Abstract
Social information can be acquired either directly or indirectly from cues inadvertently produced by individuals with similar interests and requirements (“inadvertent social information,” ISI). These inadvertent cues provide “public information” that other individuals can use to guide their behavior. We show here that female mice use olfactory ISI to determine their choice of, and responses to, males and that the use of this ISI involves the gene for oxytocin (OT). Female mice (OT wild type and CF-1 strain) displayed a significant interest in, and choice of, the odors of uninfected males of varying sexual status that were associated with the odors of an another estrous female. This recognition of, and choices for, specific, individual male odors was evident 24 h later. Female mice also distinguished between males subclinically infected with the gastrointestinal nematode parasite, Heligimosomoides polygyrus, and nonparasitized males, displaying aversive responses (analgesia, increased corticosterone) to, and avoidance of, the odors of infected males. The presence of the odors of another estrous female with that of the infected male, which are indicative of potential mate interests, attenuated these aversive responses and resulted in a choice for the odors of infected male. OT gene-deficient (knockout) females were impaired in their use of this ISI to modulate their responses to either uninfected males of differing sexual states or infected males. These findings suggest that OT genes are necessary for the processing of inadvertent social information and likely the integration of both direct and indirect social information.
Social information can be acquired either as direct social signals or indirectly as inadvertent social information (ISI) produced by the behavior and performance of others with similar requirements (1). ISI has been shown to be used in situations ranging from where and what to eat to whom to mate with (2). The use of inadvertently produced and publicly available information (“public information;” ref. 1), in which some cues of one female’s mate choice decision influences another female’s mate choice (“mate copying”), is reported in several species of birds and fish (3–7), with even reversals of mate choice decisions indicated (8). Although social influences on human mate choice are suggested, the use of ISI in determining the mating and social preferences of mammals remains to be established (1, 2, 9).
An ISI cue can be considered as any factor that provides potential inadvertent information about another females mate choice decision (1, 2). Odors guide the social behavior and mate responses of rodents, with female mice using odors to determine the quality, condition, health, and suitability of a male as a potential mate (e.g., refs. 10–15). Female mice are known to attend to male scent marks (15, 16) and to deposit scent marks in response to male odors. In addition, females have been shown to investigate the odors of other females in a number of contexts (10, 16, 17). As such, female odors that are associated with that of a male encompass a potential source of ISI that may be used to guide the social interests and mate choice of other females.
A major cost of social behavior is the increased risk of exposure to parasites. Social behaviors facilitate interactions between conspecifics, increasing the probability of exposure to, and transmission of, parasites from infected to uninfected individuals (18, 19). Parasites have been shown to affect mate choice and mating patterns with females preferentially selecting parasite-free or -resistant males on the basis of a variety of cues (e.g., refs. 20–22). Female mice can discriminate between uninfected and infected males on the basis of odor, displaying aversive and avoidance responses to infected males and their odors (e.g., refs. 13 and 23–30). Thus, the ability to use direct social information is critical for both the recognition and expression of appropriate responses to infected individuals. However, whether ISI further influences and modulates the responses of females to infected individuals remains to be determined.
The neural–hormonal systems implicated in the processing of direct (non-ISI) social information include the neuropeptides vasopressin and oxytocin (OT) (31, 32). OT is involved in the central mediation of several social behaviors, including mate and maternal bonds, as well as social recognition (33–36). Male and female mice with deletions of the OT gene [OT knockout (OTKO) mice] are impaired in tests of odor-mediated social recognition and memory despite apparently normal olfactory and learning abilities (37, 38). The gene for OT is also involved in the olfactory-mediated recognition of, and discrimination against, infected males by female mice (27, 28). However, differently to this, the neural–hormonal systems involved in processing ISI mediated social behaviors and responses are not known.
Here we assessed the use of ISI odor cues by female mice in determining their choice of male odors and how ISI affects female responses to parasitized males. We then considered the possible involvement of OT in the use of ISI in directing the social interest and mate choice of females.
Results
Effects of Female Odors on Initial Choices.
Responses to uninfected males.
When presented with equivalent male A and B stimulus odors females did not show any clear choice. However, the CF-1 and OTWT females displayed a significant choice for the odor of a male that was associated with that of an estrous female, with the OTKO females displaying a slight, nonsignificant, increase in their preference (Table 1). On day 2, OTWT and CF-1, but not OTKO, females displayed a significant choice for the odor of the male that had been previously associated with the odor of an estrous female. In all cases, equivalent patterns of choice were shown by estrous and nonestrous females. The presence of the odor of a nonestrous female had no significant effect on male odor choice. As well, OTWT, OTKO, and CF-1 females did not show any significant choice for the odor of an estrous female by itself and were able to distinguish between the odor of a male and female.
Table 1.
Initial odor choices of OTWT, OTKO, or CF-1 strain (CF-1) female mice (n = 30) presented with the odors of two equivalent males (male A and male B)
| Day | Genotype | Choice | % Initially choosing male odor A | P |
|---|---|---|---|---|
| 1 | WT | Male A − male B | 50 | NS |
| 1 | WT | Male A + ♀ − male B | 80 | 0.001 |
| 2 | WT | Male A − male B | 80 | 0.001 |
| 1 | KO | Male A − male B | 50 | NS |
| 1 | KO | Male A + ♀ − male B | 60 | NS |
| 2 | KO | Male A − male B | 50 | NS |
| 1 | CF-1 | Male A − male B | 50 | NS |
| 1 | CF-1 | Male A + ♀ − male B | 80 | 0.001 |
| 1 | CF-1 | Male A − male B | 80 | 0.01 |
On day 1, females were presented male odor A either with (♀) or without the odor of an estrous female. On day 2, females were presented with male odors A and B again. NS indicates no significant difference in choice.
The CF-1, OTWT, and OTKO females all discriminated between the odors of sexually stimulated and unstimulated males and displayed a significant initial choice for the odors of unstimulated males (Table 2). Both estrous and nonestrous females chose the odors of stimulated males, with only a few nonestrous females choosing the odors of unstimulated males. When the odor of an estrous female was associated with that of the unstimulated male, OTWT and CF-1 females displayed a significant choice for the unstimulated male. OTKO females displayed a nonsignificantly increased choice for the odors of unstimulated males. On day 2, OTWT and CF-1 females displayed a significant initial choice for the odors of the familiar unstimulated male that had been associated with the odors of the estrous female and avoidance of the odors of a novel unstimulated male. In contrast, the OTKO females avoided the odors of both the novel and familiar unstimulated males. The odors of a nonestrous female had no significant effects on initial odor choice.
Table 2.
Initial odor choices of OTWT, OTKO, or CF-1 strain (CF-1) female mice (n = 30) presented with the odors of a sexually stimulated (stim) or unstimulated (unstim) male
| Day | Genotype | Choice | % Initially choosing unstimulated ♂ odor | P |
|---|---|---|---|---|
| 1 | WT | Unstim − stim | 20 | 0.001 |
| 1 | WT | Unstim + ♀ − stim | 70 | 0.01 |
| 2 | WT | Unstim (familiar) − stim | 70 | 0.01 |
| 2 | WT | Unstim (novel) − stim | 20 | 0.001 |
| 1 | KO | Unstim − stim | 20 | 0.001 |
| 1 | KO | Unstim + ♀ − stim | 50 | NS |
| 2 | KO | Unstim (familiar) − stim | 20 | 0.001 |
| 2 | KO | Unstim (novel) − stim | 20 | 0.001 |
| 1 | CF-1 | Unstim − stim | 20 | 0.001 |
| 1 | CF-1 | Unstim + ♀ − stim | 80 | 0.001 |
| 2 | CF-1 | Unstim (familiar) − stim | 70 | 0.01 |
| 2 | CF-1 | Unstim (novel) − Stim | 30 | 0.01 |
On day 1, females were presented the odors of an unstimulated male either with (♀) or without the odor of an estrous female. On day 2, females were presented with either the odors of the same (familiar) male they were exposed to on day 1 or the odors of a different (novel) male. NS indicates no significant differences in choice.
Responses to parasitized males.
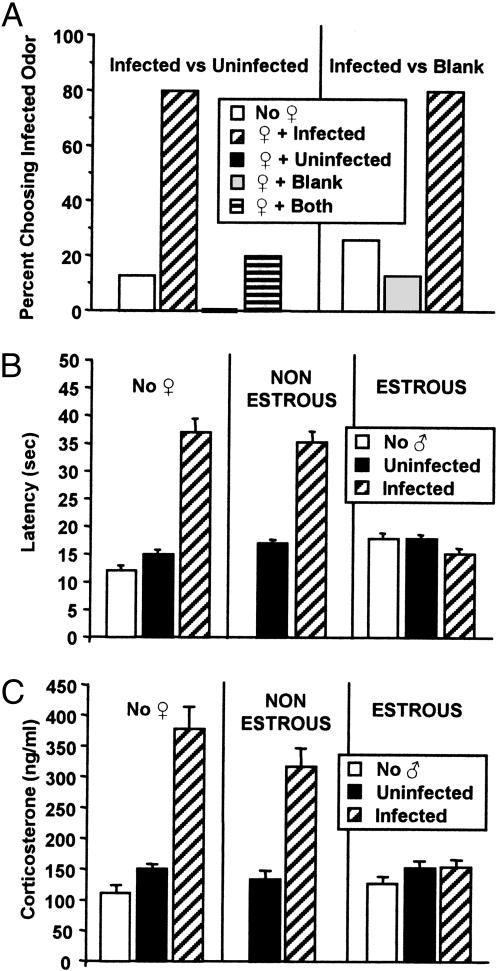
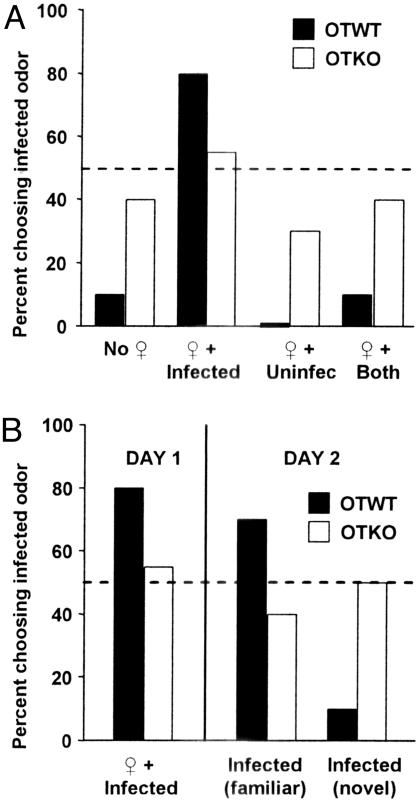
In the infected–uninfected stimulus odor combination on day 1 CF-1 and OTWT females of all estrous phases showed a significant (P < 0.001) initial choice for the odors of uninfected males and avoidance of the odors of infected males (Figs. 1A and 2A). On day 2, they still showed an overwhelming (P < 0.001) initial choice of the odors of uninfected males, regardless of whether the infected males were familiar or novel (Fig. 2B). In contrast, OTKO females at all estrous phases failed to show a significant initial choice of either odor on both days 1 and 2.
Fig. 1.
Effects of a 1 min exposure to the urine odors of male mice. (A) Initial odor choices of CF-1 females (n = 15) provided with either infected male − uninfected male or infected male − blank stimulus odor combinations. The stimulus odors presented were: (i) no female (♀) odor, (ii) estrous female + infected male, (iii) estrous female + uninfected male, (iii) estrous female + infected and uninfected male, (iv) estrous female + blank. (B) Effects of a 1-min exposure to the urine odors of either infected or uninfected males on the nociceptive responses of CF-1 female mice (n = 10). Male odors were presented either by themselves (no female, ♀) or in association with the odor of either an estrous or nonestrous female. Nociceptive responses recorded in the absence of male odors (no male, ♂) are also shown. Vertical lines denote a standard error of the mean. (C) Effects of a 1-min exposure to the urine odors of either infected or uninfected male mice on the plasma corticosterone levels of female mice (n = 10). Male odors were presented either by themselves (no female, ♀) or in association with the odor of either an estrous or nonestrous female. Corticosterone levels of females in the absence of male odors (no male, ♂) are also shown. Vertical lines denote a standard error of the mean.
Fig. 2.
Effects of a 1-min exposure to the urine odors of male mice. (A) Initial day 1 odor choices of OTWT and OTKO females (n = 30) provided with H. polygyrus-infected male − uninfected male stimulus odor combinations. The stimulus odors were presented either in association with the odor of an estrous females (female, ♀) or without a female odor (no female). (B) On day 2, the females (n = 15) were retested and given a choice between the odors of an unfamiliar infected male (infected novel) and uninfected male, or the odors of an uninfected male and a familiar infected male (male that had on day 1 been associated with a female odor).
The presence of the odor of an estrous female associated with that of an infected male significantly (P < 0.001) increased the choice for the odor of the infected male by the CF-1 and OTWT females of all estrous phases, such that the females now showed a significant (P < 0.001) initial choice for the odors of infected males and avoidance of the odors of uninfected males (Figs. 1A and 2A). On day 2 the OTWT and CF-1 females displayed a significant initial choice for the odors of familiar infected males that had been previously associated with the odors of an estrous female and a significant avoidance of the odors of uninfected males (Figs. 1A and 2B). However, the females displayed a marked avoidance of the odors of unfamiliar infected mates. In contrast, the presence of the odor of an estrous female associated with that of either an infected or uninfected male had no significant effect on the initial odor choices of OTKO females. In the infected-blank stimulus odor combination CF-1 females showed a significant initial choice for the blank odor and avoidance of the odor of the infected male. However, the presence of the odor of an estrous female with that of the infected male caused a highly significant (P < 0.001) initial choice for the odor of the infected male. The odors of a nonestrous female (not shown) had no significant effects on the day 1 initial odor choices of either the CF-1, OTWT, or OTKO females.
Female Odor and Nociceptive Responses.
The nociceptive responses of the CF-1 females that were exposed to the odors of infected and uninfected males that were associated with female odors were analyzed with a 2 × 1 ANOVA consisting of two between factors: male odor (infected, uninfected), female odor (estrous, nonestrous); and one within group factor, test time (before and after exposure). For CF-1 females, the analysis revealed significant main effects of male odor [F(2,72) = 8.972; P = 0.0003], female odor [F(2,72) = 14.99; P < 0.0001], as well as a significant interaction between these factors [F(3,72) = 40,667; P < 0.0001].
In the absence of any female odor, CF-1 females of all estrous phases that were exposed to the odors of infected males displayed a significant increase (P < 0.001) in response latency indicative of the induction of analgesia (Fig. 1B). Response latencies recorded in the absence of any male or female are indicative of, and nonsignificantly different from, the preexposure latencies of the various groups. Exposure to the odors of uninfected males had no significant effect on response latency. The presence of the odor of an estrous female with that of the infected male blocked (P = 0.02) the analgesic response, whereas the presence of the odor of a nonestrous female had no significant effect on the level of analgesia.
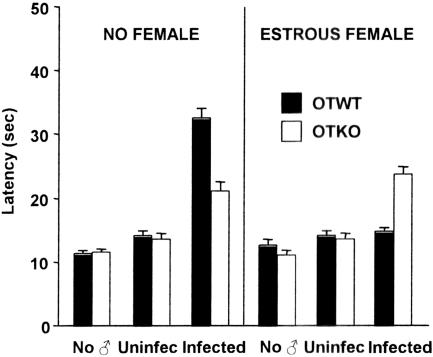
The nociceptive responses of the OTWT and OTKO females that were exposed to the odors of infected and uninfected males associated with female odors were analyzed by a 3 × 2 ANOVA consisting of two between factors; male odor (infected, uninfected), female type (OTWT, OTKO); and one within-group factor, test time (before and after exposure). The analysis revealed significant main effects of male odor [F(2,72) = 21.4, P < 0.001), female type [F(1,72) = 16.22, P < 0.001], as well as significant interaction between these factors [F(3,73) = 18.6; P < 0.001].
In the absence of any female odor the OTWT and OTKO females of all estrous phases displayed significant analgesic responses (P < 0.001), with the OTKO females displaying significantly (P < 0.01) lower levels of analgesia (Fig. 3). Exposure to the odors of uninfected males had no significant effects on response latency. The presence of the odor of an estrous female with that of the infected male blocked the analgesic responses of the OTWT females, but had no significant effect on the analgesic responses of the OTKO females. Female odors had no significant effects on basal nociceptive sensitivity.
Fig. 3.
Effects of a 1-min exposure to the urine odors of either infected or uninfected male mice on the nociceptive responses of OTWT and OTKO female mice (n = 10). Male odors were presented either by themselves (no female) or in association with the odor of an estrous female. Nociceptive responses recorded in the absence of male odors (no male, ♂) are also shown. Vertical lines denote a standard error of the mean.
Female Odor and Corticosterone Responses.
There was a significant main effect of male odor exposure [F(2,72) = 39.37; P < 0.001] and the presence of female odor [F(2,72) = 21.73; P = 0.002] on corticosterone levels, as well as a significant interaction between these factors [F(3,73) = 58.72; P < 0.001; Fig. 1C). Post hoc comparisons showed that CF-1 females that were exposed to the odors of infected males displayed a highly significant (P < 0.0001) increase in corticosterone levels. Females that were exposed to the odors of uninfected males also displayed a significant (P = 0.04) increase in corticosterone levels that was, however, markedly lower (P < 0.001) than that elicited by exposure to the odors of infected males. Estrous female odor blocked these increases in corticosterone, such that there were no longer any significant differences between basal corticosterone levels (no male) and the corticosterone levels of females that were exposed to the odors of either infected or noninfected males. Exposure to the odors of nonestrous females had no significant effect on the increases in corticosterone levels elicited by exposure to the odors of infected males. The odors of estrous and nonestrous females had no significant effects on basal corticosterone levels or the rises in corticosterone and analgesic responses elicited by exposure to predator odor.
Discussion
Our studies reveal that naive female mice can use inadvertent social information to determine their responses to males and that the processing of this ISI involves the gene for OT. We show that (i) female interest in, and choice of, the odors of individual males is affected by ISI provided in the form of the odor interests of another estrous female; (ii) the aversive and avoidance responses of females to parasitized males are attenuated by this ISI; and (iii) OT gene-deficient female mice (OTKOs) are impaired in their use of ISI.
In an odor choice test, OTWT and CF-1, but not OTKO, female mice displayed a significant interest in, and initial choice of, the odor of a male that was associated with the odor of an estrous female. Likewise, when ISI, in the form of the odor of an estrous female, was associated with that of the less attractive unstimulated male, OTWT and CF-1, but not OTKO, females reversed their choice, displaying an initial choice of the odors of the unstimulated male. These findings reveal that not only is the gene for OT involved in ISI processing, but also that it is associated with the mediation of both “true individual recognition” (i.e., discrimination between familiar individuals with differing significance) (39, 40) and, as has been shown (31–33, 37, 38), the recognition of different categories of individuals (i.e., familiar vs. novel).
Similarly, and consistent with prior findings (23, 28), females (OTWT and CF-1) discriminated between the odors of infected and uninfected males and displayed an initial choice for the odors of uninfected males and avoidance of the odors of infected males. However, when the odor of a novel estrous, but not nonestrous female, was associated with that of the infected male, females no longer displayed an avoidance of infected males. Rather, they showed a significant initial choice for the infected male. In contrast, OTKO females displayed ISI-insensitive reduced avoidance responses to, and discrimination against, infected males. On day 2, the OTWT and CF-1, but not OTKO, females still displayed a significant interest in the specific, familiar, individual male odor that had been associated with that of a female. This finding shows that female mice distinguish between particular individual infected males and do not extend and generalize their reduced aversions and use of ISI to other infected individuals.
The choice test used here does not test mating preferences directly, rather it assesses social interest. However, results of previous studies have shown that the selections made in odor choice tests are consistent with the appetitive components of mating preferences and are suggested to give reliable indications of mate choice in mice (29, 41–43). In addition, the positive reproductive consequences of such social preferences have been shown (44). As such, the alterations in odor responses induced here by the presence of the odor cues of another female can be considered indicative of shifts in female initial choice and mate preferences. However, consummatory responses may be differentially regulated and influenced by direct and inadvertent social information.
The aversive responses of females (CF-1 and OTWT) to parasitized males were also affected by ISI. Females that were exposed to the odors of infected males displayed marked analgesic responses and rise in corticosterone, both of which were blocked by the presence of the odors of an estrous female. As observed in the choice test, this attenuated analgesia and avoidance was evident 24 h later when females were reexposed to the odors of the familiar, but not a novel, infected male. These analgesic responses are important components of the suite of defensive responses to stimuli associated with actual and potential threats and, through their fear, anxiety, and stress associated motivational correlates, facilitate the recognition and avoidance of novel infected individuals.
OTKO females displayed reduced aversive responses to infected males and did not modulate their responses to the infected males on the basis of ISI. The OTKOs displayed similar levels of analgesia and odor interest regardless of the presence or absence of the odor of another female. However, the OTKO females could distinguish between the odors of intact and castrated males, as well as those of males and females of various conditions, showing that the olfactory abilities and sensitivity of the OTKO females to males of different sexual states were not impaired (27, 28). Also, females did not display any overall significant choice for the odors of females per se, and were not simply responding to an enhanced stimulus.
A number of studies with birds and fishes have suggested that the mate choice decisions of females can be influenced by the mating decisions of other females. Such nonindependent mate choice (45) has been termed “mate copying” (3–8). According to this proposal, the mate choice of a particular male by one female causes an increased preference for that same male by another female. Indeed, even the reversal of female mate choice by copying of what was previously a less preferred male has been reported in the guppy (8). It has also been further reported that females do not actually need to observe the mate-choice process, only, as implied here, the outcome of an “apparent choice” (46). Social influences on mate choice have also been speculated for humans (1, 2). However, mate choice copying was not found in another mammal, fallow deer (9). However, in the latter case, consummatory aspects of mate selection were examined, whereas the present study considers odor-based appetitive components.
The female mice in the present study can be considered to be using the odor interests of other estrous females. As such, these findings raise the possibility that female mice may use or “copy” the olfactory-based mate interests of other females in a manner evocative of mate copying reported in other species. Here “uninfected” does not necessarily imply a parasite resistant and/or better quality male. High-quality males can have both greater health and more parasites than low-quality males (47). Dominant males are more susceptible to infection by Heligimosomoides polygyrus, with reduced parasite clearance being associated with higher testosterone levels in males (48). As such, using the putative preference/interest of another female for an infected male would be appropriate.
Females display a marked preference for the odors of sexually stimulated males (males exposed for 24 h to the odors of an estrous female) over that of unstimulated, nonexposed males (28). Females have also been suggested to be better able to assess the health and infection status of sexually stimulated than unstimulated males (30). ISI and public information are suggested to be used when individual information may be ambiguous (2) such as is provided by the unstimulated uninfected and infected males used here. Inexperienced naive females may, thus, use the putative olfactory interests of other females and obtain information about male quality in a manner similar to that suggested for visually based mate copying in birds and fishes (1–8). In a broader context, females can also be considered to be “trusting” the mate interests or preferences of other females. OT involvement here can be construed as paralleling the suggestion for OT having a prosocial role in enhancing trust in humans (49).
Mice and rats can distinguish volatile urinary odors associated with differences in alleles borne at the major histocompatibility complex (MHC) and use MHC-associated odors for mediating individual recognition, determining mating preferences and condition (e.g., refs. 42 and 50–53). The MHC is part of the immune system with infection activating the release of MHC antigens and infection may alter these cues (52, 53). H. polygyrus infections stimulate host immune responses with an accompanying inflammation of mucosal tissue allowing persistence of infections for >30 weeks in certain strains of mice (48).
Mice also use nonvolatile odor cues provided by another highly polymorphic gene complex, that of the major urinary proteins (MUPs) for individual recognition (11, 12). The MUPs bind and release components that can be used for individual recognition with either the proteins themselves and/or protein–ligand complexes providing odor cues about identity and, possibly, condition (12).
This odor information regarding the condition and the identity of the scent owner (male and female odors) is assessed by the accessory olfactory system and vomeronasal organ as well as by the main olfactory systems and conveyed to the amygdala (14). OT in the medial amygdala has been shown to be critical for social recognition in male and female mice and, as such, a target for the altered responses to the odors of infected males in the OTKOs. These effects of OT on social behavior and its’ action at the level of amygdala have been further associated with estrogenic mechanisms and genes for estrogen receptors (33, 38). OT at the level of amygdala provides a target at which social information can be integrated and assessed. Together, these findings indicate that the gene for OT is not only associated with the use of direct social information (31, 32, 54), but also with the utilization of ISI and the processing and integration of social information in a broader context.
Materials and Methods
Animals.
Outbred CF-1 male and sexually naïve female mice (2–3 months of age, 25–30 g) were individually housed in clear polyethylene cages with a wood shavings bedding at 20 ± 2°C under a 12 h light/12 h dark cycle (lights off at 10:00 a.m.). Food (Purina Mouse Chow) and tap water were available ad libitum.
OTKO Mice.
Sexually naïve, gonadally intact female OTKO mice and their wild-type (OTWT) littermates of the same genetic background (8–10 months of age, 25–30 g) were obtained from the colony maintained at The Rockefeller University by mating heterozygous male and female mice whose offspring were genotyped by PCR amplification of tail DNA. Original breeding pairs (mixed background of 129/Sv and Black Swiss) were obtained from Washington University School of Medicine (55). Sibling groups of pathogen-free female mice were housed in groups of four to six in polyethylene cages (26 × 16 × 12 cm) under a 12 h light/12 h dark cycle (lights off at 10:00 a.m.) at 20 ± 2°C. Four to five days before testing, mice were individually housed in a similar manner. All procedures were approved by, and conducted in accordance with, The Institutional Animal Care and Use Committee of The Rockefeller University.
Parasitized Males.
Male CF-1 mice (2–6 months of age) were infected by oral intubation with the nematode, H. polygyrus, under light anesthesia. Each infected mouse received ≈200 infective H. polygyrus larvae (L3) suspended in 0.50 ml of water. Infection was confirmed 40 days later. Infected mice showed no signs of malaise or pathology at any stage of infection. Although the dose of L3 larvae used here is considered sufficient to elicit immune depression in male mice (48), no behavioral responses associated with “sickness” behaviors were evident. Resistant stages of this gastrointestinal nematode are shed in the feces of infected hosts, and after 6–9 days they are infective to other mice that acquire them during feeding, grooming, and other social interactions (26, 28).
Urine Collection.
During the early light period, individual infected (26 days after infection) and uninfected male and CF-1 female mice were gently held by the scruff of the neck to stimulate urine production (0.2–0.3 ml). The urine from individual mice was evenly collected onto filter papers (Whatman no. 5) and frozen immediately at −18°C until later use. There were no significant differences in the volumes of urine produced by infected and uninfected mice.
Effects of Female Odor on Initial Odor Choices.
In the mid-dark period, individual OTWT, OTKO, and CF-1 female mice (n = 30) were placed in a clean cage (25 × 15 × 20 cm) in which a vented Plexiglas tube (10 cm in length, 3 cm in diameter, sealed at each end with plastic mesh) was placed. The mesh allowed females to have nasal contact with both volatile and nonvolatile urine odor components but prevented chewing of the odor sources. Each end of the tube contained a 2-cm2 section of filter paper with a different odor source. The first odor to which a female went to, and remained at, for a minimum of 10 s was considered to be the initial odor choice (26). Females generally took 20–30 s to make their initial choice.
Responses to Uninfected Males.
The odor choices provided on day 1 to OTWT, OTKO, and CF-1 females were: (i) male A vs. male B; (ii) male A + estrous female vs. male B; (iii) male A + nonestrous female vs. male B (for CF-1 females only) (listed in Table 1). Twenty-four hours later (day 2), the initial odor choices of the females were again determined, without any accompanying female odor in the stimulus odor pairs. The male odor sources “A” and “B” came from equivalent uninfected males.
The responses of females to the odors of sexually simulated and unstimulated males were also examined. Males were sexually stimulated by exposing them overnight (16–18 h) to filter paper (3 × 3 cm) containing estrous female urine (5.0 μl), which induces a reliable increase in testosterone levels (30, 56). On day 1, the CF-1, OTWT, and OTKO females (n = 30 for each genotype) were presented with the odors of: (i) sexually stimulated male vs. unstimulated male; (ii) sexually stimulated male vs. unstimulated male + estrous female; (iii) sexually stimulated male vs. unstimulated male + nonestrous female (CF-1 females only) (listed in Table 2). Twenty-four hours later (day 2), the initial odor choices were again determined with the day 1 groups being subdivided into two experimental groups (n = 15 in each of the groups). The stimulus odor pairs for these groups on day 2 were from an unstimulated male [familiar (day 1) or novel] and a sexually stimulated male. Estrous state of the females was determined on each day after the initial choices were made. The choice tube was washed thoroughly with hot water and unscented soap (Alconnox, New York) between trials.
Responses to Parasitized Males.
The odor sources provided for the CF-1 female mice were: (i) infected male vs. uninfected male; (ii) infected male + estrous female vs. uninfected male; (iii) infected male vs. uninfected male + estrous female; (iv) infected male vs. blank (clean filter paper); (v) infected male + estrous female vs. blank; (vi) infected male + estrous female vs. blank + estrous female (Fig. 1). The odor sources for OTKO and OTWT female mice (n = 30; day 1) were: (i) infected male vs. uninfected male; (ii) infected male + estrous female vs. uninfected male; and (iii) infected male + nonestrous female vs. uninfected male (described in Fig. 2).
Twenty-four hours later (day 2), the initial odor choices of the females were redetermined with day 1 groups being subdivided into two experimental groups (n = 15 in each group). The stimulus odor pairs for these groups on day 2 were from infected males (familiar or novel) and either the original (familiar) or a different (novel) uninfected male.
Additional control determinations were made of the responses of CF-1 females to the odors of: (i) infected male + nonestrous female vs. uninfected male; (ii) infected male vs. uninfected male + nonestrous female; (iii) infected male vs. blank + estrous female; (iv) uninfected male + estrous female vs. blank; (xi) blank vs. blank. Estrous state of the females was determined after the initial odor choices were made.
Female Odors and Nociceptive Responses to Parasitized Males.
In this case each end of the tube contained the same odor. For CF-1, OTWT, and OTKO females, the odor exposures were: (i) uninfected male; (ii) infected male; (iii) infected male + estrous female; (iv) infected male + nonestrous female. CF-1 females were additionally exposed to the odors of: (v) uninfected male + nonestrous female; (vi) uninfected male + nonestrous female; (vii) blank (clean filter paper) + estrous female; (viii) blank (clean filter paper) + nonestrous female. Before (15 min), and as established (24), immediately after exposure to the odors for 1 min, the nociceptive responses of each female (n = 10, for each odor) were determined. Individual mice were placed on a metal surface (analgesiometer; Accu-Scan, Columbus, OH) maintained at 50 ± 0.5°C and the latency of a front foot-lifting response was recorded, after which the mouse was quickly removed and returned to her home cage.
Female Odors and Corticosterone Responses.
CF-1 females (n = 10) were exposed for 1 min to the preceding odors. Fifteen minutes after odor exposures, mice were killed by cervical dislocation, trunk blood was collected on ice and then centrifuged at 14,000 rpm (1,800 × g) for 10 min, and the resulting plasma was stored at –70°C until time of assay. Plasma samples (100:l) from the female mice were assayed in duplicate for corticosterone using commercially available 125I-labeled RIA kits (Coat-a-Count; Diagnostic Products, Los Angeles). The sensitivity of the assay was calculated to be 10 ng/ml, and the intrassay coefficient of variation, measured in triplicate from low, medium, and high pools, ranged from 4 to 12%.
Data Analyses.
Logistic regression analysis and χ2 analysis were used to determine whether a female’s initial odor choice was influenced by the presence of the odor of another female. The Hosmer–Lemeshow test was used to assess the goodness of fit of the logistic model, fitted by using the SAS Logistic procedure (57). Significance levels of the various regression coefficients and the effects of odor condition were obtained by comparing the change in deviance (upon adding a term to the regression model) with the χ2 distribution on 1 df. Nociceptive response values were log transformed before analysis by analysis of variance (ANOVA) and Tukey’s studentized range test. Hormonal values were square root transformed before analysis by ANOVA. A 0.05 level of significance was used throughout.
Acknowledgments
This work was supported by Agriculture and Agri-Food Canada (D.D.C.), The Natural Sciences and Engineering Research Council of Canada (M.K., W.J.B., and E.C.), and National Institute of Mental Health Grants MH38273 (to D.W.P.) and MH62147 (to S.O.).
Abbreviations
- ISI
inadvertent social information
- OT
oxytocin
- OTKO
OT knockout
- OTWT
OT wild type.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Danchlin E., Giraldeau L.-A., Valone T. J., Wagner R. H. Science. 2004;305:487–491. doi: 10.1126/science.1098254. [DOI] [PubMed] [Google Scholar]
- 2.Valone T. J., Templeton J. T. Philos. Trans. R. Soc. London B. 2002;357:1549–1553. doi: 10.1098/rstb.2002.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson R. M., Hoglund J. Trends Ecol. Evol. 1992;7:229–232. doi: 10.1016/0169-5347(92)90050-L. [DOI] [PubMed] [Google Scholar]
- 4.Pruett-Jones S. G. Am. Nat. 1992;42:10000–10009. doi: 10.1086/285452. [DOI] [PubMed] [Google Scholar]
- 5.Dugatkin L. A. Proc. Natl. Acad. Sci. USA. 1996;93:2770–2773. doi: 10.1073/pnas.93.7.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galef B. J., Jr., White D. J. Anim. Behav. 1998;55:545–552. doi: 10.1006/anbe.1997.0616. [DOI] [PubMed] [Google Scholar]
- 7.Witte K., Ryan M. J. Behav. Ecol. 1998;9:534–539. [Google Scholar]
- 8.Dugatkin L. A., Godin J.-G. J. Proc. R. Soc. London Ser. B; 1992. pp. 179–184. [Google Scholar]
- 9.Clutton-Brock T. H., McComb K. Behav. Ecol. 1993;4:191–193. [Google Scholar]
- 10.Hurst J. L. Anim. Behav. 1990;40:223–232. [Google Scholar]
- 11.Hurst J. L., Payne C. E., Nevison C. M., Maries A. D., Humphries R. E., Robertson D. H. L., Cavaggioni A., Beynon R. J. Nature. 2001;414:631–634. doi: 10.1038/414631a. [DOI] [PubMed] [Google Scholar]
- 12.Beynon R. J., Hurst J. L. Biochem. Soc. Trans. 2003;31:142–146. doi: 10.1042/bst0310142. [DOI] [PubMed] [Google Scholar]
- 13.Penn D., Potts W. K. Trends Ecol. Evol. 1998;13:391–396. doi: 10.1016/s0169-5347(98)01473-6. [DOI] [PubMed] [Google Scholar]
- 14.Dulac C., Torello T. Nat. Rev. Neurosci. 2003;4:551–562. doi: 10.1038/nrn1140. [DOI] [PubMed] [Google Scholar]
- 15.Gosling L. M., Roberts S. C. Ad. Study Behav. 2001;30:169–217. [Google Scholar]
- 16.Maruniak J. A., Owen K., Bronson F. H., Desjardins C. Behav. Biol. 1975;13:211–217. doi: 10.1016/s0091-6773(75)91920-3. [DOI] [PubMed] [Google Scholar]
- 17.Palanza P., Parmigiani S., vom Saal F. S. Anim. Behav. 1994;48:245–247. [Google Scholar]
- 18.Møller A. P., Dufva R., Allander K. Adv. Study Behav. 1993;22:65–102. [Google Scholar]
- 19.Altizer S., Nunn C. L., Dobson A. P., Ezenwa V., Jones K. E., Pedersen A. B., Poss M., Pulliam S.C. Ann. Rev. Ecol. Syst. 2003;34:517–547. [Google Scholar]
- 20.Hamilton W.D., Zuk M. Science. 1982;218:384–387. doi: 10.1126/science.7123238. [DOI] [PubMed] [Google Scholar]
- 21.Milinski M., Bakker T. C. Nature. 1990;344:330–333. [Google Scholar]
- 22.Wedekind C. Proc. R. Soc. London Ser. B; 1992. pp. 169–174. [Google Scholar]
- 23.Kavaliers M., Colwell D. D. Proc. R. Soc. London Ser. B; 1995. pp. 31–35. [Google Scholar]
- 24.Kavaliers M., Colwell D. D. Anim. Behav. 1995;50:1161–1169. [Google Scholar]
- 25.Penn D., Schneider G., White K., Slev P., Potts W. Ethology. 1998;104:685–694. [Google Scholar]
- 26.Kavaliers M., Colwell D. D., Braun W. J., Choleris E. Anim. Behav. 2003;65:59–68. [Google Scholar]
- 27.Kavaliers M., Colwell D. D., Choleris E., Agmo , Muglia L. J., Ogawa S., Pfaff D. W. Genes Brain Behav. 2003;2:220–230. doi: 10.1034/j.1601-183x.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- 28.Kavaliers M., Choleris E., Agmo A., Muglia L. J., Ogawa S., Pfaff D. W. Anim. Behav. 2005;70:693–702. [Google Scholar]
- 29.Ehman K. D., Scott M. E. Anim. Behav. 2001;62:781–789. [Google Scholar]
- 30.Zala S. M., Potts W. K., Penn D. J. Behav. Ecol. 2004;15:338–344. [Google Scholar]
- 31.Ferguson N. J., Young L., Insel T. Front. Neuroendocrinol. 2002;2:220–226. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- 32.Winsolow J. T., Insel T. R. Curr. Biol. 2004;14:1–6. [Google Scholar]
- 33.Choleris E., Kavaliers M., Pfaff D. W. J. Neuroendocrinol. 2004;16:383–389. doi: 10.1111/j.0953-8194.2004.01178.x. [DOI] [PubMed] [Google Scholar]
- 34.Carter S. C. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 35.Pedersen C. A., Boccia L. M. Horm. Behav. 2002;41:170–177. doi: 10.1006/hbeh.2001.1736. [DOI] [PubMed] [Google Scholar]
- 36.Ferguson J. N., Adag J. M., Insel T. R., Young L. J. J. Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferguson J. N., Young L. J., Hearn E. F., Matzuk M. M., Insel T. R. Nat. Genet. 2001;25:284–287. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 38.Choleris E., Gustafsson J.-A., Korach K. S., Muglia L. J., Pfaff D. W., Ogawa S. Proc. Natl. Acad Sci. USA. 2003;100:6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halpin Z. T. Adv. Study Behav. 1986;16:39–70. [Google Scholar]
- 40.Lai W.-S., Ramiro L.-L. R., Yu H. A., Johnston R. E. J. Neurosci. 2005;25:11239–11247. doi: 10.1523/JNEUROSCI.2124-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krackow S., Matuschak M. Ethology. 1991;88:99–108. [Google Scholar]
- 42.Egid K., Brown J. L. Anim. Behav. 1989;38:448–450. [Google Scholar]
- 43.Wagner W. E., Jr Anim. Behav. 1998;55:1029–1042. doi: 10.1006/anbe.1997.0635. [DOI] [PubMed] [Google Scholar]
- 44.Drickamer L. C., Gowaty P. A., Holmes C. M. Anim. Behav. 2000;59:371–378. doi: 10.1006/anbe.1999.1316. [DOI] [PubMed] [Google Scholar]
- 45.Westneat D. F., Walters A., McCarthy T. H., Hatch M. I., Hein M. K. Anim. Behav. 2000;59:467–478. doi: 10.1006/anbe.1999.1341. [DOI] [PubMed] [Google Scholar]
- 46.Swaddle J. P., Cathey M. G., Correll M., Hodkinson B. P. Proc. R. Soc. London Ser. B; 2005. pp. 1053–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Getty T. Am. Nat. 2002;199:363–371. doi: 10.1086/338992. [DOI] [PubMed] [Google Scholar]
- 48.Barnard C. J., Behnke J. M., Gage A. R., Brown H., Smithurst P. R. Proc. R. Soc. London Ser. B; 1998. p. 69300701. [Google Scholar]
- 49.Kostfeld M., Heinrichs M., Zak P. J., Fischbacher U., Fehr E. Nature. 2005;435:673–675. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 50.Yammazaki K., Boyse E. A., Mike V., Thaler H. T., Mathieson B. J., Abbot J., Boyse J., Zayas Z. A., Thomas L. J. Exp. Med. 1976;144:1324–1335. doi: 10.1084/jem.144.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Potts W. K., Manning C. J., Wakeland E. K. Philos. Trans. R. Soc. London B. 1994;346:369–378. doi: 10.1098/rstb.1994.0154. [DOI] [PubMed] [Google Scholar]
- 52.Singer A. G., Beauchamp G. K., Yamazaki K. Proc. Natl. Acad. Sci. USA. 1993;94:2210–2214. doi: 10.1073/pnas.94.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh P. B. Reproduction. 2001;121:529–539. doi: 10.1530/rep.0.1210529. [DOI] [PubMed] [Google Scholar]
- 54.Insel T. R., Fernald R. D. Annu. Rev. Neurosci. 2004;42:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- 55.Gross G. A., Imamura T., Luedke C., Vogt S. K., Olson L. M., Nelson D. M., Sadovsky Y., Muglia L. J. Proc. Natl. Acad. Sci. USA. 1998;95:11875–11879. doi: 10.1073/pnas.95.20.11875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kavaliers M., Choleris E., Colwell D. D. Horm. Behav. 2001;40:497–509. doi: 10.1006/hbeh.2001.1714. [DOI] [PubMed] [Google Scholar]
- 57.Homer D., Jr., Lemeshow S. Applied Logistic Regression. New York: Wiley; 1989. [Google Scholar]