Abstract
Voltage-gated Ca2+ channels in arterial myocytes can mediate Ca2+ release from the sarcoplasmic reticulum and, thus, induce contraction without the need of extracellular Ca2+ influx. This metabotropic action of Ca2+ channels (denoted as calcium-channel-induced calcium release or CCICR) involves activation of G proteins and the phospholipase C-inositol 1,4,5-trisphosphate pathway. Here, we show a form of vascular tone regulation by extracellular ATP that depends on the modulation of CCICR. In isolated arterial myocytes, ATP produced facilitation of Ca2+-channel activation and, subsequently, a strong potentiation of CCICR. The facilitation of L-type channel still occurred after full blockade of purinergic receptors and inhibition of G proteins with GDPβS, thus suggesting that ATP directly interacts with Ca2+ channels. The effects of ATP appear to be highly selective, because they were not mimicked by other nucleotides (ADP or UTP) or vasoactive agents, such as norepinephrine, acetylcholine, or endothelin-1. We have also shown that CCICR can trigger arterial cerebral vasoconstriction in the absence of extracellular calcium and that this phenomenon is greatly facilitated by extracellular ATP. Although, at low concentrations, ATP does not induce arterial contraction per se, this agent markedly potentiates contractility of partially depolarized or primed arteries. Hence, the metabotropic action of L-type Ca2+ channels could have a high impact on vascular pathophysiology, because, even in the absence of Ca2+ channel opening, it might mediate elevations of cytosolic Ca2+ and contraction in partially depolarized vascular smooth muscle cells exposed to small concentrations of agonists.
Keywords: L-type Ca2+ channel, vascular contractility, excitation–contraction coupling
Contraction of vascular smooth muscle cells (VSMCs) determines vessel diameter and, thus, regulates blood flow and pressure. VSMC contraction is triggered by a rise of cytosolic calcium ion concentration ([Ca2+]) due to either transmembrane Ca2+ influx or Ca2+ release from the sarcoplasmic reticulum (SR). Extracellular Ca2+ entry normally occurs through L-type voltage-dependent Ca2+ channels, although there are numerous ligand-gated membrane channels that are also Ca2+ permeable (1). Ca2+ release from the SR is mediated by inositol trisphosphate (InsP3) and ryanodine receptors. Vasoactive agents acting on the different vascular territories induce VSMC contraction by binding to membrane receptors and the subsequent activation of one or more of the Ca2+-entry and/or Ca2+-release channels (2–5).
We have described a previously unnoticed metabotropic effect of vascular L-type Ca2+ channels, denoted as calcium-channel-induced Ca2+ release (CCICR), which requires Ca2+ channel activation but is independent of extracellular Ca2+ influx (6). This new Ca2+-release mechanism depends on the conformational change of L-type Ca2+ channels and the downstream activation of the G protein/phospholipase C (PLC) cascade, leading to synthesis of InsP3 and Ca2+ release from the SR (6, 7). Although CCICR can be triggered either by direct membrane depolarization in patch-clamped myocytes or upon exposure of the cells to a high-K+ solution (6), its actual physiological significance is unknown. This research was undertaken to study the possible synergistic interaction between CCICR and vasoactive agents and to evaluate the impact of CCICR on arterial contractility. It had been reported in several cell types (including arterial myocytes) that agonist-induced Ca2+ release from internal stores can be modulated by the cell’s membrane potential. However, this phenomenon was not associated with L-type Ca2+ channel activation but was attributed to the existence of a voltage-dependent step in the PLC-activation/InsP3-synthesis pathway (8–10). Here, we report that CCICR is, indeed, selectively regulated by low concentrations of extracellular ATP, an agent coreleased with norepinephrine (NE) from sympathetic terminals and from cells (erythrocytes and platelets) damaged during vascular injury (11–13). Moreover, CCICR can produce arterial cerebral vasoconstriction in the absence of extracellular Ca2+ influx, and this effect is greatly potentiated by subthreshold concentrations of ATP. Unexpectedly, ATP directly modifies L-type Ca2+ channel gating and, thus, increases both Ca2+-current amplitude and CCICR. This metabotropic action of L-type Ca2+ channels could be of high relevance for vascular pathophysiology because, even in the absence of Ca2+ channel opening, it might mediate elevations of cytosolic Ca2+ and protracted contraction in partially depolarized VSMCs exposed to small concentrations of agonists.
Results
Extracellular ATP Potentiates Ca2+ Channel-Induced Ca2+ Release.
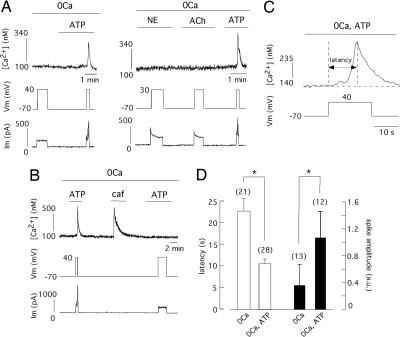
Voltage-gated Ca2+ channels of VSMCs in the different vascular territories can be regulated by numerous vasomotor agents (2, 14–17); hence, we sought synergistic interactions between some of these agents and CCICR. In voltage-clamped myocytes bathed in Ca2+-free medium that responded to step membrane depolarization with only a small increase or no change of cytosolic [Ca2+], the presence of extracellular ATP resulted in a marked augmentation of CCICR amplitude (Fig. 1A Left). Cells selected for these experiments were either unresponsive to ATP (10–100 μM) or showed only a minor Ca2+ response to the nucleotide at the holding potential of −70 mV. Application in the same cells of NE or acetylcholine (ACh), agents without vasoconstrictive action on the basilar artery (18, 19), failed to produce any effect on CCICR (n = 9) (Fig. 1A Right). Both the depolarization-induced Ca2+ signal and its potentiation by extracellular ATP disappeared when the SR Ca2+ store was depleted by the addition of caffeine (Fig. 1B). Blockade of the G protein/PLC cascade with intracellular GDPβS (1 mM), which completely suppresses CCICR (6), also abolished the potentiation by ATP (n = 4 cells tested). CCICR signals evoked by step depolarizations appeared with a latency of several seconds, probably reflecting the time required to synthesize sufficient InsP3 to release Ca2+ from the SR (Fig. 1C). This latency (measured as the time to reach the peak of the Ca2+ signal) was voltage-dependent and varied from 15.9 ± 2 s (n = 7) for small depolarizations (from −70 mV to membrane voltages between −30 and −10 mV) to 8.5 ± 1 s, (n = 12) for larger depolarizations (from −70 mV to potentials between +30 and +50 mV) (P < 0.05). Exposure to ATP significantly reduced the duration of the latency and increased the amplitude of the Ca2+ spike (Fig. 1D), thus further suggesting potentiation of CCICR by ATP.
Fig. 1.
Potentiation of Ca2+ channel-induced Ca2+ release (CCICR) by extracellular ATP. (A Left) Ca2+ release evoked by the application of depolarizing pulses from −70 mV to +40 mV in a voltage-clamped myocyte bathed in Ca2+-free solution. The amplitude of the Ca2+ signal increased in the presence of ATP (10 μM). (A Right) Lack of effect of NE (10 μM) and ACh (10 μM) on Ca2+ release. (B) Abolishment of CCICR after the intracellular reservoirs are depleted by addition of caffeine (5 mM). The fluorimetric Ca2+ signals are accompanied by the corresponding membrane voltage (Vm, mV) and holding current (Im, pA) recordings. (C) Ca2+ signal in response to a depolarizing pulse shown at an expanded time scale to illustrate the delay (latency) between the change in the membrane potential and the maximum value of the increase in the cytosolic [Ca2+]. (D) Effect of ATP on the latency (open bars) and amplitude (filled bars, arbitrary units) of depolarization-induced Ca2+ spikes. ∗, P < 0.05. External solution: nominal 0Ca2+ plus 2 mM EGTA added. The number of cells studied is indicated in parentheses. a.u., arbitrary units; caf, caffeine.
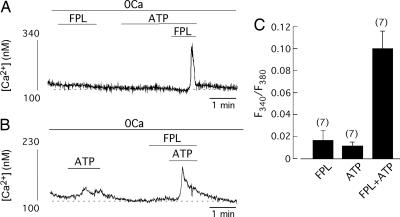
Because, in the absence of membrane depolarization, CCICR can be triggered by pharmacological activation of Ca2+ channels with 2,5-dimethyl-4-[2-(phenylmethyl)benzoil]-1H-pyrrole-3-carboxylic acid methyl ester (FPL)-64176, a well known Ca2+ channel agonist (20–22), we tested to see the interaction between FPL and ATP. In intact cells where FPL or ATP alone evoked only small Ca2+ signals, simultaneous exposure to both agents markedly increased the amplitude of the Ca2+ spikes independently of their order of application (Fig. 2A–C). Therefore, these data indicate that ATP potentiates CCICR evoked by either step membrane depolarization or FPL.
Fig. 2.
Synergistic interaction of extracellular ATP and FPL-induced CCICR. (A and B) Measurement of Ca2+ release evoked by the exposure to FPL (2.5 μM) and/or ATP (25 μM). The amplitude of Ca2+ spikes markedly increased when both agonists were present simultaneously. (C) Summary of the effects of ATP and FPL on cytosolic [Ca2+] in undialyzed myocytes in a 0Ca solution containing 2 mM EGTA. The number of cells studied is indicated in parentheses.
Direct and Selective Activation of L-Type Ca2+ Channels by ATP in Arterial Myocytes.
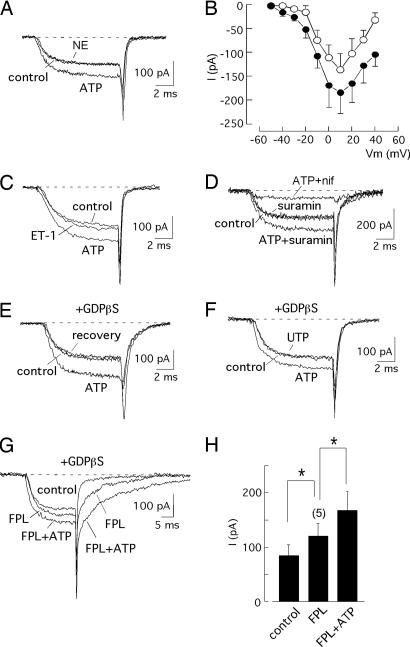
Because CCICR requires Ca2+ channel activation, we investigated the effect of ATP on the macroscopic voltage-dependent Ca2+ currents recorded in patch-clamped myocytes. These currents were unaffected by NE (10 μM, n = 7) (Fig. 3A) or ACh (10 μM, n = 6), agents that, as shown above (see Fig. 1A), did not influence the CCICR signals (Table 1). However, in the same cells, ATP (10 μM) elicited an increase in the amplitude of the Ca2+ currents of ≈30–40% in the entire range of membrane potentials (Fig. 3 A and B). At +20 mV, the peak Ca2+ current increased significantly in the presence of ATP and was almost completely blocked by nifedipine (Table 1). Besides the increase in the Ca2+ conductance, ATP accelerated activation and slowed inactivation and closing kinetics (see Fig. 5, which is published as supporting information on the PNAS web site). To further evaluate the specificity of the effect of ATP on rat basilar myocytes, we tested the action of endothelin-1 (ET-1), an efficacious constrictor in the basilar artery (23, 24). ET-1 had no significant effect on the amplitude of the macroscopic Ca2+ current (Fig. 3C). Peak Ca2+ current amplitude recorded during depolarizations to +20 mV before and after application of ET-1 (5–10 nM) were 99.6 ± 16.9 pA and 98.8 ± 19.8 pA, respectively (n = 5). In these same cells, current amplitude significantly increased in the presence of ATP (10 μM; 170.9 ± 26.9 pA). To distinguish between a possible direct effect of ATP on Ca2+ channels and the indirect modulation mediated by activation of metabotropic P2Y receptors, we performed experiments using the ATP-receptor antagonist suramin (25 μM). This concentration was sufficient to block 93 ± 5% of the Ca2+-release signal in intact cells (n = 22), where ATP (10 μM) produced a large Ca2+ transient. Nevertheless, suramin neither had an effect on the amplitude of the Ca2+ current nor prevented the increase of current amplitude induced by ATP (Fig. 3D and Table 1). Moreover, simultaneous addition of suramin and pyridoxal phosphate 6-azophenyl-2′,4′-disulfonic acid (another P2 antagonist) (17) yielded similar results (see Fig. 6A, which is published as supporting information on the PNAS web site). The reversible augmentation of Ca2+ current amplitude by ATP occurred in cells dialyzed with 1 mM GDPβS (Fig. 3E, Table 1) and was not mimicked by either other adenine nucleotides (ADP) or the P2Y receptor agonist UTP (Fig. 3F, Table 1). However, the nonhydroxylable analogue ATPγS produced a current potentiation similar to ATP (see Fig. 6B). As expected, ATP markedly enhanced the FPL-induced activation of Ca2+ channels (Fig. 3 G and H). These data strongly suggest that, in basilar myocytes, ATP enhances Ca2+ current amplitude through direct interaction with the channels, thus explaining the potentiation of CCICR described in the preceding section. This effect of ATP on vascular L-type Ca2+ channels does not seem to be restricted to basilar myocytes, because it was also observed with similar characteristics (≈40% increase of peak Ca2+-current amplitude at +20 mV, n = 5) in experiments performed on dispersed pulmonary artery myocytes.
Fig. 3.
Activation of Ca2+ channels by ATP in voltage-clamped myocytes. (A) Increase in Ca2+ current by external application of ATP (10 μM) and lack of effect of NE (10 μM). (B) Current amplitude (measured at the end of depolarizing pulses) as a function of the membrane potential (Vm) in control myocytes (open symbols) and after exposure to ATP for 2–4 min (filled symbols). Each point is the mean ± SE of measurements done in five myocytes. (C) Increase in Ca2+ current by external application of ATP (10 μM) and lack of effect of endothelin-1 (10 nM). In most experiments, NE and endothelin-1 were applied for at least 3 min. (D) Increase of Ca2+-current amplitude by ATP in the presence of suramin (25 μM). The Ca2+ current was unaffected by suramin but was almost completely abolished by nifedipine (1 μM). (E) Reversible potentiation of Ca2+ current by ATP in myocytes internally dialyzed by GDPβS (1 mM) to block G protein. (F) The effect of ATP on Ca2+ current is not mimicked by the P2-receptor agonist UTP (10 μM). (G) ATP (10 μM) markedly enhanced the effect of FPL (50 nM) on Ca2+ currents in patch-clamped myocytes. All current traces were obtained by depolarization to +20 mV from −70 mV. (H) Summary of ATP and FPL effects on Ca2+-current amplitude. ∗, P < 0.05. The number of cells studied is indicated in parentheses.
Table 1.
Regulation of L-type Ca2+-channel activity by vasoactive agents
| Internal Cs solution | Control | ACh (10 μM) | NE (10 μM) | ATP (10 μM) | Suramin (25 μM) | ATP + suramin | ATP + nifed (1 μM) | UTP (10 μM) | ADP (10 μM) |
|---|---|---|---|---|---|---|---|---|---|
| Control | 141 ± 23 | 184 ± 22* | 29.8 ± 4.7* | ||||||
| n = 12 | |||||||||
| Control | 107.5 ± 15.3 | 104.7 ± 14.4 | 131 ± 19* | ||||||
| n = 9 | |||||||||
| Control + GDPβS | 177.1 ± 26 | 235.9 ± 30* | |||||||
| n = 13 | |||||||||
| Control + GDPβS | 184.6 ± 35.7 | 181 ± 36 | |||||||
| n = 6 | |||||||||
| Control + GDPβS | 123.86 ± 45 | 126.69 ± 44.7 | |||||||
| n = 7 | |||||||||
| Control + GDPβS | 87 ± 11.2 | 94.36 ± 12.3 | 139.3 ± 14* | ||||||
| n = 6 | |||||||||
| Control + GDPβS | 107.13 ± 13.3 | 103.36 ± 14.4 | 141.4 ± 13.4* | ||||||
| n = 7 |
The data show the effect of ACh, NE, ATP, UTP, ADP, nifedipine, and suramin (added to the external solution; concentrations are in parentheses) and GDPβS (1 mM; added to the internal solution) on macroscopic Ca2+ currents (expressed in pA as mean ± SE). Experiments were done in voltage-clamped myocytes, and current was measured at the end of the depolarizing pulses to +20 mV from a holding potential of −70 mV. Asterisks indicate statistically significant differences with respect to the values obtained with control solutions. nifed, nifedipine.
Extracellular ATP Potentiates Contraction Induced by CCICR in Intact Arterial Rings.
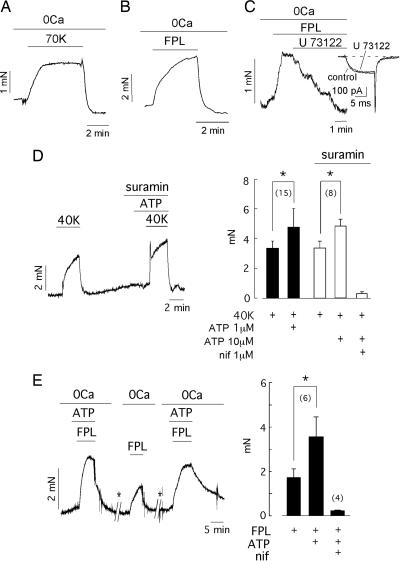
Because CCICR can induce contraction in isolated basilar myocytes (6), we studied whether the metabotropic activation of Ca2+ channels can, indeed, produce contraction in intact arterial rings and whether ATP can modulate the CCICR-dependent vasomotor responses. Exposure of basilar arterial rings to a depolarizing solution with 70 mM K+ produced a powerful and reversible contractive response (7.02 ± 0.37 mN, n = 8). Although it is generally thought that much of the K+-induced contractility depends on extracellular Ca2+ influx, it is noteworthy that the response to high K+ was not completely abolished by using external solutions without Ca2+ plus 0.2–0.5 mM EGTA (0Ca solution). In these conditions, depolarization with 70 mM K+ produced an appreciable vasoconstriction of ≈1/3 the amplitude of that produced with normal extracellular [Ca2+] (2.4 ± 0.5 mN, n = 9) (Fig. 4A). To ascertain what signal (either membrane depolarization per se or Ca2+ channel activation) triggers SR Ca2+ release and contraction of basilar arterial rings, we performed experiments with FPL. Pharmacological activation of Ca2+ channels with FPL elicited a powerful contraction of arterial rings (7.01 ± 1.9 mN, n = 21), which was partially preserved (2.85 ± 0.38 mN, n = 23) after removal of extracellular Ca2+ (Fig. 4B). Interestingly, the FPL-induced arterial contraction almost disappeared (≈95% inhibition, n = 5) in the presence of U73122 (1–5 μM), a selective blocker of PLC (25) without effects on Ca2+ currents (Fig. 4C). The isoform U73433, inactive on the enzyme, did not alter the FPL-dependent contraction. These observations indicate that, in the absence of extracellular Ca2+ influx, FPL can produce vasoconstriction due to Ca2+ release from internal stores. Because FPL activates Ca2+ channels without primarily altering the membrane potential, the data strongly suggest that it is Ca2+ channel activation, independent of any change in membrane voltage, that induces Ca2+ release and vasoconstriction.
Fig. 4.
CCICR-induced contraction in intact arterial rings and potentiation by external ATP. (A) Contractive response of a basilar arterial ring exposed to 70 mM K+ in the absence of extracellular Ca2+ (Ca2+-free external solution plus 500 μM EGTA added). (B) FPL (1 μM)-induced contraction in the absence of extracellular Ca2+ plus 500 μM EGTA added. (C) Suppression of contraction evoked with FPL (1 μM) by application of an inhibitor of PLC (U73122, 2 μM). (Inset) The Ca2+ current (generated by depolarization to +20 mV from −70 mV) is not significantly affected by the presence of U73122 (10 μM). (D Left) Low concentrations of ATP (1 μM) markedly induced a potentiation of vasoconstriction induced by 40 mM K+ in the presence of the ATP-receptor antagonist suramin (25 μM). (D Right) Comparison of vasoconstriction in arteries exposed to 40 mM K+ and ATP (1 μM, filled bars; 10 μM, open bars) and suramin. (E Left) In 0Ca solutions, ATP (10 μM) markedly increased isometric force induced by FPL (10 μM). During the time indicated by the asterisks, intracellular stores were refilled by application of 40 mM K+ solution in the presence of Ca2+. (E Right) Summary of ATP (1–10 μM) and nifedipine (0.5–2 μM) effects on isometric force developed in arterial rings exposed to FPL (1–20 μM). In all cases, the 0Ca solution contained 200–500 μM EGTA. ∗, P < 0.05. The number of arterial rings studied is indicated in parentheses.
Given that a significant fraction of L-type Ca2+ channel-dependent contraction in arterial myocytes seems to be mediated through CCICR, we investigated, in arterial rings, the interaction between ATP and CCICR described in isolated myocytes. First, we analyzed the contractive responses of arterial rings exposed to low concentrations of ATP (1–10 μM) and 40 mM K+. It was tested before that, with this concentration of extracellular K+, the membrane potential of isolated patch-clamped myocytes was depolarized to −25.4 ± 5.2 mV (n = 5) and that, at high concentrations, ATP contracted arterial rings (2.9 ± 1.6 mN; n = 47). Although low concentrations of ATP (1 μM) generally failed to evoke any arterial-ring contraction, the isometric force induced by 40 K+ (3.3 ± 0.5 mN) was significantly increased (4.9 ± 0.56 mN, n = 15) in the presence of ATP, even after purinergic-receptor blockade with suramin (Fig. 4D). Note that there was no statistically significant difference between the contraction induced by 40 K+ in the presence and absence of suramin. These experiments on arterial rings indicate that, in fair agreement with the data in single cells, isometric force elicited by 40 K+-induced depolarization is markedly potentiated by small concentrations of ATP, below those required to evoke vasoconstriction.
Because ATP can enhance the FPL-dependent potentiation of both Ca2+ currents and Ca2+ release in isolated myocytes (see Figs. 2 and 3), we also analyzed the interaction between these agents using arterial rings. In the absence of extracellular Ca2+, ATP reversibly increased the vasoconstriction induced by FPL (Fig. 4E). This additive effect of FPL and ATP was observed even with concentrations of the two agents below those required to induce a contractive response separately. In contrast, vasoconstriction induced by FPL was not potentiated by the simultaneous addition of small concentrations of ET-1 (data not shown), which is compatible with the lack of effect of ET-1 on the macroscopic Ca2+ current in basilar myocytes (see Fig. 3C). Because FPL induced Ca2+ release in the absence of membrane depolarization (6), these data further support the view that ATP can potentiate FPL-induced CCICR and, thus, arterial ring contraction without the need of large changes in membrane voltage.
Discussion
The main observations in this study are: (i) CCICR is markedly and selectively increased by extracellular ATP; (ii) the effect of ATP is mediated through the direct activation of Ca2+ channels, leading to Ca2+ release from the SR and vasoconstriction; (iii) CCICR, a phenomenon studied before in isolated arterial myocytes (6), can elicit contraction in arterial basilar rings in the absence of extracellular Ca2+ and is potentiated by ATP; and (iv) the interaction among ATP, membrane depolarization, and CCICR represents a new form of eliciting arterial-myocyte contraction, even in the absence of Ca2+ channel opening, which could contribute to pathophysiological conditions presenting persistent vasoconstriction.
We have shown that CCICR, indeed, has physiological significance, because it is differentially modulated by vasoactive agents. NE is known to increase the open probability of Ca2+ channels in mesenteric myocytes (14) and increased Ca2+ currents in rabbit ear artery (15). However, the effect of NE varies in different vascular territories, because we have observed that NE (up to 10 μM) does not influence the contractility of the basilar arterial rings and the absence effect of this drug on the basilar artery studied in vivo has been reported (26). In accord with these results, we have also shown that NE has no effect on Ca2+currents in basilar myocytes and, therefore, does not modulate CCICR. ACh, a vasoactive agent in some vascular territories (27, 28), and ET-1, an efficacious constrictor in the rat basilar artery (24), also failed to induce potentiation of CCICR or Ca2+ currents in our preparation. In contrast, ATP, a vasoactive agent in the mammalian cerebral circulation (12, 13, 29), exerts a powerful facilitatory role on CCICR and rat basilar myocyte contractility. ATP is known to elicit, in VSMCs, either Ca2+ influx through ligand-gated calcium channels or Ca2+ release due to activation of metabotropic P2Y receptors (3, 30). These effects of ATP are abolished by blockade of the receptors with suramin or pyridoxal phosphate 6-azophenyl-2′,4′,-disulfonic acid, in the case of effects mediated by P2Y receptors, or after G protein inhibition by the intracellular application of GDPβS. Interestingly, we describe here that, after blockade of purinergic receptors, ATP (even at subthreshold concentration) reversibly increases Ca2+-current amplitude and, in the absence of extracellular Ca2+, enhances CCICR and, subsequently, arterial contractility. These phenomena appear to be highly selective, because other nucleotides (ADP or UTP) or the vasoactive agents tested (NE, ACh, or ET-1) did not produce the same effect as ATP. ATP and FPL had an additive effect on myocyte Ca2+ channels, thus suggesting that they act through separate mechanisms to increase the macroscopic current amplitude. ATP’s effects on Ca2+ channels were unaltered by intracellular dialysis with GDPβS but were mimicked by extracellular ATPγS. Altogether, these data strongly suggest that ATP directly interacts with a receptor site in the channel molecule to favor Ca2+ channel activation. A qualitatively similar phenomenon has been observed in ventricular myocytes, where direct interaction of ATP with the channels was suggested, because the effect was maintained in single L-type Ca2+ channels reconstituted into planar lipid bilayers (31, 32). Therefore, two observations in our study are the direct and selective facilitatory effect of ATP on L-type Ca2+currents in vascular myocytes and, more significantly, the potentiation of CCICR by ATP. CCICR is inhibited by nifedipine and other Ca2+ antagonists (6), thus explaining why ATP-induced Ca2+ release in basilar myocytes is abolished after application of these drugs (3).
Besides the effect of ATP on myocyte Ca2+ channels, we have demonstrated that, without extracellular Ca2+ influx, basilar arterial smooth muscle can contract in response to Ca2+ channel activation by either membrane depolarization or direct pharmacological modulation. These results agree with previous studies in intestine (33), aorta (34), coronary artery (35), human umbilical artery (36), and portal vein (37), in which contraction of smooth muscle cells by a Ca2+-free depolarizing solution was reported. Contraction of the basilar artery elicited upon Ca2+ channel activation does not necessarily require membrane depolarization, because FPL, a Ca2+ channel agonist that directly modifies Ca2+ channel gating, (20–22) also produced contraction of arterial rings bathed in Ca2+-free solution. These findings are in excellent agreement with the data obtained in single myocytes, demonstrating that CCICR (the coupling of Ca2+ channels to the SR through the activation of the PLC/InsP3 pathway) can be triggered by either membrane depolarization or FPL. The CCICR helps to explain the dependence of InsP3-dependent Ca2+ release on the membrane potential observed in smooth muscle (8, 9) and other cell types (7, 10); however, the possibility that there is a voltage-dependent step at the level of either G protein or PLC activation (8–10) cannot be fully excluded.
The potentiation of CCICR and vasoconstriction by ATP described in this article might have a significant role in physiological and pathophysiological conditions presenting myocyte depolarization and increase of external ATP concentration. ATP is coreleased with NE from sympathetic terminals and also from cells (erythrocytes and platelets) that are damaged during vascular injury (11–13). In this regard, a most representative condition is the delayed cerebral vasoconstriction characteristic of patients with subarachnoid hemorrhage (SAH). Although the cause of this type of cerebral vasospasm is likely of multiple origin, there is a wealth of data suggesting that ATP released from blood clots (erythrocytes and platelets) might have an important contribution (13, 38, 39). We have shown here that, at concentrations that cannot evoke contraction, ATP potentiates both CCICR and the vasoconstriction induced by depolarization with high K+. It has been shown that, in experimental SAH, cerebral arterial myocytes are depolarized (40, 41) therefore, it is possible that the conjunction of membrane depolarization and ATP release (12, 13) (even at low concentrations) leads to cerebral vasospasm. The fact that organic Ca2+ channel antagonists inhibit not only extracellular Ca2+ influx but also CCICR (6) would explain the beneficial effect of these drugs in the prevention or treatment of ischemia secondary to SAH (42). In conclusion, our data support the view that L-type Ca2+ channels in basilar VSMCs are not only mediators of Ca2+ influx, but they also have a metabotropic action, leading to Ca2+ release from the SR. This metabotropic effect of Ca2+ channels, markedly potentiated by even low concentration of ATP acting directly on Ca2+ channels, could contribute to vascular pathophysiology.
Materials and Methods
Preparation of Dispersed Basilar Myocytes.
Isolated rat basilar arterial myocytes were obtained from anesthetized Wistar rats (250–300 g). Basilar arteries were dissected from the brain, cut into pieces, and incubated at 4°C for 12–14 h in Hanks’ solution containing 1.3 mg/ml papain, 1.2 mg/ml collagenase, 40 IU/ml elastase, and 0.75 mg/ml BSA. Cells were mechanically dispersed and plated on coverslips. Myocytes were easily distinguished by their size and typical elongated shape or by their immunoreactivity to α-actin (6).
Cytosolic Ca2+ Measurements and Patch-Clamp Recordings.
Cytosolic [Ca2+] was estimated in intact and dialyzed cells. In the first case, myocytes were incubated in Hanks’ solution with 1 μM Fura 2-AM added for 15 min at room temperature. In dialyzed cells, Fura 2 potassium salt (50 μM) was added to the patch pipette solution. For the experiments, a coverslip with cells was placed on a recording chamber mounted on the stage of an inverted microscope (Axiovert 35, Zeiss) equipped with epifluorescence and photometry. Alternating excitation wavelengths of 340 and 380 nm were used, and background fluorescence was subtracted before obtaining the F340/F380 ratio. Calibration of Ca2+ signals was done in vitro as described in ref. 43. Voltage- and current-clamp recordings were performed by using the whole-cell configuration of the patch-clamp technique (6, 43). When cytosolic [Ca2+] and membrane holding potential or current were recorded simultaneously, the signals were digitized at a sampling interval of 500 ms and filtered at 3 KHz. Ca2+ currents were digitized by using a sample interval of 50 μs. All the experiments were performed at room temperature (≈22°C).
Solutions.
The composition of Hanks’ solution was 125 mM NaCl, 5.36 mM KCl, 5 mM NaHCO3, 0.34 mM Na2HPO4, 0.44 mM KH2PO4, 10 mM glucose, 1.45 mM sucrose, and 10 mM Hepes, pH 7.4. The recording chamber was continuously superfused with a standard external solution with 140 mM NaCl, 2.7 mM KCl, 2.5 mM CaCl2, 1 mM MgCl2, and 10 mM Hepes, pH 7.4. Unless otherwise noted, all the drugs used were added to this solution at the indicated concentration. The 70 mM K+ solution was obtained by replacing 70 mM of NaCl with KCl. The 0Ca solution was obtained by substituting Mg2+ for Ca2+ and with 2 mM EGTA added. Cytosolic Ca2+ measurements and patch-clamp recordings were done by dialyzing the cells with a standard internal solution containing 83 mM potassium glutamate, 47 mM KCl, 1 mM MgCl2, 10 mM Hepes, 4 mM ATP-Mg, and 50 μM Fura 2-potassium salt, pH 7.2. To record whole-cell Ca2+ currents, the solutions used were as follows: external (140 mM NaCl, 2.7 mM KCl, 10 mM BaCl2, and 10 mM Hepes, pH = 7.4); and internal (100 mM CsCl, 30 mM CsF, 2 mM MgCl2, 4 mM ATP-Mg, 10 mM EGTA, and 10 mM Hepes, pH 7.2).
Measurement of Contractility in Arterial Rings.
Basilar arteries were cleaned of connective tissue, cut in rings (≈2 mm) and mounted on a small-vessel myograph (JP Trading, Aarhus, Denmark) to measure isometric tension connected to a digital recorder (Myodataq-2.01, Myodata-2.02 Multi Myograph System). The rings were placed on a chamber filled with the standard external solution (10 mM glucose added) and bubbled with 95% O2 and 5% CO2 at pH 7.4. Before the experiments, the segments were subjected to an optimal tension (90% of tension equivalent to an intramural pressure of 100 mm Hg) and stabilized for at least 30 min. For Ca2+-free solution, CaCl2 was omitted, and 200–500 μM EGTA was added. After the equilibration period, we discarded arterial rings that developed a tension of <1 mN in response to KCl (40–70 K+). Experiments in the absence of Ca2+ were done after incubating arterial rings in Ca2+-free solution for 15 min and washing the external solution at least four times. All the drugs used were added directly to the chamber while vessel tension was monitored. Experiments were performed at 37°C.
Statistical Analysis.
Data are expressed as mean ± SE, and the statistical significance was estimated by using the Student t test. Values of P < 0.05 were considered significant.
Supplementary Material
Acknowledgments
This work was supported by Spanish Ministry of Science and Technology Grant PM99-0120 and Redes Red de Investigacion en Enfermedades Cardiovasculares (RECAVA) and Centro de Investigacion de Enfermedades Neurologicas (CIEN) of the Spanish Ministry of Health. J.L.-B. received the Ayuda a la Investigación 2000 from the Juan March Foundation.
Abbreviations
- ACh
acetylcholine
- [Ca2+]
calcium ion concentration
- CCICR
calcium-channel-induced calcium release
- ET-1
endothelin-1
- FPL
2,5-dimethyl-4-[2-(phenylmethyl)benzoil]-1H-pyrrole-3-carboxylic acid methyl ester
- InsP3
inositol trisphosphate
- NE
norepinephrine
- PLC
phospholipase C
- SR
sarcoplasmic reticulum
- VSMC
vascular smooth muscle cell.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Van Breemen C., Saida K. Annu. Rev. Physiol. 1989;51:315–329. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]
- 2.Worley J. F., Quayle J. M., Standen N. B., Nelson M. T. Am. J. Physiol. 1991;261:H1951–H1960. doi: 10.1152/ajpheart.1991.261.6.H1951. [DOI] [PubMed] [Google Scholar]
- 3.Sima B., Weir B. K., Macdonald R. L., Zhang H. Stroke. 1997;28:2053–2058. doi: 10.1161/01.str.28.10.2053. [DOI] [PubMed] [Google Scholar]
- 4.Gokina N. I., Bevan J. A. Am. J. Physiol. 2000;278:H2094–H2104. doi: 10.1152/ajpheart.2000.278.6.H2094. [DOI] [PubMed] [Google Scholar]
- 5.Kamishima T., Quayle J. M. Am. J. Physiol. 2004;286:H535–H544. doi: 10.1152/ajpheart.00506.2003. [DOI] [PubMed] [Google Scholar]
- 6.del Valle-Rodríguez A., Lopez-Barneo J., Ureña J. EMBO J. 2003;22:4337–4345. doi: 10.1093/emboj/cdg432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Araya R., Liberona J. L., Cardenas J. C., Riveros N., Estrada M., Powell J. A., Carrasco M. A., Jaimovich E. J. Gen. Physiol. 2003;121:3–16. doi: 10.1085/jgp.20028671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itoh T., Seki N., Suzuki S., Ito S., Kajikuri J., Kuriyama H. J. Physiol. 1992;451:307–328. doi: 10.1113/jphysiol.1992.sp019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganitkevich V. Ya., Isenberg G. J. Physiol. 1993;470:35–44. doi: 10.1113/jphysiol.1993.sp019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mason M. J., Mahaut-Smith M. P. J. Physiol. 2001;533:175–183. doi: 10.1111/j.1469-7793.2001.0175b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burnstock G., Kennedy C. Circ. Res. 1986;58:319–330. doi: 10.1161/01.res.58.3.319. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H., Weir B., Marton L. S., Macdonald R. L., Bindokas V., Miller R. J., Brorson J. R. Am. J. Physiol. 1995;269:H1874–H1890. doi: 10.1152/ajpheart.1995.269.6.H1874. [DOI] [PubMed] [Google Scholar]
- 13.Sima B., MacDonald L., Marton L. S., Weir B., Zhang J. Neurosurgery. 1996;39:815–821. doi: 10.1097/00006123-199610000-00034. [DOI] [PubMed] [Google Scholar]
- 14.Nelson M. T., Standen N. B., Brayden J. E., Worley J. F. Nature. 1988;36:382–385. doi: 10.1038/336382a0. [DOI] [PubMed] [Google Scholar]
- 15.Benham C. D., Tsien R. W. J. Physiol. 1988;404:767–784. doi: 10.1113/jphysiol.1988.sp017318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goto K., Kasuya Y., Matsuki N., Takuwa Y., Kurihara H., Ishikawa T., Kimura S., Yanagisawa M., Masaki T. Proc. Natl. Acad. Sci. USA. 1989;86:3915–3918. doi: 10.1073/pnas.86.10.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morita H., Sharada T., Takewaki T., Ito Y., Inoue R. J. Physiol. 2002;539:805–816. doi: 10.1113/jphysiol.2001.013029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirst G. D., Neild T. O., Silverberg G. D. J. Physiol. 1982;328:351–360. doi: 10.1113/jphysiol.1982.sp014268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sobey C. G., Heistad D. D., Faraci F. M. Am. J. Physiol. 1996;271:H126–H132. doi: 10.1152/ajpheart.1996.271.1.H126. [DOI] [PubMed] [Google Scholar]
- 20.Zheng W., Rampe D., Triggle D. J. Mol. Pharmacol. 1991;40:734–741. [PubMed] [Google Scholar]
- 21.Fan J., Yuan Y., Palade P. Am. J. Physiol. 2001;280:C565–C572. doi: 10.1152/ajpcell.2001.280.3.C565. [DOI] [PubMed] [Google Scholar]
- 22.McDonough S. I., Mori Y., Bean B. P. Biophys. J. 2005;88:211–223. doi: 10.1529/biophysj.104.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi H., Hayashi M., Kobayashi S., Kabuto M., Handa Y., Kawano H. Neurosurgery. 1990;27:357–361. doi: 10.1097/00006123-199009000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Zuccarello M., Lee B. H., Rapoport R. M. Gen. Pharmacol. 2000;35:11–15. doi: 10.1016/s0306-3623(01)00083-0. [DOI] [PubMed] [Google Scholar]
- 25.Smith R. J., Sam L. M., Justen J. M., Bundy G. L., Bala G. A., Bleasdale J. E. J. Pharmacol. Exp. Ther. 1990;253:688–697. [PubMed] [Google Scholar]
- 26.Kitazono T., Faraci F. M., Heistad D. D. Am. J. Physiol. 1993;264:H178–H182. doi: 10.1152/ajpheart.1993.264.1.H178. [DOI] [PubMed] [Google Scholar]
- 27.Masumoto A., Mohri M., Shimokawa H., Urakami L., Usui M., Takeshita A. Circulation. 2002;105:1545–1547. doi: 10.1161/hc1002.105938. [DOI] [PubMed] [Google Scholar]
- 28.Chrissobolis S., Sobey C. G. Stroke. 2004;35:747–752. doi: 10.1161/01.STR.0000116867.28589.3A. [DOI] [PubMed] [Google Scholar]
- 29.Miyagi Y., Zhang J. H. Am. J. Physiol. 2004;286:H1546–H1551. doi: 10.1152/ajpheart.00926.2003. [DOI] [PubMed] [Google Scholar]
- 30.Benham C. D., Tsien R. W. Nature. 1987;328:275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- 31.Scamps F., Legssyer A., Mayoux E., Vassort G. Circ. Res. 1990;67:1007–1016. doi: 10.1161/01.res.67.4.1007. [DOI] [PubMed] [Google Scholar]
- 32.Liu Q. Y., Rosenberg R. L. Am. J. Physiol. 2001;280:C1107–C1113. doi: 10.1152/ajpcell.2001.280.5.C1107. [DOI] [PubMed] [Google Scholar]
- 33.Mangel A. W., Nelson D. O., Connor J. A., Prosser C. L. Nature. 1979;281:582–583. doi: 10.1038/281582a0. [DOI] [PubMed] [Google Scholar]
- 34.Bozler E. Am. J. Physiol. 1969;216:671–674. doi: 10.1152/ajplegacy.1969.216.3.671. [DOI] [PubMed] [Google Scholar]
- 35.Kalsner S. J. Pharmacol. Exp. Ther. 1997;281:634–642. [PubMed] [Google Scholar]
- 36.Tufan H., Ayan-Polat B., Tecder-Ünal M., Polat G., Kayhan Z., Ögüs E. Life Sci. 2003;72:1321–1329. doi: 10.1016/s0024-3205(02)02382-2. [DOI] [PubMed] [Google Scholar]
- 37.Burt R. P. Am. J. Physiol. 2003;284:H1808–H1817. doi: 10.1152/ajpheart.00637.2002. [DOI] [PubMed] [Google Scholar]
- 38.Macdonald R. L., Weir B., Zhang J., Marton L. S., Sajdak M., Johns L. M. Neurosurg. Focus. 1997;3:1–7. doi: 10.3171/foc.1997.3.4.6. [DOI] [PubMed] [Google Scholar]
- 39.Carpenter R. C., Miao L., Miyagi Y., Bengten E., Zhang J. H. Stroke. 2001;32:516–522. doi: 10.1161/01.str.32.2.516. [DOI] [PubMed] [Google Scholar]
- 40.Harder D. R., Dernbach P., Waters A. J. Clin. Invest. 1987;80:875–880. doi: 10.1172/JCI113146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aihara Y., Jahromi B. S., Yassari R., Nikitina E., Agbaje-Williams M., Macdonald R. L. J. Cereb. Blood Flow Metab. 2004;24:75–83. doi: 10.1097/01.WCB.0000095803.98378.D8. [DOI] [PubMed] [Google Scholar]
- 42.van Gijn J., Rinkel G. J. Brain. 2001;124:249–278. doi: 10.1093/brain/124.2.249. [DOI] [PubMed] [Google Scholar]
- 43.Franco-Obregón A., Ureña J., López-Barneo J. Proc. Natl. Acad. Sci. USA. 1995;92:4715–4719. doi: 10.1073/pnas.92.10.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.