Abstract
In cortex and hippocampus, protracted (>4 weeks) social isolation of adult male mice alters the subunit expression of GABA type A receptors (GABAA-Rs) as follows: (i) the mRNAs encoding GABAA-R α1, α2, and γ2 subunits are decreased by ≈50%, whereas those encoding α4 and α5 subunits are increased by ≈100%; (ii) similarly, the synaptic membrane expression of the α1 subunit protein is down-regulated, and that of the α5 subunit protein is up-regulated; and (iii) the binding of [3H]flumazenil to hippocampal synaptic membranes is decreased. Behaviorally, socially isolated (SI) mice are resistant to the sedative effects of the positive allosteric GABAA-R modulators diazepam (DZP) and zolpidem. This resistance seems to be attributable to the decrease of α1-containing GABAA-Rs. Paradoxically, DZP, which, unlike zolpidem, acts at α5-containing GABAA-Rs, increases the locomotor activity of SI mice. Imidazenil, which fails to modulate α1-, α4-, and α6-containing GABAA-Rs but is a selective positive allosteric modulator of α5-containing GABAA-Rs, also increases locomotor activity in SI mice. Importantly, SI mice responded to muscimol, 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3(2H)-one, and allopregnanolone similar to group-housed mice. These data suggest that a switch (a decrease in α1/α2 and γ2 and an increase in α4 and α5 subunits) in the composition of the heteropentameric GABAA-R subunit assembly without a change in total GABAA-R number occurs during social isolation. Thus, the repertoire of DZP and imidazenil actions in SI mice appears to be elicited by the allosteric modulation of GABAA-Rs overexpressing α5 subunits. Benzodiazepine response mediated by α1-containing GABAA-Rs is expected to be silent or reduced.
Keywords: GABAA receptors, nested RT-PCR
Prolonged or repeated environmental stressors or stimuli such as maternal imprinting, are known to trigger changes in the expression of many genes, including those regulating GABA-mediated neurotransmission in the mammalian brain (1–5).
In rodents, protracted social isolation (SI) is a powerful environmental stimulus that down-regulates GABA-mediated signal transduction at GABA type A receptors (GABAA-Rs). In male mice, SI lasting 4–6 weeks causes a marked decrease in the expression of 5α-reductase type I, the rate-limiting step enzyme for neurosteroid biosynthesis, thereby reducing brain allopregnanolone (Allo) content (4, 5). Because Allo is a potent endogenous positive allosteric modulator of GABA action at several GABAA-R subtypes (6, 7), this deficit is associated with down-regulation of GABAergic tone. Hence, socially isolated (SI) mice express a reduction of the sedative/hypnotic actions of the GABAA-R modulator pentobarbital (8). Administration of Allo or drugs such as fluoxetine or norfluoxetine that increase Allo brain levels normalizes the pentobarbital-induced sedative/hypnotic action in SI mice, but these agents do not affect the sedative/hypnotic action of pentobarbital in group-housed (GH) mice (8).
Flumazenil, a benzodiazepine (BZ) recognition site ligand that acts as an antagonist of the full positive allosteric modulators of GABA-evoked Cl− currents, blocks the anxiolytic, sedative, muscle relaxant, and amnestic actions of clinically used BZs and facilitates pentobarbital-induced sedation in SI mice without potentiating or inhibiting pentobarbital action in GH mice (9).
In a resident-intruder test in SI mice (10, 11), BZs that are full positive allosteric modulators of GABA action at GABAA-Rs (e.g., midazolam and triazolam) in low micromolar doses may increase aggression, mimicking the “paradoxical” increase in aggressive outburst behavior observed sporadically in human subjects receiving BZs (12, 13).
Furthermore, in the cortex or hippocampus of SI rats, diazepam (DZP)-induced positive allosteric modulation of GABA-evoked Cl− currents is reduced (14).
The goal of our research was to establish whether the changes in GABAA-R response to GABAA-R modulators in SI mice are associated with changes in the expression of GABAA-R subunits and/or with compensatory changes in the heteropentameric assembly of subunits in various GABAA-R subtypes. We tested this hypothesis in SI mice by measuring: (i) mRNA levels for α1, α2, α3, α4, α5, β3, γ2L+S, and δ subunits with quantitative-competitive RT-PCR; (ii) the expression of α1 and α5 subunit polypeptides in the cortex with Western blot; and (iii) the behavioral correlates of GABAA-R function after stimulation of GABAA-Rs with modulators or direct agonists. We administered: (i) full (DZP) or selective [zolpidem (ZOL) and imidazenil (IMD)] positive allosteric modulators of the action of GABA at GABAA-Rs acting at BZ-binding sites (15–19), (ii) Allo that allosterically potentiates the action of GABA acting at binding sites on the GABAA-Rs other than the sites at which BZs bind (6, 7), and (iii) muscimol and 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3(2H)-one (THIP), which act as direct GABAA-R agonists (20).
Classical BZs bind and exert their allosteric modulatory function at the sites on GABAA-Rs that are at the interface of the γ2 subunits with α1, α2, or α3 subunits (21) and are inactive at GABAA-Rs containing α4 and α6 subunits (22–24). Moreover, the potency of BZs and related drugs at the GABAA-Rs depends on specific α subunit subtypes assembled in the pentameric structure of GABAA-Rs (18).
We have selected ZOL, because it acts as a sedative/hypnotic BZ endowed with positive allosteric selectivity for GABAA-Rs containing α1 subunits (15, 16); DZP, because it acts as a classical anxiolytic, anticonvulsant, and sedative BZ with full positive allosteric modulatory action without receptor subunit subtype selectivity (17–19); and IMD, because it is endowed with strong anxiolytic and anticonvulsant actions and high affinity and intrinsic activity at α5-containing GABAA-Rs but is devoid of sedative action due to its minimal intrinsic action at α1-containing GABAA-Rs (17–19). Importantly, IMD binding with low intrinsic activity at α1 GABAA-Rs antagonizes the sedative, amnestic, and ataxic actions of DZP, midazolam, and alprazolam in rats and non-human primates (25–27).
By targeting GABAA-Rs containing specific α subunits with selective allosteric modulators or selective direct agonists, our experiments provide unique information on SI-induced GABAA-R structural and functional alterations.
Results
DZP and IMD but Not ZOL Increase Rather Than Decrease Exploratory Activity in SI Mice.
DZP and ZOL elicit a dose-related decrease of exploratory activity in GH mice exposed to a novel arena (Fig. 1). In GH mice, the ED50 dose to decrease locomotor activity or induce ataxia is ≈15 μmol/kg for DZP and 5 μmol/kg for ZOL (Fig. 1 A and C). In contrast, IMD fails to change exploratory behavior (Fig. 1B) or induce ataxia or sedation even in doses (3.3 μmol/kg) that consistently produce maximal anticonflict, anxiolytic, and anticonvulsant actions (25).
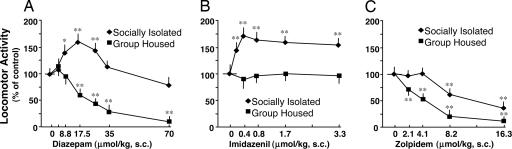
Fig. 1.
Paradoxical increase of locomotor activity in DZP- (A) or IMD- (B) and reduced impairment in locomotor activity in ZOL- (C) treated SI mice for 4 weeks. DZP, IMD, or ZOL was administered s.c. in oil 45 min before locomotor activity tests. Concentration 0 indicates a vehicle-treated group. Two-way ANOVA with factors for housing, treatment, and their interactions with locomotor activity revealed a significant effect of treatment [A, F(1,126) = 24.10, P < 0.001; B, F(1,62) = 12.08, P < 0.001; and C, F(1,54) = 57.83 P < 0.001]. ∗, P < 0.01; ∗∗, P < 0.001 vs. vehicle-treated group, two-way ANOVA followed by Bonferroni t test. Mean ± SEM of 6–10 mice.
In SI mice, DZP fails to reduce exploratory activity even for doses of 70 μmol/kg and paradoxically produces a behavioral stimulatory effect (Fig. 1A). The increase in exploratory activity elicited by DZP follows the profile of a “bell-shaped curve” with a nadir at doses between 8.8 and 26.3 μmol/kg. IMD, which is inactive on locomotor activity in GH mice, acquires a potent behavioral stimulatory action in SI mice; it is already active at 0.2 μmol/kg, reaches a maximum at 0.4 μmol/kg, and remains at this level up to 3.3 μmol/kg (Fig. 1B).
In contrast, in GH mice, ZOL induces a behavioral inhibitory activity that is greatly curtailed in SI mice (Fig. 1C)
Fig. 2 shows that the behavioral stimulatory action of DZP is blocked by the BZ recognition site antagonist flumazenil, suggesting that the paradoxical action of DZP in SI mice is mediated via BZ recognition sites.
Fig. 2.
Flumazenil (FMZ), a BZ recognition site antagonist, blocks the paradoxical effects of DZP on the locomotor activity of SI mice. Mice were pretreated with flumazenil 15 min before DZP and 60 min before locomotor activity tests. [F(3,16) = 17.21; ∗, P < 0.001 vs. vehicle-treated group, one-way ANOVA followed by Bonferroni t test. Mean ± SEM of five mice.]
We also studied the effects of the direct GABAA-R agonist muscimol (a full agonist at several GABAA-R subtypes) and THIP (an α4/δ selective GABAA-R agonist) (20) on locomotor activity in GH and SI mice. THIP (9, 18, 36, 72, or 144 μmol/kg, s.c., in oil, 45 min before test) and muscimol (2.2, 4.4, 8.8, or 17.5 μmol/kg, s.c., in oil, 45 min before test) failed to produce any stimulatory activity and, with the highest doses, caused a similarly potent sedation in GH and SI mice [locomotor activity (% of control), GH = 55 ± 8.5, SI = 48 ± 7.7 (72 μmol/kg THIP, n = 5); GH = 15 ± 2.5, SI = 19 ± 3.6 (144 μmol/kg THIP, n = 5); GH = 75 ± 11, SI = 78 ± 17 (8.8 μmol/kg muscimol, n = 5); GH = 17 ± 6.1, SI = 12 ± 2.5 (17.5 μmol/kg muscimol, n = 5)]. Because brain Allo content is decreased in SI mice, we administered Allo in a dose (8 μmol/kg) that is known to potentiate the sedative action of muscimol or pentobarbital in Allo-depleted mice (28). In this dose, Allo failed to produce an increase in locomotor activity in GH or SI mice [locomotor activity (% of control), GH = 95 ± 9.4, SI = 102 ± 13.7 (Allo, 8 μmol/kg s.c. in oil, 45 min before test, n = 5)].
Radioligand-Binding Studies.
Table 1 indicates that the Bmax but not the Kd of [3H]flumazenil binding to thoroughly washed, frozen, and thawed crude synaptic membranes prepared from the hippocampus is significantly decreased in SI compared with GH mice. Similarly, the binding of [3H]flumazenil to frontal cortical synaptic membranes measured with a concentration of 10 nM of [3H]flumazenil, which approximates the apparent Bmax of this ligand for the binding sites, tends to be decreased, although not significantly (GH = 2.0 ± 0.22 pmol/mg protein, SI = 1.6 ± 0.11 pmol/mg protein; n = 5, P = 0.16).
Table 1.
Binding of [3H]-flumazenil to hippocampal synaptic membranes of SI or GH mice
| Mice | [3H]-flumazemil binding |
|
|---|---|---|
| Kd, nM | Bmax, pmol/mg protein | |
| GH + VH | 1.2 ± 0.46 | 2.2 ± 0.10 |
| SI + VH | 0.72 ± 0.18 | 1.3 ± 0.21* |
[3H]-flumazenil-binding experiments were performed in 4-week SI mice. After SI, mice were killed, and [3H]-flumazenil-specific binding to hippocampal synaptic membranes was measured. Bmax and Kd values were calculated by Scatchard blot analyses of the binding data. Mean ± SEM of four mice. (t6 = 3.9; ∗
*, P < 0.01 vs. GH mice, Bonferroni t test.)
Incorporation of [13C]Glucose into GABA.
To study whether the differential response of GH and SI mice to BZs could be related to a difference in the biosynthesis and metabolism of GABA, we examined the initial incorporation rate of uniformly labeled [13C]glucose into [13C]glutamate and [13C]GABA in the frontal cortex and striatum (29). The steady-state levels of both GABA and the [13C]-GABA conversion index from [13C]glutamate were similar in GH and SI mice (Table 2). This rate of conversion is decreased in mice in which GAD67 has been inhibited by administration of the GAD67 inhibitor isoniazid (29).
Table 2.
GABA content and conversion index of glutamate into GABA in the frontal cortex and striatum of SI and GH mice
| Mice | GABA, nmol/mg prot | Glutamate/GABA conversion index, % [13C]-GABA/% [13C]-glutamate |
|---|---|---|
| Motor cortex | ||
| GH + VH | 22 ± 2.7 | 0.27 ± 0.04 |
| SI + VH | 19 ± 2.7 | 0.25 ± 0.06 |
| Striatum | ||
| GH + VH | 29 ± 3.0 | 0.23 ± 0.03 |
| SI + VH | 29 ± 2.1 | 0.24 ± 0.02 |
Each value is the mean ± SEM of four mice infused for 5 min with [13C]-glucose (4 mg per mouse). The percent incorporation of [13C] into glutamate or GABA was measured 2 min after [13C]-glucose infusion was terminated. No significant differences were observed in any of the parameters analyzed.
Changes in GABAA-R Subunit Expression in the Frontal Cortex and Hippocampus of SI Mice.
In the frontal cortex of GH mice, the rank order of GABAA-R subunit mRNA content is α1 > β3 > α2 > α3 > γ2L+S > α5 > δ, and > α4 (Fig. 3). In the hippocampus, the content of α5 subunits is approximately four times higher than in the frontal cortex, and it equals that of the α1 subunit (Fig. 4).
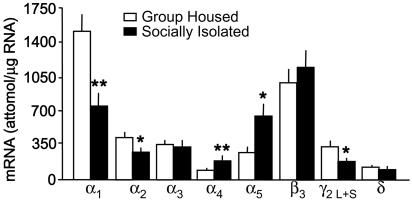
Fig. 3.
GABAA-R subunit mRNA expression in the frontal cortex of 4-week SI and GH mice. (For α1, t8 = 3.54; α2, t8 = 2.41; α4, t8 = 3.61; α5, t8 = 3.23; and γ2, t8 = 2.56; ∗, P < 0.05; ∗∗, P < 0.01 vs. GH mice, Bonferroni t test. Mean ± SEM of five mice.)
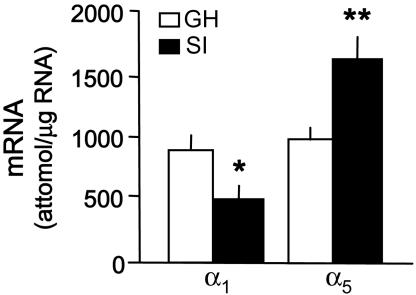
Fig. 4.
GABAA-R subunit mRNA expression in the hippocampus of 4-week SI and GH mice. (For α1, t8 = 2.84; and α5, t7 = 3.61; ∗, P < 0.05; ∗∗, P < 0.01 vs. GH mice, Bonferroni t test. Mean ± SEM of four to five mice.)
In the frontal cortex of SI mice, there is a 40–50% decrease in α1, α2, and γ2 subunit mRNA expression; in contrast, α4 and α5 are increased by 80% and 100%, respectively (Fig. 3).
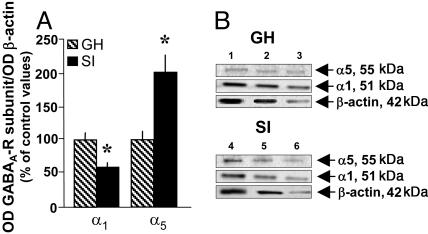
In the hippocampus of SI mice, α1 is also decreased by ≈50%, and α5 is increased by 70% (Fig. 4). Western blotting of crude synaptic membrane preparations obtained from the frontal cortex of SI mice expresses a down-regulation of α1 (−40%) and an increase in the expression of α5 subunit proteins (+100%) (Fig. 5).
Fig. 5.
GABAA-R α1 and α5 subunit protein expression in the frontal cortex of 4-week SI and GH mice. (A) Mean values ± SEM of α1 and α5 subunit proteins, n = 5. (For α1, t8 = 2.91; and α5, t8 = 3.00; ∗, P < 0.05 vs. GH mice, Bonferroni t test. Mean ± SEM of five mice.) (B) A typical Western immunoblot of α1 and α5 in a SI mouse and a GH mouse. Lanes 1–3, serial dilutions of a GH frontal cortex extract; lanes 4–6, serial dilution of a frontal cortex SI extract.
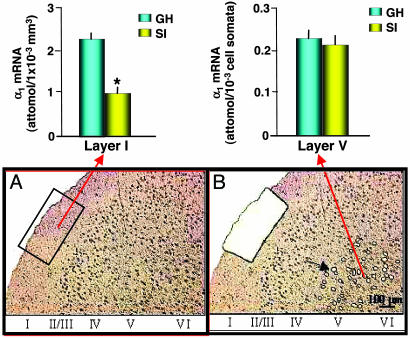
Quantification of GABAA-R α1 Subunit mRNA Expression in Frontal Cortex Layer I GABAergic Interneurons and Neuropil and Layer V Pyramidal Neurons.
To evaluate whether the decrease of GABAA-R α1 subunit expression occurring in the frontal cortex in SI mice is layer- or cell-specific, we used a laser microdissection technique coupled to nested RT-PCR amplification (19). Fig. 6 shows the expression of GABAA-R α1 subunit mRNA in layer I (which includes GABAergic interneurons and neuropil formed by the convergence of afferent thalamic fibers with apical dendrites of pyramidal neurons) and in layer V microdissected pyramidal neurons. A 50% decrease in α1 subunit expression is present in layer I of SI mice. These data suggest a layer-specific regulation of GABAA-R α1 subunit expression.
Fig. 6.
Quantitative nested RT-PCR assay of α1 subunit mRNA in layers I and V of mouse frontal cortex; comparison between 4-week SI and GH mice. (Upper) GABAA-R α1 subunit mRNA expression in layer I, including GABAergic interneurons and neuropil (Left), and layer V pyramidal neurons (Right) microdissected as described in Materials and Methods. (Layer I: t6 = 5.25; ∗, P < 0.01 vs. GH mice, Bonferroni t test. Mean ± SEM of four mice.) (Lower) Illustrations of layers I and V dissected with laser-assisted microscopy. In A, the rectangular box indicates the area to be dissected from layer I neuropil. B shows the space remaining after a portion of layer I is dissected, and the black arrow in layer V points to dissected pyramidal neuronal somata (open circle). Note the almost complete absence of toluidine blue-stained nuclei in layer I.
Discussion
SI Mice Show Changes of GABAA-R Subunit Expression.
GABAA-R is a heteropentameric structure that is formed by the coassembly of five polypeptides, including 2α, 2β, and 1γ subunits (22, 23, 30).
The dynamic mechanisms operative in the sorting out of various subunits (6α, 3β, 3γ, and one δ) and their assembly into the pentameric structure of the GABAA-Rs is not a completely understood process, but it is clear that these mechanisms may be influenced by various pharmacological treatments (e.g., long-term treatment with BZ, ethanol, and steroids) (31–33) or environmental factors (e.g., long-term stress) (1–5).
In the frontal cortex of SI mice for 4 weeks, there is a decrease in mRNA levels for α1, α2, and γ2 subunits by 40–50%, and an increase of α4 and α5 subunits by 80–100%, respectively (Fig. 3). In the hippocampus of the same mice, the α1 subunit is also decreased, and the α5 subunit mRNA is increased (Fig. 4). The changes of brain α1 and α5 mRNA subunit expression are paralleled by similar changes in GABAA-R α1 and α5 subunit polypeptides (Fig. 5). No changes were detected in α3, β3, and δ subunit expression (Fig. 3).
Because γ2 subunits are a necessary prerequisite for the formation of BZ-sensitive GABAA receptors (24, 34), it is not surprising that, in SI mice in which the expression of α1 and γ2 subunits is decreased, the binding of [3H]flumazenil to the hippocampal, and to a smaller extent to the cortical crude synaptic membranes, is also decreased (see Table 1), despite a 100% increase in α5 subunit expression.
In view of the changes in the pharmacological responses to anxiolytic BZs in SI mice, it is important to stress that in the frontal cortex of SI mice, the decrease in α1 subunit mRNA is very selective for layer I, suggesting that the changes in subunit mRNA expression are not uniformly distributed but instead are brain-region specific. We believe that this brain-region selectivity may contribute to differences in the responses of SI mice to the administration of allosteric modulators or direct GABAA receptor agonists.
Functional Consequences of Changes in GABAA Receptor Subunit Expression.
In SI mice, the changes in α and γ subunit expression that occur without changes in β and δ subunits may lead to a decrease in GABAA-R number or to the assembly of GABAA-R with a constant amount of β but with different combinations of α, γ, or δ subunits.
Mutational investigations of GABAA-R subtypes have increased our understanding of the contributions of GABAA-R subunits to the regulation of GABAA-R function in response to agonists, antagonists, or allosteric modulators (34). For example, in point-mutated α1 (H101R) knockin mice, the number of GABAA-R measured with [3H]-muscimol or [3H]-RO15-4513, which does not distinguish between α1 and α1 (H101R), is normal, but DZP-sensitive flumazenil-binding sites are decreased (≈50%). When point-mutated α1 (H101R) knockin mice are injected with DZP or ZOL, these mice are resistant to the sedative effect of these drugs. The selectivity of this effect is highlighted by the unaltered responsivity of α1 (H101R) mice to the sedative/hypnotic effects of non-BZ site ligands, such as phenobarbital or Allo (34). In addition, this selectivity was confirmed by the observation that the anxiolytic and anticonvulsant actions of DZP were not impaired in α1 (H101R) mice, supporting the view that these pharmacological actions of DZP can be attributed to GABAA-Rs containing α2, α3, and α5 subunits (34).
Based on this information, we tested whether the decrease in α1, α2, and γ2 subunits and the increase in α4 and α5 subunit expression measured in SI mice are translated into changes in GABAA-R responses to allosteric modulators or to direct GABAA-R agonists. In GH or SI mice, we compared the sedative action elicited by the BZ recognition site ligands with the sedative action elicited by the direct GABAA-R agonists muscimol and THIP or by the endogenous positive allosteric modulator of the action of GABA at GABAA-Rs, Allo.
Among the BZ recognition site ligands, we selected: (i) DZP for its high affinity and full intrinsic activity at α1-, α2-, α3-, and α5-containing GABAA-R subtypes (18). (ii) ZOL, for its high affinity and intrinsic activity for α1-containing GABAA-Rs. ZOL shows ≈20-fold lower affinity for α2- and α3-containing GABAA-Rs and no affinity for α5-containing receptors (15). (iii) IMD, which unlike DZP or ZOL fails to change GABA-mediated activity at α1-containing GABAA-Rs but selectively and with high efficacy modulates the action of GABA at GABAA-R, including α5 subunits, through an allosteric positive action (17–19). Because of the particularity of IMD’s pharmacological profile [i.e., it fails to stimulate GABAA-R-containing α1 (19), α4, and α6 (24) subunits], this drug is devoid of sedative and amnestic actions but is a potent anxiolytic and anticonvulsant agent, selectively acting as a positive allosteric modulator at α5- and perhaps α2- and α3-containing GABAA-Rs (17, 18).
Whereas muscimol, THIP, and Allo are equally active in GH and SI mice, we observed that the pharmacological profile of the three selected BZ recognition site ligands was completely modified by SI. In SI mice, ZOL exhibited a 3-fold right shift in the dose–response curve for inhibiting locomotor activity. DZP, given in doses ranging from 8.8 to 26.3 μmol/kg, rather than inhibiting locomotor activity elicits a significant paradoxical increase of locomotor activity that was blocked by the administration of flumazenil, suggesting that GABAA-Rs are operative in enhancing locomotion. IMD, which fails to alter locomotor activity in GH mice, produced a strong and sustained increase of locomotor activity in SI mice. These data suggest that in the brain of SI mice, there is a change in the assembly of the subunits included in the pentameric structure of the GABAA-Rs but the number of GABAA-Rs fails to change.
The Paradoxical Action of DZP and IMD Is Likely Mediated by α5 GABAA-R Subunit Overexpression in SI Mice.
GABAA-R-expressing α5 and α4 subunits are increased in the cortex and hippocampus of SI mice, whereas the expression of α1, α2, and γ2 subunits is decreased. Therefore, one could hypothesize that the increased locomotor activity observed in SI mice after IMD or DZP administration is due to an allosteric modulation by these anxiolytic BZs of the overexpressed α5-containing-GABAA-R subtypes integrated in neurons strategically positioned in the neuronal network that controls anxiety and motor activity.
A significant increase of locomotor activity is induced by DZP in rats that have been treated repeatedly and for protracted periods of time with large doses of DZP (35). These rats exhibit a decrease of α1 and an increase of α5 GABAA-R subunit expression in the cortex and hippocampus but no changes in GABAA-R number (31). Thus, one could infer that the paradoxical locomotor hyperactivity elicited by IMD or DZP in SI mice is due to a reduction of neophobia, probably elicited by stimulation of the overexpressed α5-containing GABAA-Rs (15, 35–38) in the absence of sedative action due to the decrease of GABAA-R-containing α1 subunits.
Because IMD fails to activate α1-, α4-, and α6-containing GABAA-Rs (17, 24) and exerts anxiolytic/antineophobic actions, probably by acting at α5-containing GABAA-Rs in GH mice, one would expect that the reduction of neophobia by IMD would also induce hyperlocomotion in GH mice. The failure of IMD to induce locomotor hyperactivity in GH mice suggests that the reduction of neophobia may not be the only mechanism that explains the increased locomotor activity elicited by IMD in SI mice.
Is the Up-Regulation of α4 GABAA-R Subunit Expression Responsible for Behavioral Alterations in SI Mice?
An increased expression in the levels of α4 GABAA-R subunits is observed in animal models of premenstrual and postpartum syndrome after withdrawal of progesterone treatment (32, 39), in animal models of epilepsy (40, 41), or during alcohol withdrawal (42). Although GABAA-R-containing α4 subunits are expressed at low levels in the brain, except in the thalamus, dentate gyrus, and outer layers of the cortex, the increased expression of these subunits in the cortex during SI invites speculation on whether the increase in this subunit expression has any consequence for the behavioral alterations observed in SI mice.
GABAA-Rs containing α4 subunits have an unusual pharmacological profile and are distinct from those receptors containing the more common α1, α2, α3, and α5 subunits. These differences include (i) insensitivity to classical BZs and IMD; (ii) high sensitivity to bretazenil, RO 15-4513, and flumazenil; (iii) high affinity for GABA; (iv) slower desensitization kinetics; and (v) higher sensitivity to alcohol (20, 24, 43).
To test whether increased α4 subunit expression contributes to the behavioral alterations observed in SI mice, we compared the action of the large-spectrum GABAA-R agonist muscimol, the more selective α4/δ subunit GABAA-R agonist THIP (20), and the positive allosteric modulator of GABAA-Rs, Allo, which is essentially equipotent in α1-, α2-, α3-, or α5-containing GABAA-R subtypes. However, Allo is more potent at α4-containing GABAA receptors (7). In small doses, none of these agents produces any stimulatory or inhibitory locomotor activity action, and at higher doses, they cause a sedative action of similar potency in GH and SI mice.
Conclusion
The results of this and previous studies (4, 5, 8, 28) suggest that prolonged SI in male mice triggers changes in GABAergic signal transduction by two mechanisms: (i) reduction of the levels of the potent GABAA-R-positive allosteric modulator Allo and (ii) alteration of the telencephalic expression of α/γ-containing GABAA-R subtypes.
These changes impair the pharmacology of GABAA-R modulators (i.e., BZ and barbiturates) and GABAA-R agonists or antagonists (i.e., muscimol and picrotoxin) (28, 44, 45) and provide initial insights into the molecular architecture and diversity of GABAA-R subtypes expressed in the brain of SI mice.
In addition to pharmacological changes in response to drugs, one of the most characteristic behavioral abnormalities of SI mice is the development of aggression (5). This behavior can be reduced by enhancing GABAA-R function with GABAA-R agonists or modulators (i.e., increasing the brain levels of Allo), suggesting that abnormalities of GABAA-R signal transduction contribute to the expression of aggression. However, further studies are necessary to establish whether the changes in GABAA-R subtype expression observed in the cortex and hippocampus are the consequence or the cause of the behavioral alterations observed in SI mice.
There is growing interest in the potential role of brain GABAergic transmission impairments in the pathophysiology of anxiety, panic, schizophrenia, and bipolar and depressive disorders. SI mice, which model abnormalities in the molecular architecture of GABAA-Rs, exhibit higher levels of fearfulness (46) and increased susceptibility to seizures (45), aggression (5), and lower sensitivity to BZs (44) than GH mice. Thus, these mice may represent a model to target specific GABAA-R subtypes with drugs expected to provide novel treatment opportunities for psychiatric disorders associated with a dysfunction of GABAA-R signal transduction.
Materials and Methods
Animals and Drug Treatment.
Adult male Swiss–Webster mice (Harlan Breeders), 18–20 g of body weight, maintained under a 12-hour dark/light cycle, and food and water ad libitum were used. Mice were housed either in groups of five per cage (24 × 17 × 12 cm) or individually for 4–6 weeks preceding behavioral and biochemical measurements (5).
Measurement of Locomotor Activity in a Novel Cage.
A computerized AccuScan 12 Animal Activity Monitoring System (Columbus Instruments, Columbus, OH) assisted by versamax software (AccuScan Instruments, Columbus, OH) monitored locomotor activity in mice (47). Each activity cage consisted of a Perspex box (20 × 20 × 20 cm) surrounded by horizontal and vertical infrared sensor beams. The locomotor activity of GH and SI mice was recorded between 1:00 and 3:00 p.m. in the animal facility room in which the mice had been housed.
Quantitative RT-PCR Analyses of GABA-R Subtype mRNAs.
mRNAs were quantified with competitive RT-PCR (48). Primers for GABAA-R subtype mRNA quantification were: α1, reverse 1458–1482, forward 1178–1202; α2, reverse 1780–1803, forward 1482–1505; α3, reverse 1737–1760, forward 1401–1424; α4, reverse 1686–1705, forward 1315–1339, α5, reverse 1502–1526; forward 1315–1339; β3, reverse 1530–1554, forward 1199–1223; γ2, reverse 1211–1235, forward 697–721; δ, reverse 1167–1190, forward 1477–1500. Templates for GABAA-R subtype α1, α2, α3, α4, α5, β3, and δ internal standards contained a restriction endonuclease site, BglII, and for the γ2 internal standard, an XbaI restriction endonuclease site. After ethidium bromide staining, the pixel intensity of the bands resulting from the native target mRNA and internal standard cRNA was determined with a Kodak DC290 camera and with Kodak d1 image analysis software.
Laser-Capture Microdissection and Quantitative Nested RT-PCR Analysis.
Using a Leica (Deerfield, IL) laser-assisted microdissection system, we isolated the somata of layer V pyramidal neurons and the corresponding layer I neuropil, which include apical dendrites of pyramidal neurons and horizontal GABAergic neurons (19).
RNA was extracted from layer I (≈0.02 mm3) or 500 cells of layer V by using a modified NIH total RNA extraction protocol (Arcturus Engineering, Mountain View, CA). A first round of RT-PCR amplification was performed with the respective specific GABAA-R subunit primer pair. For nested PCR, the first-round RT-PCR products were diluted 200-fold and run with nested primers designed so that the primer pairs were located inside the first set of primers (19).
Western Blot Protein Analysis.
The levels of GABAA-R subunits were measured by quantitative Western blot (31). We used: (i) an affinity-purified rabbit polyclonal (IgG) anti-GABA-R α1 subunit (1:1,000; Upstate Biotechnology, Lake Placid, NY); and (ii) a rabbit polyclonal antibody for the α5 subunit (1:1,000; Novus Biologicals, Littleton, CO). Crude synaptosomal membrane preparations obtained by gradient fractionation were denaturated in SDS loading buffer and electrophorized on a 10–20% SDS polyacrylamide gel. Proteins were then blotted onto a nitrocellulose membrane. Blots were then washed in PBS-Tween for 10 min × 3 and incubated for 2 h at room temperature with horseradish-peroxidase-linked secondary antibodies. The blots were developed with an enhanced chemiluminescent Western blot detection kit (Amersham Pharmacia Biosciences). The band-intensity ratios for each sample relative to the β-actin expression were calculated in three to five serial dilutions and expressed as percents of their respective control samples.
Flumazenil-Binding Assay.
Membranes were prepared from frozen hippocampi or frontal cortices through homogenization in ice-cold H2O. The homogenates were centrifuged at 20,000 × g for 20 min, and the pellets were resuspended in 50 volumes of 50 mM Tris citrate buffer, pH 7.1, yielding a final protein concentration of 0.5 mg/ml. The binding assay was carried out with 25 μg of membrane protein in a final volume of 500 μl in the presence of 0.1–20 nM [3H]flumazenil [80 Ci/mmol (1 Ci = 37 GBq), NEN] (49). Specific binding represented ≈95% of total binding. Protein concentration was measured by the method of Bradford et al. (50).
Ex Vivo Measurement of GABA Turnover Rate.
The dynamics of GABA neuronal pool renovation rates in the motor cortex and striatum of SI and GH mice were estimated as described (29) in mice infused with [13C6]glucose at a constant rate (20 μl/min) over 5 min. At various times after glucose infusion termination (1, 2, 3.5, 5, 7.5, and 10 min), mice were killed in 0.30 sec by using a focused microwave beam (10 kW) delivered to the head. Brains were sliced into 20-μm coronal sections, and the motor cortex and striatum were dissected by a Leica laser microdissection system (29).
To calculate the conversion index of glutamate into GABA, we used the ratio of [13C] enrichment into GABA over that of glutamate, measured at a time interval of 2 min after the termination of [13C6]glucose infusion. The conversion index of glutamate into GABA was used to estimate GABA neuronal pool renovation dynamics.
Statistical Analysis.
Data are given as means ± SEMs. Comparisons between the control group and each of the treatment groups were performed by one- or two-way ANOVA followed by Bonferroni t tests, as indicated in the legends to Figs. 1–6. The ED50 values were calculated from dose–response curves analyzed by the quantal dose–response: probits test using the computer program of Tallarida and Murray (51) equipped with a statistical package. The Bmax and Kd values were determined by data analyses and curve fitting with ebda (Elsevier-Biosoft, Cambridge, U.K.). Differences were considered significant at P < 0.05.
Acknowledgments
We thank Drs. Hanns Möhler (Institute of Pharmacology and Toxicicology, University of Zurich, Zurich) and Richard W. Olsen (Department of Molecular and Medical Pharmacology, University of California, Los Angeles) for insightful comments on the manuscript. This work was supported by Grants MH 56890 (to A.G.) and MH 071667-01A1 (to E.C.) and by Campus Research Board Award 2-611185 (to G.P.).
Abbreviations
- SI
social isolation
- SI
socially isolated
- GH
group housed
- Allo
allopregnanolone
- DZP
diazepam
- IMD
imidazenil
- ZOL
zolpidem
- GABAA-R
GABA type A receptor
- BZ
benzodiazepine
- THIP
4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3(2H)-one.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Orchinik M., Weiland N. G., McEwen B. S. Mol. Brain Res. 1995;34:29–37. doi: 10.1016/0169-328x(95)00118-c. [DOI] [PubMed] [Google Scholar]
- 2.De Kloet E. R., Joels M., Holsboer F. Nat. Rev. Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 3.Caldji C., Diorio J., Anisman H., Meaney M. J. Neuropsychopharmacology. 2004;29:1344–1352. doi: 10.1038/sj.npp.1300436. [DOI] [PubMed] [Google Scholar]
- 4.Dong E., Matsumoto K., Uzunova V., Watanabe H., Costa E., Guidotti A. Proc. Natl. Acad. Sci. USA. 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinna G., Dong E., Matsumoto K., Costa E., Guidotti A. Proc. Natl. Acad. Sci. USA. 2003;100:2035–2040. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puia G., Vicini S., Seeburg P. H., Costa E. Mol. Pharmacol. 1991;39:691–696. [PubMed] [Google Scholar]
- 7.Belelli D., Lambert J. J. Nat. Rev. Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 8.Pinna G., Costa E., Guidotti A. Proc. Natl. Acad. Sci. USA. 2004;101:6222–6225. doi: 10.1073/pnas.0401479101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ojima K., Matsumoto K., Watanabe H. Brain Res. 1997;745:127–133. doi: 10.1016/s0006-8993(96)01136-5. [DOI] [PubMed] [Google Scholar]
- 10.Molodavkin G. M., Voronina T. A., Aldarmaa Z. H., Meletova O. K. Eksp. Klin. Farmakol. 2004;67:3–6. [PubMed] [Google Scholar]
- 11.Gourley S. L., Debold J. F., Yin W., Cook J., Miczek K. A. Psychopharmacology. 2005;178:232–240. doi: 10.1007/s00213-004-1987-3. [DOI] [PubMed] [Google Scholar]
- 12.Woods J. H., Katz J. L., Winger G. Pharmacol. Rev. 1992;44:151–347. [PubMed] [Google Scholar]
- 13.Ben-Porath D. D., Taylor S. P. Addict. Behav. 2002;27:167–177. doi: 10.1016/s0306-4603(00)00175-1. [DOI] [PubMed] [Google Scholar]
- 14.Serra M., Pisu M. G., Littera M., Papi G., Sanna E., Tuveri F., Usala L., Purdy R. H., Biggio G. J. Neurochem. 2000;75:732–740. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- 15.Crestani F., Martin J. R., Mohler H., Rudolph U. Br. J. Pharmacol. 2000;131:1251–1254. doi: 10.1038/sj.bjp.0703717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arbilla S., Allen J., Wick A., Langer S. Z. Eur. J. Pharmacol. 1986;130:257–263. doi: 10.1016/0014-2999(86)90276-1. [DOI] [PubMed] [Google Scholar]
- 17.Guidotti A., Auta J., Davis J. M., Dong E., Grayson D. R., Veldic M., Zhang X., Costa E. Psychopharmacology. 2005;180:191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- 18.Costa E., Guidotti A. Trends Pharmacol. Sci. 1996;17:192–200. doi: 10.1016/0165-6147(96)10015-8. [DOI] [PubMed] [Google Scholar]
- 19.Costa E., Auta J., Grayson D. R., Matsumoto K., Pappas G. D., Zhang X., Guidotti A. Neuropharmacology. 2002;43:925–937. doi: 10.1016/s0028-3908(02)00199-5. [DOI] [PubMed] [Google Scholar]
- 20.Brown N., Kerby J., Bonnert T. P., Whiting P. J., Wafford K. A. Br. J. Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunther U., Benson J., Fritschy J. M., Reyes G., Knoflach F., Crestani F., Aguzzi A., Arigoni M., Lang Y., et al. Proc. Natl. Acad. Sci. USA. 1995;92:7749–7753. doi: 10.1073/pnas.92.17.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnard E. A., Skolnick P., Olsen R. W., Mohler H., Sieghart W., Biggio G., Braestrup C., Bateson A. N., Langer S. Z. Pharmacol. Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 23.Olsen R. W., Homanics G. E. In: GABA in the Nervous System. Martin D. L., Olsen R. W., editors. Philadelphia: Lippincott William & Wilkins; 2000. pp. 81–96. [Google Scholar]
- 24.Knoflach F., Benke D., Luddens H., Hamilton B. J., Carter D. B., Mohler H., Benson J. A. Mol. Pharmacol. 1996;50:1253–1261. [PubMed] [Google Scholar]
- 25.Giusti P., Ducic I., Puia G., Guidotti A., Costa E. J. Pharmacol. Exp. Ther. 1993;266:1018–1028. [PubMed] [Google Scholar]
- 26.Thompson D. M., Auta J., Guidotti A., Costa E. J. Pharmacol. Exp. Ther. 273:1307–1312. [PubMed] [Google Scholar]
- 27.Auta J., Faust W. B., Lambert P., Guidotti A., Costa E., Moerschbaecher J. M. Behav. Pharmacol. 1995;6:323–332. [PubMed] [Google Scholar]
- 28.Pinna G., Uzunova V., Matsumoto K., Puia G., Mienville J. M., Costa E., Guidotti A. Neuropharmacology. 2000;39:440–448. doi: 10.1016/s0028-3908(99)00149-5. [DOI] [PubMed] [Google Scholar]
- 29.Carboni G., Tueting P., Tremolizzo L., Sugaya I., Davis J., Costa E., Guidotti A. Neuropharmacology. 2004;46:1070–1081. doi: 10.1016/j.neuropharm.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Sieghart W. Pharmacol. Rev. 1995. 1995;47:181–234. [PubMed] [Google Scholar]
- 31.Impagnatiello F., Pesold C., Longone P., Caruncho H., Fritschy J. M., Costa E., Guidotti A. Mol. Pharmacol. 1996;49:822–831. [PubMed] [Google Scholar]
- 32.Smith S. S., Gong Q. H., Hsu F. C., Markowitz R. S., ffrench-Mullen J. M., Li X. Nature. 1998;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S., Fleming R. L., Morrow A. L. Pharmacol. Ther. 2004;101:211–226. doi: 10.1016/j.pharmthera.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Rudolph U., Mohler H. Annu. Rev. Pharmacol. Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- 35.Sansone M. Psychopharmacology. 1979;66:109–110. doi: 10.1007/BF00431999. [DOI] [PubMed] [Google Scholar]
- 36.McKernan R. M., Rosahl T. W., Reynolds D. S., Sur C., Wafford K. A., Atack J. R., Farrar S., Cook G., Ferris P., et al. Nat. Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 37.Crestani F., Martin J. R., Mohler H., Rudolph U. Nat. Neurosci. 2000;3:1059. doi: 10.1038/80553. [DOI] [PubMed] [Google Scholar]
- 38.Crestani F., Lorez M., Baer K., Essrich C., Benke D., Luscher B., Mohler H. Nat. Neurosci. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- 39.Concas A., Mostallino M. C., Porcu P., Follesa P., Barbaccia M. L, Trabucchi M., Purdy R. H., Grisenti P., Biggio G. Proc. Natl. Acad. Sci. USA. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banerjee P. K., Tillakaratne N. J. K., Brailowsky S., Olsen R. W., Tobin A. J., Snead O. C., 3rd Exp. Neurol. 1998;154:213–223. doi: 10.1006/exnr.1998.6928. [DOI] [PubMed] [Google Scholar]
- 41.Brooks-Kayal A. R., Shumate M. D., Jin H., Lin D. D., Rikhter T. Y., Holloway K. L., Coulter D. A. J. Neurosci. 1999;19:8312–8318. doi: 10.1523/JNEUROSCI.19-19-08312.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cagetti E., Liang J., Spigelman I., Olsen W. Mol. Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- 43.Sundstrom-Poromaa I., Smith D. H., Hua Gong O., Sabado T. N., Li X., Light A., Wiedmann M., Williams K., Smith S. S. Nat. Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guidotti A., Dong E., Matsumoto K., Pinna G., Rasmusson A. M., Costa E. Brain Res. Rev. 2001;37:110–115. doi: 10.1016/s0165-0173(01)00129-1. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto K., Nomura H., Murakami Y., Taki K., Takahata H., Watanabe H. Pharmacol. Biochem. Behav. 2003;75:831–835. doi: 10.1016/s0091-3057(03)00169-2. [DOI] [PubMed] [Google Scholar]
- 46.Pinna G., Costa E., Guidotti A. Psychopharmacology. 2006 doi: 10.1007/s00213-005-0213-2. in press. [DOI] [PubMed] [Google Scholar]
- 47.Pinna G., Galici R., Schneider H. H., Stephens D. N., Turski L. Proc. Natl. Acad. Sci. USA. 1997;94:2719–2723. doi: 10.1073/pnas.94.6.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grayson D. R., Ikononmovic S. In: Vitro Neurochemical Techniques, Neuromethods. Boulton A. A., Baker G. B., Bateson A. N., editors. Vol. 34. Totowa, NJ: Humana; 1998. pp. 127–151. [Google Scholar]
- 49.Massotti M., Schlichting J. L., Giusti P., Memo M., Costa E., Guidotti A. J. Pharmacol. Exp. Ther. 1991;256:1154–1160. [PubMed] [Google Scholar]
- 50.Bradford M. M. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 51.Tallarida R. J., Murray R. B. Manual of Pharmacologic Calculations with Computer Programs. 2nd Ed. New York: Springer; 1987. [Google Scholar]