Abstract
Phosphodiester linkages, including those that join the nucleotides of DNA, are highly resistant to spontaneous hydrolysis. The rate of water attack at the phosphorus atom of phosphodiesters is known only as an upper limit, based on the hydrolysis of the dimethyl phosphate anion. That reaction was found to proceed at least 99% by C–O cleavage, at a rate suggesting an upper limit of 10−15 s−1 for P–O cleavage of phosphodiester anions at 25°C. To evaluate the rate enhancement produced by P–O cleaving phosphodiesterases such as staphylococcal nuclease, we decided to establish the actual value of the rate constant for P–O cleavage of a simple phosphodiester anion. In dineopentyl phosphate, C–O cleavage is sterically precluded so that hydrolysis occurs only by P–O cleavage. Measurements at elevated temperatures indicate that the dineopentyl phosphate anion undergoes hydrolysis in water with a t1/2 of 30,000,000 years at 25°C, furnishing an indication of the resistance of the internucleotide linkages of DNA to water attack at phosphorus. These results imply that staphylococcal nuclease (kcat = 95 s−1) enhances the rate of phosphodiester hydrolysis by a factor of ≈1017. In alkaline solution, thymidylyl-3′-5′-thymidine (TpT) has been reported to decompose 105-fold more rapidly than does dineopentyl phosphate. We find however that TpT and thymidine decompose at similar rates and with similar activation parameters, to a similar set of products, at pH 7 and in 1 M KOH. We infer that the decomposition of TpT is initiated by the breakdown of thymidine, not by phosphodiester hydrolysis.
Keywords: DNA hydrolysis, DNA stability, nuclease, rate enhancement, phosphate ester
Phosphoric acid diesters are, in general, exceedingly unreactive in water (1–3), so that the phosphodiester linkages that join the nucleotides of DNA are highly resistant to spontaneous hydrolysis. By extrapolation of earlier model experiments at elevated temperatures, the uncatalyzed hydrolysis of dimethyl phosphate in neutral solution was found to proceed with an estimated rate constant of ≈2 × 10−13 s−1 at 25°C, corresponding to a half-time of 140,000 years. That reaction was found to proceed at least 99% by C–O cleavage, suggesting an upper limit of ≈1 × 10−15 s−1 at 25°C on the rate constant for spontaneous P–O cleavage of a phosphodiester anion, the reaction that is catalyzed by many phosphodiesterases (4).
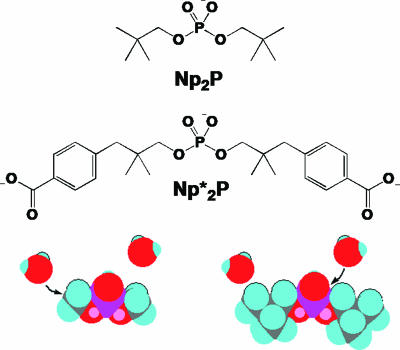
More recently, a rate constant of 6 × 10−7 s−1 has been reported for the decomposition of thymidylyl-3′-5′-thymidine (TpT) at 80°C in 1 M KOH (5). Extrapolation of the results obtained earlier for dimethyl phosphate hydrolysis in neutral solution (4), to 80°C, would indicate a rate ≈105-fold slower. That discrepancy might indicate a major role for catalysis by hydroxide, but the hydrolysis of another dialkyl phosphodiester, bis-3-(4-carboxyphenyl)neopentyl phosphate (Np2*P), in which γ-branching of the leaving alcohol prevents C–O cleavage (Fig. 1), also proceeds ≈105-fold more slowly in 1 M KOH (6).
Fig. 1.
Phosphodiesters used in the present work, in which water attack is sterically confined to the phosphorus atom. The space-filling models indicate the preferred site of attack for nucleophilic water on dimethyl and Np2P, respectively.
In an effort to resolve that discrepancy, and to evaluate the approximate rate enhancement produced by phosphodiesterases such as staphylococcal nuclease (7), we sought to establish the value of the rate constant for P–O cleavage of a simple phosphodiester anion. In the present work, we determined the rate of spontaneous hydrolysis of dineopentyl phosphate (Np2P), in which P–O cleavage occurs, but C–O cleavage is precluded by steric effects (Fig. 1) and cannot occur through elimination. We also measured the reactivity of a methyl triester analogue and reinvestigated the decomposition of TpT in 1 M KOH at 80°C.
Results
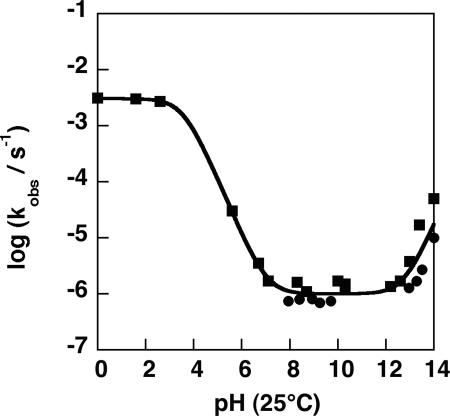
Rate constants were obtained for Np2P (0.01 M) hydrolysis at 250°C in anion-forming buffers (0.1 M potassium formate, acetate, phosphate, borate, and carbonate) whose pH had been determined at 25°C and also in solutions containing HCl (0.1–1.0 M) and KOH (0.1–1.0 M). For Np2P, C–O cleavage by nucleophilic substitution is sterically precluded and cannot occur through elimination. Hence, only P–O cleavage occurs, as was demonstrated by mass spectrometric analysis of the products of hydrolysis in H218O. Very similar rate constants were obtained for hydrolysis over the range from pH 6.5 to 13 (Fig. 2). Fig. 3 shows an Arrhenius plot of typical results obtained in 0.1 M potassium phosphate buffer (pH 6.8). Extrapolation of those results indicates that, at 25°C, the apparent first-order rate constant is 7 × 10−16 s−1 for hydrolysis of the Np2P anion. A more complete Arrhenius plot, based on 68 data points gathered over the range between pH 6.5 and 13, indicates that ΔH‡ = 29.5 ± 0.7 kcal/mol for this reaction, and TΔS‡ = −8.5 ± 1.0 kcal/mol. Data for the hydrolysis of Np2*P at 250°C agree closely with these data (Fig. 2).
Fig. 2.
Influence of buffer pH, measured at 25°C, on rate constants (s−1) for hydrolysis of Np2P (squares) and Np2*P (circles) at 250°C.
Fig. 3.
Influence of temperature on rate constants (s−1) for hydrolysis of Np2P in 0.1 M potassium phosphate buffer (pH 6.8).
In strongly alkaline solution, hydroxide ion catalysis became apparent at KOH concentrations >0.1 M. The results of an Arrhenius plot of rate constants obtained in 1 M KOH were extrapolated to give k25 = 1.4 × 10−15 s−1 for this reaction, with ΔH‡ = 29.5 kcal/mol and TΔS‡ = −8.0 kcal/mol, very similar to those observed for the water reaction (data not shown). This rate is closely comparable with the rate of hydrolysis in 1 M KOH of Np2*P in which the leaving alcohol similarly prevents C–O cleavage, but P–O cleavage is expected to be unimpeded (6). The rate of Np2P hydrolysis also increased at pH values < 6 (Fig. 2), consistent with water attack on uncharged Np2P. Solubility limitations precluded determination of rate constants for Np2P hydrolysis at low pH values where the ester is mostly protonated, except at high temperatures near 250°C. For that reason, the thermodynamics of activation could not be determined for the reaction of the neutral species.
To estimate the difference in reactivity between the neutral and anionic diesters, we measured the rate of hydrolysis of the triester methyl Np2*P at 25°C in 1 M NaOH, using the methyl group as a surrogate for protonation of the phosphate. The phosphate diester products were methyl Np*P and Np2*P in a ratio of 1:4. Partitioning the observed rate constant (5 × 10−6 s−1) between Np* and methanol displacement gives a rate constant of 1 × 10−6 s−1 for Np* expulsion. The loss of methanol from Np2*P might in principle proceed by either C–O or P–O bond cleavage; that distinction was not investigated.
As noted earlier, a surprisingly rapid rate of internucleotide hydrolysis (k = 6 × 10−7 s−1) has been reported for TpT (5). In 1 M KOH at 80°C, TpT was found to decompose ≈5 orders of magnitude more rapidly than would be expected from the present findings for Np2P as well as the earlier findings for Np2*P (6). To investigate the source of that discrepancy, we examined the reaction of TpT in 1 M KOH at 80°C and also in potassium phosphate buffer (0.1 M, pH 6.8).
We were able to duplicate the findings of Takeda et al. (5), observing decomposition of TpT at 80°C in 1 M KOH. We also observed decomposition, proceeding at a similar rate, in potassium phosphate buffer (0.1 M, pH 6.8) at 80°C. We found, however, that under both sets of conditions, thymidine itself is unstable, and that both thymidine and TpT decompose at comparable rates in such a way as to obscure the rate of cleavage (if any) of the phosphodiester bond of TpT. Moreover, at pH 14, both thymidine and TpT decompose at 80°C, with similar pseudo-first-order rate constants and activation parameters, to roughly the same set of products, as indicated by proton NMR analysis (see Fig. 5, which is published as supporting information on the PNAS web site). UV analysis indicated that opening of the pyrimidine ring proceeds with the same rate constant.
At pH 6.8 (0.1 M potassium phosphate buffer), thymidine and TpT decomposed with rate constants and activation parameters that were identical within experimental error. However, at pH 6.8, decomposition leads to a different set of products from those observed in 1 M KOH. Under these conditions, proton NMR and UV spectroscopic analysis showed that both substrates are converted quantitatively to thymine, with no significant degradation of the pyrimidine ring. The hydrolysis of thymidine to thymine, as indicated by UV analysis, was observed earlier in neutral and acid solution (8).
Discussion
Table 1summarizes the rate constants, enthalpies, and entropies of activation for diester hydrolysis observed in this study and earlier work. The similarity between the extrapolated value of k25 = 7 × 10−16 s−1 for Np2P and the approximate upper limit on the rate constant for P–O cleavage of dimethyl phosphate (≈1 × 10−15 s−1 at 25°C) that was indicated by earlier experiments on dimethyl phosphate (1) suggests that P–O cleavage is not sterically impeded in Np2P. It seems reasonable to infer that the extrapolated rate constant of k25 = 7 × 10−16 s−1, equivalent to a half-time of 31,000,000 years at 25°C, can be considered typical of apparent water attack on the phosphorus atom of simple dialkyl phosphate anions. Because the activation parameters for the hydroxide-catalyzed and spontaneous reactions are very similar, the form of the pH rate profile at high pH will not change at lower temperature. Hydroxide attack at the diester anion appears to become a significant contributor to hydrolysis only at very high pH.
Table 1.
Kinetics of phosphate ester hydrolysis
| Ester | Ion | Ref. | Cleavage | k25 °C, s−1 or M−1·s−1 | ΔG‡ 25°C, kcal/mol | ΔH‡, kcal/mol | TΔS‡, 25°C, kcal/mol |
|---|---|---|---|---|---|---|---|
| Monoester | |||||||
| H2O + MeP− | Monoanion | 9 | P–O | 2.4 × 10−10 | +30.6 | +30.0 | −0.6 |
| H2O + MeP= | Dianion | 10 | P–O | 2 × 10−20 | +44.3 | +47.0 | +2.7 |
| Diester | |||||||
| H2O + Me2P | Neutral | 1 | C–O | 6 × 10−10 | +30.0 | +25.0 | −5.0 |
| H2O + Me2P− (or HO− + Me2P) | Anion (neutral) | 4 | C–O | 1.6 × 10−13 | +34.9 | +25.9 | −9.0 |
| HO− + Me2P− | Anion | 2 | C–O | 3 × 10−11 | +31.7 | +27.6 | −4.1 |
| H2O + Np2P− | Anion | This work | P–O | 7 × 10−16 | +38.1 | +29.5 | −8.6 |
| HO− + Np2P− | Anion | This work | P–O | 1.4 × 10−15 | +37.7 | +29.5 | −8.0 |
| HO− + Np2*P− | Anion | 6 | P–O | 1 × 10−15 | +37.9 | +30.8 | −7.1 |
| Triester | |||||||
| H2O + Me3P | 11 | C–O | 2 × 10−8 | +28.1 | +22.6 | −5.5 | |
| HO− + Me3P | 12 | P–O | 1.4 × 10−4 | +22.7 | +15.4 | −7.3 | |
| HO− + Et3P | 11 | P–O | 9 × 10−6 | +24.3 | +14.1 | −10.2 | |
| Diesterase | |||||||
| Staph. nuclease | 7 | P–O | 95 | +14.7 | +10.8 | −3.9 |
As to the actual mechanism by which Np2P is hydrolyzed, it is of interest that the rate constant for Np2P hydrolysis, extrapolated to 100°C from the present results, is larger by 3 orders of magnitude than would be expected by extrapolation to pKa 15.5 of a Brønsted plot based on the rates of hydrolysis of anions of diaryl phosphate esters of alcohols with pKa values ranging from 4 to 8.5 (3). In further contrast to our observations (Fig. 2), these data also show that hydroxide-catalyzed hydrolysis dominates over the spontaneous reaction as the leaving groups become poorer. It is possible that the dialkyl diester is not hydrolyzed through water attack on the monoanion but through the kinetically equivalent mechanism of hydroxide attack on the neutral diester (Fig. 4). We can estimate the rate of the latter reaction by assuming that the methyl triester of Np2*P is a reasonable model for the neutral diester; at 25°C, the rate of reaction of Np2*P with the hydroxide ion is 1 × 10−6 M−1·s−1. Taking into account the unfavorable equilibrium (K = Kw/Ka ≈ 3 × 10−13; Fig. 4) involved in transferring a proton from water to the diester anion, the predicted rate for the reaction through this mechanism is ≈3 × 10−19 s−1. These comparisons and the associated errors do not allow a clear distinction to be made between the two mechanisms using our data, although recent calculations (13) give very good agreement with the triester-like mechanism. Comparing the rate of reaction of the methyl triester and corresponding diester with hydroxide, we note that the effect of neutralizing the phosphoryl group is to accelerate this reaction by ≈109 fold. That effect is substantially greater than the effect (≈106-fold) of the analogous change in RNA models that undergo transesterification with an intramolecular nucleophile (14). Interestingly, like the diaryl diesters and in contrast to the observations reported here, RNA cleavage is dominated by the base-catalyzed reaction from pH 5 with a minimal contribution from any pH-independent reaction (15).
Fig. 4.
Equilibrium between alternate reactants in the apparently uncatalyzed hydrolysis of the Np2P anion.
It is of interest to consider these observations in relation to the lifetime of the backbone of DNA. Because C–O cleavage competes effectively with P–O cleavage (Table 1), it seems reasonable to suppose that the predominant mode of phosphodiester hydrolysis of DNA might involve C–O cleavage by water (or hydroxide) attack at the relatively unhindered 5′-carbon atom of the nucleoside to which the phosphoryl group is attached. The possibility of confirming that conjecture by experiment is clouded, however, by the likelihood that other modes of decomposition transpire far more rapidly than ester hydrolysis, as discussed below.
A surprisingly rapid rate of internucleotide hydrolysis (k = 6 × 10−7 s−1) has been reported for TpT at 80°C (5). At pH 14 at 80°C, TpT was found to decompose ≈5 orders of magnitude more rapidly than would be expected from the behavior of Np2*P (6) or the present findings for Np2P. We found, however, that under both neutral and basic conditions, thymidine itself is unstable, and that both thymidine and TpT decompose at the same rate in such a way as to obscure any cleavage of the phosphodiester bond of TpT. In 1 M KOH, both thymidine and TpT decompose at 80°C with approximately the same pseudo-first-order rate constants and activation parameters, to a similar set of products, as indicated by proton NMR analysis (see, which are published as supporting information on the PNAS web site). UV analysis indicates that opening of the pyrimidine ring proceeds with the same rate constant. Because both thymidine and TpT decompose at similar rates in 1 M KOH at 80°C, whereas Np2P and Np2*P are hydrolyzed 5 orders of magnitude more slowly, we infer that ring-opening in base opens pathways for further decomposition (e.g., by elimination) that were not available before ring-opening and are not available to a simple phosphodiester. Thus, the spontaneous cleavage of DNA is likely to be dominated by pathways that occur through the formation of abasic sites, rather than by P–O bond breaking as catalyzed by many phosphodiesterases. From these results, it is evident that DNA cleavage can be initiated in a variety of ways, suggesting the need for caution in attributing the decomposition (e.g., by novel artificial catalysts) of long strands of DNA to any particular mechanism.
If the rate constant for the uncatalyzed hydrolysis of the Np2P− anion, extrapolated to 25°C (7 × 10−16 s−1) is compared with kcat for staphylococcal nuclease at 25°C (95 s−1) (7), the rate enhancement produced by staphylococcal nuclease is found to be 1.4 × 1017-fold, corresponding to a 23.2 kcal/mol reduction in ΔG‡. That value is comparable in magnitude with the rate enhancements produced by orotidine 5′-monophosphate decarboxylase (1.4 × 1017-fold) (16) and β-amylase (7 × 1017-fold) (17) and is presently exceeded only by the values that have been recorded for fructose 1,6-bisphosphatase (1.1 × 1021-fold) (10) and arginine decarboxylase (7 × 1019-fold) (18).
Comparison of ΔH‡ = 27.8 for water attack on the Np2P− anion (Table 1) with ΔH‡ = 10.8 for reaction of the enzyme–substrate complex of staphylococcal nuclease indicates that most of the 23.2 kcal/mol reduction in ΔG‡ by staphylococcal nuclease is accounted for by a reduction in the heat of activation for phosphodiester hydrolysis. That tendency, also observed in the other slow reactions mentioned in the previous paragraph, may be understandable in view of the fact that most of the very high activation barrier to product formation is enthalpic rather than entropic in these reactions (19). A major reduction in enthalpy of activation might be expected for a reaction in which polar forces of attraction, especially ionic H-bonds (20, 21), are at work between an enzyme and the altered substrate in stabilizing the transition state. In the particular case of staphylococcal nuclease (7), ionized functional groups have been shown to line the active site cavity (22).
Materials and Methods
Dimethyl phosphate was synthesized by the alkaline hydrolysis of trimethyl phosphate, and Np2P was synthesized by the alkaline hydrolysis of 4-nitrophenyl dineopentyl phosphate. Methyl dialkyl triesters were synthesized from methyl dichlorophosphate and the corresponding alcohol. TpT was purchased from Sigma-Aldrich. The hydrolysis of phosphodiesters of methanol and neopentanol (0.01 M) was conducted in HCl (1–0.1 M), in 0.1 M buffers containing potassium formate, acetate, phosphate, borate, or carbonate, and in KOH (0.1–1 M). These buffers were chosen because the heats of ionization of the conjugate acids, like those of phosphoric acid esters, are <4 kcal/mol (23), and their pH values are correspondingly insensitive to changing temperature. [The heat of ionization of 0.1 M potassium borate buffers, which we determined by measuring the variation of buffer pH (8.45 at 25°C) over the temperature range from 20° to 70°C with the glass electrode, was 2.4 kcal/mol.] Reaction mixtures were introduced into polytetrafluoroethylene-lined stainless steel bombs (model no. 276AC, Parr Instruments, Moline, IL) that were placed in convection ovens (model no. 47900, Barnstead/Thermolyne, Dubuque, IA), and maintained at constant temperatures ranging from 150° to 260°C (temperature variation ± 1.5°C) for varying periods of time. Approximately 120 min elapsed before the contents of the polytetrafluoroethylene-lined reaction vessels arrived within 2°C of the target temperature, as determined by using a bomb and liner that had been drilled to accommodate a thermometer inserted through the top of the oven. To minimize errors arising from that time lag, all reactions were conducted for a minimum of 16 h, and 2 h were subtracted from the total elapsed time in calculating rate constants. The progress of reaction was followed by monitoring the disappearance of starting material by proton NMR. The integrated intensities of the resonances of the reactants and products were compared with those of pyrazine, a known concentration of which had been added just before analysis as an integration standard. Each of these reactions yielded the alcohol and inorganic phosphate without significant accumulation of monoester. In contrast to Np2P, dimethyl phosphate undergoes hydrolysis in 1 M KOH much more rapidly than does methyl phosphate, except at very high temperatures (compare the first two entries in Table 1). As a practical consequence of the present observations, either trimethyl or dimethyl phosphate can be converted entirely to the monoester overnight in 1 M KOH at 150–170°C, with no trace of contaminating dimethyl phosphate or inorganic phosphate. The reactions of Np2*P and methyl Np2*P were measured by monitoring the changes in concentration of starting diester and product alcohols relative to an internal standard of 4-methylbenzoic acid by HPLC analysis.
For the reactions of Np2*P at 250°C, the reaction mixtures were divided between several small polytetrafluoroethylene-lined stainless steel bombs that were completely immersed in a vigorously circulating bath containing silicon oil. The temperature was continuously monitored with an electronic contact thermometer, and individual samples were withdrawn at various time intervals. When using the circulating oil bath, thermal equilibration was achieved in ≈15 min.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant GM-18325 and by the Engineering and Physical Sciences Research Council (P.W.) and the Biotechnology and Biological Sciences Research Council (C.L.).
Abbreviations
- TpT
thymidylyl-3′-5′-thymidine
- Np2P
dineopentyl phosphate
- Np2*P
bis-3-(4-carboxyphenyl)neopentyl phosphate.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Bunton C. A., Mhala M. M., Oldham K. G., Vernon C. A. J. Chem. Soc. 1960;1960:3293–3301. [Google Scholar]
- 2.Kumamoto J., Cox J. R., Jr., Westheimer F. H. J. Am. Chem. Soc. 1956;78:4858–4860. [Google Scholar]
- 3.Kirby A. J., Younas M. J. Chem. Soc. B. 1970;1970:510–513. [Google Scholar]
- 4.Wolfenden R., Ridgway C., Young G. J. Am. Chem. Soc. 1998;120:833–834. [Google Scholar]
- 5.Takeda N., Shibata M., Tajima N., Hirao K., Komiyama M. J. Org. Chem. 2000;65:4391–4396. doi: 10.1021/jo000323d. [DOI] [PubMed] [Google Scholar]
- 6.Williams N. H., Wyman P. Chem. Commun. 2001;2001:1268–1269. [Google Scholar]
- 7.Serpersu E., Shortle D., Mildvan A. S. Biochemistry. 1987;26:1289–1300. doi: 10.1021/bi00379a014. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro R., Kang S. Biochemistry. 1969;8:1806–1810. doi: 10.1021/bi00833a004. [DOI] [PubMed] [Google Scholar]
- 9.Bunton C. A., Llewellyn D. R., Oldham K. G., Vernon C. A. J. Chem. Soc. 1958;1958:3574–3587. [Google Scholar]
- 10.Lad C., Williams N. H., Wolfenden R. Proc. Natl. Acad. Sci. USA. 2003;100:5607–5610. doi: 10.1073/pnas.0631607100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aksnes G., Bergesen K. Acta Chem. Scand. 1966;30:2508–2514. [Google Scholar]
- 12.Barnard P. W. C., Bunton C. A., Llewellyn D. R., Vernon C. A., Welch V. A. J. Chem. Soc. 1961;1961:2670–2676. [Google Scholar]
- 13.Iché-Tarrat N., Barthelat J.-C., Rinaldi D., Vigroux A. J. Chem. Phys. B. 2005;109:22570–22580. doi: 10.1021/jp0550558. [DOI] [PubMed] [Google Scholar]
- 14.Oivanen M., Kuusela S., Lönnberg H. Chem. Rev. 1998;98:961–990. doi: 10.1021/cr960425x. [DOI] [PubMed] [Google Scholar]
- 15.Järvinen P., Oivanen M., Lönnberg H. J. Org. Chem. 1991;56:5396–5401. [Google Scholar]
- 16.Radzicka A., Wolfenden R. Science. 1995;267:90–93. doi: 10.1126/science.7809611. [DOI] [PubMed] [Google Scholar]
- 17.Wolfenden R., Lu X., Young G. J. Am. Chem. Soc. 1998;120:6814–6815. [Google Scholar]
- 18.Snider M. J., Wolfenden R. J. Am. Chem. Soc. 2000;122:833–834. [Google Scholar]
- 19.Wolfenden R., Snider M., Ridgway C., Miller B. J. Am. Chem. Soc. 1999;121:7419–7420. [Google Scholar]
- 20.Kauzmann W. Adv. Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- 21.Meot-Ner M. Chem. Rev. 2005;105:213–284. doi: 10.1021/cr9411785. [DOI] [PubMed] [Google Scholar]
- 22.Cotton F. A., Hazen E. E., Jr., Legg M. J. Proc. Natl. Acad. Sci. USA. 1979;76:2551–2555. doi: 10.1073/pnas.76.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edsall J. T., Wyman J. Biophysical Chemistry. Vol. 1. New York: Academic; 1958. pp. 452–453. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.