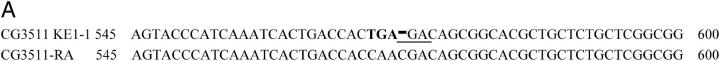Abstract
Mutations that inactivate the retinoblastoma (Rb) pathway are common in human tumors. Such mutations promote tumor growth by deregulating the G1 cell cycle checkpoint. However, uncontrolled cell cycle progression can also produce new liabilities for cell survival. To uncover such liabilities in Rb mutant cells, we performed a clonal screen in the Drosophila eye to identify second-site mutations that eliminate Rbf− cells, but allow Rbf+ cells to survive. Here we report the identification of a mutation in a novel highly conserved peptidyl prolyl isomerase (PPIase) that selectively eliminates Rbf− cells from the Drosophila eye.
AN important goal of novel cancer therapy is to elicit the death of mutant tumor cells in the patient, while allowing normal cells to survive. The identification of gene products required for tumor cell survival can provide highly validated drug targets for the development of therapeutic inhibitors. Ideally, targets could be identified that would kill cancer cells while sparing normal cells. A synthetic lethal screen is one method of identifying such targets. In this type of screen, cells are genetically altered to model tumor cells and one then screens for mutations that eliminate the model tumor cells but have little or no effect on wild-type cells.
One way to model tumor cells is to functionally inactivate the RB1 gene. In addition to being mutated in retinoblastomas, where it was initially discovered, RB1 is mutated in many other cancers including prostate (Kubota et al. 1995), bladder (Miyamoto et al. 1995), parathyroid (Cryns et al. 1994), and 90% of small cell lung cancers (SCLCs) (Minna et al. 2002). RB1 is also functionally inactivated in tumors that do not harbor mutations in the RB1 locus itself, but do carry mutations that target the pathway through the loss of cyclin-dependent kinase (Cdk) inhibitors or overexpression of Cyclin D1 or Cdk4 (reviewed in Sherr and McCormick 2002). Additionally, the transforming activities of DNA tumor virus oncoproteins are mediated via their interaction with RB1 (Helt and Galloway 2003).
The RB1 protein acts as a critical regulator of G1/S phase progression by binding to members of the E2F family of transcription factors (Dyson 1998; Nevins 2001). E2F-RB1 complexes prevent entry into S phase by actively repressing transcription through the recruitment of histone deacetylases and other chromatin modifiers to E2F-responsive promoters (Harbour and Dean 2000; Ogawa et al. 2002). Progression from G1 through S phase occurs when RB1 is inactivated through phosphorylation by the Cdk complexes Cyclin D/Cdk4 or Cyclin D/Cdk6 and Cyclin E/Cdk2 (Lundberg and Weinberg 1998). Phosphorylation relieves transcriptional repression and allows E2F-dependent transcription of target genes required for S phase progression, such as Cyclin E (Morris et al. 2000) as well as enzymes required for DNA synthesis and metabolism (Stevaux and Dyson 2002). In addition to its effects on cell proliferation, loss of RB1 predisposes cells to apoptosis through the actions of E2F on p53 (reviewed in Chau and Wang 2003), thereby creating a selective pressure for tumors to accumulate mutations in p53.
Components of the RB1 pathway are being investigated as potential anticancer targets. These include the upstream kinases, Cdk2, Cdk4, and Cdk6, and the downstream effector of retinoblastoma (Rb), E2F (McLaughlin et al. 2003; Vermeulen et al. 2003). These targeted approaches could lead to therapies with an improved profile of efficacy vs. toxicity compared to conventional treatment. It would also be of interest to identify novel targets involved in RB1 biology, especially those necessary for the viability of cells mutant for RB1. We therefore carried out a synthetic lethal screen in Drosophila to look for RB1-interacting genes.
Like its mammalian counterpart, Drosophila Rbf (CG7413) binds to E2F1 and regulates E2F target gene expression (Du et al. 1996; Du and Dyson 1999; Datar et al. 2000; Dick and Dyson 2003) and is regulated by the Cdk complexes Cyclin D/Cdk4 and Cyclin E/Cdc2c (Xin et al. 2002), indicating that the function of RB1 is conserved between Drosophila and mammals.
To identify novel therapeutic targets in the RB1 pathway, we performed a synthetic lethal genetic screen in Drosophila to identify recessive mutations that result in the loss of cells that lack dRB1 (Rbf−), but allow wild-type cells (Rbf+) to survive. The synthetic lethal approach is commonplace in unicellular organisms such as yeast, where synthetic lethality is scored via organismal death. In multicellular organisms, however, synthetic lethality cannot be scored simply by organismal lethality, because desired mutations may cause organismal lethality on their own due to their function in essential tissues or cell types. An additional complication in the case of Rbf is that it itself is required for embryonic survival. To circumvent this issue, we generated mosaic animals that carry clones of Rbf− tissue in the eye, whereas the rest of the animal is Rbf+. We then generated overlapping clones of homozygous induced mutations in the eye and screened for potential synthetic lethality by scoring for the absence of clones carrying both the induced mutation and the Rbf− mutation. We report the identification of a mutation in a novel highly conserved peptidyl prolyl isomerase that preferentially eliminates Rbf mutant cells.
MATERIALS AND METHODS
Drosophila stocks and handling:
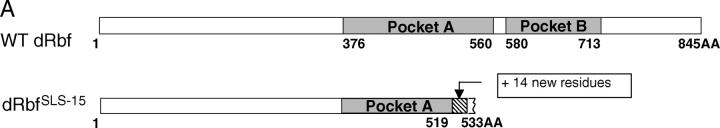
All fly stocks and crosses were handled using standard procedures at 25°. Rbf alleles and rescue lines used in this study have been deposited at the Bloomington Drosophila Stock Center. RbfSLS-15 (Figure 1A) was generated in a suppressor screen as being able to reverse the G1 arrest conferred by the overexpression of human p21 in the Drosophila eye (data not shown). PExp{FRT2.1 [Rbf+, w+, 3.5ey-FLP]} was inserted on the X chromosome and recombined onto the RbfSLS-15 chromosome to rescue the embryonic lethal phenotype while generating Rbf− cells in the eye. The subsequent RbfSLS-15, PExp{FRT2.1[Rbf+, w+, 3.5ey-FLP]} chromosome was crossed to Minute-FRT, w+ lines for each individual chromosome arm (MFRT2R, MFRT2L, MFRT3R, and MFRT3L) to generate the female “screening stocks” (RbfSS2R, RbfSS2L, RbfSS3R, and RbfSS3L) that allowed the generation of marked homozygous clones in a single generation (full stock genotypes used in the screen are provided in Table 1). The screening males used in mutagenesis were constructed by recombining an unmarked isogenic chromosome arm onto each FRT arm to facilitate the creation of w homozygous clones when crossed to screening stock females. This was done by recombining a P-element insertion from the Exelixis collection, which was inserted in an isogenic chromosome just proximal to the FRT, onto [P-ry FRT]. The presence of the P element was identified using w+, and the presence of the FRT was monitored by PCR using primers Neo2F (ATCTGGACGAAGAGCATCAGGG) and Neo2Ra (CGATACCGTAAAGCACGAGGAAG). The isogenic arm was then recombined onto the FRT line by monitoring the absence of w+ and the presence of the FRT by PCR. Males also carried an exogenous source of ey-FLP on the non-FRT autosome to create more robust homozygous clones than those produced by the PExp{FRT2.1[Rbf+, w+, 3.5ey-FLP]} construct alone.
Figure 1.—
Schematics of the Rbf protein and Rbf rescue construct. (A) Diagram of the wild-type Rbf and RbfSLS-15 mutant proteins. The mutation analysis of the RbfSLS-15 transcripts revealed an 11-bp deletion resulting in a frameshift at amino acid residue 519, followed by the addition of 14 novel residues and truncation of the Rbf protein at residue 533. The truncated protein lacks Pocket B, a highly conserved RBF domain that is required for interactions with partner proteins and the execution of RBF function. (B) Diagram of the Rbf rescue construct and Rbf− clone generation. The RbfSLS-15 mutation combined with a Rbf rescue construct allows for the generation of Rbf− clones specifically in the eye, due to eye-specific FLP expression followed by recombination between the FRT sites and subsequent loss of the Rbf+ and w+ genes. All other tissues, which do not express FLP, remain Rbf+, resulting in a rescue of the organismal lethality normally associated with Rbf-deficient flies.
TABLE 1.
List of strains constructed for use in dRbf synthetic lethal screen
| Stock description | Abbreviation | Genotype |
|---|---|---|
| dRbf alleles | CAS-21 | Su(p21)CAS-21/FM7c; +/CyO, P{p21-pGMR-33B} |
| SLS-15 | w, Su(p21)SLS-15, P{ry[+t7.2] = neopFRT}18A/FM7c, ftz lacZ | |
| Rescue construct | RbfR | y, w, P{dRbf-pExP-FRT2.1-3.5ey-FLP-5}/FM7c |
| Rescued dRbf alleles |
CAS-21+dRbR | w, Su(p21)CAS-21, PExp{FRT2.1[dRbf+, w+, 3.5ey-FLP]}; sp; e |
| SLS-15+dRbfR | w, Su(p21)S RbfSS2L LS-15, PExp{FRT2.1[dRbf+, w+, 3.5ey-FLP]}; sp; e | |
| dRbf screening stocks |
RbfSS2L | w, Su(p21)SLS-15, PExp{FRT2.1[dRbf+, w+, 3.5ey-FLP]}; M(2)24F[1]P{w[+mC] = piM}36F P{ry[+t7.2] = neoFRT}40A/CyO |
| RbfSS2R | w, Su(p21)SLS-15, PExp{FRT2.1[dRbf+, w+, 3.5ey-FLP]}; P{ry[+t7.2] = neoFRT}42D P{w[+mC] = piM}45F M(2)53[1]/CyO | |
| RbfSS3R | w, Su(p21)SLS-15, PExp{FRT2.1[dRbf+, w+, 3.5ey-FLP]}; P{ry[+t7.2] = neoFRT}82B P{w[+mC] = piM}87E RpS3[*]/TM6B, Tb[1] | |
| RbfSS3L | w, Su(p21)SLS-15, PExp{FRT2.1[dRbf+, w+, 3.5ey-FLP]}; Dp(1;3)sc[j4], y+, P{w+}, M(3)67C, pi75c, P[ry+, hs-neo, FRT]80B; ry/TM6B | |
| Male stocks | IsoFS2L | iso2 P{ry[+t7.2] = neoFRT}40A; P{ry[+7.2] = ey-FLP.N}6, ry[506] |
| IsoFS2R | P{ry[+t7.2] = neoFRT}42D iso2; P{ry[+7.2] = ey-FLP.N}6, ry[506] | |
| IsoFS3L | P{ry[+7.2] = ey-FLP.N}5/CyO; iso3 P[ry+, hs-neo, FRT]80B/TM6B | |
| IsoFS3R | P{ry[+7.2] = ey-FLP.N}5; P{ry[+t7.2] = neoFRT}82B iso3 | |
| dRbf+ MFRT lines for counterscreen |
MFRT2L | w[*]; M(2)24F[1] P{w[+mC] = piM}36F P{ry[+t7.2] = neoFRT}40A/CyO |
| MFRT2R | w[*]; P{ry[+t7.2] = neoFRT}42D P{w[+mC] = piM}45F M(2)53[1]/CyO | |
| MFRT3L | yw; Dp(1;3)sc[j4], y+, P{w+}, M(3)67C, pi75c, P[ry+, hs-neo, FRT]80B; ry/TM6B | |
| MFRT3R | w[*]; P{ry[+t7.2] = neoFRT}82B P{w[+mC] = piM}87E RpS3[*]/TM6B, Tb[1] | |
| ey-flp lines | EFL2 | P{ry[+t7.2] = ey-FLP.N}6, ry[506] |
| EFL3 | P{ry[+t7.2] = ey-FLP.N}5, ry[506] | |
| CyO-GFP source | CyO-GFP | w; L[2] Pin[1]/CyO, P{w[+mC] = GAL4-Kr.C}DC3, P{w[+mC] = UAS− GFP.S65T}DC7 |
| KE1 alleles | KE1-1 | yw, FRT(2R)KE1-1/CyO |
| KE1-2 | yw, FRT(2R)KE1-2/CyO |
Primary genetic screen:
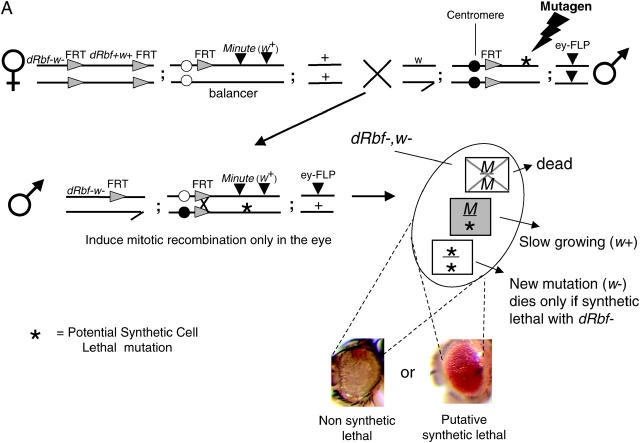
Males were mutagenized by feeding them 5 mm EMS for 20–24 hr (in a 1% sucrose solution) after a 4-hr starvation period. Batches of 40 mutagenized males were mated to 30–50 virgin females (Figure 2A). The low EMS concentration was determined to induce only 0.8 lethal mutations per autosomal arm, which was essential to the success of identifying synthetic loci, since any additional mutations that caused cell lethality would have led us to discard the hit during the counterscreen. The mutagenesis rates for each round were confirmed by monitoring the segregation of X-linked lethals in the F1 generation: these were 2L = 0.289, 2R = 0.289, 3L = 0.141, and 3R = 0.221, respectively. Additional mutagenesis was performed via gamma-ray irradiation at 1.625 krad using a Cobalt-60 source Gammacell 220 Irradiator. Crosses were flipped daily for 3 consecutive days. Progeny were scored for the absence of w tissue in the eye, leaving the w+ (Minute) tissue to populate the eye. Candidate mutations that resulted in the elimination of 90% of the w tissue were selected for further testing and crossed to balancer stocks. Five of the resulting progeny were subsequently retested to ensure the passage of the mutation and the validity of the phenotype.
Figure 2.—
Schematic of the primary screen and counterscreen. (A) Schematic of the primary screen. Rbf+ screening-stock virgin females were crossed to mutagenized male stocks. Male progeny were assayed for mutations that resulted in the loss of w eye clones, causing the eyes to be w+. Two separate FLP/FRT recombination events are initiated by the eyeless promoter. First, the FRTs flanking the Rbf rescue construct recombine in cis, eliminating the Rbf+ and w+ genes, resulting in a large Rbf−, w clone in the eye. Second, the trans recombination between the two autosomal FRTs results in the generation of three different cell types:
1. Minute/Minute (M/M): This cell type is cell lethal because M/M cells die, regardless of the Rbf status of the cell.
2. Minute/mutation (M/*): This cell type is viable and marked with w+. When cells are heterozygous for Minute they are slow growing and are easily outcompeted.
3. mutation/mutation (*/*): This cell type is viable if the mutation is not synthetic lethal with Rbf−, since this outcompetes the M/* clone, resulting in a 90–95% w eye. When there is a synthetic lethal interaction with Rbf−, the clone is unable to populate the eye and M/* is the only cell type that survives, resulting in a w+ eye.
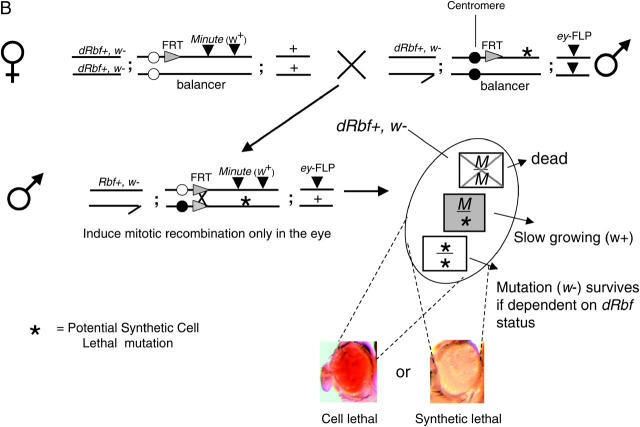
(B) Schematic of the counterscreen. To eliminate those mutations that are not dependent upon Rbf status, hits from the primary screen were crossed to Rbf+ MFRT line virgins. The FRT/FLP recombination events under the direction of the eyeless promoter result in the generation of three different cell types: (1) M/M, as described above; (2) M/*, as described above; and (3) */*, if the previously observed synthetic lethal phenotype is indeed Rbf− specific, this cell type will be able to populate the eye in a Rbf+ background, resulting in a w eye. Conversely, if these cells are absent, resulting in a w+ eye, then there is no Rbf− synthetic interaction and the previously observed phenotype was due to nonspecific cell lethality.
Counterscreen:
Individual modifiers were subsequently mated to a corresponding counterscreen stock (Rbf+, Minute-FRT, w+ lines: MFRT2R, MFRT2L, MFRT3R, and MFRT3L) and assayed for w tissue viability in the eye to demonstrate a specific interaction dependent on Rbf− (Figure 2B). Confirmed synthetic modifiers were stocked over CyO or TM6B balancer chromosomes.
Genetic mapping of modifiers:
Only synthetic lethal modifiers that were also homozygous organismal lethal were mapped. Recombination mapping of the synthetic lethal phenotype was conducted using al1 dpov1 b1 pr1 cn1 c1 px1 sp1 for hits on the second chromosome or ru1 h1 th1 st1 cu1 sr1 es ca1 for hits on the third chromosome and selecting for recombinants that retained a FRT. A copy of ey-FLP (EFL2 or EFL3) was crossed in and recombinants were scored for organismal lethality and synthetic lethality (Table 3). The organismal lethal phenotype was further mapped using deficiencies obtained from the Bloomington Stock Center and deficiencies created by Exelixis (Parks et al. 2004) that span the region identified by the recombination mapping (Table 4). Homozygous lethal transposons residing within interacting deficiencies were assayed for lethality in conjunction with our screen hits. Candidate loci within the mapped regions were analyzed by DNA sequencing.
TABLE 3.
Visible recombination mapping ofKE1-1
| Recombinant | Synthetic lethal |
Organismal lethal |
Large larvae |
|---|---|---|---|
| al, dp, b, FRT(42D) [*] | Y | Y | Y |
| FRT(42D), [*], c, px, sp | N | N | N |
| FRT(42D), [*], px, sp | N | N | N |
| FRT(42D), [*], sp | N | N | N |
| FRT(42D), c, [*] | Y | Y | Y |
| FRT(42D), c, px, [*] | Y | Y | Y |
Recombinants bearing the visible chromosomal markers shown in column 1 were scored for synthetic lethality with Rbf− in eye clones (column 2), organismal lethality as homozygotes (column 3), and the presence of the large larva phenotype as homozygotes (column 4). Y, the phenotype is present; N, phenotype absent. [*], portion of mutant chromosome.
TABLE 4.
Mapping ofKE1 organismal lethality using chromosomal deficiencies
| Deficiency stock | Left end | Right end | Viability with KE1-1 and KE1-2 |
|---|---|---|---|
| BL-1682 | 59D5–10 | 60B3–8 | Viable |
| BL-2355 | 59D8–11 | 60A7 | Viable |
| BL-1587 | 59E2 | 60B1 | Viable |
| Df(2R)Exel7180 | 59E3 | 59F6 | Viable |
| Df(2R)Exel7182 | 60A13 | 60A16 | Viable |
| Df(2R)Exel9024 | 60A16 | 60A16 | Viable |
| Df(2R)Exel6080 | 60A6 | 60B5 | Viable |
| Df(2R)Exel7184 | 60B12 | 60C4 | Viable |
| Df(2R)Exel6082 | 60B4 | 60C6 | Viable |
| Df(2R)Exel6281 | 60C4 | 60C7 | Viable |
| BL-1473 | 60C5–6 | 60D1 | Viable |
| BL-2604 | 60C6 | 60D9–10 | Lethal |
| BL-3157 | 60E6 | 60F1–2 | Viable |
| BL-2471 | 60E6–9 | 60E11 | Viable |
| BL-2528 | 60E9 | 60F1 | Viable |
Deficiency name or stock number tested is given in column 1. The left- and right-hand cytogenetic locations are given in columns 2 and 3, and the lethality or viability when the deficiency was scored with KE1-1 and KE1-2 is given in column 4.
F2 lethal noncomplementation screen for additional KE1 alleles:
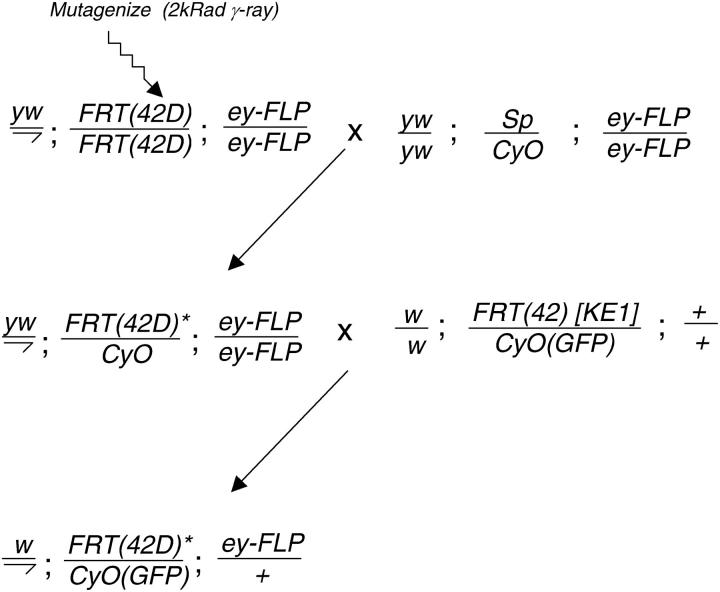
FRT(42D); ey-FLP males were mutagenized via gamma-ray irradiation at 2.0 krad. Batches of 40 mutagenized males were mated to 30 yw; Sp/CyO; ey-FLP virgin females. Individual male progeny were mated to +; FRT [KE1-1]/CyO-GFP virgin females and progeny were scored for the absence of straight wings. Putative KE1 allele-carrying males were crossed to the 2R screening stock (RbfSS2R) to ensure the absence of w clones and crossed to the 2R counterscreening stock (MFRT2R) to ensure the presence of w clones and confirm synthetic lethality. We scored 5000 individual male crosses and isolated one new allele of KE1 (referred to as KE1-2), which was lethal in trans to KE1-1, irrespective of the presence of ey-FLP.
Mutation detection of KE1 alleles:
Staggered sequencing primers, spaced at 120- to 150-bp intervals and facing both directions, were designed for all open reading frames and their flanking regions throughout the genomic region of interest: coordinates 20047526–20093250 (FlyBase release 4.0). The selected forward and reverse PCR primer pairs were then used to amplify the regions of interest, using genomic DNA prepared from five individual larvae (large larvae in the case of homozygous mutants or the parental mutagenized strain for controls). Using this procedure, we were able to obtain high-quality fragments of genomic DNA up to 10 kb in length, although the usual product length was ∼7 kb. Products were amplified for 30 cycles using a modified long-range PCR protocol with Takara (Berkeley, CA) LA Taq polymerase, checked on agarose gels, and purified with the Millipore (Bedford, MA) MultiScreen PCR cleanup kit. Purified PCR products were used as templates for sequencing, using the above-designed staggered sequencing primers and primer walking in both directions to obtain full-length sequence. ABI (Columbia, MD) BigDye sequencing reactions were performed according to manufacturer's protocol using 20–80 ng PCR product. Reactions were ethanol precipitated and loaded onto an ABI 3700 sequencer. Sequencing traces were uploaded to a Unix workstation, assembled with the PhredPhrap package, and viewed and analyzed with Consed. Of the nine currently annotated open reading frames in this region (FlyBase release 4.0), five were sequenced in entirety: CG3511, CG12252, Nurf-38, CG12252, and CG3522. Additionally, in KE1-2 mutants, we sequenced the entire upstream region of CG3511, through to the adjacent locus of CG12252.
Taqman analysis of transcripts:
Both KE1-1 and KE1-2 were stocked over marked CyO-GFP balancer chromosomes (Table 1). Triplicate groups of 10 third instar larvae negative for GFP were collected from isoFS2R, KE1-1, and KE1-2 animals (Table 1). Total RNA was collected using QIAGEN's (Valencia, CA) RNeasy kit for total RNA isolation from animal tissue. The RNA was reverse transcribed into cDNA [Applied Biosystems (Foster City, CA) Multiscribe reverse transcriptase—random hexamer primed]. TaqMan primer/probe assays were carried out for 18S ribosomal RNA, CG3511, and the adjacent locus CG3522. Relative quantity values were obtained for each sample compared to a cDNA standard curve. Standard cDNA was created by reverse transcribing total RNA from an isogenic w fly strain (Exelixis strain A5001, BL-6326). TaqMan assays were run on the ABI PRISM 7900HT sequence detection system. Normalized values for the quantity of CG3511 transcript levels were generated by dividing the CG3511 values by the 18S values for each sample.
Protein sequence data mining:
Protein sequences related to the CG3511 protein were found by a combination of BLAST and Smith-Waterman pairwise analyses against human sequence databases and all sequence databases from the National Center for Biotechnology Information. Sequences were additionally mined solely on the basis of being predicted to contain the Pfam domain models found in CG3511; sequences containing the prolyl isomerase domain (model PF00160) either alone or following three to four WD domains (model PF0400) were identified and analyzed. Only sequences with Pfam scores >0 and E-values <1 were used in the analyses. All sequences data mined were analyzed against the fly genome to select those with top BLAST scores to CG3511 and not to another fly protein sequence. Those meeting BLAST requirements were termed orthologs. All mined sequences that conserved the PF00400 and PF00160 domain organization met orthology criteria, while none of the PF00160 only sequences did. Sequence alignments were performed using Clustal W and visualized by a tree diagram for multiple sequence alignments or by BOXSHADE for pairwise alignments.
RESULTS
Stock generation and synthetic lethal screen:
Inactivating mutations in Rbf were isolated in a suppressor screen for genes able to overcome the G1 arrest caused by the overexpression of human p21 in the Drosophila eye [Su(p21)SLS-15 and Su(p21)CAS-21; data not shown]. Su(p21)SLS-15 (Rbf SLS-15) mutant flies were subsequently used as the starting point for a Rbf synthetic lethal screen. Sequencing of the mutant chromosome and RT-PCR analysis of RbfSLS-15 transcripts revealed an 11-bp deletion resulting in a frameshift mutation at amino acid residue 519 and the addition of 14 novel residues before ending at residue 533 (Figure 1A). This generates a truncated protein lacking the highly conserved Rbf-binding pocket, which is required for interactions with partner proteins and RBF function (Helt and Galloway 2003). Like reported null alleles of Rbf (Du and Dyson 1999; Datar et al. 2000), our alleles confer embryonic lethality as homozygous mutations.
To circumvent the requirement for Rbf during development, we constructed a transgenic Rbf+ screening strain bearing a FLP-FRT rescue transgene to provide wild-type Rbf to all cells and to mark Rbf+ cells in the developing eye with w+ (Figure 1B, Table 1). This transgenic strain is rescued to complete viability and fertility and generates marked viable clones of Rbf−, w cells where FLP recombinase is expressed. To generate homozygous clones of newly induced mutations in the F1 progeny, these flies also carried a FRT at the base of one of the autosomal chromosomal arms in cis to a Minute mutation (MFRTs) (Figure 2; Lambertsson 1998) to generate the Rbf screening stocks (Table 1). For the screen, a low frequency of mutations was induced by EMS in w males carrying an autosomal FRT chromosome plus ey-FLP. These flies were then crossed to the transgenic Rbf screening stock females. ey-FLP generates overlapping clones of both Rbf−, w (from the screening stock females) and the mutagenized FRT autosome (from males) in the eyes of the F1 progeny, thereby enabling us to screen for recessive synthetic lethal mutations in a single generation. Putative synthetic lethal progeny were identified by the presence of solid red eyes (Rbf−, M, w+), indicating that the mutant cells (Rbf−, w) are absent. We screened through individual progeny from crosses generating mitotic clones on the second and third autosomes, which constitute ∼80% of the genome. We screened 342,000 mutagenized chromosomes and initially identified 1585 chromosomes bearing putative synthetic lethal mutations in combination with Rbf− (Table 2), for retest and counterscreening in the following generation.
TABLE 2.
Summary of screen hit rates
| Chromosome arm screened | Mutant chromosomes scored |
Primary screen hits |
Confirmed synthetic lethal hits |
|---|---|---|---|
| 2L | 132,708 | 222 | 0 |
| 2R | 49,216 | 220 | 2 |
| 3L | 43,621 | 896 | 5 |
| 3R | 116,915 | 247 | 3 |
| Total | 342,560 | 1,585 | 10 |
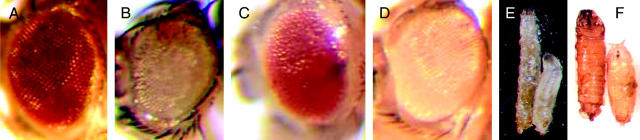
To eliminate those mutations that cause cell lethality independent of Rbf status, we counterscreened the 1585 chromosomes in Rbf+ eye clones induced under similar conditions (Figure 2B) and reconfirmed their ability to reduce the viability of Rbf− cells. Ten of the 1585 mutations were found to be bona fide synthetic lethals, reducing the viability of Rbf−, but not Rbf+, cells (Table 2). Nine of these were developmentally lethal and complemented one another. One of these 9, on the right arm of the second chromosome, was designated KE1-1. When homozygous KE1-1 mutant clones are induced in the developing eye in a Rbf− background, the resulting adult eyes lack the Rbf−, w clonal tissue (Figure 3, B and C). However, when KE1-1 mutant clones are generated in a Rbf+ background, the tissue is viable (Figure 3D), demonstrating that KE1-1 is homozygous viable in cells in the presence of wild-type Rbf. Thus, the lethal interaction is specific to Rbf− cells, and KE1-1 is a true synthetic lethal mutation. When KE1-1 is homozygous in all tissues throughout development, homozygous larvae display an enlarged body phenotype compared to their heterozygous KE1-1/+ siblings (Figure 3E). These “large larvae” wander for an extended period before death, although rare escapers can progress to giant pupae that fail to eclose as adults (Figure 3F).
Figure 3.—
Phenotypes of KE1-1 eye clones, mutant larvae, and pupae. (A) Wild-type Drosophila eye. (B) Rbf−, w clone generated in the screening stock. (C) Clone of KE1-1 generated in the Rbf− w screening stock. The KE1-1, Rbf−, w cells die due to synthetic lethality, leaving the eye populated with Rbf−, M, w+ cells. (D) Clone of KE1-1 generated in the Rbf+ counterscreen stock. The KE1-1, Rbf+, w cells are viable, demonstrating that KE1-1 is not cell lethal on its own. (E) Large larva phenotype of a KE1-1/KE1-1 wandering third instar larva (left) compared to a KE1-1/+ larva (right). (F) Rare KE1-1/KE1-1 escaper pupae (left) are also large compared to KE1-1/+ pupae (right). Full genotypes of flies shown in B–D are: (B) RbfSLS-15, PExp{FRT2.1[Rbf+, w+, 3.5ey-FLP]}; P{ry[+t7.2] = neoFRT}42D P{w[+mC] = piM}45F M(2)53[1]/P{ry[+t7.2] = neoFRT}42D iso2; P{ry[+7.2] = ey-FLP.N}6, ry[506}]; (C) w, RbfSLS-15, Pexp{FRT2.1 [Rbf+, w+, 3.5ey-FLP]}; P{ry[+t7.2] = neoFRT}42D, P{w[+mC] = piM}45F, M(2)53[1]/ P{ry[+t7.2] = neoFRT}42D, iso2[KE1-1]; P{ry[+7.2] = ey-FLP.N}6, ry[506]/+; (D) w; P{ry[+t7.2] = neoFRT}42D, P{w[+mC] = piM}45F, M(2)53[1]/P{ry[+t7.2] = neoFRT}42D, iso2[KE1-1]; P{ry[+7.2] = ey-FLP.N}6, ry[506]/+.
To identify the KE1-1 locus, we defined the chromosomal region sufficient to confer synthetic lethality in eye clones using standard recombination mapping with visible markers (Table 3). This analysis defined a region at the tip of 2R distal to sp at 60C as necessary and sufficient to confer the Rbf− synthetic lethal phenotype. When homozygous, this chromosomal region also produced a lethal phenotype with large larvae and delayed pupation. This demonstrated that the Rbf−-dependent synthetic lethality, large larval phenotype, developmental delay, and organismal lethality all cosegregate with the region distal to 60C and suggested that a single locus might be responsible for all the observed phenotypes. Organismal lethality was used for further mapping and revealed that KE1-1 failed to complement an existing chromosomal deletion spanning 60C6 to 60D9–10 (Table 4, BL-2604). Using the targeted deletion strategy previously described (Parks et al. 2004), this large deficiency was then subdivided into five small overlapping deletions with molecularly defined endpoints. Only one of the small deletions generated, Df(2)Exel8091 (60D4–60D14), failed to complement the organismal lethality present on the KE1-1 chromosome (Table 5), placing the locus responsible for homozygous lethality between genomic coordinates 20047526 and 20093250 (FlyBase v4.0).
TABLE 5.
Fine-scale mapping ofKE1 organismal lethality using custom-generated deficiencies
| Deficiency stock | Left end | Right end | Viability with KE1-1 and KE1-2 |
|---|---|---|---|
| BL-2604 | 60C6 | 60D9–10 | Lethal |
| Df(2R)Exel6278 | 60C7 | 60D4 | Viable |
| Df(2R)Exel6278 | 60C7 | 60D4 | Viable |
| Df(2R)Exel9043 | 60C7 | 60C7 | Viable |
| Df(2R)Exel7185 | 60C8 | 60D3 | Viable |
| Df(2R)Exel7186a | 60D10 | 60E1 | Viable |
| Df(2R)Exel8091a | 60D4 | 60D14 | Lethal |
Deficiency name or stock number tested is given in column 1. The left- and right-hand cytogenetic locations are given in columns 2 and 3, and the lethality or viability when the deficiency was scored with KE1-1 and KE1-2 is given in column 4.
Df was not permanently stocked.
Confirmation that CG3511 mutations confer the Rbf− synthetic interaction phenotype:
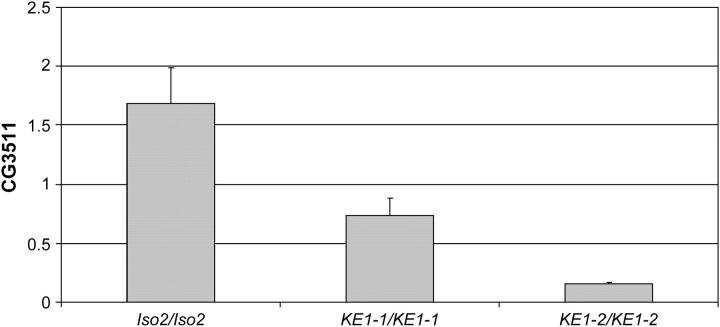
We sequenced several candidate open reading frames between these coordinates and identified lesions in one open reading frame, CG3511, which is predicted to encode a previously uncharacterized protein with similarities to cyclophilins. The predicted KE1-1 cDNA contains a pair of missense mutations at nucleotides 569 and 570, followed by a single-base-pair deletion at nucleotide 572 (Figure 4A). These changes are predicted to cause a frameshift at amino acid 133 and the early truncation of the protein at residue 158 (Figure 4, B and C). While the mutations in CG3511 confer organismal lethality, proof that this mutation alone was sufficient to cause the synthetic interaction with Rbf− in eye clones remained to be shown. We therefore conducted a noncomplementation screen to identify additional mutations in CG3511 and tested their ability to prevent the survival of Rbf− clones (Figure 5, materials and methods). From this screen we isolated KE1-2, which also displayed the large larva phenotype when homozygous or when in trans to KE1-1 (data not shown). KE1-2 was also lethal over Df(2)Ex8091 and BL-2604 (Table 5), confirming that KE1-2 likely represents a second allele of CG3511. Sequencing of the open reading frame of CG3511 and its adjacent 5′ region (into CG12252) did not reveal any mutations, so we analyzed transcript levels in mutant larvae by RT-PCR. Transcript levels of CG3511 were reduced >90% in KE1-2 larvae compared to the parental strain (Figure 6). A reduction in transcript levels of ∼50% was also observed in homozygous KE1-1 larvae. This reduction in mRNA levels in mutants was specific to CG3511, since transcript levels of adjacent genes were present at normal levels (data not shown). A likely explanation is that the KE1-2 mutant contains an aberration in a distant cis-regulatory element controlling the transcript levels of CG3511. KE1-2 was introduced into our screening and counterscreening strains to test its interactions with Rbf in the eye. Clones homozygous for KE1-2 failed to survive in Rbf− but not Rbf+ eyes, confirming that the mutation on the KE1-2 chromosome is sufficient to confer the Rbf− synthetic phenotype (data not shown). As with KE1-1, recombination mapping using visible markers demonstrated that the Rbf−-dependent synthetic lethality, large larvae phenotype, and organismal lethality of KE1-2 all cosegregated with the region distal to 60C, containing CG3511. Thus, even though we were unable to define the nucleotide changes in KE1-2 mutants, these mapping data suggest that the KE1-2 chromosome contains a lesion that cosegregates with the same narrowly defined region containing CG3511 and that causes a reduction in the levels of this transcript. The most plausible explanation is that the KE1-2 mutant chromosome bears a lesion in a cis-regulatory element in CG3511, and that the observed reduction in transcript levels is sufficient to confer the Rbf−-dependent phenotype.
Figure 4.—
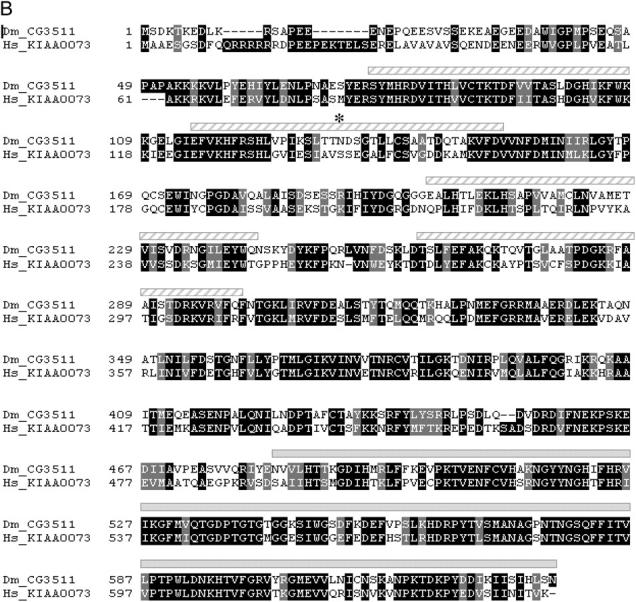
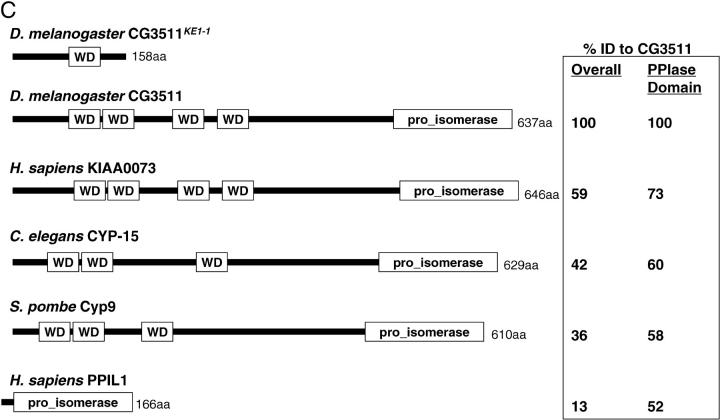
CG3511 encodes a unique and highly conserved peptidyl prolyl isomerase protein. (A) The KE1-1 mutant contains a two-nucleotide substitution and a single-base-pair deletion in the transcript of CG3511-RA, when compared to wild type. A partial sequence of the transcript between nucleotides 545 and 600 is shown, with the changes present in the KE1-1 mutant given in boldface type. (B) Protein sequence alignment of CG3511 and its predicted human ortholog KIAA0073. Identical residues are shaded in black, similar residues are shaded gray. The WD domains and prolyl isomerase domain predictions are graphically represented above the alignment by hatched bars and solid bars, respectively. An asterisk denotes the location of the first frameshifted residue in the KE1-1 mutant. (C) Conservation of predicted proteins and domains encoded by the KE1-1 allele, wild-type CG3511, and selected eukaryotic orthologs. PPIL1 represents the next closest PPIase to CG3511 and is shown for comparison. The organization of WD motifs and the peptidyl prolyl isomerase within the proteins is depicted by boxes. Percentage sequence identities throughout the proteins and within the conserved peptidyl prolyl isomerase domains are shown.
Figure 5.—
F2 lethal noncomplementation screen for additional KE1 alleles. Mutagenized yw; FRT(42D); ey-FLP males were mated to females bearing additional copies of ey-FLP. Single male F1 progeny, heterozygous for the newly induced mutations, were mated to KE1-1 females and the F2 progeny were scored for the absence of [FRT(42D)*/KE1-1] flies.
Figure 6.—
CG3511 is underexpressed in KE1 mutants. Quantitative analysis of CG3511 transcript levels in larvae is shown. The y-axis shows normalized CG3511 transcript levels (see materials and methods) present in wild-type (IsoFSR), KE1-1, and KE1-2 mutant third instar larvae. The reduction in transcript levels observed in the KE1-2 larvae is >10-fold.
The product of CG3511 is predicted to encode a protein of 637 amino acids. The N terminus of the protein contains four WD domains, which often impart protein interaction and scaffolding functions to proteins (Smith et al. 1999). At the carboxyl terminus is a cyclophilin-type peptidyl prolyl isomerase (PPIase) domain. Sequence analysis reveals that CG3511 is 59% identical overall to its predicted human ortholog, KIAA0073, and 73% identical within its prolyl isomerase domain (Figure 4, B and C). The peptidyl prolyl isomerase-like (PPIL) proteins are the next most closely related PPIases, but they lack WD domains and are predicted to be orthologs of other fly proteins. Single CG3511 orthologs are found throughout eukaryotes, including nematode CYP-15 and fission yeast Cyp9. The inclusion of multiple WD domains distinguishes these unique PPIases from others described to date.
DISCUSSION
We have designed and carried out a screen in which overlapping clones of mutant cells are generated in the eye in such a way as to allow screening of recessive mutations for synthetic lethality in the F1 generation. This scheme made it possible to screen through large numbers of mutations without having to set up individual lines and therefore allowed for the isolation of the very rare Rbf synthetic lethal mutations.
Peptidyl prolyl isomerases belong to an extended protein superfamily whose members all catalyze the cis-trans isomerization of proline imidic bonds in polypeptides. The superfamily includes the cyclophilin-like peptidyl prolyl isomerases (Cyp), the FK-506-binding proteins (immunophilin/FKBP), and the parvulin/Pin proteins (Shaw 2002). In addition to sequence and structural divergence, differences in substrates and sensitivity to inhibitors distinguish members within these families (Harrison and Stein 1990; Hennig et al. 1998). Mechanistically, interconversion of x-Pro bond cis-trans conformation can alter protein folding and the conformation of the native state, leading to potential effects on protein function and regulation of serine/threonine phosphorylation events (Andreotti 2003; Weiwad et al. 2004). PPIases have been shown to play diverse functional roles in the cell and some, like Pin1, have been implicated in cellular transformation and human cancer (Bao et al. 2004; Yeh et al. 2004).
There is considerable evidence in the literature to support a mechanistic link between the PPIase Pin1 and its regulation of the cell cycle and apoptosis (Lu 2003; Urist and Prives 2004). Pin1 alters the conformation of the p53 family members p53 and p73 and is required for them to induce the DNA damage checkpoint in response to genotoxic stress (Zacchi et al. 2002; Zheng et al. 2002; Urist and Prives 2004). Pin1 has also been shown to interact with Cdc25 and Plk1 and to modulate Cyclin D1 expression levels and activity and Rb phosphorylation (Liou et al. 2002; Shaw 2002; You et al. 2002). In turn, Pin1 itself is a direct target of E2F activity, participating in a positive feedback loop involving cyclin D1/Cdks, E2F, and RB1 (Ryo et al. 2002). Loss of Pin1 in mouse embryonic fibroblasts causes cell cycle defects and decreases the levels of cyclinD1 and phosphorylated RB1 (You et al. 2002). Similarly, Pin1 knockout mice display a range of proliferative defects, many of which are attributed to its effects on Cyclin D1 (Liou et al. 2002). Although KIAA0073, the human ortholog of CG3511, has not been studied as extensively as Pin1, it is possible that KIAA0073 and other PPIases aside from Pin1 might also interact with components of the cell cycle and checkpoint pathways, as was previously suggested from the comparatively mild knockout phenotype observed for Pin1 (Liou et al. 2002).
In summary, we describe a novel conserved gene, CG3511, which when mutated (as in KE1-1) or when its transcript levels are reduced in abundance (as in KE1-2) results in the specific loss of Rbf− cells in the Drosophila eye. Future experiments will elucidate how the PPIase protein family may interact with RB1 to regulate cell survival and/or proliferation. KIAA0073 may represent an efficacious and novel anti-cancer drug target whose inhibition might result in the specific death of RB1 mutant cells. Such a synthetic lethal target would have applications in several RB1 pathway-dependent cancers, such as SCLC (Sherr and McCormick 2002), and may represent a unique opportunity for targeted therapeutics.
Acknowledgments
The authors acknowledge the members of the Exelixis Flytech and Flycore teams for their role in the establishment and maintenance of stocks used as mapping tools in this screen; members of the Genome Biochemistry department, particularly Damien Curtis and Michael Cancilla, for developing the mutation detection protocols; and members of the Bioinformatics team for their role in database and informatics tool development. We also thank members of the Genetics department, in particular the oncology team for their helpful discussions and participation in the screens, especially Daniel Curtis for guidance and Mike Costa for his continuous intellectual input and critical reading of the manuscript. This work was carried out as part of the oncology alliance between Exelixis and Bristol-Myers Squibb.
References
- Andreotti, A. H., 2003. Native state proline isomerization: an intrinsic molecular switch. Biochemistry 42: 9515–9524. [DOI] [PubMed] [Google Scholar]
- Bao, L., A. Kimzey, G. Sauter, J. M. Sowadski, K. P. Lu et al., 2004. Prevalent overexpression of prolyl isomerase Pin1 in human cancers. Am. J. Pathol. 164: 1727–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau, B. N., and J. Y. Wang, 2003. Coordinated regulation of life and death by RB. Nat. Rev. Cancer 3: 130–138. [DOI] [PubMed] [Google Scholar]
- Cryns, V. L., A. Thor, H. J. Xu, S. X. Hu, M. E. Wierman et al., 1994. Loss of the retinoblastoma tumor-suppressor gene in parathyroid carcinoma. N. Engl. J. Med. 330: 757–761. [DOI] [PubMed] [Google Scholar]
- Datar, S. A., H. W. Jacobs, A. F. de la Cruz, C. F. Lehner and B. A. Edgar, 2000. The Drosophila cyclin D-Cdk4 complex promotes cellular growth. EMBO J. 19: 4543–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick, F. A., and N. Dyson, 2003. pRB contains an E2F1-specific binding domain that allows E2F1-induced apoptosis to be regulated separately from other E2F activities. Mol. Cell 12: 639–649. [DOI] [PubMed] [Google Scholar]
- Du, W., and N. Dyson, 1999. The role of RBF in the introduction of G1 regulation during Drosophila embryogenesis. EMBO J. 18: 916–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, W., M. Vidal, J. E. Xie and N. Dyson, 1996. RBF, a novel RB-related gene that regulates E2F activity and interacts with cyclin E in Drosophila. Genes Dev. 10: 1206–1218. [DOI] [PubMed] [Google Scholar]
- Dyson, N, 1998. The regulation of E2F by pRB-family proteins. Genes Dev. 12: 2245–2262. [DOI] [PubMed] [Google Scholar]
- Harbour, J. W., and D. C. Dean, 2000. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 14: 2393–2409. [DOI] [PubMed] [Google Scholar]
- Harrison, R. K., and R. L. Stein, 1990. Substrate specificities of the peptidyl prolyl cis-trans isomerase activities of cyclophilin and FK-506 binding protein: evidence for the existence of a family of distinct enzymes. Biochemistry 29: 3813–3816. [DOI] [PubMed] [Google Scholar]
- Helt, A. M., and D. A. Galloway, 2003. Mechanisms by which DNA tumor virus oncoproteins target the Rb family of pocket proteins. Carcinogenesis 24: 159–169. [DOI] [PubMed] [Google Scholar]
- Hennig, L., C. Christner, M. Kipping, B. Schelbert, K. P. Rucknagel et al., 1998. Selective inactivation of parvulin-like peptidyl-prolyl cis/trans isomerases by juglone. Biochemistry 37: 5953–5960. [DOI] [PubMed] [Google Scholar]
- Kubota, Y., K. Fujinami, H. Uemura, Y. Dobashi, H. Miyamoto et al., 1995. Retinoblastoma gene mutations in primary human prostate cancer. Prostate 27: 314–320. [DOI] [PubMed] [Google Scholar]
- Lambertsson, A., 1998. The minute genes in Drosophila and their molecular functions. Adv. Genet. 38: 69–134. [DOI] [PubMed] [Google Scholar]
- Liou, Y. C., A. Ryo, H. K. Huang, P. J. Lu, R. Bronson et al., 2002. Loss of Pin1 function in the mouse causes phenotypes resembling cyclin D1-null phenotypes. Proc. Natl. Acad. Sci. USA 99: 1335–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, K. P., 2003. Prolyl isomerase Pin1 as a molecular target for cancer diagnostics and therapeutics. Cancer Cell 4: 175–180. [DOI] [PubMed] [Google Scholar]
- Lundberg, A. S., and R. A. Weinberg, 1998. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol. Cell. Biol. 18: 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, F., P. Finn and N. B. La Thangue, 2003. The cell cycle, chromatin and cancer: mechanism-based therapeutics come of age. Drug Discov. Today 8: 793–802. [DOI] [PubMed] [Google Scholar]
- Minna, J. D., J. A. Roth and A. F. Gazdar, 2002. Focus on lung cancer. Cancer Cell 1: 49–52. [DOI] [PubMed] [Google Scholar]
- Miyamoto, H., T. Shuin, S. Torigoe, Y. Iwasaki and Y. Kubota, 1995. Retinoblastoma gene mutations in primary human bladder cancer. Br. J. Cancer 71: 831–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, L., K. E. Allen and N. B. La Thangue, 2000. Regulation of E2F transcription by cyclin E-Cdk2 kinase mediated through p300/CBP co-activators. Nat. Cell Biol. 2: 232–239. [DOI] [PubMed] [Google Scholar]
- Nevins, J. R., 2001. The Rb/E2F pathway and cancer. Hum. Mol. Genet. 10: 699–703. [DOI] [PubMed] [Google Scholar]
- Ogawa, H., K. Ishiguro, S. Gaubatz, D. M. Livingston and Y. Nakatani, 2002. A complex with chromatin modifiers that occupies E2F- and Myc-responsive genes in G0 cells. Science 296: 1132–1136. [DOI] [PubMed] [Google Scholar]
- Parks, A. L., K. R. Cook, M. Belvin, N. A. Dompe, R. Fawcett et al., 2004. Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat. Genet. 36: 288–292. [DOI] [PubMed] [Google Scholar]
- Ryo, A., Y. C. Liou, G. Wulf, M. Nakamura, S. W. Lee et al., 2002. PIN1 is an E2F target gene essential for Neu/Ras-induced transformation of mammary epithelial cells. Mol. Cell. Biol. 22: 5281–5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, P. E., 2002. Peptidyl-prolyl isomerases: a new twist to transcription. EMBO Rep. 3: 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr, C. J., and F. McCormick, 2002. The RB and p53 pathways in cancer. Cancer Cell 2: 103–112. [DOI] [PubMed] [Google Scholar]
- Smith, T. F., C. Gaitatzes, K. Saxena and E. J. Neer, 1999. The WD repeat: a common architecture for diverse functions. Trends Biochem. Sci. 24: 181–185. [DOI] [PubMed] [Google Scholar]
- Stevaux, O., and N. J. Dyson, 2002. A revised picture of the E2F transcriptional network and RB function. Curr. Opin. Cell Biol. 14: 684–691. [DOI] [PubMed] [Google Scholar]
- Urist, M., and C. Prives, 2004. The linchpin? Pin1 meets p73. Cancer Cell 5: 515–517. [DOI] [PubMed] [Google Scholar]
- Vermeulen, K., D. R. Van Bockstaele and Z. N. Berneman, 2003. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 36: 131–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiwad, M., A. Werner, P. Rucknagel, A. Schierhorn, G. Kullertz et al., 2004. Catalysis of proline-directed protein phosphorylation by peptidyl-prolyl cis/trans isomerases. J. Mol. Biol. 339: 635–646. [DOI] [PubMed] [Google Scholar]
- Xin, S., L. Weng, J. Xu and W. Du, 2002. The role of RBF in developmentally regulated cell proliferation in the eye disc and in Cyclin D/Cdk4 induced cellular growth. Development 129: 1345–1356. [DOI] [PubMed] [Google Scholar]
- Yeh, E., M. Cunningham, H. Arnold, D. Chasse, T. Monteith et al., 2004. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat. Cell Biol. 6: 308–318. [DOI] [PubMed] [Google Scholar]
- You, H., H. Zheng, S. A. Murray, Q. Yu, T. Uchida et al., 2002. IGF-1 induces Pin1 expression in promoting cell cycle S-phase entry. J. Cell. Biochem. 84: 211–216. [DOI] [PubMed] [Google Scholar]
- Zacchi, P., M. Gostissa, T. Uchida, C. Salvagno, F. Avollo et al., 2002. The prolyl isomerase Pin1 reveals a mechanism to control p53 functions after genotoxic insults. Nature 419: 853–857. [DOI] [PubMed] [Google Scholar]
- Zheng, H., H. You, X. Z. Zhou, S. A. Murray, T. Uchida et al., 2002. The prolyl isomerase Pin1 is a regulator of p53 in genotoxic response. Nature 419: 849–853. [DOI] [PubMed] [Google Scholar]