Abstract
Swarming and mass migration are spectacular and sometimes devastating features of the biology of various animal species. These phenomena are typically associated with actual or anticipated depletion of food resources after an increase in population density, but the mechanisms driving such collective movements are poorly understood. Here we reveal that insects in large, coordinated migratory bands consisting of millions of Mormon crickets in western North America were deprived of two essential nutritional resources: protein and salt. The insects themselves provided a major source of these nutrients, and cannibalism was rife. We show that protein and salt satiation reduced cannibalism and that protein satiation inhibited walking. Additionally, experimentally reducing the motility or mobility of crickets substantially increased their risk of being cannibalized by other band members. As a result, the availability of protein and salt in the habitat will influence the extent to which bands march, both through the direct effect of nutrient state on locomotion and indirectly through the threat of cannibalism by resource-deprived crickets approaching from the rear. The crickets are, in effect, on a forced march. Migratory band formation and subsequent mass movement, therefore, are manifestations of specific tradeoffs between the costs and benefits of group living. Bands afford antipredator benefits to individual group members. Group movement then mitigates the resulting costs of intraspecific competition, namely local depletion of nutritional resources and the associated increased risk of cannibalism.
Keywords: cannibalism, migration, Mormon cricket, swarming
The mechanisms that drive collective mass animal movements remain very poorly understood (1, 2), and existing models of such phenomena are largely untested. Outbreaks of Mormon crickets, Anabrus simplex (Orthoptera: Tettigoniidae), in western North America provide a striking example of collective mass animal movement, with extensive marching bands comprising millions of these large flightless insects moving en masse. Such bands can extend up to 10 km in length and travel 2 km per day (3, 4). Recent radio-tracking studies demonstrated that when Mormon crickets were isolated from a band, they suffered high levels of predation (5), indicating that band formation serves as an antipredator strategy.
Once groups form under conditions conducive to population growth, group movement should enhance the likelihood of leaving areas that have been depleted of resources and encountering more favorable habitats, thereby alleviating the cost of intraspecific competition for resources within the group. Despite being omnivorous, Mormon crickets do not strip the environment bare of vegetation as they go (6–8). Thus, resource depletion, if it occurs, must be selective. Understanding what these key resources are will illuminate the mechanisms, function, and ecological correlates of collective movement in Mormon crickets, as well as for other similar phenomena such as mass migrations of locusts (9).
Candidate nutritional resources that might drive mass movement when limited are protein (nitrogen), carbohydrate, water, and micronutrients such as minerals. Our aim was to establish which of these resources contribute to mass migration and how their effects are mediated. Our results indicated that protein and salt are the key limiting resources and that crickets themselves provided a major source of these nutrients in the field, which would explain the high incidence of cannibalism in Mormon cricket bands (10, 11). This finding led us to explore the relationships among protein and salt deprivation, cannibalism, and mass movement and to test the prediction that movement by individual Mormon crickets within migratory bands serves to reduce their risk of being cannibalized.
Results
Initial Field Observations.
Detailed observations of feeding within an ≈1-km-long band of late-instar Mormon crickets in the Curlew National Grasslands of southern Idaho (May 19–25, 2005; N42°12′52.44″, W112°34′55.56″) were consistent with previous observations of Mormon cricket resource use (6, 12, 13) and indicate that the insects selectively feed on high-protein food sources: seed heads and seed pods, flowers, foliage of nitrogen-rich legumes, carrion, and mammal feces (see Fig. 5, which is published as supporting information on the PNAS web site). They also ingested their own shed exoskeleton after molting, which is a sign of protein limitation (14). Additionally, crickets were seen to ingest soil soaked in cattle urine, suggesting nitrogen or salt deprivation. Other potential sources of plant food were abundant in the habitat but seldom eaten. We found that crickets avidly ate each other, however, demonstrating active predation and consumption of molting, wounded, and freshly dead insects. Cannibalism has previously been described in this species (3, 10, 11, 13), as in other omnivores (15–17), and can lead to dangerously slick driving conditions for automobiles as Mormon crickets stop to eat crushed conspecifics on roadways and get run over themselves.
Preference for Protein Versus Carbohydrate.
To test unequivocally whether mass-moving crickets select protein over carbohydrate, dishes containing chemically defined artificial diets [as used extensively in previous experiments on other Orthoptera (18)] were placed on a cattle trail in the face of a marching band and videotaped in 10-min trials. Four dry, granular diets were presented in 4-cm Petri dishes placed 4 cm apart perpendicular to the flow of insects: diet P contained 42% protein but no digestible carbohydrate, diet C had 42% carbohydrate but no protein, diet PC contained 21% of both nutrients, and diet O contained neither macronutrient. The relative positions of the diets were randomized across eight trials.
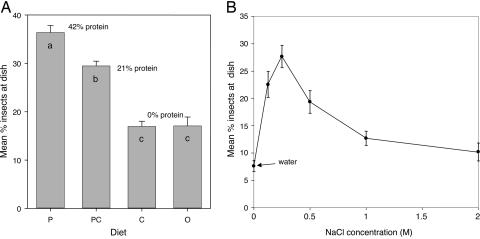
There was a clear preference for protein-containing diets, with the highest-protein diet (P) arresting the movement of the most insects upon tarsal contact, there being as many as 13 crickets clustered and jostling on the P dish at any one time (Fig. 1A; also see Movie 1, which is published as supporting information on the PNAS web site). Carbohydrate had no effect, with insects stopping as readily to feed on the carbohydrate- and protein-free O diet as the C diet.
Fig. 1.
Response of Mormon crickets to nutrient resources placed in the path of the marching band. (A) Mean + SE numbers of crickets that were at each of four diet dishes throughout 10-min observation periods (see Materials and Methods). The number of crickets arrested at each dish was a function of the protein content of the diet, with no indication of responsiveness to carbohydrate. Bars marked with different letters differ in a least significant difference post hoc test at P < 0.0005, after ANOVA in which the effect of treatment was highly significant (F3,28 = 51.3, P < 0.0005; Levene’s test of equality of variances, F3,28 = 1.57, P = 0.218). (B) As for A, but showing responses to a graded series of NaCl concentrations placed in front of the marching band. There was a strong preference for 0.25 M NaCl, with numbers falling at lower and higher concentrations. ANOVA results, F5,54 = 18.64, P < 0.0005; Levene’s test of equality of variances, F5,54 = 1.12, P = 0.359.
To establish whether patterns of nutrient preference found in marching bands persisted under environments in which protein and carbohydrate were abundant, crickets were captured from the band and housed individually in 30- × 15- × 9-cm clear plastic boxes with a plastic mesh lid, containing two preweighed dishes of dry diet, one P and the other C, along with a water-filled glass vial with a cotton wick as a water source. The boxes were left in the field under ambient conditions, and diet intake was measured daily over 48 h, with fresh diets being provided after 24 h. The same experiment was repeated 25 days later (June 20–23, 2005) at the same site with adult insects from a different band.
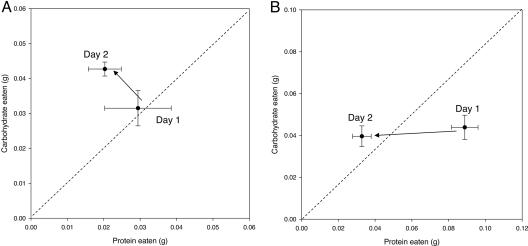
Whereas the data in Fig. 1A indicate a marked preference for diet P over diet C in the short term, over the first full day of access to both diets, crickets selected slightly more of the C than the P diet (a 1.1:1.0 ratio). Over the next day, they ate somewhat less protein and substantially more carbohydrate (a 2.1:1.0 ratio of C/P) (Fig. 2A). This pattern was even more pronounced in the second band studied. Insects from this band were protein-deprived to an even greater extent on the first day of the assay but also increased the relative amount of carbohydrate in their diets over time (Fig. 2B).
Fig. 2.
Means ± bivariate SEs for daily intake of protein and carbohydrate. (A) Six field-collected Mormon cricket nymphs (three male, three female) confined for 48 h with P and C diets. Protein intake declined somewhat across the 2 days, whereas carbohydrate intake increased, indicating compensation for prior protein deficiency. The C/P intake ratio increased significantly from day 1 to day 2 [two-tailed, one-sample t test with test value of 0 (no change between days 1 and 2), t = 2.80, df = 5, P = 0.038]. (B) Twenty field-collected Mormon cricket adults (10 male, 10 female) from a different band assayed as above 25 days later. The C/P intake ratio increased significantly from day 1 to day 2 (t = 4.82, df = 19, P < 0.0005). The diagonal dashed lines in A and B indicate a 1:1 C/P ratio.
Preference for Salt Versus Water.
We tested next the response to a concentration series of NaCl. Six 4-cm Petri dishes were placed abreast 4 cm apart and contained cotton wool soaked in water (no salt added), 0.125, 0.25, 0.5, 1.0, and 2.0 M NaCl. The water dish arrested fewest insects, and there was a sharp preference peak for 0.25 M NaCl (up to 12 crickets fighting over the dish), with the numbers of insects stopping to drink falling away at lower and higher concentrations [Fig. 1B; also see Movie 2 (which is published as supporting information on the PNAS web site) for a living histogram].
Protein Satiation and Its Effect on Cannibalism.
Results from the preceding experiments indicated that crickets were protein- and salt-deprived. To test whether protein satiation reduced the propensity to cannibalize, freshly captured crickets were individually housed in the field for 5 h in 2.5-liter white plastic buckets containing a preweighed dish of diets P, C, PC, or O. One-half of the 80 insects (40 males, 40 females) had a separate water source, and the others were water-deprived. After the pretreatment period, crickets were placed into clean buckets with a live but freshly incapacitated cricket (with its cervical membrane ruptured) of equivalent size. Cricket and victim were left for 50 min, after which the extent of cannibalism was scored.
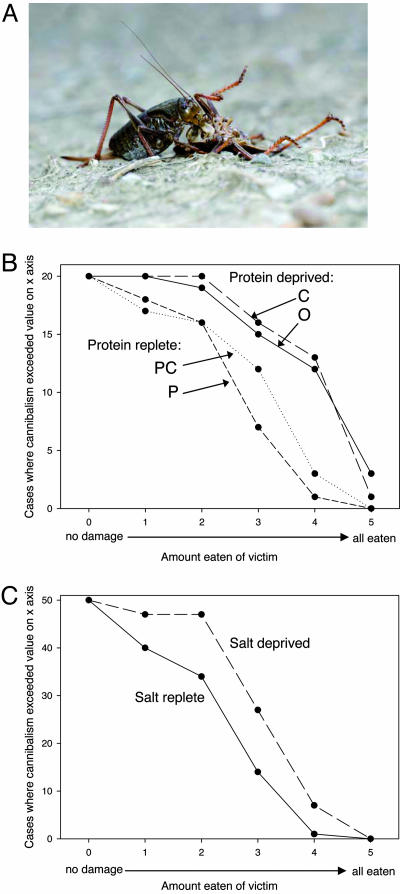
Protein prefeeding substantially reduced the amount of cannibalism, whereas neither carbohydrate nor water deprivation had any effect (Fig. 3A). Some protein-deprived insects consumed an entire similarly sized conspecific in a single meal. A meal of this size is extraordinary and in our experience unique for an animal that chews its food.
Fig. 3.
State-dependent changes in cannibalism (A) in field-collected Mormon cricket nymphs after 5 h of pretreatment on either one of four synthetic diets (P, C, PC, or O; n = 20) (B) or grass seed heads with or without a separate source of 0.25 M NaCl (n = 50) (C). Plots indicate the cumulative frequency of damage to the victim (see Materials and Methods) after a 50-min exposure, with more steeply declining curves indicating less cannibalism. (B) Cannibalism data are pooled within diets across treatments in which crickets had access to or were deprived of water during the pretreatment (n = 10). ANOVA results: effect of pretreatment diet, F3,72 = 8.64, P < 0.0005; effect of water, F1,72 = 0.262, P = 0.610; diet by water interaction, F3,72 = 1.21, P = 0.312; Levene’s test of equality of variances, F7,72 = 1.14, P = 0.349. Means for P- and PC-pretreated crickets did not differ, nor did those from C- and O-prefed insects, whereas the former two differed from both of the latter two treatments at P < 0.003 (least significant difference post hoc test). Although the artificial diets all included 1.8% salt, these differences were due to protein consumed in the pretreatment and not to differences in salt intake between the diets. Thus, in a generalized linear model with diet treatment as a main effect (excluding water) and no covariates, F3,76 = 8.66 for the diet pretreatment effect (P < 0.0005). When salt eaten during the pretreatment (calculated from the mass difference in the food dish and the known level of salt in the diet) was included as a covariate, the effect of diet remained significant (F3,75 = 5.875, P = 0.001). However, when protein eaten was also included in the model, having corrected for differences in salt eaten, the effect of diet became nonsignificant (F3,74 = 2.467, P = 0.069). (C) There was a strong effect of salt status on propensity to cannibalize in the absence of variation in dietary protein. ANOVA results, F1,98 = 14.21, P < 0.0005; Levene’s test of equality of variances, F7,72 = 1.41, P = 0.237.
Salt Satiation and Its Effect on Cannibalism.
The same experiment was conducted for salt satiation, with crickets being pretreated for 5 h with bulbous bluegrass (Poa bulbous) seed heads (the most abundant high-protein food source they had been observed to feed on) augmented by a cotton swab soaked in either water or 0.25 M NaCl. There was a strong effect of salt balance on cannibalism, with salt-replete insects eating significantly less of the victim than those deprived of extra salt (Fig. 3B).
Protein Satiation and Its Effect on Locomotion.
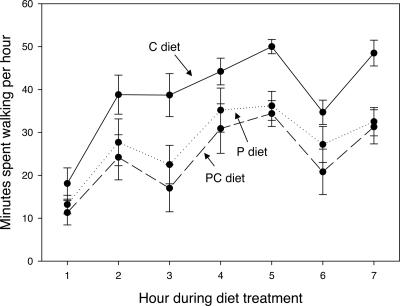
To establish whether protein satiation inhibited the tendency to march, crickets were isolated in 30- × 15- × 9-cm plastic cages with a water source and observed for locomotion at 1-min intervals for 7 h after the addition of a 4-cm Petri dish containing one of three artificial diets (P, C, or PC) ad libitum. Protein consumption inhibited locomotion. Over the observation period, crickets without access to protein spent 50% more time walking than did those with protein (46 ± 4.6%, 41 ± 6.2%, and 65 ± 4.3% of time spent walking for P, PC, and C diets, respectively; Fig. 4).
Fig. 4.
Protein satiety inhibits locomotion. Crickets were fed one of three diets (P, C, or PC), and their level of locomotory activity was recorded over the next 7 h. ANOVA results (totals across 7 h) for the effect of diet were as follows: F2,27 = 6.28, P = 0.006. Levene’s test of equality of variances, F2,27 = 0.903, P = 0.417. Means for diets P and PC did not differ (P = 0.425), although both were significantly lower than diet C (P = 0.016 and 0.002, respectively, least significant difference post hoc test). That the effect was due specifically to protein intake is indicated by the fact that including protein consumption by each insect during the assay as a covariate in the model removed the significance of diet treatment (diet effect, F2,26 = 2.16, P = 0.135), whereas including either carbohydrate intake (F2,26 = 8.95, P = 0.001) or salt intake (F2,26 = 4.64, P = 0.019) did not.
Movement as a Defense Against Cannibalism.
To test the effect of limiting mobility and motility on cannibalism, we affixed the ventral surface of test crickets to a small flat stone with a drop of hot glue. One-half of the crickets were alive and able freely to move their legs (immobile but motile), whereas the remainder were freshly killed (i.e., immobile and immotile) by suffocation. Insects were aligned facing downstream in the flow of crickets in the band. Seven trials were conducted in which one live and one dead cricket were placed 10 cm apart on a cattle trail.
Consumption of freshly dead individuals began within 21 s after contact with a cricket from the band (median, 15 s), whereas the median latency to attack for motile individuals that were able to move their appendages when contacted by oncoming crickets was 165 s (z = −2.37, P < 0.018). Video observations showed that potential victims repelled attacking crickets by kicking them with their hind legs.
In another experiment, we allowed limited mobility by tethering crickets and also varied motility by compromising leg kicking. Fourteen replicate trials were conducted, with each replicate placed at 10-m intervals along a cattle trail running perpendicular to the flow of a migratory band. Replicates consisted of placing four potential victims tethered ≈30 cm apart. Tethered insects were (i) dead with all legs intact, (ii) alive with all legs intact, (iii) alive and missing one rear leg, or (iv) alive and missing both rear legs. Position within replicates was varied across trials. Insects were left for 2 h, after which they were recorded as either still present or cannibalized.
All of the 14 intact but dead tethered insects were consumed by other band members within 2 h. In contrast, 13 of 14 live and fully intact crickets survived (Fisher’s exact test, P < 0.0001). Restricted motility in terms of missing rear legs had a major effect on survival, despite the fact that these insects were mobile (albeit restricted by being tethered). Insects missing both back legs were cannibalized significantly more often than completely intact insects (only 5 of 14 survived, P = 0.002) and more often than those missing one leg (of which 11 of 14 survived, P = 0.02).
To assess the effects of limited motility on crickets under natural conditions, we quantified the frequency of insects within the band that were missing one rear leg, both rear legs, or fully intact. Observations were made by counting the number of insects crossing 1-m transects for 15 min at four different positions within the band. Only 2% of 2,522 observed insects were missing one leg. Insects missing both back legs were never observed.
Discussion
Our results clearly indicated that marching Mormon crickets foraged preferentially for protein and salt, not water or carbohydrate. Regarding salt, a 0.25 M NaCl solution was maximally stimulating. The fact that Mormon crickets are high in protein (19) and the sodium concentration in the hemolymph of omnivorous exopterygote insects is typically 0.15–0.2 meq·liter−1 (20) explains their taste for conspecifics. In short, Mormon crickets are walking packages of protein and salt. They constitute an abundant potential source of these vital nutrients for hungry conspecifics in migratory bands.
A key question is whether crickets were specifically deprived of protein and salt as a result of their recent nutritional history or perhaps hardwired as omnivores to find them highly and consistently stimulating as a result of an evolutionary history of nitrogen and salt limitation (17, 21). Distinguishing between these explanations requires investigating whether state-dependency exists in the response to protein and salt (22, 23). We addressed this issue by testing (i) whether crickets confined with P and C diets changed the balance of protein and carbohydrate eaten over consecutive days and (ii) whether altering the nutrient status of crickets affected their propensity to cannibalize. Data from two different Mormon cricket bands indicated that the crickets in the field were protein-deprived and that when allowed free access to a high-protein source, they soon redressed this imbalance and selected a carbohydrate-biased diet (Fig. 2). Additionally, providing constant access to protein or salt for 5 h significantly reduced cannibalism (Fig. 3).
Without discounting other nutrients, we conclude from these experiments that the marching Mormon crickets were protein and salt deprived and that they fed selectively to redress these nutrient imbalances when the opportunity arose. Although we have shown that crickets can remedy their nutritional deficiencies in a relatively short period given access to the lacking resource, our findings also indicate that most individuals in the band had not done so and were currently resource-deprived. In fact, unless crickets are in the vanguard of the band as it encounters new habitat, cannibalism may provide the only means of achieving nutrient balance.
As a result, we predict that band members must continually move to avoid being eaten by similarly deprived conspecifics, a prediction that was clearly met by the results of experiments in which we compromised the motility and mobility of crickets within marching bands. The safest cricket from cannibal attacks is one that is mobile and able to fend off approaches with its two hind legs. The immediate presence of conspecifics is already known to induce locomotion in individual Mormon crickets (24), and the threat of cannibalism likely underlies this response.
Current models of collective motion fail to consider this “forced march” mechanism. Attraction and alignment among near neighbors are currently considered the principal driving forces, with the resultant direction of mobile animal groups being largely determined by the “pull” from those ahead as opposed to the “push” from those behind (25) (although see ref. 26 for how alignment can occur over long-length scales with just repulsion).
We also found a direct effect of protein status on the tendency of isolated individual crickets to march (Fig. 4). This result is consistent with extensive research on locusts (27) showing that protein satiety results in inhibition of locomotion, with consequent inhibition of aggregation behavior in hopper groups (28). Given that protein-deprived individuals are more likely to move as well as cannibalize each other (Figs. 3B and 4), an increase in locomotion will concomitantly increase the frequency of potential cannibalistic interactions within the group.
The heterogeneous nutritional environments encountered by animals can have major effects on their ecology and evolution. Variation in resource availability is already known to mediate a remarkable shift in Mormon cricket mating strategies, with protein deprivation inducing a reversal of courtship roles among insects in high-density migratory band-forming populations (4, 29). Our results show that understanding the nutritional status of these organisms explains yet another extraordinary facet of their biology, namely mass migration.
In conclusion, we have demonstrated how understanding the nutritional ecology of migratory band-forming insects provides a unifying framework that explains both how and why interindividual interactions can lead to landscape-scale mass movement. Under outbreak conditions, the availability of protein and salt in the habitat (a joint function of habitat quality and cricket population density) is likely to determine the extent to which migratory bands march, both through the direct influence of nutrient state on locomotion and through the indirect effect of contact by resource-deprived cannibalistic crickets approaching from behind. Increases in locomotion among nutritionally deprived individuals synergistically interact with their increased propensity to cannibalize to induce mass movement. Specific nutrient resource deprivation and resulting cannibalism are, therefore, important costs of migratory band formation in Mormon crickets and probably also in other systems, such as locusts.
Why do Mormon cricket bands persist as cohesive units in the face of the risk of cannibalism? The mark-recapture study of Sword et al. (5) demonstrated the severe cost of leaving a migratory band. Insects removed from a band and transplanted to nearby sites suffered 50–60% mortality due to predation in just 2 days. Presumably, the risk of predation upon leaving the group outweighs the combined fitness costs of migratory band membership. Living and moving together in a migratory band is a compromise that makes the best of a seemingly very bad situation.
Materials and Methods
Preference for Protein Versus Carbohydrate.
The protein used for the P and PC diets contained a 3:1:1 mix of casein/peptone/albumen. The digestible carbohydrate used in the C and PC diets comprised a 1:1 mix of sucrose/dextrin. P, C, and PC contained 54% cellulose, whereas the O diet was 96% cellulose. All four diets contained 1.8% Wesson’s salt mixture and 2.2% vitamins and essential lipids.
Data were derived from videotapes by counting the number of crickets with at least one foretarsus or midtarsus contacting the contents of each dish at 30-s intervals for 10 min, beginning once the first cricket arrived at any of the dishes. These 30-s records (20 total) were summed and normalized by dividing the total for each dish by the sum across all four dishes (which ranged from 295 to 427). These values were then averaged across the eight independent diet trials.
Preference for Salt Versus Water.
The relative positions of the six dishes containing water or salt solutions were randomized in the 10 trials. Trials were videotaped for 10 min, and data were scored as described above. The total numbers of crickets contacting any of the dishes ranged from 124 to 872.
Protein and Salt Satiation and Their Effect on Cannibalism.
The extent of cannibalism was scored on a scale of 0–5 by two observers, with 0 being no cannibalism and 5 the consumption of the entire victim. An independent sample of 25 crickets was run in which actual meal size (measured by weight change in the cannibal) was regressed against scored damage to the victim, yielding R2 = 0.89, F1,24 = 193.5, and P < 0.0005.
Protein Satiation and Its Effect on Locomotion.
Crickets were collected on June 27, 2005 from marching bands south of Dinosaur National Monument in northwest Colorado (N40°26′16.68″, W109°01′44.64″) and transported to the Northern Plains Agricultural Research Laboratory in Sidney, MT. They were housed in a 0.3-m3-screen cage and fed ad libitum with flowers of arrowleaf balsamroot (Balsamoriza sagittata), lupine (Lupinus sp.), and Senecio sp., as well as wild onion (Allium sp.). At the Northern Plains Agricultural Research Laboratory, they were maintained at 30°C under ambient light for 20 h before testing individually.
Movement as a Defense Against Cannibalism.
These experiments were conducted on the same population as the protein and salt experiments. Encounters with advancing Mormon crickets were videotaped for 20 min per trial. The latency of attack was quantified from the videos as the time elapsed between initial contact with a cricket from the band and the point at which the focal insect was being consumed by one or more attacking crickets. Difference in latency to attack between motile versus immotile individuals across all pairwise comparisons was assessed with the Wilcoxon signed rank test.
In the tethering experiment, crickets had a loop of nylon monofilament fishing line tied around the thorax between the forelegs and midlegs. The line extended 10 cm from the loop knot on the dorsal surface of the pronotum and was fastened to a 5-cm nail driven into the ground.
Supplementary Material
Acknowledgments
We thank L. Senior and F. Clissold for critical logistical support; D. Roberts for assistance locating field sites; and Jerome Buhl, Joe Hale, and Gabriel Miller for valuable comments. This work was supported by the Agricultural Research Service of the U.S. Department of Agriculture. S.J.S. was also supported by Engineering and Physical Sciences Research Council (EPSRC) Grant GR/SO4765/01 and by an Australian Research Council Federation Fellowship. I.D.C. was supported by EPSRC Grants GR/S04765/01 and GR/T11241/01 and also by Balliol College and the Royal Society.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Krause J., Ruxton G. D. Living in Groups. Oxford: Oxford Univ. Press; 2002. [Google Scholar]
- 2.Conradt L., Roper T. J. Trends Ecol. Evol. 2005;20:449–456. doi: 10.1016/j.tree.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Gwynne D. T. Katydids and Bush-Crickets: Reproductive Behavior and Evolution of the Tettigoniidae. Ithaca, NY: Cornell Univ. Press; 2001. [Google Scholar]
- 4.Lorch P. D., Sword G. A., Gwynne D. T., Anderson G. A. Ecol. Entomol. 2005;30:548–555. [Google Scholar]
- 5.Sword G. A., Lorch P. D., Gwynne G. T. Nature. 2005;433:703. doi: 10.1038/433703a. [DOI] [PubMed] [Google Scholar]
- 6.Swain R. B. Nature and Extent of Mormon Cricket Damage to Crop and Range Plants. Washington, DC: U.S. Dept. Agric; 1944. Technical Bulletin 866. [Google Scholar]
- 7.MacVean C. Rangelands. 1990;12:234–235. [Google Scholar]
- 8.Redak R. A., Capinera J. L., Bonham C. D. Environ. Entomol. 1992;21:94–102. [Google Scholar]
- 9.Uvarov B. Grasshoppers and Locusts. Vol. 2. London: Centre for Overseas Pest Research; 1977. [Google Scholar]
- 10.Cowan F. T. Life History, Habits, and Control of the Mormon Cricket. Washington, DC: U.S. Dept. Agric; 1929. Technical Bulletin 161. [Google Scholar]
- 11.Wakeland C. Mormon Crickets in North America. Washington, DC: U.S. Dept. Agric; 1959. Technical Bulletin 1202. [Google Scholar]
- 12.Ueckert D. N., Hansen R. M. J. Econ. Entomol. 1970;63:96–98. [Google Scholar]
- 13.MacVean C. M. In: Integrated Pest Management on Rangeland: a Shortgrass Prairie Perspective. Capinera J. L., editor. Boulder, CO: Westview; 1987. pp. 116–135. [Google Scholar]
- 14.Mira A. J. J. Insect. Physiol. 2001;46:605–610. doi: 10.1016/s0022-1910(99)00146-8. [DOI] [PubMed] [Google Scholar]
- 15.Polis G. A., Myers C. A., Holt R. D. Annu. Rev. Ecol. Syst. 1989;20:297–330. [Google Scholar]
- 16.Elgar M. A., Crespi B. J. Cannibalism: Ecology and Evolution Among Different Taxa. Oxford: Oxford Univ. Press; 1992. [Google Scholar]
- 17.Denno R. F., Fagan W. F. Ecology. 2003;84:2522–2531. [Google Scholar]
- 18.Simpson S. J., Abisgold J. D. Physiol. Entomol. 1985;10:443–452. [Google Scholar]
- 19.DeFoliart G. R., Finke M. D., Sunde M. L. J. Econ. Entomol. 1982;75:848–852. [Google Scholar]
- 20.Chapman R. F. The Insects Structure and Function. Cambridge, U.K: Cambridge Univ. Press; 1998. [Google Scholar]
- 21.Coll M., Guershon M. Annu. Rev. Entomol. 2002;47:267–297. doi: 10.1146/annurev.ento.47.091201.145209. [DOI] [PubMed] [Google Scholar]
- 22.Simpson S. J., Raubenheimer D. Entomol. Exp. Appl. 1996;80:55–64. [Google Scholar]
- 23.Mayntz D., Raubenheimer D., Salomon M., Toft S., Simpson S. J. Science. 2005;307:111–113. doi: 10.1126/science.1105493. [DOI] [PubMed] [Google Scholar]
- 24.Sword G. A. Anim. Behav. 2005;69:437–444. [Google Scholar]
- 25.Couzin I. D., Krause J. Adv. Study Behav. 2003;32:1–75. [Google Scholar]
- 26.Couzin I. D., Franks N. R. Proc. R. Soc. London Ser. B; 2003. pp. 139–146. [Google Scholar]
- 27.Simpson S. J., Raubenheimer D. Adv. Study Behav. 2000;29:1–44. [Google Scholar]
- 28.Despland E., Simpson S. J. Anim. Behav. 2000;59:643–652. doi: 10.1006/anbe.1999.1335. [DOI] [PubMed] [Google Scholar]
- 29.Gwynne D. T. Ecology. 1993;74:1406–1413. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.