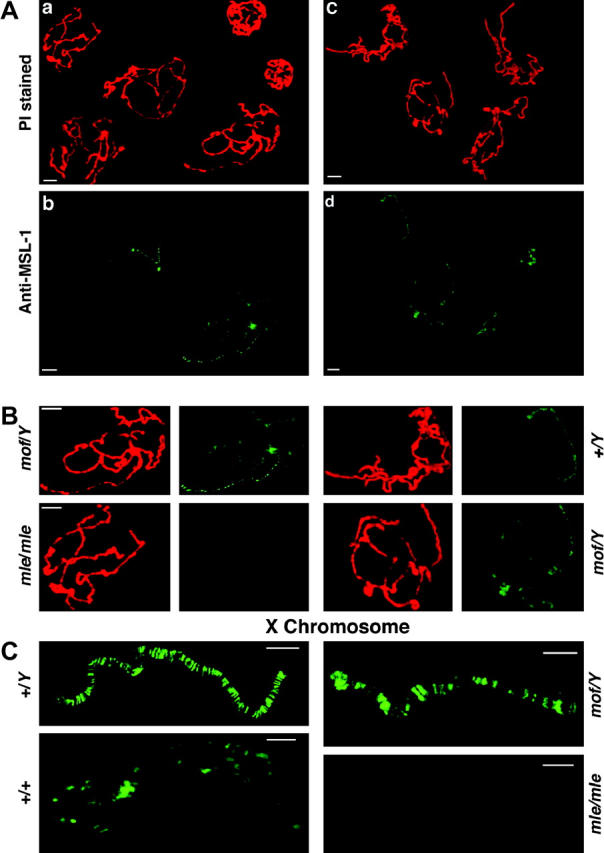
Distribution of the MSL complex in the mof mutant as revealed by MSL-1 labeling. (A) (a) A mixture of polytene chromosomes from mof males and mle/mle females stained with propidium iodide (PI). (b) The same nuclei probed for MSL-1. The females have no detectable MSL labeling on their chromosomes (Bhadra et al. 1999) and serve as a background control. The mof males show a destabilization of the MSL complex such that a low level of binding is now present on the autosomes, although a stronger labeling still occurs on the X. (c) A mixture of polytene chromosomes from mof and normal males stained with PI. (d) The same nuclei probed for MSL-1. The normal males show the usual strong labeling on the X chromosome and no detectable autosomal labeling. In contrast, the mof males exhibit some degree of MSL labeling on the autosomes. (B) Magnified views for comparison of single nuclei from the two mixtures in A. (C) Magnified views of the X chromosome from four genotypes. The normal male (+/Y) has strong labeling on the X. The normal female (+/+) (from a labeling not shown above) has a lower level of labeling, which is similar to the autosomes in the same nucleus (Hiebert and Birchler 1994). The mof male (mof/Y) retains strong binding at the high-affinity sites and weaker binding between them. The mle/mle female (mle/mle) shows no detectable labeling and illustrates that the signal between the strongly bound sites in the mof male is above background.
Sequestration of JIL-1 kinase:
The expression of the autosomes is likely elevated in mle/mle males because of the release of MOF from the X, resulting in uniform genome-wide histone modification (Bhadra et al. 1999; Birchler et al. 2001), but with no MSL complex to override an effect on gene expression. However, in the case of the mof mutant males, a lesser, but nevertheless significant, increase in expression of some autosomal genes was observed (Bhadra et al. 1999), but MOF activity is depleted in this case. One possible explanation is that the low amount of MSL complex that returns to the autosomes in the mof mutant allows a response of some autosomal genes to the negative dosage effect of the X chromosome. Alternatively, this effect could be explained by the return of the JIL-1 histone kinase (Jin et al. 1999) to all chromosomes due to the partial destabilization of the MSL complex. To address this question, we examined mixtures of normal and mof mutant salivary gland chromosomes probed with antibodies against JIL-1 kinase. As a control we also examined the distribution of JIL-1 kinase in mixtures of normal male and female polytene chromosome spreads. The kinase is distributed on all chromosomes in females (Figure 6), as is MOF (Bhadra et al. 1999). In normal males, the kinase is preferentially sequestered to the X, but in the mof1 mutant, it is redistributed to all chromosomes. This lack of sequestration is analogous to the distribution of MOF in the mle mutant males (Bhadra et al. 1999). The dispersal of JIL-1 kinase in the mof mutant males appears more uniform in contrast to the pattern of MSL1, suggesting that the kinase is not associated with the MSL complex under these circumstances. Thus, in the mof mutant, the kinase also becomes a candidate to influence autosomal expression. However, in the mof; mle double mutant, JIL-1 kinase would be expected to still be uniformly present, but autosomal gene expression is less affected compared to normal males, as described above. Thus, the most likely explanation for the changes in gene expression in the mof mutant is the autosomal localization of the MSL complex.
Figure 6.—
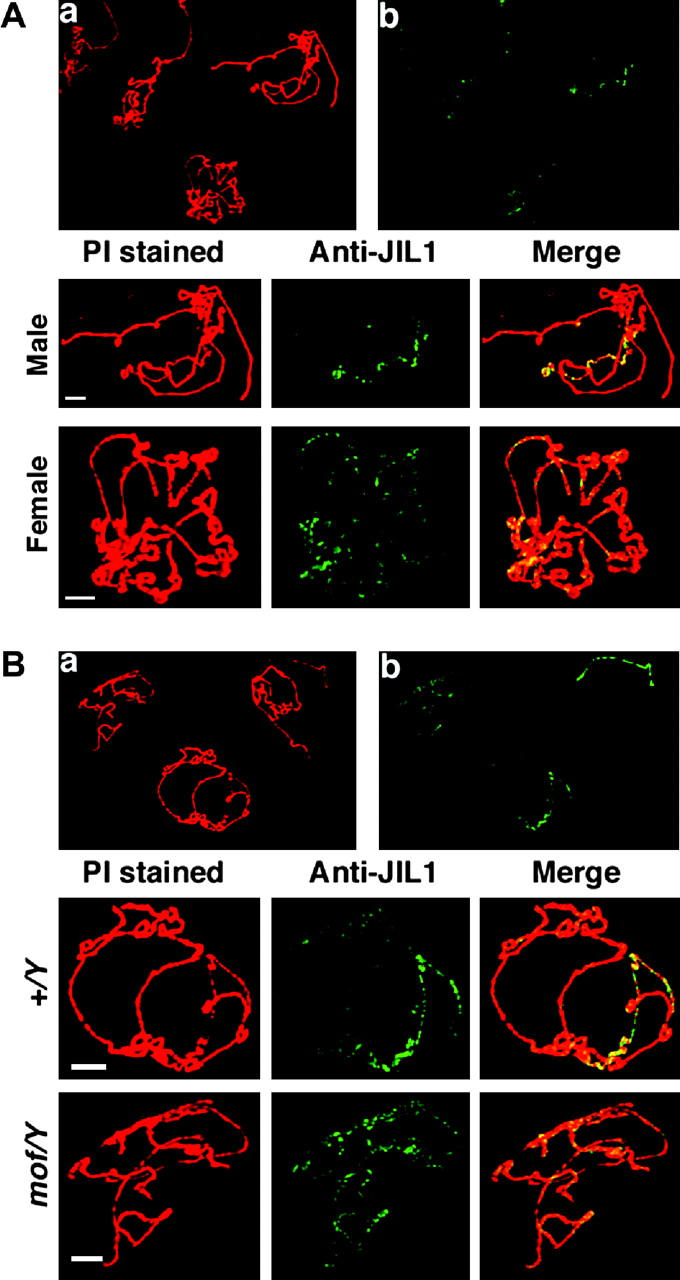
Distribution of JIL-1 kinase in the mof and normal males. (A) A mixture of polytene chromosomes from normal males and females was probed with antibody against JIL-1 kinase. (a) PI stained chromosomes. (b) Probings for JIL-1 kinase. Bottom, enlargement of a male and a female nucleus illustrating the enrichment of JIL-1 kinase on the X chromosome in males and the uniform distribution in females. (B) A mixture of polytene chromosomes from normal and mof mutant males stained with PI (a) and probed for JIL-1 kinase (b). Bottom, enlargements of single nuclei illustrating that the kinase is sequestered to the X in normal males, but released in the mof mutants to a uniform distribution on all chromosomes.
Targeting the MSL complex and MOF to the X chromosomes in females lowers autosomal gene expression:
The SXL protein, which is present only in females, blocks the translation of the MSL2 gene product, which prevents the formation of the MSL complex (Kelley et al. 1995). In females mutant for Sxl, MSL2 is translated and the complex is organized and targeted to the two X chromosomes (Kelley et al. 1995). We examined gene expression in embryos mutant for Sxl to determine the effect of this ectopic MSL sequestration. Representative X-linked genes were changed very little in expression, but the trend for autosomal genes showed reductions (with one gene showing increased expression) (Figure 7). Thus, the spectrum of gene expression in embryos of this genotype is consistent with that of a larger sample of genes assayed at the larval stage (Bhadra et al. 2000). In the genotype examined, the MSL complex and histone acetylation is increased on the two X chromosomes at the expense of the autosomes (Bhadra et al. 2000), in some but not all cells (Kelley et al. 1995). Thus, any observed effect on gene expression will appear to be of a lesser magnitude than that which occurs in the affected cells. The combined data (Bhadra et al. 2000; see results) suggest that on the autosomes, the reduction of histone acetylation causes lowered gene expression, but the increase of acetylation on the X's, in the presence of the MSL complex, is without major consequence in terms of gene expression. Experiments described above demonstrate that a reduction of histone acetylation on the X chromosome in the presence of the MSL complex is also without major effect on gene expression.
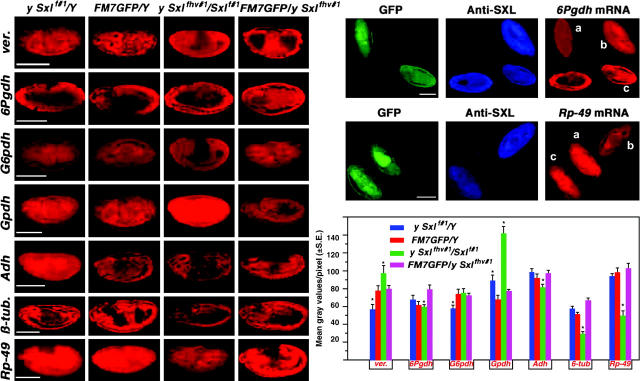
Figure 7.—
Gene expression analysis in Sxl− embryos. From a cross of Sxlf1/FM7 GFP × Sxlfhv/Y, embryos were subjected to the fluorescent gene expression assay. This cross produces a segregating population in which mutant females are represented with greatly reduced SXL protein. This mutant combination was chosen to be in parallel with previous Northern analyses at the third instar larval stage (Bhadra et al. 2000). In the Sxl mutant cells, the MSL complex is formed and sequestered to the two X chromosomes, where the H4Lys16 acetylation is increased concomitant with a reduction of acetylation on the autosomes (Bhadra et al. 2000). Some genotypes in the progeny carry mutations for yellow and white, so they were not included in the analysis. A representative embryo (at approximately stage 13) for each gene in each genotype is shown. On the right, two fields of mixed stages probed for the X-linked 6Pgdh and the autosomal Rp49 genes are shown. a, FM7 GFP male; b, Sxlf1/Sxlfhv female; c, Sxlfhv/FM7 GFP female. A histogram of the seven genes analyzed is shown at the bottom right. A range of 10 to 36 embryos were analyzed for each value. The expression patterns of these representative genes are quite similar to a previous survey at the third instar larval stage (Bhadra et al. 2000). Generally, the X-linked genes are similar in expression to normal females, whereas autosomal gene expression is reduced. An asterisk denotes those means that are significantly different from the respective FM7-bearing genotype of the same sex.
ISWI is required for the override of histone acetylation:
Mutations in several chromatin protein genes result in a chromosomal phenotype in which the male X is much wider than normal, although other cytological defects have been noted. These genes include JIL1 kinase (Wang et al. 2001), ISWI (Corona et al. 2002), nurf301 (Badenhorst et al. 2002), l(3)tl (Zhimulev et al. 1976), and In(1)BM2(rv) (Bose and Duttaroy 1986). These gene products may be required for the override process, directly or indirectly, thus producing a similar bloated X chromosome phenotype when mutant. Their mutant form would allow the X chromosome to respond to the high level of histone acetylation. In particular, the ISWI protein is an ATPase subunit of several chromatin remodeling complexes and the X chromosomal phenotype is modulated by the amount of histone acetylation present on the X chromosome (Corona et al. 2002). This mutant effect is likely to be partial, given a maternal contribution of ISWI to the embryo. The expression of selected genes was determined in embryos segregating for two different ISWI alleles and their heteroallelic combination. The results showed that X-linked genes in males are increased in expression in the ISWI mutant individuals (Figure 8), but autosomal genes are unchanged. Five of six genes, regardless of X or autosomal location, are increased in expression to a slight degree in mutant females. This pattern parallels the level and distribution of H4 acetylation present in the two sexes and suggests the possibility that in normal genotypes, the ISWI gene product is required for the MSL-mediated override of the effect of H4 Lys16 acetylation on gene expression.
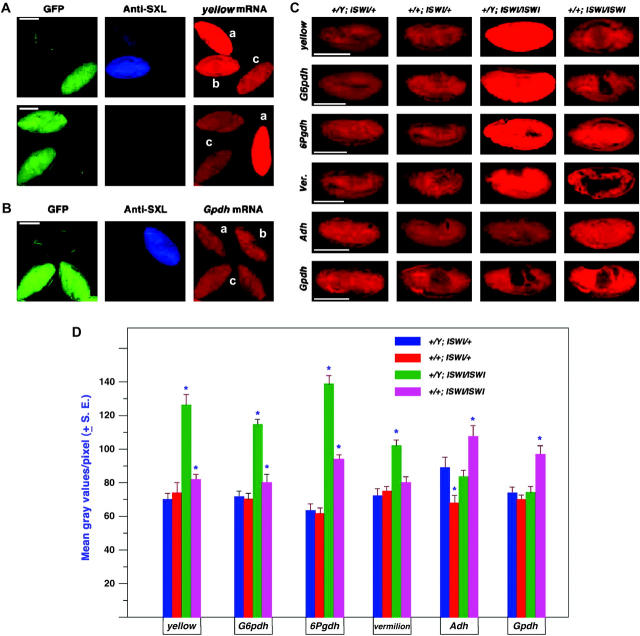
Figure 8.—
Gene expression in the ISWI segregating population of embryos. Embryos were collected from a cross of w−/Y; CyO, GFP/ISWI1 × w−/w−; CyO GFP/ISWI2. Genotypes were classified on the basis of antibody labeling against SXL to determine sex and GFP excitation to determine the ISWI constitution. (A) Mixed stage embryos in the same field probed for the expression of the X-linked yellow gene. a, male ISWI mutant homozygote; b, female mutant homozygote; c, normal male. (B) Mixed stage embryos in the same field probed for the autosomal gene, α-Gpdh. a, male mutant homozygote; b, normal female; c, normal male. (C) Representative embryos at approximately stage 13 for four X-linked genes (y, G6pdh, 6Pgdh, and v) and two autosomal genes (Adh and α-Gpdh). The white gene expression was not tested because the X chromosome in these stocks carries a mutant allele. (D) Histogram of the mean gray values of embryos at approximately stage 13 from the segregating ISWI population. A range of 10 to 20 embryos were analyzed for each mean. An asterisk denotes those means that are significantly different from the normal genotype of the same sex.
DISCUSSION
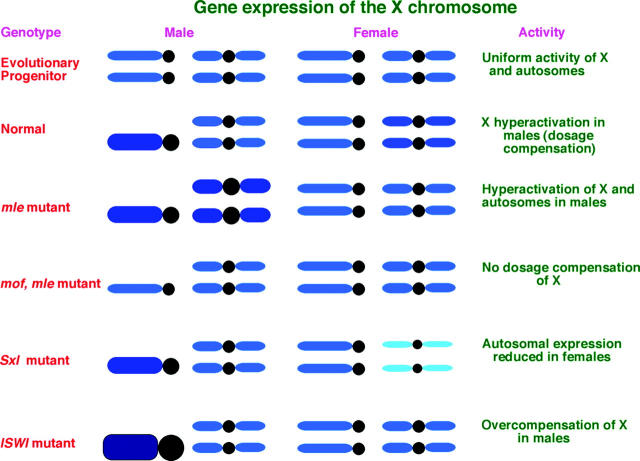
In normal males, the majority of genes on the X chromosome are hyperactivated approximately twofold to achieve the same level of expression as the two X chromosomes in females (Arkhipova et al. 1997). We believe that this twofold increase in expression is caused by an imbalance of transcription factors that occurs due to the reduced dosage of the X chromosome relative to the autosomes in males, in much the same manner as the control of sex determination (Erickson and Cline 1993), except that the latter is converted to an all or none mechanism. Changing the stoichiometry of members of transcription factor complexes causes modulations of target gene expression and haplo-insufficiency (Birchler et al. 2001; Veitia 2002; Papp et al. 2003). This process is commonly manifested in chromosomal monosomics in which the most common effect is an approximate twofold increase of target gene expression throughout the genome (Birchler 1979; Birchler and Newton 1981; Guo and Birchler 1994). The single X chromosome in male flies is similar to this situation and is thought to capitalize on this “inverse dosage effect” of transcription factors to bring about the proper level of X chromosomal dosage compensation (Birchler et al. 2001, 2003). The dosage of the X chromosome has been shown to produce an inverse dosage effect on white transgenes inserted into the autosomes (Birchler 1992). The twofold reduction in copy number of the target structural genes on the X appears to be counteracted by the twofold increase in gene expression; acting simultaneously, these effects result in dosage compensation (Birchler 1981, 1992; Devlin et al. 1982; Birchler et al. 1990). Because the balance of regulatory factors is postulated to cause the dosage effect on the target loci, the modulation of the latter is usually within the inverse limits regardless of the level of individual expression.
The MSL complex accumulates on the X in males at high levels at the expense of its presence on the autosomes. The consequence of this sequestration is a higher histone modification, i.e., H4 Lys16 acetylation and H3 phosphorylation, on the X and lowered amounts on the autosomes compared to females (Turner et al. 1992; see results). This phenomenon appears to have evolved to modify the dosage effects of the X-linked haplo-insufficient transcription factors, which themselves must be among the uncompensated X-linked genes of the sexually dimorphically expressed loci in the genome (Jin et al. 2001). The depletion of histone modifications on the autosomes is postulated to mitigate the approximately twofold increase of the target genes there that would otherwise occur (Figure 9). On the X chromosome in normal males, there are increased histone modifications, but the MSL complex is thought to counteract the expected response of target genes (Figure 9). This process allows the proper twofold hyperactivation rather than any greatly elevated levels of X-linked gene expression that would otherwise occur, for example, in the ISWI mutants (Figure 9).
Figure 9.—
Summary of gene expression. The evolutionary progenitor situation to the heteromorphic sex chromosomes in Drosophila is assumed to involve a pair of homologous chromosomes with similar gene expression between the sexes. The current karyotype shows dosage compensation of the single X chromosome in males compared to two in females and nearly equal autosomal expression. Because the gene expression in the msl mutants shows hyperactivation of both the X and the autosomes, it is postulated that a global inverse dosage effect, typical of monosomic situations, is operating and that the MSL sequestration evolved to counteract this dosage effect on the autosomes. Retention of dosage compensation in the mof mutant males, but not in the double mutant, indicates that the MSL complex overrides the impact of histone acetylation, in this case when acetylation on the X is reduced. The loss of dosage compensation in the double-mutant mof; mle indicates that the MSL chromatin remodeling complex is necessary for the inverse dosage effect to operate. The failure to increase X expression when the MSL complex and increased H4 acetylation is targeted to the X's in females indicates the override also operates with increased acetylation. The overcompensation of the male X in the ISWI mutants suggests that this chromatin remodeling subunit is required for the override process.
An alternative proposal states that the MSL complex brings the histone acetylase and kinase to the X to open the chromatin in such a manner as to create a twofold increase in expression (Belote and Lucchesi 1980; Kuroda et al. 1991; Bone et al. 1994; Jin et al. 1999). A critical issue to determine the function of the MSL genes is their mutant effect on gene expression. The original studies analyzed the autoradiographic grain counts from tritiated uridine incorporated into nascent RNA on third larval instar polytene chromosomes, as a measure of the rate of transcription (Belote and Lucchesi 1980; Okuno et al. 1984). The data were treated as a relative number from the X chromosome compared to the autosomes. This ratio was diminished to nearly one-half in males mutant for the mle or msl3 genes.
This ratio change in the msl males could occur if the X is reduced in expression due to a loss of dosage compensation and autosomal expression is not altered. The assumption at the time was that this relative change would be an indication of a loss of dosage compensation, but no determination of absolute levels of gene expression was made. Alternatively, the altered ratio could result if the X basically retains dosage compensation and the autosomes are generally increased in expression. Inspection of the grain count data for the X and autosomes (Belote and Lucchesi 1980; Okuno et al. 1984) favors the latter interpretation. This result matches the type of gene expression profile typical of an inverse dosage effect of the X chromosome operating on many loci in the genome. A survey of 39 X and autosomal genes in mle and normal males, assayed at the larval stage, with absolute determinations of expression relative to DNA, demonstrated that the latter situation was the case (Hiebert and Birchler 1994). The results reported here confirm this relationship in embryos in which the absolute expression is obvious visually. This conclusion is further bolstered by our finding of conditions that do in fact result in a loss of X dosage compensation, namely the mof; mle double mutant. These results demonstrate that the phenotypic effect of a loss of X chromosomal dosage compensation is lethality at the late embryonic stage rather than the larval-pupal transition at which the msl mutants die. The accumulated data suggest that the MSL complex does not directly mediate dosage compensation of the X chromosome in males and that the basis of compensation must reside in a different mechanism.
An explanation for the increased autosomal expression in msl mutants came from studies of MSL and histone acetylase distribution in males, females, and mle males (Bhadra et al. 1999). The MSL2 gene product, a key component of the MSL complex for X sequestration, is expressed only in males, being suppressed in females at translation by the Sxl gene product (Kelley et al. 1995). Other members of the complex are present in females and are located on all chromosomes at a uniform low level (Bhadra et al. 1999). Mutations in the various msl genes disassociate all MSL proteins from the chromosomes in females, indicating that a partial complex is present uniformly throughout the genome in normal females. The MOF acetylase associates with all chromosomes in females whether or not the MSL complex is present (Bhadra et al. 1999) and is capable of acetylating H4 in both situations. Thus, it is postulated that there is little mutational effect in females because there is no obvious redistribution of acetylation levels and there is no chromosomal imbalance present in this sex as well. The JIL-1 kinase (Jin et al. 1999, 2000; Wang et al. 2001) phosphorylates H3 and follows a similar, but distinct, type of sequestration.
In normal males, the complex is sequestered to the X chromosome, accumulating MOF and JIL-1 kinase in the process. Thus, the X is increased for histone modifications, as measured on the chromosomal level, relative to females and the autosomes have reduced levels of these modifications. In the mle mutant males, the complex is removed from the X and MOF is released to be associated with all chromosomes, which produces a more uniform genome-wide H4 acetylation (Bhadra et al. 1999). Therefore, the acetylation level drops, but is not eliminated, on the X, whereas on the autosomes, the acetylation changes from very low to a level similar to that in females. Two other situations produce a related type of effect. An analysis of roX− flies, which lack the RNA component of the complex, also demonstrated that when the MSL complex is disrupted, histone acetylation becomes distributed to all chromosomes (Meller and Rattner 2002). Second, in the germline there is no MSL complex and the histone acetylation is distributed throughout the nucleus (Rastelli and Kuroda 1998). Thus, male nuclei lacking the MSL complex have an increased level of H4 Lys16 acetylation on the autosomes relative to the normal somatic situation, providing a potential explanation for the autosomal increased expression. In general, gene expression is correlated with the level of histone acetylation (Jenuwein and Allis 2001), which is involved in enhancing transcription factor binding to DNA (Vettese-Dadey et al. 1996).
In the mof mutant males, the Adh gene was significantly increased in expression and α-Gpdh was reduced. A previous study found an increase in expression to be the case for a few additional autosomal genes (Bhadra et al. 1999). This effect cannot readily be attributed to increased autosomal H4 Lys16 acetylation because MOF activity is depleted in this genotype. In this study it was found that the MSL complex is destabilized in the mof mutant and becomes associated with the autosomes at a low level. This redistribution may allow autosomal genes to respond to some degree to the dosage effect of the X chromosome.
Alternatively, JIL-1 kinase redistribution may be responsible for this effect. Here we showed that its sequestration to the X is eliminated in the mof mutant males such that its distribution becomes uniform in the genome. However, because the double mutant shows a slight reduction for autosomal gene expression, it seems likely that the autosomal increases of gene expression in the mof mutant are due to the newly distributed MSL complex and that the JIL-1 kinase alone may have a slightly negative impact on gene expression. The X chromosome morphology phenotype of the JIL1 kinase mutants (Wang et al. 2001) is similar, although not identical, to those of ISWI, which might suggest a role for JIL1 kinase in the override of histone acetylation.
On the X chromosome, dosage compensation is maintained for the genes examined without MOF activity or without the MSL complex. If dosage compensation is caused by the twofold inverse dosage effect of the X, then either histone acetylation or the MSL complex allows this effect to occur. An alternative explanation, namely that both histone acetylation and the MSL complex are required together for X hyperactivation, is not supported by the observation that targeting the MSL complex with an active MOF to the X chromosomes in females by a variety of methods does not cause a generalized increase in gene expression, but does lower overall autosomal expression by depleting MOF, the MSL complex, and histone acetylation there (Bhadra et al. 1999, 2000; Figure 7). The data suggest that the MSL complex alone will permit the dosage effect of transcription factors to operate. When acetylation is not counteracted, the level of gene expression is positively correlated with the amount of acetylation rather than negatively correlated with the chromosomal dosage.
This relationship helps explain the need for an override system of the high levels of acetylation on the normal male X. Indeed, targeting of MOF to a transgene in yeast causes a 10-fold increase in expression (Akhtar and Becker 2000), which is far greater than the 2-fold effect required to explain dosage compensation. The increased expression of X-linked genes in males mutant for ISWI shows a related type of overexpression and suggests a role for this gene product in the override process (Figure 8).
The ISWI chromatin remodeling component is present throughout the nucleus, but primarily it is the X chromosome in males that acquires a bloated appearance in the mutants for this gene (Corona et al. 2002). Modulation of the amount of histone acetylation on the X chromosome in the ISWI mutant background causes a positive correlation between the amount of acetylation and the severity of this phenotype (Corona et al. 2002). Thus, ISWI is implicated in interacting with the MSL complex to override the effect of histone acetylation on gene expression.
Analysis of global patterns of gene expression in the germline of Drosophila has indicated a generalized presence of dosage compensation and a male-biased autosomal expression (Parisi et al. 2003). The germline does not express most of the components of the MSL complex and H4Lys16Ac is not concentrated on the X chromosome (Rastelli and Kuroda 1998). This study and our previous studies (Hiebert and Birchler 1994; Bhadra et al. 1999, 2000) suggest that a related situation occurs in the somatic tissues of mle mutant males. Thus, the available data indicate that in all cases in which the MSL complex is uncoupled from the X chromosome, generalized dosage compensation still occurs.
As the sex chromosomes evolve, one member of a homologous pair degenerates (Charlesworth 1978). This circumstance is formally analogous to a monosomic situation, leading one to expect that the inverse effect would become prevalent on gene expression throughout the genome (Hiebert and Birchler 1994; Bhadra et al. 1999, 2000; Birchler et al. 2001). A plausible evolutionary scenario might begin with the presence of a small hemizygous region in males that is targeted for upregulation by accumulation of greater histone acetylase. The hemizygous region on the X would result from the inactivation of a segment of the incipient Y chromosome due to inhibition of recombination and accumulation of mutations. As the hemizygous region lengthens, the probability of trans-acting dosage effects occurring increases. The dosage effects would foster a more comprehensive compensation of the hemizygous region and tend to increase gene expression of the whole genome. As the latter effect increases, selection would favor greater depletion of the acetylase from the autosomes to diminish this effect. Thus once initiated, the sequestration of acetylase to the X and differentiation of the Y chromosome would accelerate. As the amount of acetylase continues to increase on the X, an override mechanism might evolve to prevent overcompensation. Whatever the evolutionary progression, the current circumstances suggest that interactions of chromatin remodeling factors in somatic cells appear to have evolved to equalize both X and autosomal gene expression in response to changes in X chromosomal dosage.
Acknowledgments
We thank M. Kuroda for MSL1 and MOF antibodies and mutant stocks, K. Johansen for JIL-1 kinase antibodies, J. Tamkun for ISWI alleles, B. Turner for H4Ac16 antibodies, and J. Lucchesi for the mof mutant stock. We thank V. B. Narmadha for excellent assistance. Research was supported by a grant from the National Science Foundation (MCB 0211376) and the National Institutes of Health (GM-068042) to J.B. U.B. was supported by a grant from Indo-France collaborative research, a Human Frontier Young Investigator grant, and a Wellcome Trust Senior International Research Fellowship.
References
- Akhtar, A., and P. B. Becker, 2000. Activation of transcription through histone H4 acetylation by MOF, an acetyltransferase essential for dosage compensation in Drosophila. Mol. Cell 5: 367–375. [DOI] [PubMed] [Google Scholar]
- Arkhipova, I., J. Li and M. Meselson, 1997. On the mode of gene-dosage compensation in Drosophila. Genetics 145: 729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badenhorst, P., M. Voas, I. Rebay and C. Wu, 2002. Biological functions of the ISWI chromatin remodeling complex NURF. Genes Dev. 16: 3186–3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn, S., M. Mimmack, M. Ryan, M. A. Caldwell, E. Jauniaux et al., 2002. Neuronal target genes of the neuron-restrictive silencer factor in neurospheres derived from fetuses with Down's syndrome: a gene expression study. Lancet 359: 310–315. [DOI] [PubMed] [Google Scholar]
- Belote, J. M., and J. C. Lucchesi, 1980. Control of X chromosome transcription by the maleless gene in Drosophila. Nature 285: 573–575. [DOI] [PubMed] [Google Scholar]
- Bhadra, U., M. Pal Bhadra and J. A. Birchler, 1999. Role of the male specific lethal (msl) genes in modifying the effects of sex chromosomal dosage in Drosophila. Genetics 152: 249–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadra, U., M. Pal Bhadra and J. A. Birchler, 2000. Histone acetylation and gene expression analysis of Sex lethal mutants in Drosophila. Genetics 155: 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler, J. A., 1979. A study of enzyme activities in a dosage series of the long arm of chromosome one in maize. Genetics 92: 1211–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler, J. A., 1981. The genetic basis of dosage compensation of alcohol dehydrogenase-1 in maize. Genetics 97: 625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler, J. A., 1992. Expression of cis-regulatory mutants of the white locus in metafemales of Drosophila melanogaster. Genet. Res. 59: 11–18. [DOI] [PubMed] [Google Scholar]
- Birchler, J. A., and K. J. Newton, 1981. Modulation of protein levels in chromosomal dosage series of maize: the biochemical basis of aneuploid syndromes. Genetics 99: 247–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler, J. A., J. C. Hiebert and K. Paigen, 1990. Analysis of autosomal dosage compensation involving the Alcohol dehydrogenase locus in Drosophila melanogaster. Genetics 124: 677–686. [PMC free article] [PubMed] [Google Scholar]
- Birchler, J. A., U. Bhadra, M. Pal Bhadra and D. L. Auger, 2001. Dosage-dependent gene regulation in multicellular eukaryotes: implications for dosage compensation, aneuploid syndromes, and quantitative traits. Dev. Biol. 234: 275–288. [DOI] [PubMed] [Google Scholar]
- Birchler, J. A., M. Pal-Bhadra and U. Bhadra, 2003. Dosage dependent gene regulation and the compensation of the X chromosome in Drosophila males. Genetica 117: 179–190. [DOI] [PubMed] [Google Scholar]
- Bone, J. R., R. J. Lavender, R. Richman, M. J. Palmer, B. M. Turner et al., 1994. Acetylated histone H4 on the male X chromosome is associated with dosage compensation in Drosophila. Genes Dev. 8: 96–104. [DOI] [PubMed] [Google Scholar]
- Bose, D., and A. Duttaroy, 1986. A case of variegation at the level of chromosome organization. Chromosoma 94: 87–93. [Google Scholar]
- Campos-Ortega, J. A., and V. Hartenstein, 1985 The Embryonic Development of Drosophila melanogaster. Springer-Verlag, Berlin.
- Charlesworth, B., 1978. Model for evolution of Y chromosomes and dosage compensation. Proc. Natl. Acad. Sci. USA 75: 5618–5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona, D. F. V., C. R. Clapier, P. B. Becker and J. W. Tamkun, 2002. Modulation of ISWI function by site-specific histone acetylation. EMBO Rep. 3: 242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin, R. H., D. G. Holm and T. A. Grigliatti, 1982. Autosomal dosage compensation in Drosophila melanogaster strains trisomic for the left arm of chromosome 2. Proc. Natl. Acad. Sci. USA 79: 1200–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin, R. H., D. G. Holm and T. A. Grigliatti, 1988. The influence of whole-arm trisomy on gene expression in Drosophila. Genetics 118: 87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson, J. W., and T. W. Cline, 1993. A bZIP protein, sisterless-a, collaborates with bHLH transcription factors in Drosophila development to determine sex. Genes Dev. 7: 1688–1702. [DOI] [PubMed] [Google Scholar]
- FitzPatrick, D. R., J. Ramsay, N. I. McGill, M. Shade, A. D. Carothers et al., 2002. Transcriptome analysis of human autosomal trisomy. Human Mol. Genet. 11: 3249–3256. [DOI] [PubMed] [Google Scholar]
- Franke, A., A. Dernberg, G. J. Bashaw and B. S. Baker, 1996. Evidence that MSL-mediated dosage compensation in Drosophila begins at blastoderm. Development 122: 2751–2760. [DOI] [PubMed] [Google Scholar]
- Gu, W., X. Wei, A. Pannuti and J. C. Lucchesi, 2000. Targeting the chromatin-remodeling MSL complex of Drosophila to its sites of action on the X chromosome requires both acetyl transferase and ATPase activities. EMBO J. 19: 5202–5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, M., and J. A. Birchler, 1994. Trans-acting dosage effects on the expression of model gene systems in maize aneuploids. Science 266: 1999–2002. [DOI] [PubMed] [Google Scholar]
- Hiebert, J. C., and J. A. Birchler, 1994. Effects of the maleless mutation on X and autosomal gene expression in Drosophila melanogaster. Genetics 136: 913–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker, A. D., D. Hilfiker-Kleiner, A. Pannuti and J. C. Lucchesi, 1997. mof, a putative acetyl transferase gene related to the Tip60 and MOZ human genes and to the SAS genes of yeast, is required for dosage compensation in Drosophila. EMBO J. 16: 2054–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein, T., and C. D. Allis, 2001. Translating the histone code. Science 293: 1074–1080. [DOI] [PubMed] [Google Scholar]
- Jin, W., R. Riley, R. D. Wolfinger, K. P. White, G. Passador-Gurgel et al., 2001. Contributions of sex, genotype, and age to transcriptional variance in Drosophila melanogaster. Nat. Genet. 29: 389–395. [DOI] [PubMed] [Google Scholar]
- Jin, Y., Y. Wang, D. L. Walker, H. Dong, H. C. Conley et al., 1999. JIL-1: a novel chromosomal tandem kinase implicated in transcriptional regulation in Drosophila. Mol. Cell 4: 129–135. [DOI] [PubMed] [Google Scholar]
- Jin, Y., Y. Wang, J. Johansen and K. M. Johansen, 2000. JIL-1, a chromosomal kinase implicated in regulation of chromatin structure, associates with the male specific lethal (MSL) dosage compensation complex. J. Cell Biol. 149: 1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama, Y., G. Mengus, G. Gilfillan, H. G. Kennedy, C. Stuckenholz et al., 2001. Association and spreading of the Drosophila dosage compensation complex from a discrete roX1 chromatin entry site. EMBO J. 20: 2236–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal, D., J. J. A. Contes, K. Treuner, A. H. Yang, M. A. Kingsbury et al., 2003. Alteration of gene expression by chromosome loss in the postnatal mouse brain. J. Neurosci. 23: 5599–5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley, R. L., and M. I. Kuroda, 2000. The role of chromosomal RNAs in marking the X for dosage compensation. Curr. Opin. Genet. Dev. 10: 555–561. [DOI] [PubMed] [Google Scholar]
- Kelley, R. L., I. Solovyeva, L. M. Lyman, R. Richman, V. Solovyev et al., 1995. Expression of msl-2 causes assembly of dosage compensation regulators on the X chromosomes and female lethality in Drosophila. Cell 81: 867–877. [DOI] [PubMed] [Google Scholar]
- Kuroda, M. I., M. Kernan, R. Kreber, B. Ganetzky and B. S. Baker, 1991. The maleless protein associates with the X chromosome to regulate dosage compensation in Drosophila. Cell 66: 935–947. [DOI] [PubMed] [Google Scholar]
- Lyle, R., C. Gehrig, C. Neergaard-Henrichsen, S. Deutsch and S. E. Antonarakis, 2004. Gene expression from the aneuploid chromosome in a trisomy mouse model of Down syndrome. Genome Res. 14: 1268–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman, L. M., K. Copps, L. Rastelli, R. L. Kelley and M. I. Kuroda, 1997. Drosophila male-specific lethal-2 protein: structure/function analysis and dependence on MSL-1 for chromosome association. Genetics 147: 1743–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke, M. A., M. F. Mette, T. Kanno and A. J. Matzke, 2003. Does the intrinsic instability of aneuploid genomes have a causal role in cancer? Trends Genet. 19: 253–256. [DOI] [PubMed] [Google Scholar]
- Meller, V. H., and B. P. Rattner, 2002. The roX genes encode redundant male-specific lethal transcripts required for targeting of the MSL complex. EMBO J. 21: 1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, H. J., 1932. Further studies on the nature and causes of gene mutations. Proc. Int. Congr. Genet. 1: 213–255. [Google Scholar]
- Okuno, T., T. Satou and K. Oishi, 1984. Studies on sex-specific lethals of Drosophila melanogaster. Jpn. J. Genet. 59: 237–247. [DOI] [PubMed] [Google Scholar]
- Pal Bhadra, M., U. Bhadra and J. A. Birchler, 1997. Cosuppression in Drosophila: gene silencing of Alcohol dehydrogenase by white-Adh transgenes is Polycomb dependent. Cell 90: 479–490. [DOI] [PubMed] [Google Scholar]
- Pal Bhadra, M., U. Bhadra and J. A. Birchler, 1999. Cosuppression of nonhomologous transgenes in Drosophila involves mutually related endogenous sequences. Cell 99: 35–46. [DOI] [PubMed] [Google Scholar]
- Palmer, M. J., R. Richman, L. Richter and M. I. Kuroda, 1994. Sex-specific regulation of the male-specific lethal-1 dosage compensation gene in Drosophila. Genes Dev. 8: 698–706. [DOI] [PubMed] [Google Scholar]
- Papp, B., C. Pal and L. D. Hurst, 2003. Dosage sensitivity and the evolution of gene families in yeast. Nature 424: 194–197. [DOI] [PubMed] [Google Scholar]
- Parisi, M., R. Nutell, D. Naiman, G. Bouffard, J. Malley et al., 2003. Paucity of genes on the Drosophila X chromosome showing male-biased expression. Science 299: 697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, J. L., S. W. Hayward, Y. Wang, J. Vasselli, C. Pavlovich et al., 2001. The consequences of chromosomal aneuploidy on gene expression profiles in a cell line model for prostate carcinogenesis. Cancer Res. 61: 8143–8149. [PubMed] [Google Scholar]
- Rastelli, L., and M. I. Kuroda, 1998. An analysis of maleless and histone H4 acetylation in Drosophila melanogaster spermatogenesis. Mech. Dev. 71: 107–117. [DOI] [PubMed] [Google Scholar]
- Saran, N. G., M. T. Pletcher, J. E. Natale, Y. Cheng and R. H. Reeves, 2003. Global disruption of the cerebellar transcriptone in a Down syndrome mouse model. Hum. Mol. Genet. 12: 2013–2019. [DOI] [PubMed] [Google Scholar]
- Tautz, D., and C. Pfeifle, 1989. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma 98: 81–85. [DOI] [PubMed] [Google Scholar]
- Turner, B. M., A. J. Birley and J. Lavender, 1992. Histone H4 isoforms acetylated at specific lysine residues define individual chromosomes and chromatin domains in Drosophila polytene nuclei. Cell 69: 376–384. [DOI] [PubMed] [Google Scholar]
- Veitia, R. A., 2002. Exploring the etiology of haploinsufficiency. BioEssays 24: 175–184. [DOI] [PubMed] [Google Scholar]
- Vettese-Dadey, M., P. A. Grant, T. R. Hebbes, C. Crane-Robinson, C. D. Allis et al., 1996. Acetylation of histone H4 plays a primary role in enhancing transcription factor binding to nucleosomal DNA in vitro. EMBO J. 15: 2508–2518. [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., W. Zhang, Y. Jin, J. Johansen and K. M. Johansen, 2001. The JIL-1 tandem kinase mediates histone H3 phosphorylation and is required for maintenance of chromatin structure in Drosophila. Cell 105: 433–443. [DOI] [PubMed] [Google Scholar]
- Wieschaus, E., and C. Nusslein-Volhard, 1986 Looking at embryos, pp. 199–227 in Drosophila: A Practical Approach, edited by D. B. Roberts. IRL Press, Oxford.
- Zhimulev, I. F., E. S. Belyaeva and V. A. Lychev, 1976. Comparative study of the function of polytene chromosomes in laboratory stocks of Drosophila melanogaster and the l(3)tl mutant (lethal tumorous larvae). Chromosoma 55: 121–136. [DOI] [PubMed] [Google Scholar]