Abstract
Disruption of the leptin signaling pathway within the heart causes left ventricular hypertrophy (LVH). Because human obesity is a syndrome of leptin resistance, which is not amenable to leptin treatment, the identification of parallel signal transduction pathways is of potential therapeutic value. Ciliary neurotrophic factor (CNTF), which acts parallel to leptin in the hypothalamus, is not previously recognized to have cardiac activity. We hypothesized that CNTF receptors are present on cardiomyocytes and their activation reverses LVH in both leptin-deficient ob/ob and leptin-resistant db/db mice. The localization of CNTF receptors (CNTFRα) to the sarcolemma in C57BL/6, ob/ob and db/db was confirmed in situ with immunohistochemistry, and immunoblotting (60 and 40 kDa) on isolated myocytes. ob/ob mice were randomly assigned to receive s.c. recombinant CNTF (CNTFAx15; 0.1 mg·kg−1 per day; n = 11) calorie-restriction (n = 9), or feeding ad libitum (n = 11). db/db mice were allocated to three similar groups (n = 8, 7, and 8, respectively) plus a leptin group (1 mg·kg−1 per day; n = 7). Echocardiography showed that CNTFAx15 reduced cardiac hypertrophy [posterior wall thickness decreased by 29 ± 8% (P < 0.01) in ob/ob and by 21 ± 3% in db/db mice (P < 0.01)], which was consistent with the reduction of myocyte width. Western blotting showed that leptin and CNTFAx15 activated Stat3 and ERK1/2 pathway in cultured adult mice cardiomyocytes and cardiac tissue from in ob/ob and db/db mice. Together, these findings support the role of a previously undescribed signaling pathway in obesity-associated cardiac hypertrophy and have therapeutic implications for patients with obesity-related cardiovascular disease and other causes of LVH.
Keywords: signal transduction, cardiac remodeling, mouse, heart, Axokine
Left ventricular hypertrophy (LVH) and its subsequent progression to congestive heart failure represents a major cause of morbidity and mortality in the United States (1). Obesity, an important mediator of LVH (2), results from either deficiency of or receptor insensitivity to leptin (3–5), a hormone that regulates appetite and energy metabolism (6). We have previously demonstrated that both leptin deficiency and resistance contribute to LVH in murine models (7). Additionally, we showed regression of LVH with leptin repletion in leptin-deficient ob/ob mice. However, because the majority of human obesity is associated with hyperleptinemia and leptin resistance (4, 8, 9) that is unresponsive to leptin treatment, we sought to identify an alternate signaling axis that regulates cardiac architecture. Here we address this issue with ciliary neurotrophic factor (CNTF), which activates a related signaling pathway to leptin and has similar effects on body weight and metabolism (10–12). The CNTF receptor (CNTFR) complex closely resembles the leptin receptor (ObR) structurally and has a similar distribution in the hypothalamic nuclei associated with regulation of feeding and body weight (13, 14). Both receptors are members of the gp130 cytokine family of receptors. The CNTF receptor is a trimeric receptor complex with a CNTF binding component (CNTFRα), a leukemia inhibitory factor β subunit, and the signal transducer of IL-6 (gp130) (15). Support for parallels between leptin and CNTF signaling pathways include structural homology between ObR and the gp130 subunit of the CNTFR (16) as well as activation of similar signal transduction pathways such as the Janus kinase-signal transducer and activator of transcription pathway (17–19). Thus, we tested whether CNTF receptors are present and functional in the heart and whether their activation would reverse established LVH in both ob/ob and db/db mice (10).
Results
Presence of CNTFRα Receptors in Cardiac Tissue.
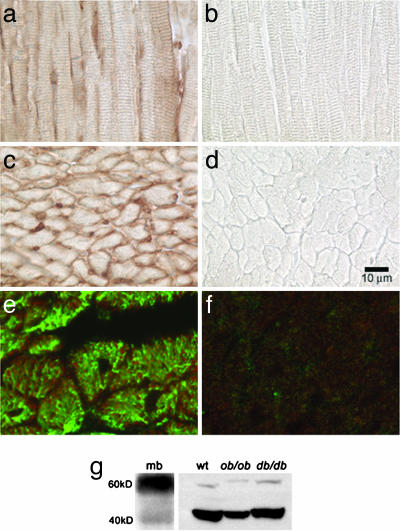
To determine whether CNTFRs were present in the heart, we initially performed in situ peroxidase staining. The CNTFR (CNTFRα) was visualized on myocytes in both longitudinal and transverse sections of C57bl/6 wt mice hearts (Fig. 1a and c). Double immunofluorescence staining for desmin and CNTFRα localized its presence to the sarcolemma (Fig. 1e), which was further confirmed by immunoblots (60-kDa glycosylated and 40-kDa nonglycosylated bands) on isolated myocytes (Fig. 1g). Mouse brain was used as positive control.
Fig. 1.
Demonstration of CNTFRα in the heart. (a–f) Immunohistochemistry with peroxidase staining performed on longitudinal (a and b) and transverse (c and d) sections of the heart showing the presence of CNTFRα in the heart. Double immunofluorescence staining for desmin (green) and CNTFRα (red) localized its presence to the myocyte (e). b, d, and f are negative controls omitting the primary antibody. (g) Western blot on isolated cardiomyocytes from C57BL/6 (wt), ob/ob and db/db mice confirming the presence of CNTFRα in the myocyte. Bands corresponding to the 60-kDa glycosylated and 40-kDa nonglycosylated portions of the receptor can be seen. Mouse brain (mb) was used as a positive control.
Regression of LVH in the ob/ob Mice with CNTFAx15.
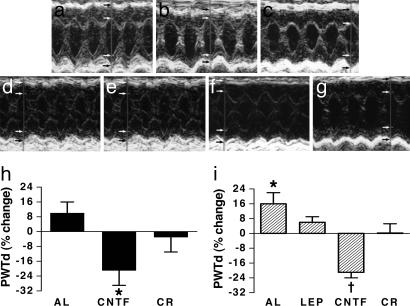
Based on our earlier finding that leptin regresses established LVH in ob/ob mice (ref. 7; Table 1), we hypothesized that CNTFAx15 would mimic these effects on cardiac structure. CNTFAx15 regressed cardiac hypertrophy, decreasing interventricular septal thickness by 20 ± 3% (P = 0.000035) posterior wall thickness by 29 ± 8%, (P = 0.000022), (Fig. 2b and h), and calculated left ventricular (LV) mass by 21 ± 9%, (P = 0.0077). In contrast and as previously shown, calorie restriction did not reduce LVH in these mice (Table 2). The degree of regression with CNTFAx15 restored wall thickness and left ventricular mass (LVM) essentially to normal. (Table 2) Interestingly, a reduction in LVM was noted in the calorie restricted group, but this decrease was due entirely to a smaller chamber size in these animals and, in the absence of reduced wall thickness, is unlikely to represent true regression of LVH.
Table 1.
Baseline body weights and echocardiographic parameters in WT, ob/ob, and db/db mice prior to the start of intervention
| Parameter | WT | ob/ob | db/db |
|---|---|---|---|
| No. of mice | 15 | 31 | 30 |
| Age, months | 5 | 5–6 | 5–6 |
| BW, g | 22 ± 0 | 70 ± 1* | 59 ± 1*,† |
| IVSd, mm | 0.76 ± 0.03 | 1.10 ± 0.02* | 1.03 ± 0.03* |
| PWTd, mm | 0.85 ± 0.02 | 1.03 ± 0.02* | 0.96 ± 0.02* |
| LVEDd, mm | 3.26 ± 0.10 | 3.51 ± 0.07 | 3.59 ± 0.07‡ |
| LVEDs, mm | 1.63 ± 0.06 | 1.51 ± 0.07 | 1.51 ± 0.07 |
| LVM, mg | 87 ± 6 | 143 ± 4* | 138 ± 5* |
BW, body weight; IVSd, interventricular septal thickness in diastole; PWTd, posterior wall thickness in diastole; LVEDd, left ventricular end-diastolic diameter; LVEDs, left ventricular end-systolic diameter.
*, P < 0.01 vs. WT;
†, P < 0.01 vs. ob/ob; and
‡, P < 0.05 vs. WT by one-way ANOVA.
Fig. 2.
Regression of LVH with CNTF in ob/ob and db/db mice. (a–g) M-mode echocardiograms after 4 weeks after intervention of 5–6 months old ob/ob mice fed ad libitum (a), treated with CNTFAx15 (b), or calorie-restricted (c) and db/db mice fed ad libitum (d), treated with CNTFAx15 (e), calorie restricted (f), and treated with leptin (g). Notable findings include reduction in both septal and posterior wall thickness in the CNTFAx15-treated animals compared with other groups. (h and i) Graphical demonstration of significant decreases in posterior wall thickness in diastole (PWTd) in ob/ob mice (h; ∗, P = 0.000022) and db/db mice (i; ∗, P = 0.029; †, P = 0.00019) with CNTFAx15 treatment. AL, ad libitum; LEP, leptin; CR, calorie restriction. Arrows denote endo- and epicardial borders of heart wall.
Table 2.
Changes in echocardiographic parameters after intervention in ob/ob mice
| Parameter | Fed ad libitum (n = 11) |
CNTFA×15-treated (n = 11) |
Calorie-restricted (n = 9) |
|||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| BW, g | 70 ± 1 | 72 ± 1 | 70 ± 2 | 43 ± 2* | 71 ± 2 | 46 ± 2* |
| IVSd, mm | 1.11 ± 0.04 | 1.15 ± 0.04 | 1.13 ± 0.04 | 0.90 ± 0.03* | 1.05 ± 0.04 | 0.95 ± 0.04 |
| PWTd, mm | 1.02 ± 0.03 | 1.12 ± 0.03 | 1.10 ± 0.03 | 0.86 ± 0.03* | 0.97 ± 0.02 | 0.90 ± 0.04 |
| LVEDd, mm | 3.60 ± 0.14 | 3.29 ± 0.10 | 3.38 ± 0.09 | 3.20 ± 0.09 | 3.56 ± 0.16 | 3.22 ± 0.08 |
| LVEDs, mm | 1.49 ± 0.10 | 1.19 ± 0.06† | 1.50 ± 0.11 | 1.12 ± 0.03† | 1.54 ± 0.15 | 1.23 ± 0.05† |
| LVM, mg | 149 ± 7 | 144 ± 6 | 147 ± 10 | 106 ± 14* | 135 ± 5 | 100 ± 5* |
*, P < 0.01 vs. preintervention baseline and
†, P < 0.05 vs. preintervention baseline by paired t tests. Abbreviations are as per Table 1.
Regression of Myocyte Width in the ob/ob Mice with CNTFAx15.
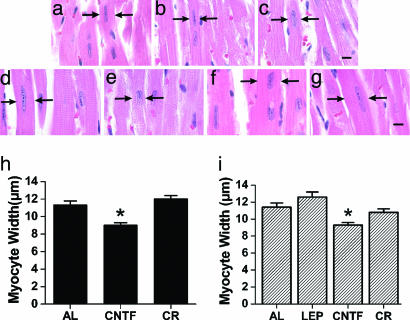
To determine whether the observed impact of CNTFAx15 on LVH could be attributed to a regression of myocyte hypertrophy, we performed histological studies with hematoxylin/eosin staining on ob/ob mouse hearts (n = 3–5) from each of the three groups (controls fed ad libitum, calorie restricted, or CNTFAx15 treated), at the end of their treatment period. Myoycte width with CNTFAx15 in the ob/ob mice was reduced to 9.0 ± 0.3 μm compared to 11.3 ± 0.5 μm in those fed ad libitum (P < 0.001), and, in the calorie-restricted mice, 12.0 ± 0.4 μm (P < 0.001). There was no significant difference in cell size between the calorie restricted and ad libitum groups (Fig. 3b and h). Myocyte width in age-matched C57bl/6 wt mice is 8.2 ± 0.2 μm (20).
Fig. 3.
CNTFAx15 mediated reduction of myocyte width in ob/ob and db/db mice. (a–g) Histology sections stained with hematoxylin/eosin from ob/ob mice fed ad libitum (a), treated with CNTFAx15 (b), or calorie-restricted (c) and db/db mice fed ad libitum (d), treated with CNTFAx15 (e), calorie-restricted (f), and treated with leptin (g). (h and i) Graphical demonstration that myocytes from CNTFAx15-treated ob/ob (h) and db/db (i) mice have decreased cell width compared to the control groups (∗, P < 0.0001). AL, ad libitum; LEP, leptin; CR, calorie restriction. (Scale bar: 5 microns.) Arrows denote borders of cardiac myocytes.
Regression of LVH in db/db Mice with CNTFAx15.
We next assessed the impact of CNTFAx15 on LVH in leptin-receptor deficient db/db mice. CNTFAx15 reduced septal thickness by 27 ± 6% (P = 0.0068), posterior wall thickness by 21 ± 3% (P = 0.00019) (Fig. 2 e and i), and calculated LV mass by 41 ± 6% (P = 0.00064), restoring cardiac architecture toward normal. CNTFAx15 also significantly reduced LV end-systolic and -diastolic dimensions (P = 0.042 and 0.013, respectively) (Table 3). Importantly, as predicted on the basis of an absent leptin receptor, leptin did not change LV wall thickness or LVM in db/db mice (Table 3). However, LVM was decreased in the calorie-restricted group, but, as discussed earlier, this reduction is most likely attributable to a decrease in chamber size.
Table 3.
Changes in echocardiographic parameters after intervention in db/db mice
| Parameter | Fed ad libitum (n = 7) |
CNTFA×15-treated (n = 8) |
Calorie-restricted (n = 8) |
Leptin-treated (n = 7) |
||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| BW, g | 59 ± 1 | 62 ± 2 | 60 ± 2 | 43 ± 1* | 60 ± 2 | 41 ± 1* | 58 ± 2 | 61 ± 2 |
| IVSd, mm | 0.94 ± 0.06 | 1.08 ± 0.06 | 1.11 ± 0.07 | 0.79 ± 0.05* | 1.02 ± 0.02 | 0.93 ± 0.06 | 1.05 ± 0.06 | 1.11 ± 0.02 |
| PWTd, mm | 0.90 ± 0.04 | 1.04 ± 0.05† | 1.02 ± 0.02 | 0.80 ± 0.04* | 0.94 ± 0.02 | 0.94 ± 0.05 | 1.02 ± 0.04 | 1.08 ± 1.02 |
| LVEDd, mm | 3.29 ± 0.17 | 3.33 ± 0.15 | 3.65 ± 0.13 | 3.31 ± 0.08* | 3.80 ± 0.12 | 3.22 ± 0.08* | 3.56 ± 0.12 | 3.31 ± 0.08 |
| LVEDs, mm | 1.21 ± 0.11 | 1.09 ± 0.18 | 1.50 ± 0.13 | 1.20 ± 0.02† | 1.72 ± 0.14 | 1.25 ± 0.05† | 1.53 ± 0.06 | 1.23 ± 0.04* |
| LVM, mg | 107 ± 11 | 135 ± 6 | 150 ± 7 | 87 ± 8* | 148 ± 7 | 103 ± 8* | 143 ± 5 | 137 ± 4 |
*, P < 0.01 vs. preintervention baseline and
†, P < 0.05 vs. preintervention baseline by paired t tests. Abbreviations are as per Table 1.
Regression of Myocyte Width in the db/db Mice with CNTFAx15.
Assessment of myocyte width in db/db mice by hematoxylin/eosin staining showed that only CNTFAx15 regressed toward normal (9.3 ± 0.3 microns), in comparison to leptin-treated (12.6 ± 0.6 microns; P < 0.001), calorie-restricted (10.8 ± 0.4 microns; P < 0.05) and ad libitum controls (11.4 ± 0.5 microns; P < 0.01) (Fig. 3 e and i).
CNTFAx15 Signal Transduction.
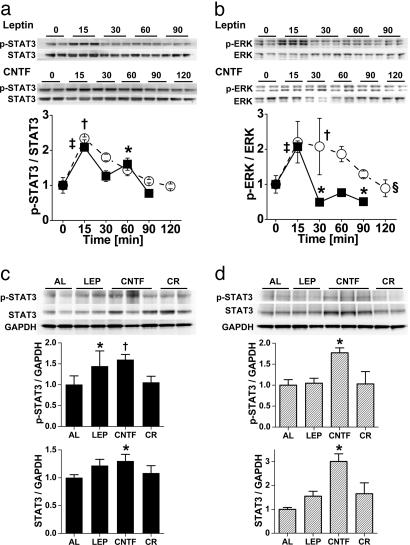
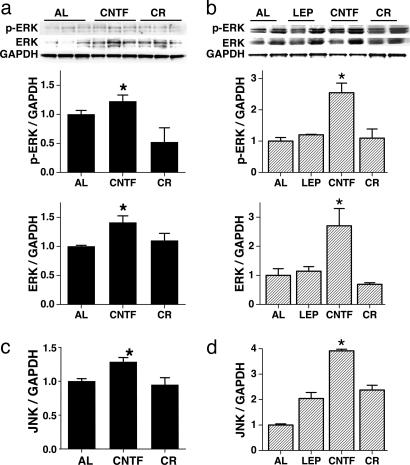
To determine whether CNTFAx15 and leptin activated similar signal transduction pathways in isolated cardiac myocytes, the activation of STAT3, ERK1/2 and JNK pathways, and calcineurin (CaN) signaling was determined. CNTFAx15 and leptin produced near-identical phosphorylation of STAT3 (Fig. 4a). Similarly, CNTFAx15 and leptin both led to the phosphorylation of ERK1/2, although the potency of this effect was greater with CNTFAx15 (Fig. 4b). Neither CNTFAx15 nor leptin affected JNK phosphorylation or CaN levels in isolated myocytes (Fig. 5). In terms of chronic 4-week therapy, the predominant effect was an increase in STAT3 phosphorylation and protein expression after CNTFAx15 treatment. Although the p-STAT3/STAT3 ratio was not increased with CNTFAx15, the overall STAT3 phosphorylation and total STAT3 abundance were higher in cardiac tissue from Axokine-treated ob/ob (Fig. 4c) and db/db mice (Fig. 4d). CNTFAx15 treatment resulted in a similar effect on the ERK1/2 pathway (Fig. 6a and b). As expected, leptin did not produce this effect in db/db mice lacking the leptin receptor. Consistent with the isolated cardiomyocyte data, JNK phosphorylation and CaN protein level were not increased in the cardiac tissue. However, total JNK protein expression was augmented (Fig. 6 c and d).
Fig. 4.
Leptin and CNTFAx15 activate STAT3 and ERK pathways. (a) Western blot analysis demonstrates time-dependent STAT3 phosphorylation in isolated adult mouse cardiomyocytes after exposure to leptin (■; 50 ng/μl) or CNTFAx15. (○; 50 ng/μl). STAT3 phosphorylation occurred within 15 min and subsided over time. (b) Similar activation occurred in the ERK1/2 pathway, with the effect of CNTFAx15 being more prolonged. [∗, P < 0.05; †, P < 0.01; ‡, P < 0.001 vs. time 0; §, P < 0.05 between groups (two-way ANOVA).] (c and d) Immunoblots from ob/ob mice (filled bars) and db/db mice (hatched bars) demonstrate increased abundance in both phosphorylated STAT3 and STAT3 expression with 4 weeks of CNTFAx15 (CNTF) treatment. Leptin (LEP) increased STAT3 abundance in ob/ob but not db/db mice. CR, calorie restriction. [∗, P < 0.05; †, P < 0.01 vs. ad libitum (AL).]
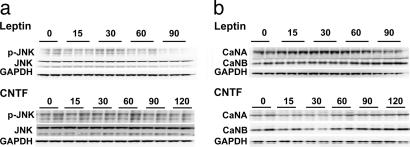
Fig. 5.
Impact of leptin and CNTFAx15 on pJNK and CaN signaling in cultured cardiomyocytes, ob/ob, and db/db mice. (a and b) Western blot analysis shows no induction of JNK phosphorylation (a) or altered calcineurin (CaN) protein expression (b) in isolated adult mouse cardiomyocytes with leptin (Upper) or CNTFAx15 (Lower) treatment.
Fig. 6.
Impact of CNTFAx15 on ERK and JNK signal transduction pathways in ob/ob and db/db mice. (a and b) Western blot analysis depicts increased ERK phosphorylation and ERK protein expression in cardiac tissue of ob/ob (filled bars) and db/db mice (hatched bars) with CNTFAx15 (CNTF) treatment but not with leptin (LEP) in db/db mice. (c and d) CNTFAx15 increased JNK protein abundance in heart tissue in ob/ob and db/db mice. However, calorie restriction (CR) does not affect JNK protein expression in cardiac tissue of ob/ob and db/db mice. (∗, P < 0.05.)
Discussion
The major finding of this study is that the CNTF signaling pathway, which is known to activate a parallel signal transduction pathway to leptin in the hypothalamus, has important cardiac bioactivity. The CNTF receptor is present in the cardiac sarcolemma, and its activation, like leptin (7), regresses established LVH in leptin-deficient mice. Importantly, CNTFAx15 signaling has similar cardiac effects in mice with leptin receptor dysfunction. CNTFAx15 influences signal transduction in isolated cardiac myocytes supporting a role for direct effects on the heart, activating both the phosphorylation of STAT3 and ERK1/2. These findings have therapeutic implications given that the majority of human obesity is due to leptin resistance rather than leptin deficiency (4, 8, 9).
The role of leptin or its deficiency in mediating cardiac hypertrophy is controversial. We (7) have previously demonstrated that leptin deficiency contributes to LVH in vivo in intact ob/ob mice with established LVH. On the other hand, in normal neonatal myocytes, exposure to leptin in vitro stimulates growth or hypertrophy (21, 22). However, these findings are not incompatible with each other, as the observed dichotomy could easily arise because of differences in milieu. For example, neonatal cells physiologically undergo rapid growth under the influence of normal trophic factors, unlike adult myocytes. Syed et al. (23) recently provided proof of principle for this concept and showed that although certain genes produced changes in ventricular structure and function in neonatal cells, they had no impact on the adult myocardium. These findings suggest that physiologic differences in characteristics between neonatal and adult myocytes play an important role in their response to external stimuli and the net result may even be the opposite (23).
CNTFAx15 and leptin stimulated the phosphorylation of STAT3 acutely in cardiac myocytes and augmented total STAT3 levels in association with regression of hypertrophy in the ob/ob and db/db mice. STAT3 phosphorylation is attributed with a prohypertrophic effect in neonatal myocytes (24, 25) but also a cardioprotective antiremodeling effect (26, 27). The present data support the dominance of the cardioprotective effects in this setting. Importantly, these results also demonstrate that CNTFAx15 and leptin directly activate similar signal transduction cascades within cardiac myocytes.
Recent epidemiologic studies support our paradigm as well. In a study conducted in healthy individuals free from cardiovascular disease, Pladevall et al. (28) showed that leptin deficiency was associated with increased left ventricular mass index, thus demonstrating that leptin has an antihypertrophic effect in the presence of an intact signaling pathway. Although some older studies showed a positive correlation between leptin and LVH when adjusting for body mass index (BMI) (29), others have highlighted that this positive correlation is completely abrogated after adjusting for BMI (30). Nonetheless, it is well established that obesity in clinical populations is associated with elevated leptin primarily due to leptin resistance, implying depressed downstream signaling despite increased leptin levels (4, 8, 9). Our current results further substantiate that like leptin, the CNTF signaling pathways regress established LVH, probably by restoring downstream leptin or leptin-like signaling, and offer additional support to the paradigm that leptin signaling deficiency contributes to the development of LVH in obesity in vivo.
Several points warrant mention. The potential mechanism of action of CNTFAx15 is complex. Although we have demonstrated direct actions within the heart (such as STAT3 and ERK1/2 activation), we cannot exclude the possibility of systemic effects. For example, central nervous system effects of CNTFAx15 may directly influence the heart. Indeed, the leptin/leptin-receptor axis is known to activate the central nervous system (31, 32) and to mediate metabolic actions by acting centrally. Additionally, it is possible that the receptors are not present solely on cardiac myocytes but also on other tissues, such as cardiac ganglia, implying the possibility of paracrine signaling effects within the heart. Nonetheless, our finding that CNTFAx15 causes a regression of established LVH not only in leptin-deficient animals but also in those lacking a functional leptin receptor in the context of stimulating signal transduction within cardiac myocytes establishes the existence of a previously unrecognized pathway in the regulation of LVH.
Our findings identify a cardiac signal transduction pathway in obesity related cardiac hypertrophy. Because the majority of human obesity is associated with leptin resistance, further evaluation of CNTFAx15 for potential therapeutic applications in the treatment of LVH in patients with leptin resistance may be fruitful.
Materials and Methods
Animals.
We used ob/ob, db/db, and C57BL/6 WT mice obtained from The Jackson Laboratory. Animal treatment and care was provided in accordance with institutional guidelines. All animals were housed under diurnal lighting conditions and allowed food and tap water ad libitum except the calorie-restricted groups, where each mouse received 1 g of food per day as described below. The Animal Care and Use Committee of The Johns Hopkins University approved all protocols.
Isolated Myocyte Preparation.
Cardiac myocytes were isolated and prepared from mouse hearts as described in detail by Khan et al. (33). Myocytes were plated with 80% confluency into 35-mm dishes (Falcon) containing a serum-free 500 μM Ca2+ containing isolation solution for 30 min. Myocytes were then treated with 50 ng/μl leptin and 50 ng/μl CNTFAx15 for various periods of time.
Treatment by Administration of Exogenous Leptin, CNTFAx15, and Calorie Restriction.
Five- to 6-month-old ob/ob and db/db mice were randomly assigned to three or four groups, respectively, and treated for a period of 4 weeks. The first group received CNTFAx15, the second group was calorie-restricted so as to lose similar weight as treated animals, and the third group was fed ad libitum as controls. Additional db/db mice were assigned to a fourth group to receive leptin. Mice in the CNTFAx15 group were injected daily with recombinant mouse CNTFAx15 (Regeneron Pharmaceuticals, Tarrytown, NY, 0.1 mg·kg−1 per day), whereas the leptin group were injected with recombinant mouse leptin (R & D Systems, 1 mg·kg−1 per day). The calorie restriction regimen consisted of 1 g of food per day, which was previously determined to result in a similar rate of weight loss to leptin or CNTFAx15 administration at these doses (7). The calorie-restricted groups and the ad libitum controls were injected with Dulbecco’s PBS.
Echocardiography.
To examine the impact of CNTFAx15 and leptin on established LVH (Table 1) in leptin-deficient ob/ob mice (7), we performed echocardiograms on mice randomized to one of three treatment groups: CNTFAx15 (n = 11, 5–6 months old, weight: 70 ± 2 g), calorie restriction (n = 9, 5–6 months old, weight: 71 ± 2 g), and controls fed ad libitum (n = 11, 5–6 months old, weight: 70 ± 1 g). We also investigated the effects of CNTFAx15 (n = 8, 5–6 months old, weight: 60 ± 2 g), calorie restriction (n = 8, 5–6 months old, weight: 60 ± 2 g), leptin (n = 7, 5–6 months old, weight: 58 ± 2 g), and controls fed ad libitum (n = 7, 5–6 months old, weight: 59 ± 1 g) on LVH after a 4-week treatment period in db/db mice. Echocardiography was performed on conscious unanesthetized mice at baseline and repeated at the end of the treatment period. The individual performing the echocardiograms was blinded to the group assignments. Mice were trained before each study until they were relaxed for the procedure. Studies were performed by using a Sequoia C256 (Siemens, Mountain View, CA) echocardiogram with a 15 MHz linear array transducer. Interventricular septal and posterior wall thicknesses, as well as diastolic and systolic LV dimensions were recorded from M-mode images by using averaged measurements from three to five consecutive cardiac cycles. LVM was calculated by using LVM = 1.055 × (interventricular septal thickness in diastole thickness + posterior wall thickness + left ventricular end diastolic diameter)3 − (left ventricular end diastolic diameter)3.
Histology.
Mice hearts were harvested, washed with ice-cold PBS, sectioned, and either fixed in 10% formalin or in Streck’s tissue fixative (Streck Laboratories, Omaha, NE) overnight and were paraffin embedded the next morning. Histochemical and immunohistochemical studies were carried out on 4-μm-thick sections.
Histochemical Staining.
Hematoxylin/eosin staining were performed to evaluate cell sizes. Myocyte diameters were measured in regions of myocardium with parallel myocyte fascicles in longitudinal sections by using imagej (National Institutes of Health). Representative high-powered-fields distributed around the myocardium were used to measure 7–29 cells from each heart. The individual analyzing histology data were blinded to the group assignments.
Immunohistochemistry.
For antigen retrieval, the sections were steamed with Target Retrieval System (DAKO) for 20 min, before blocking with avidin and biotin blocking solutions (DAKO) and 10% normal goat serum. The receptor was stained with a monoclonal mouse anti-CNTFR-α (BD Pharmingen, no. 558783; 1:100 dilution). After washing, the secondary antibody (biotinylated goat anti-mouse Ig; E0433, DAKO; 1:400 dilution) and streptavidin peroxidase (Vectastain, Vector Laboratories) were applied.
The peroxidase activity was developed 5 min with 0.066% diaminobenzidine/0.01% H2O2/2.5% NiSO4. Slides were cleared in xylene, coverslipped, and viewed on a Zeiss Axiovert 200 microscope equipped with 510-Meta confocal laser scanning module.
Fluorescence.
Tissue section were rinsed with ice-cold PBS twice for 2 min and were blocked with 10% goat serum for 30 min. The slides were incubated with rabbit anti-human desmin antibody (Accurate Chemical & Scientific, no. YMPS31) and monoclonal mouse anti-CNTFR-α antibody (BD Pharmingen, no. 558783) at 4°C overnight. After washing, FITC and rhodamine coupled secondary antibodies (Vector Laboratories) were applied for 45 min at room temperature. Tissue slide were washed and coverslipped with mounting medium and viewed.
Western Blots.
Hearts from ob/ob, db/db, and WT mice were harvested after cervical dislocation and rinsed in isolation buffer to remove excess blood. Cells were isolated as described by Minhas et al. (34). Isolated myocytes were homogenized in cold cell lysis buffer (Cell Signaling Technology, Beverly, MA) with one tablet of complete protease inhibitors (Roche Diagnostics) per 10 ml of buffer. The protein lysate was quantitated with the bicinchonic assay (Pierce), and 30–50 μg of total protein was separated in 4–12% Bis-Tris·HCl polyacrylamide gels (Invitrogen). The proteins were transferred onto nitrocellulose membranes and blocked for 1 h with 5% nonfat milk. After blocking, the membranes were incubated with monoclonal anti-CNTFRα antibody (1:1,000, BD Pharmingen), polyclonal anti-phospho-STAT3 antibody, polyclonal anti-phospho-ERK1/2 antibodies, polyclonal anti-phospho-JNK antibodies (all 1:1,000, Cell Signaling Technology), or monoclonal anti-calcineurin antibodies (1:2,000, Chemicon International) overnight at 4°C. Immunoblots were detected by using enhanced chemiluminescent kits (SuperSignal, Pierce) and analyzed with a densitometer (Bio-Rad). The membranes were then stripped and reprobed with polyclonal anti-STAT3 antibody, polyclonal anti-ERK1/2 antibodies, polyclonal anti-JNK antibodies (all 1:1,000, Cell Signaling Technology), or monoclonal anti-GAPDH antibodies (1:10,000, Research Diagnostics). Mouse brain was used as a positive control for calcineurin.
Sample Size and Statistical Analysis.
Sample size was estimated as five to seven animals in each group for statistical power >0.80 to determine regression of hypertrophy and LVM. Data are reported as mean ± SEM. Statistical significance was determined by paired t tests (GraphPad (San Diego) instat statistical software), or by two-way ANOVA with Bonferroni correction [stata (StataCorp LP, College Station, TX) statistical software]. P values <0.05 were considered significant.
Acknowledgments
This work was supported in part by the Donald W. Reynolds Foundation (L.A.B. and J.M.H.), National Institutes of Health Grants R01 HL-65455 and National Institute on Aging Grant R01AG025017 (to J.M.H.), National Institutes of Health Grant K08 Hl 076220-01 (to L.A.B.), and the Talles Family Fund for Cardiomyopathy Research (to L.A.B.). CNTFAx15 (Axokine) was generously provided by Regeneron Pharmaceuticals, Tarrytown, NY, through a materials transfer agreement with The Johns Hopkins University.
Abbreviations
- CNTF
ciliary neurotrophic factor
- CNTFR
ciliary neurotrophic factor receptor
- LV
left ventricle
- LVH
left ventricular hypertrophy
- LVM
left ventricular mass
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kaplinsky E. Cardiovasc. Drugs Ther. 1994;8(Suppl. 3):549–556. doi: 10.1007/BF00877223. [DOI] [PubMed] [Google Scholar]
- 2.Eckel R. H., Barouch W. W., Ershow A. G. Circulation. 2002;105:2923–2928. doi: 10.1161/01.cir.0000017823.53114.4c. [DOI] [PubMed] [Google Scholar]
- 3.Bray G. A., York D. A. J. Clin. Endocrinol. Metab. 1997;82:2771–2776. doi: 10.1210/jcem.82.9.4224. [DOI] [PubMed] [Google Scholar]
- 4.Considine R. V., Sinha M. K., Heiman M. L., Kriauciunas A., Stephens T. W., Nyce M. R., Ohannesian J. P., Marco C. C., McKee L. J., Bauer T. L., et al. N. Engl. J. Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 5.Montague C. T., Farooqi I. S., Whitehead J. P., Soos M. A., Rau H., Wareham N. J., Sewter C. P., Digby J. E., Mohammed S. N., Hurst J. A., et al. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 6.Sweeney G. Cell. Signalling. 2002;14:655–663. doi: 10.1016/s0898-6568(02)00006-2. [DOI] [PubMed] [Google Scholar]
- 7.Barouch L. A., Berkowitz D. E., Harrison R. W., O’Donnell C. P., Hare J. M. Circulation. 2003;108:754–759. doi: 10.1161/01.CIR.0000083716.82622.FD. [DOI] [PubMed] [Google Scholar]
- 8.Scarpace P. J., Matheny M., Tumer N. Neuroscience. 2001;104:1111–1117. doi: 10.1016/s0306-4522(01)00142-7. [DOI] [PubMed] [Google Scholar]
- 9.Scarpace P. J., Tumer N. Physiol. Behav. 2001;74:721–727. doi: 10.1016/s0031-9384(01)00616-3. [DOI] [PubMed] [Google Scholar]
- 10.Sleeman M. W., Garcia K., Liu R., Murray J. D., Malinova L., Moncrieffe M., Yancopoulos G. D., Wiegand S. J. Proc. Natl. Acad. Sci. USA. 2003;100:14297–14302. doi: 10.1073/pnas.2335926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gloaguen I., Costa P., Demartis A., Lazzaro D., Di Marco A., Graziani R., Paonessa G., Chen F., Rosenblum C. I., Van der Ploeg L. H., et al. Proc. Natl. Acad. Sci. USA. 1997;94:6456–6461. doi: 10.1073/pnas.94.12.6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert P. D., Anderson K. D., Sleeman M. W., Wong V., Tan J., Hijarunguru A., Corcoran T. L., Murray J. D., Thabet K. E., Yancopoulos G. D., et al. Proc. Natl. Acad. Sci. USA. 2001;98:4652–4657. doi: 10.1073/pnas.061034298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elias C. F., Aschkenasi C., Lee C., Kelly J., Ahima R. S., Bjorbaek C., Flier J. S., Saper C. B., Elmquist J. K. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 14.Elmquist J. K., Elias C. F., Saper C. B. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 15.Sleeman M. W., Anderson K. D., Lambert P. D., Yancopoulos G. D., Wiegand S. J. Pharm. Acta Helv. 2000;74:265–272. doi: 10.1016/s0031-6865(99)00050-3. [DOI] [PubMed] [Google Scholar]
- 16.Davis S., Aldrich T. H., Stahl N., Pan L., Taga T., Kishimoto T., Ip N. Y., Yancopoulos G. D. Science. 1993;260:1805–1808. doi: 10.1126/science.8390097. [DOI] [PubMed] [Google Scholar]
- 17.Boulton T. G., Stahl N., Yancopoulos G. D. J. Biol. Chem. 1994;269:11648–11655. [PubMed] [Google Scholar]
- 18.Bjorbaek C., Elmquist J. K., Frantz J. D., Shoelson S. E., Flier J. S. Mol. Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- 19.Ziotopoulou M., Erani D. M., Hileman S. M., Bjorbaek C., Mantzoros C. S. Diabetes. 2000;49:1890–1896. doi: 10.2337/diabetes.49.11.1890. [DOI] [PubMed] [Google Scholar]
- 20.Barouch L. A., Cappola T. P., Harrison R. W., Crone J. K., Rodriguez E. R., Burnett A. L., Hare J. M. J. Mol. Cell. Cardiol. 2003;35:637–644. doi: 10.1016/s0022-2828(03)00079-8. [DOI] [PubMed] [Google Scholar]
- 21.Rajapurohitam V., Gan X. T., Kirshenbaum L. A., Karmazyn M. Circ. Res. 2003;93:277–279. doi: 10.1161/01.RES.0000089255.37804.72. [DOI] [PubMed] [Google Scholar]
- 22.Xu F. P., Chen M. S., Wang Y. Z., Yi Q., Lin S. B., Chen A. F., Luo J. D. Circulation. 2004;110:1269–1275. doi: 10.1161/01.CIR.0000140766.52771.6D. [DOI] [PubMed] [Google Scholar]
- 23.Syed F., Odley A., Hahn H. S., Brunskill E. W., Lynch R. A., Marreez Y., Sanbe A., Robbins J., Dorn G. W. Circ. Res. 2004;95:1200–1206. doi: 10.1161/01.RES.0000150366.08972.7f. [DOI] [PubMed] [Google Scholar]
- 24.Kunisada K., Tone E., Fujio Y., Matsui H., Yamauchi-Takihara K., Kishimoto T. Circulation. 1998;98:346–352. doi: 10.1161/01.cir.98.4.346. [DOI] [PubMed] [Google Scholar]
- 25.Pan J., Fukuda K., Kodama H., Sano M., Takahashi T., Makino S., Kato T., Manabe T., Hori S., Ogawa S. Heart Vessels. 1998;13:199–208. doi: 10.1007/BF01745045. [DOI] [PubMed] [Google Scholar]
- 26.Harada M., Qin Y. J., Takano H., Minamino T., Zou Y. Z., Toko H., Ohtsuka M., Matsuura K., Sano M., Nishi J., et al. Nat. Med. 2005;11:305–311. doi: 10.1038/nm1199. [DOI] [PubMed] [Google Scholar]
- 27.Oshima Y., Fujio Y., Nakanishi T., Itoh N., Yamamoto Y., Negoro S., Tanaka K., Kishimoto T., Kawase I., Azuma J. Cardiovasc. Res. 2005;65:428–435. doi: 10.1016/j.cardiores.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 28.Pladevall M., Williams K., Guyer H., Sadurni J., Falces C., Ribes A., Pare C., Brotons C., Gabriel R., Serrano-Rios M., et al. J. Hypertens. 2003;21:1467–1473. doi: 10.1097/00004872-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Paolisso G., Tagliamonte M. R., Galderisi M., Zito G. A., Petrocelli A., Carella C., de Divitiis O., Varricchio M. Hypertension. 1999;34:1047–1052. doi: 10.1161/01.hyp.34.5.1047. [DOI] [PubMed] [Google Scholar]
- 30.Tritos N. A., Kissinger K. V., Manning W. J., Danias P. G. Clin. Endocrinol. 2004;60:60–66. doi: 10.1111/j.1365-2265.2004.01944.x. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz M. W., Woods S. C., Porte D., Jr, Seeley R. J., Baskin D. G. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 32.Haynes W. G., Sivitz W. I., Morgan D. A., Walsh S. A., Mark A. L. Hypertension. 1997;30:619–623. doi: 10.1161/01.hyp.30.3.619. [DOI] [PubMed] [Google Scholar]
- 33.Khan S. A., Skaf M. W., Harrison R. W., Lee K., Minhas K. M., Kumar A., Fradley M., Shoukas A. A., Berkowitz D. E., Hare J. M. Circ. Res. 2003;92:1322–1329. doi: 10.1161/01.RES.0000078171.52542.9E. [DOI] [PubMed] [Google Scholar]
- 34.Minhas K. M., Khan S. A., Raju S. V., Phan A. C., Gonzalez D. R., Skaf M. W., Lee K., Tejani A. D., Barouch L. A., O’Donnell C. P., et al. J. Physiol. 2005;565:463–474. doi: 10.1113/jphysiol.2005.084566. and erratum (2005) 566. 999. [DOI] [PMC free article] [PubMed] [Google Scholar]