Abstract
We identified the Drosophila melanogaster Signal peptide peptidase gene (Spp) that encodes a multipass transmembrane aspartyl protease. Drosophila SPP is homologous to the human signal peptide peptidase (SPP) and is distantly related to the presenilins. We show that, like human SPP, Drosophila SPP can proteolyze a model signal peptide and is sensitive to an SPP protease inhibitor and that it localizes to the endoplasmic reticulum. Expression of Drosophila SPP was first apparent at germ band extension, and in late embryos it was robust in the salivary glands, proventriculus, and tracheae. Flies bearing mutations in conserved residues or carrying deficiencies for the Spp gene had defective tracheae and died as larvae.
THE distribution of proteins in cell membranes can shift rapidly in response to changes in metabolic or developmental conditions. Among the enzymes that help to produce these rapid changes are proteases that bring about the maturation, activation, or relocalization of membrane proteins. Some of these proteases are themselves membrane proteins that cleave within the membrane anchoring transmembrane domain (TMD) of their substrates (Rudner et al. 1999; Weihofen and Martoglio 2003). These proteases, called intramembrane-cleaving proteases (I-CLiP's), are thought to have a catalytic domain within the plane of the lipid bilayer. Here we report the identification of a Drosophila signal peptide peptidase (SPP), an I-CLiP with activity against the TMD of signal peptides.
Intramembrane proteases have been implicated in developmental and disease processes, including cholesterol biosynthesis (Brown and Goldstein 1999), Notch signaling (Levitan and Greenwald 1995; De Strooper et al. 1999; Struhl and Greenwald 1999; Ye et al. 1999; Saxena et al. 2001), EGF signaling (Lee et al. 2001; Urban et al. 2001, 2002), signal peptide processing (Weihofen et al. 2002), and Alzheimer's disease (Scheuner et al. 1996; De Strooper et al. 1998; Kimberly et al. 2001). Presenilin is a member of this class of protease. It is an I-CLiP that is thought to constitute the catalytic core of the γ-secretase complex (Wolfe et al. 1999), a multisubunit enzyme first identified genetically by association with and thereby implicated in familial early onset Alzheimer's disease (Levy-Lahad et al. 1995; Sherrington et al. 1995). Predictions based on hydrophobicity calculations suggest that presenilin has eight TMDs, two of which contain aspartyl residues that lie within the plane of the bilayer and that form a catalytic site. Among the known substrates of presenilin are amyloid precursor protein (APP) (Kimberly et al. 2001), Notch (Levitan and Greenwald 1995; De Strooper et al. 1999; Struhl and Greenwald 1999; Ye et al. 1999; Saxena et al. 2001), and the Notch ligands, Delta and Jagged (Bland et al. 2003; Ikeuchi and Sisodia 2003; LaVoie and Selkoe 2003).
SPP is an intramembrane aspartyl protease with structural and catalytic similarities to presenilins (Weihofen et al. 2002). The human enzyme has been identified and characterized and has been shown to cleave signal peptide fragments that are released during maturation of precursor proteins. Its kinship with presenilin is revealed by its sensitivity to inhibitors directed against the active site of presenilin/γ-secretase (Weihofen et al. 2003; Nyborg et al. 2004). Like presenilin, SPP has multiple transmembrane regions that may assemble its catalytic site in the plane of the lipid bilayer. The active site motifs YD and LGLGD are in the center of adjacent TMDs (Weihofen et al. 2002; Urny et al. 2003). However, the orientation of the SPP active site is apparently inverted relative to the active site of presenilin, and it is predicted to cleave type II transmembrane domains that have their amino termini on the cytoplasmic side of the membrane, as opposed to presenilin substrates that have a type I orientation. Although the role of SPP is not fully understood, it seems likely that its function is not solely to cleanse ER membranes of released signal peptides. Peptides produced by SPP cleavage have been shown to bind and regulate calmodulin and class I major histocompatibility molecules (Martoglio et al. 1997; Braud et al. 1998; Lemberg et al. 2001), so it is possible that SPP functions to produce and release functional peptides or proteins from membranes and could be regulated to target specific substrates.
Our goal in this study was to determine whether SPP plays a role in animal development. We identified the Drosophila SPP ortholog and found that, like the human SPP, this enzyme has proteolytic activity in vitro. We also identified a number of mutants defective in Drosophila SPP and found that SPP has an essential role during larval development.
MATERIALS AND METHODS
Cloning of Spp and rescue construct:
The proximal 5-kb EcoRI fragment of the λ-phage clone S2-6 (Schneitz et al. 1993) was used to isolate CG11840 cDNA clones from a library of embryo cDNA's (Brown and Kafatos 1988); this gene was initially named shanti, but with the demonstration that it encodes a signal peptide peptidase, is henceforth called Spp. Two Spp cDNA's (LD08101 and CK00414) were also identified in the EST database of Berkeley Drosophila Genome Project; sequence analysis revealed that LD08101 is full length. Comparison of LD08101 and the 5-kb EcoRI genomic fragment revealed the presence of two small introns (65 and 66 bp), which have consensus 5′ and 3′ splice sites.
The pP(W8, Spp+) rescue construct was made by inserting a 5.3-kbp genomic DNA fragment containing the Spp gene into the P-element vector P(W8) (Klemenz et al. 1987). This genomic region includes the complete SPP coding sequence, beginning 3677 bases upstream of the initiating methionine and extending 381 nucleotides (5360–4979) beyond the stop codon. This region does not include the protein coding sequence from lwr. The pP(W8, SppM) rescue construct is identical except that the two catalytic site aspartates were mutated to alanines. For pP(H-Pelican, lwr+), a 2.4-kb lwr genomic sequence was amplified from genomic DNA using oligo lwrF1 (CCATCTACCGCAGTCCATAGCTC) and oligo lwr1214-220 (CGTTGGTAGCCTACTAGAAG). The amplified fragments were cloned into the pCR 2.1 vector (TOPO-TA cloning kit, Invitrogen, Carlsbad, CA) and then into the P-element vector pP(H-Pelican) (Barolo et al. 2000). pP(H-Pelican, lwr+) contains the entire lwr coding region, but not the Spp coding region. Germline transformation was performed as described by Spradling (1986).
A second chromosome integrant of P(H-Pelican, lwr+) was recombined with the Df(2L)lwr14 deficiency. This chromosome was viable in trans with both lwr05486 and lwr5, indicating that P(H-Pelican, lwr+) can rescue mutations in lwr; however, Df(2L)lwr14 is not rescued by one or two copies of P(H-Pelican, lwr+), indicating that this deficiency removes at least one other essential gene. A second chromosome integrant of pP(W8, Spp+) was recombined with Df(2L)lwr14. The resulting Df(2L)lwr14, pP(W8, Spp +) chromosome was viable with Df(2L)lwr14, P(H-Pelican, lwr+), indicating that the lwr14 deficiency carries essential mutations in both the lwr and the Spp genes. Germline clones were made from Df(2L)lwr14, P(H-Pelican, lwr+), P(ry+r7.2=neoFRT)40A females.
Mutagenesis of Spp and sequence analysis:
Flies were cultured on standard molasses/cornmeal medium at 25°. Mutagenesis was carried out by feeding EMS to 150 isogenized cn1 bw1 males as by the method of Lewis and Bacher (Lewis 1968). These males were mated en masse for 3 days with Sco/Cyo females and individual F1 progeny were tested for complementation with Df(2L)lwr14, P(H-Pelican, lwr+). Df(2L)lwr14 removes part of both the lwr and the Spp genes. Five second chromosomes were recovered that failed to complement Df(2L)lwr14, P(H-Pelican, lwr+), and the recovered alleles defined a single complementation group; three of these were characterized further.
To analyze the lesions in the Spp5 and Spp7 mutants, genomic DNA was isolated from homozygous mutant larvae, which were identified as the nonfluorescent progeny in cultures of Spp5/CKG19 and Spp7/CKG19 flies (CKG19: CyO, Kr-GAL4, UAS-GFPS65T; Casso et al. 2000). Genomic DNA was isolated from extracts of 10 homozygous mutant Spp5 and Spp7 larvae using a protocol for DNA extraction from single flies (Ashburner protocol 48). The Spp6 mutation was characterized using DNA extracted from Spp6/SM6a adult flies. The entire Spp coding region was amplified in three independent PCR reactions using oligos F (ggcgattttcaggaacggattggattgg) and R (aaagtcgagggaacattttctacaattg), and sequences were obtained from cloned PCR products.
Cell culture and histology:
c-MYC epitope tags (EQKLISEEDL) were added to the N terminus (using oligos aaaATGgaacaaaaacttatttctgaagaagacctgGCGGAGGAAGTCATCGGAACCG and taactcgagCTACTTGCCCTTTTTCGACTCC) and to the C terminus (using oligos ttagaattcgtATGGCGGAGGAAGTCATCG and acaagcttctaCAGGTCTTCTTCAGAGATGAGTTTTTGTTCCTTGCCCTTTTTCGACTCC) of SPP by PCR. After the sequences were confirmed by DNA sequence analysis, the fusion constructs were cloned into vector pUAST (Brand and Perrimon 1993) in which expression is controlled by the Saccharomyces cerevisiae enhancer binding protein GAL4 to create both pUAS-SppCmyc, which has a carboxy-terminal cMyc tag, and pUAS-SppNmyc, which has an amino-terminal tag.
S2 cells were grown in Shields and Sang M3 Media (Sigma-Aldrich) supplemented with 12.5% heat-inactivated fetal bovine serum, 2.5 g/liter Bacto-peptone, and 1 g/liter yeastolate. Cells were cotransfected with pA5c-GAL4, a plasmid in which the expression of GAL4 is controlled by the Drosophila actin Act5C promoter (Ishikawa et al. 1999), and either pUAS-SppNmyc or pUAS-SppCmyc using Effectene (QIAGEN, Valencia, CA). Cotransfected with these was marker plasmid pA5cGG105, expressing a fusion of calreticulin (Crc, CG9429), GFP, and KDEL, which marks ER only, or marker plasmid pA5cGG112, expressing a fusion of KDEL receptor (KdelR, CG5183) with GFP, which localizes to the Golgi and ER. In our experiments, KdelR-GFP localization in punctate cytoplasmic structures characteristic of the Golgi was the most prominent. Two days after transfection, cells were plated on Permanox chamber slides coated with concanavalin-A to facilitate adherence to the substrate (Rogers et al. 2002), fixed in phosphate-buffered saline (PBS) containing 4% formaldehyde for 20 min, washed in PBS, permeabilized in PBS containing 0.1% Tween-20 and 3% BSA for 30 min (PBSTw), and washed. Slides were then incubated with mouse anti-Myc antibody at 1:1000 (sc-789, Santa Cruz Biotechnology) followed by Cy3-conjugated donkey anti-mouse secondary antibody at 1:1000 (Jackson Laboratory), both in PBSTw.
In situ hybridizations were carried out as described (O'Neill and Bier 1994; Biehs et al. 1998) using a digoxygenin-labeled Spp anti-sense riboprobe to the entire 1.1-kb coding region. Embryos were from an overnight collection from y w or Scr2/TM3-ftz-lacZ flies, and salivary glands were from wandering stage third instar y w larvae. Control hybridizations with a sense Spp digoxygenin-labeled riboprobe showed no specific staining.
The state of air filling in the dorsal trunks of the trachea of homozygous mutant first instar larvae was assessed just after hatching using standard optics on a Leica DMR compound microscope. Upon air filling, the trachea become more refractive and appear dark. Mutant phenotypes ranged from breaks in normal air filling to larvae without any discernible air filling (Figure 4, K and L). The percentage of larvae with normal air filling was determined (Table 1). The non-air-filled portions of the tracheal trunks were visualized using Nomarski optics (data not shown).
Figure 4.—
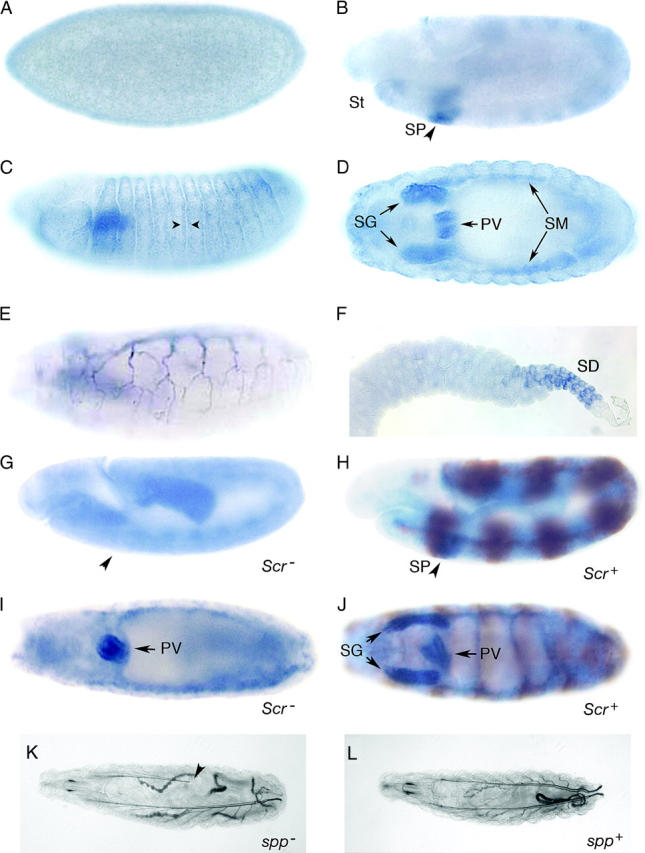
Spp expression in embryos and larvae. (A–D) Expression in embryos was not detectable at cellularization (A), but at germ band extension was present (B, arrowhead) in the salivary primordium (SP) posterior to the stomodeal invagination (St). Enhanced levels of expression are seen in older embryos adjacent to the segmental involutions (C, arrowheads) and in the salivary glands (SG), proventriculus (PV), and somatic musculature (SM). (E) Expression in the tracheal branches of a late stage embryo. (F) Expression in the salivary gland duct cells (SD) of a third instar larva. Expression in Scr2 mutants (G and I) and their Scr2/+ siblings (H and J). Brown stripes indicate anti-β-galactosidase staining from a balancer chromosome. Light micrograph showing the longitudinal dorsal tracheal trunks of an Spp mutant (K) and wild-type (L) first instar larva. Incomplete tracheal air filling in the tracheal dorsal trunks in the Spp mutant is indicated with an arrow. Anterior is left except in F.
TABLE 1.
SPP mutants: lesions and tracheal phenotypes
| Spp allele | Protein mutation | DNA mutation | Tracheal air filling (%) |
|---|---|---|---|
| Df(2L)lwr14 | Fusion of aa 1–282 to 21 novel residues | 1137-base deletion | 15 |
| Spp5 | L → F 95 | C → T 283 | 70 |
| Spp6 | K → stop 295 | 29-base deletion | 0 |
| Spp7 | P → L 248 | C → T 743 | 38 |
Four recessive lethal alleles of the Drosophila Spp gene are shown, along with a description of their genetic lesions and predicted effects on the resulting SPP proteins. The percentage of larvae with normal air filling is shown. A total of 95% of both wild-type and heterozygous Spp mutant animals had normal tracheal air filling.
Proteolysis assays:
The Spp gene coding region was cloned into p426gal1 (Mumberg et al. 1995) in which its expression is controlled by a GAL4-dependent promoter. This plasmid (p426gal-Spp), p426gal1, and pDAW300 [p426gal carrying human SPP (Weihofen et al. 2002)] were transformed into S. cerevisiae (MATα; his3Δ, leu2Δ0; lys2Δ0; ura3Δ0) and expression was induced in SC media containing 2% galactose overnight at 20° to an OD of 0.6. SPP assays were done as described (Weihofen et al. 2002), with extracts prepared from crude microsomes by solubilizing in buffer containing CHAPS. The model human HLA-E signal sequence substrate (HLA-A/24) was resolved by SDS-PAGE.
Manipulation of SPP levels by ectopic expression and RNA interference:
Wild-type Spp cDNA and SppAS, an Spp cDNA in which the putative catalytic aspartates were replaced with alanine (228) and serine (274), were subcloned into pUAST (Brand and Perrimon 1993). An 1167-bp fragment comprising the entire Spp coding region minus the initiating methionine codon was subcloned in a tail-to-tail orientation in pWIZ, a P-element vector for the GAL4-dependent expression of double-stranded RNA hairpins (Lee and Carthew 2003). The sequence of these constructs was confirmed by sequence analysis. The two insertions with strongest expression were both mapped to the second chromosome and then recombined onto a single second chromosome.
RESULTS
Identification of Drosophila Spp:
This investigation began as a search for the gene altered in the oroshigane mutant strain, which has a phenotype that resembles hedgehog loss of function (Epps et al. 1997). Although we did not identify a gene in the oroshigane region that could be correlated with the mutant phenotype, an unrelated gene in the region (CG11840) that encodes a candidate I-CLiP was found in the course of these studies. This article describes the general properties and preliminary genetic characterization of this gene, which we call signal peptide peptidase (Spp).
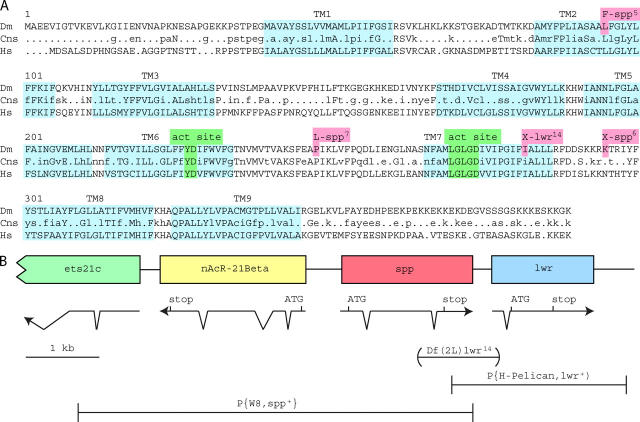
DNA sequence was obtained from wild-type flies for the Spp genomic region and for two Spp cDNA's (Figure 1). The Spp transcription unit spans 1757 bases and has two small introns (65 and 66 nucleotides). The encoded protein has 389 residues that topology predictions estimate will span the membrane nine times. TMDs 6 and 7 have centrally located aspartates, which may comprise active site residues in the catalytic domain of the enzyme in a manner similar to intramembrane cleaving aspartyl proteases such as presenilin. A BLASTP score of 439 for SPP (Altschul et al. 1990) suggests that SPP shares functional homology with human aspartyl protease SPP (Weihofen et al. 2002) and kinship with a family of I-CliP's that has known members in plants, vertebrates, and invertebrates (Ponting et al. 2002). Figure 1A illustrates the apparent consensus sequence for this family and shows that the regions with greatest conservation reside in the portions that are predicted to form the active site, helices 6 and 7. The consensus sequences in helices 6 and 7, YD and LGLGD, respectively, are also found in the active site of presenilin. The C terminus of SPP has a likely ER retention sequence, KKXX. Assuming that KKXX is directed to the cytoplasmic compartment, the orientation of helices 6 and 7 and of the active site is predicted to be opposite to that of presenilin and to be the same as that of human SPP.
Figure 1.—
The Drosophila Spp gene. (A) The protein sequence of Drosophila SPP (Dm) aligned with human SPP (Hs) and an SPP family consensus sequence derived from four species [D. melanogaster (CG11840), human (NP_110416), Mus musculus (BAC25752), and Arabidopsis thaliana (NP_565294)] using the program MultAlin (Corpet 1988). Capitalized amino acids indicate absolute conservation among the four species, while lowercase characters represent amino acids conserved in three of the four. Residues mutated in alleles 5, 6, and 7 (red) and those that make up the catalytic core (green) are highlighted. Transmembrane regions (TM1–9) shown in blue are based on predictions by the algorithm TopPred II (Claros and von Heijne 1994). TM5 was predicted for Drosophila and Arabidopsis SPP, but not for the human or the mouse sequences. (B) The Spp locus and neighboring genes are shown. Predicted translational start and stop codons are shown above a diagram of the intron/exon structures. The regions used in two genomic rescue constructs P(W8, Spp+) and P(H-Pelican, lwr+) as well as the sequence deleted in Df(2L)lwr14 are shown.
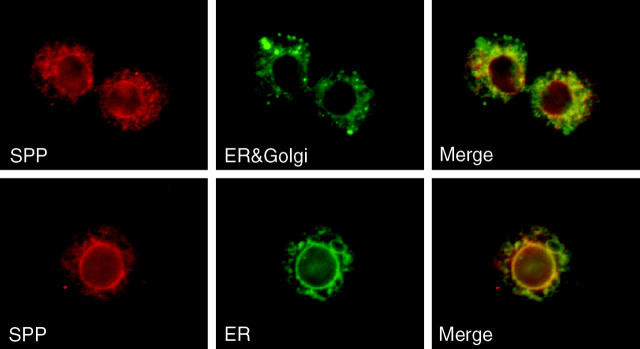
When a MYC-SPP fusion was expressed in cultured Drosophila S2 cells, it distributed both in a perinuclear ring and in a lacy reticular pattern outside the nucleus. This distribution is consistent with ER localization (Figure 2). In cells that were cotransfected with both MYC-SPP and a calreticulin-GFP-KDEL fusion protein (a marker for the ER), significant colocalization was observed (Figure 2, bottom). However, MYC-SPP only weakly colocalized with a KDEL-Receptor-GFP fusion, a marker for Golgi (see materials and methods; Figure 2, top). Similar patterns of subcellular localization were observed with SPP that had an epitope tag on either the amino or the carboxy terminus. Human SPP also terminates with KKXX and localizes to the ER as a C-terminally tagged protein (Nyborg et al. 2004). These data are consistent with the ER localization of SPP and with similar studies showing that murine (Urny et al. 2003) and human (Friedmann et al. 2004) SPP also localize to the ER. We suggest that SPPs may contain ER localization signals in addition to KKXX or that ectopically expressed SPP may associate with endogenous SPP to form a dimer that is retained in the ER/pre-Golgi.
Figure 2.—
Immunofluorescence of Schneider-2 cells expressing MYC-tagged Drosophila SPP. Drosophila SPP that was tagged on either the N terminus (top) or the C terminus (bottom) revealed perinuclear and reticular localization. Left, SPP (red); middle, KDEL-receptor-GFP fusion protein (top) and calreticulin-GFP-KDEL fusion protein (bottom), labeled ER + Golgi and ER, respectively (green); right, merged images.
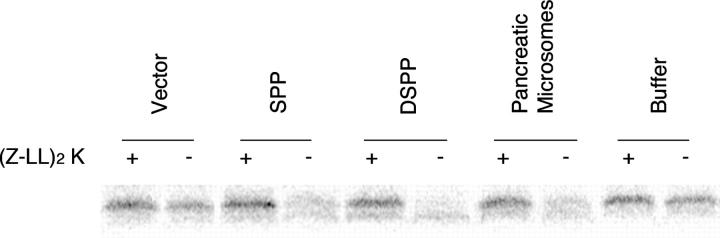
To establish whether the protein encoded by Spp has enzymatic activity as a signal peptide peptidase, we expressed both the human and the Drosophila SPP in S. cerevisiae and assayed extracts from these yeast strains for proteolytic activity on a 24-amino-acid signal peptide derived from the human HLA-A signal sequence. Previous studies showed that this peptide is a substrate for human SPP and that human SPP proteolytic activity is sensitive to 1,3-di-(N-carboxybenzoyl-l-leucyl-l-leucyl)amino acetone [(Z-LL)2-ketone] (Weihofen et al. 2002). This protease inhibitor was shown to block signal peptide processing, but not to affect signal peptidase or other proteases such as cathepsin or the proteasome (Weihofen et al. 2000). We found that the HLA-A derived signal peptide, a previously described substrate of human SPP (Lemberg et al. 2001; Weihofen et al. 2002), was quantitatively cleaved by extracts that contained either human or Drosophila SPP (Figure 3). The peptide was also cleaved by an extract of canine pancreatic microsomes, which has been shown previously to be a source of SPP activity (Weihofen et al. 2002). In contrast, the peptide was stable after incubation with buffer or control yeast extracts or after incubation in the presence of the SPP inhibitor (Z-LL)2-ketone. We conclude that the Drosophila Spp gene encodes a functional SPP enzyme.
Figure 3.—
Signal peptide peptidase activity of Drosophila SPP. In this assay, SDS-PAGE resolves the test peptide but cleavage products could not be identified. In the left six lanes, extracts of solubilized crude microsomes from S. cerevisiae expressing either human SPP or Drosophila SPP or carrying the empty expression vector (vector) were added to radiolabeled human HLA signal peptide (top band) in either the presence or the absence of the SPP inhibitor (Z-LL)2-ketone. In the right four lanes, a solubilized extract of canine pancreatic microsomes (pancreatic microsomes) or solubilization buffer (buffer) as a control were assayed with the same substrate and inhibitor. SPP activity is indicated by the (Z-LL)2-ketone-sensitive loss of the HLA signal peptide band.
Expression and role of Spp during Drosophila development:
We monitored expression of Spp mRNA in Drosophila embryos and larvae by in situ hybridization (Figure 4). No expression was observed during the cellularization or gastrulation stages. Hybridization to Spp probes was first apparent during germ band extension in a region caudal to the stomodeal invagination in the approximate location of invaginating salivary placodes, and it was enhanced very slightly in the epidermal cells adjacent to the segmental folds. Expression increased during germ band retraction and, in later stages, was most prominent in the salivary glands, proventriculus, and trachea. Expression of Spp in the salivary placodes and the embryo salivary glands was dependent on Sex combs reduced (Scr), a homeotic gene whose function is necessary for salivary gland fate (Figure 4, G–J). Expression of Spp was observed in the embryo proventriculus but not the salivary placodes and salivary glands of scr mutant embryos (Figure 4, G–J). In third instar larvae, prominent expression was observed in stalk cells of salivary glands (Figure 4F) and in a nonuniform pattern in wing and leg imaginal discs (not shown). Although murine Spp transcripts have been reported to be abundant in both CNS and PNS tissues (Urny et al. 2003), we did not detect Spp mRNA at significant levels in either the Drosophila CNS or PNS.
To investigate the role of SPP, we generated and analyzed mutants that delete or otherwise alter the Spp gene. A synthetic deletion of the C-terminal 106 residues was constructed using Df(2L)lwr14, which deletes 1136 bp from the 5′ proximal region of Spp and extends into the neighboring lesswright (lwr) gene (Figure 1B) (Apionishev et al. 2001). lwr encodes an essential SUMO-transferase, and its loss results in embryonic lethality and cuticle defects (Epps and Tanda 1998). We restored lwr function with P(H-Pelican, lwr+), which contains a 2-kb genomic fragment that rescues the lethality of lwr mutants carrying Df(2L)lwr14, lwr05486, and lwr5. Df(2L)lwr14, P(H-Pelican, lwr+) homozygous animals, which should lack Spp function only, failed to develop beyond the larval period. These animals arrested growth soon after embryo hatching, surviving for as much as several weeks as small second instar larvae. This phenotype was unchanged in animals that developed from Df(2L)lwr14, P(H-Pelican, lwr+), P(ry+r7.2=neoFRT)40A germline clones, indicating that the survival of the mutants to the larval period is not due to partial rescue by maternal product.
To verify that the lethality of the Spp deficiency was due to loss of SPP, we introduced a 5360-bp genomic fragment containing the Spp gene. Flies homozygous for Df(2L)lwr14 with either the P(H-Pelican, lwr+) or the pP(W8, Spp+) were not viable. However, homozygotes harboring both rescue constructs developed to adults. This result demonstrates that the lwr and Spp functions are the only ones defective in Df(2L)lwr14 and indicates that Spp encodes an essential function for normal development. To determine whether the predicted aspartyl protease activity of SPP was responsible for this rescue, the catalytic site aspartyl diad was mutated to alanines to create pP(W8, SppM). Unlike its wild-type counterpart, pP(W8, SppM) was unable to rescue Df(2L)lwr14 in combination with P(H-Pelican, lwr+), implying a critical role for the protease activity of SPP.
New alleles of Spp were generated by chemical mutagenesis. Five mutants were isolated that failed to complement the Df(2L)lwr14, P(H-Pelican, lwr+) synthetic deficiency or the large deficiency Df(2L)BSC16 that deletes the Spp gene. All were larval lethal and no complementation was observed between any two mutants. We analyzed the three with the most severe larval growth phenotypes: Spp5, Spp6, and Spp7 (Table 1; Figure 1A). Spp6 is the most severe; it truncates SPP at residue 322. Spp6 mutants died as first instar larvae, showing a greater degree of growth retardation than Df(2L)lwr14. Spp5 and Spp7 have point mutations in conserved residues. The leucine residue mutated in Spp5 is conserved in the Psn/SPP superfamily (Ponting et al. 2002); the proline residue mutated in Spp7 is conserved in the SPP family and is in a putative membrane-spanning domain.
Mutant Spp larvae had tracheal air-filling defects. During stage 16 of normal embryogenesis, the luminal cavities of the tracheal tubes evacuate liquid and fill with air. We monitored the extent of air filling in the dorsal trunks of the trachea of first instar larvae. Compared to either wild-type larvae or heterozygous siblings, the trachea of Spp homozygous mutants often failed to fill with air either partially or entirely (Figure 4, K and L; Table 1). This defect in tracheal physiology was rescued in Spp mutants carrying the Spp genomic rescue construct pP(W8, Spp+). Partial rescue was achieved by heat-shock-dependent expression of the Spp cDNA (data not shown). This phenotype is consistent with both the expression of Spp in the trachea and the observation that Spp larvae are markedly lethargic compared to their heterozygous siblings. Using the extent of air filling in mutant larvae to rank the relative severity of the Spp6, Spp7, and Spp5 mutants, Spp5 was the least severe and Spp6 was the most severe.
Growth arrest of strong mutant combinations occurred predominantly in the first and second instars as determined by mouth hook morphology. Growth arrest in Spp mutants was not apparently caused by molting defects, since none of the larvae had two pairs of mouth hooks (a characteristic of mutants defective in the ecdysone pathway). The mutant larvae frequently lived for several weeks and were able to ingest food, but did not grow notably in size and did not progress beyond the larval stages. This “failure to thrive” phenotype suggests a defect in feeding or digestion, but we have no direct evidence for this. To determine if growth arrest in the mutant animals was caused by generalized cell death, we stained both wild-type and mutant Spp6 larvae with propidium iodide, which is excluded by live cells. No differences in number or type of cells stained with propidium iodide were detected between normal and mutant larvae (not shown).
Ectopic expression of SPP in the wing:
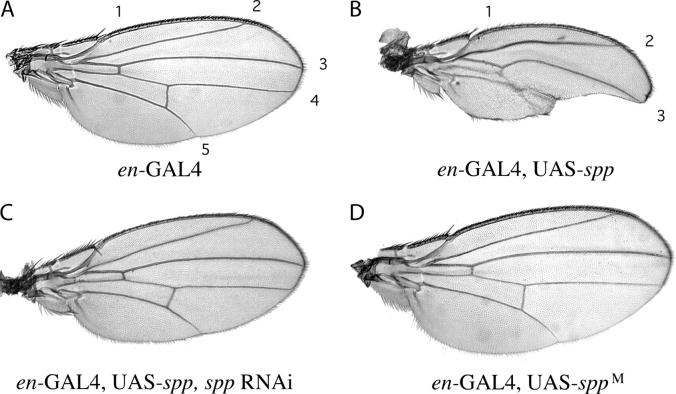
To determine if the level of SPP is important to the normal development of the adult animal, we ectopically expressed SPP in the wing disc using the Gal4 UAS system (Brand and Perrimon 1993). When SPP was ectopically expressed at high levels in the posterior compartment, significant loss of tissue resulted and the size of this region was dramatically reduced (Figure 5, A and B). Notching of these wings was similar to the phenotype caused by ectopic apoptosis that has been observed after dREF overexpression (Yoshida et al. 2001), but we did not find evidence indicating that wing notching caused by ectopic expression of SPP had a similar etiology. It was not suppressed by coexpression of the antiapoptotic protein p35 or by reduction to one copy of the genes reaper, grim, or head involution defective, which are required for induction of apoptosis. The wing notching phenotype was enhanced at higher temperature, suggesting that it is sensitive to levels of Spp expression, and it was suppressed by coexpression of a double-stranded RNAi that targets Spp (Figure 5C). Induction of this Spp RNAi transgene alone had no phenotypic consequence (not shown).
Figure 5.—
Ectopic expression of Spp in the posterior compartment of the wing. Genotypes were as follows: en-GAL4 alone (A); en-Gal4, UAS-Spp (B); en-Gal4, UAS-Spp, Spp RNAi (C); and en-Gal4, UAS-SppM (D). Numbers refer to the five wing veins.
To obtain additional evidence that the wing notching phenotype was caused by the activity of SPP, we engineered a gene encoding mutant protein in which the putative catalytic aspartyl residues at positions 228 and 272 were replaced with alanine and serine, respectively. Expression of this mutated gene in the posterior compartment did not cause loss of tissue (Figure 5D). This result supports the conclusion that the wing notching phenotype was caused by elevated levels of SPP activity.
DISCUSSION
Our goal in this work was to determine if signal peptide peptidase is necessary for normal animal development. We present data showing that Drosophila Spp encodes the fly ortholog of human signal peptide peptidase and show that Drosophila Spp provides an essential function required during the larval stages. We also show that SPP is strongly expressed in only a limited set of cells and that the mutant phenotype is consistent with a need for its function in these tissues. Further work will be needed to establish whether the role of Drosophila SPP is a general one that cleanses membranes of signal peptides or if it has specific targets and generates essential products through its action.
Human SPP is an intramembrane aspartyl protease whose active site is predicted to be buried within the lipid bilayer. It belongs to a family of enzymes conserved among animals, plants, and fungi (Ponting et al. 2002; Weihofen et al. 2002). The Drosophila and human SPPs have strong sequence similarity, with the strongest conservation near the catalytic aspartyl residues. The similarity between the human and fly enzymes also includes the transmembrane topology of these proteins, as both are predicted to span the lipid bilayer nine times (Friedmann et al. 2004). In addition, the SPPs contain a conserved carboxy-terminal ER retention signal KKXX. Human SSP localizes to the ER (Urny et al. 2003) and we found that the Drosophila SPP does as well. The strongest evidence that Drosophila SPP is a signal peptide peptidase is its enzymatic activity. We show that the Drosophila SPP can proteolyze a model signal peptide and that this activity is sensitive to the SPP protease inhibitor, (Z-LL)2-ketone.
We examined the expression of Spp during Drosophila embryogenesis. Earliest expression was during germ band extension. Expression in the germ band was nonuniform, with areas of weak enhancement that had a segmental periodicity; the most pronounced expression was in the salivary placodes. Later in embryogenesis, prominent expression was in three tubular organ systems: the salivary glands, the proventriculus (an anterior segment of the gut), and the trachea. In preliminary experiments with an Spp promoter-GFP fusion reporter, Spp-dependent GFP fluorescence was observed in similar patterns in the salivary glands, proventriculus, and trachea of both embryos and larvae (data not shown). Although other tissues such as wing and leg imaginal discs also expressed low levels of Spp, increasing levels of SPP by ectopic expression caused malformations in adult wings, legs, eyes, and sensory structures (Figure 5; data not shown). We conclude that expression of Spp is predominantly in salivary glands, proventriculus, and trachea and that normal development in other tissues requires that expression not rise above low levels.
We showed that Spp expression is necessary for Drosophila development by identifying recessive lethal mutations in Spp. These Spp alleles define a single complementation group. We identified two chromosomes with small deletions in the Spp gene. These deletions remove the two final transmembrane helices containing the QPALLY region highly conserved throughout evolution (Figure 1). In addition, the two missense mutations that were characterized (Spp5 and Spp7) changed conserved residues in the second transmembrane domain and third intracellular loop, respectively. Our molecular analysis therefore confirms the importance of these residues to SPP function that is implied by their conservation. We also introduced mutations in the conserved catalytic aspartyl diad. The aspartates in this domain are essential for the protease activity of human SPP as well as for the related polytopic intramembrane aspartyl protease, presenilin (Wolfe et al. 1999; Weihofen et al. 2002). We found that a wild-type Spp construct could rescue Spp mutants, but that a similar construct encoding a protein with mutated catalytic domain aspartates could not. This result implies that the proteolytic activity of SPP is critical to its function. Our analysis of Spp mutants suggests that this activity is required specifically in the tracheae. We found that the dorsal trunks of mutant embryos were abnormal, with mutant phenotypes ranging from entire tracheal systems devoid of air to dorsal trunks that did not completely fill with air. Defects in this essential oxygen delivery system are consistent with the lethargic nature of Spp mutant larvae.
Having established that SPP has an essential role, we now hope to identify and characterize the critical processes and substrates it controls. SPP was originally identified as an enzyme that cleaves signal peptides, potentially cleansing the ER membrane of the signal sequence remnants of secreted proteins. It also appears to regulate vital systems by producing bioactive peptides, as is evident in the metabolism of human MHC (Lemberg et al. 2001). It is also possible that SPP could function to release proteins from the membrane by cleaving their transmembrane domain tether. Intramembrane proteolysis by presenilin has been implicated in the processing and reverse signaling of a number of type I transmembrane proteins such as Notch, Delta, and Jagged (Levitan and Greenwald 1995; De Strooper et al. 1999; Struhl and Greenwald 1999; Ye et al. 1999; Saxena et al. 2001; Bland et al. 2003; LaVoie and Selkoe 2003; Ikeuchi and Sisodia 2003). Presenilin cleaves the transmembrane domains of these proteins, releasing their respective cytoplasmic domains to migrate to the nucleus where they function as transcriptional regulators. Since the active site of SPP is predicted to be inverted within the bilayer by 180° compared to the presenilin active site, the substrates of SPP would be type II transmembrane proteins, inverted in the membrane compared to the type I presenilin targets. In support of this type of activity, human SPP can activate an artificial ATF6 transcription factor construct that is tethered to the membrane in a type II orientation (Nyborg et al. 2004).
The essential nature of the SPP function is not limited to Drosophila. A recent publication describes the Caenorhabditis elegans spp homolog imp-2, which is required for normal molting in nematode development (Grigorenko et al. 2004). It appears that cholesterol and lipid metabolism are involved in this SPP-dependent process. It will be interesting to investigate whether there is also a connection between these processes and spp function in Drosophila, especially since intramembrane proteolysis plays a critical role in the activation of the SREBP pathway (Rawson 2003). More generally, we are most interested in identifying the targets of Drosophila SPP whose cleavage is necessary for normal development. To this end, we have begun to analyze Spp mutant animals using DNA microarray expression profiling. Preliminary comparisons of mutant and wild-type larvae revealed significant differences in transcription profiles (data not shown).
Finally, we note that SPP has been defined in part by its membership in a conserved family of intramembrane proteases (Ponting et al. 2002; Friedmann et al. 2004) and by its activity directed against signal peptides (Weihofen et al. 2000). Animal genomes are known to encode several different proteins with high sequence identity to SPP, and the Drosophila genome encodes two: CG11840 (Spp) and CG17030. While we have clearly established that SPP has an essential role in Drosophila development, characterization of CG17030 mutants and of Spp CG17030 double mutants will be important to further define the roles they play and to establish whether these genes have redundant functions. The results presented in this article suggest that this system holds great promise to help define the roles and mechanisms of action of this recently identified and interesting family of intramembrane proteases.
Acknowledgments
We are especially grateful to Helen Salz for her willingness to allow us to name the Drosophila Spp gene as we have. We thank the following for reagents and help with experiments: Chen-ming Fan, Elena Friedman, Songmei Liu, Andreas Weihofen, Marius Lemberg, Joy Gu, Heying Lin, Susan Younger, Janet Epps, Steven Beckendorf, Vidya Chandrasekaran, Deepak Malhotra, Robert Threlkeld, Gohta Goshima, and Ron Vale. We also thank the following for helpful discussions: Kevin Hill, Brenda Ng, Gretchen Ehrenkaufer, Felipe-Andrés Ramírez-Weber, David Iwaki, Frank Hsiung, Xingwu Lu, Francoise Chanut, and Arjun Guha. We acknowledge Hee Jae and the University of California-San Francisco Genomics Core Facility for DNA sequencing. D.J.C. received support from National Institute on Aging Training grant AG00278; this work was supported by grants from the National Institutes of Health to S.T. and T.B.K.
References
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers and D. J. Lipman, 1990. Basic local alignment search tool. J. Mol. Biol. 215: 403–410. [DOI] [PubMed] [Google Scholar]
- Apionishev, S., D. Malhotra, S. Raghavachari, S. Tanda and R. S. Rasooly, 2001. The Drosophila UBC9 homologue lesswright mediates the disjunction of homologues in meiosis I. Genes Cells 6: 215–224. [DOI] [PubMed] [Google Scholar]
- Barolo, S., L. A. Carver and J. W. Posakony, 2000. GFP and beta-galactosidase transformation vectors for promoter/enhancer analysis in Drosophila. Biotechniques 29: 726, 728, 730, 732. [DOI] [PubMed] [Google Scholar]
- Biehs, B., M. A. Sturtevant and E. Bier, 1998. Boundaries in the Drosophila wing imaginal disc organize vein-specific genetic programs. Development 125: 4245–4257. [DOI] [PubMed] [Google Scholar]
- Bland, C. E., P. Kimberly and M. D. Rand, 2003. Notch-induced proteolysis and nuclear localization of the Delta ligand. J. Biol. Chem. 278: 13607–13610. [DOI] [PubMed] [Google Scholar]
- Brand, A. H., and N. Perrimon, 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Braud, V. M., D. S. Allan, C. A. O'Callaghan, K. Soderstrom, A. D'Andrea et al., 1998. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 391: 795–799. [DOI] [PubMed] [Google Scholar]
- Brown, M. S., and J. L. Goldstein, 1999. A proteolytic pathway that controls the cholesterol content of membranes, cells and blood. Proc. Natl. Acad. Sci. USA 96: 11041–11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, N. H., and F. C. Kafatos, 1988. Functional cDNA libraries from Drosophila embryos. J. Mol. Biol. 203: 425–437. [DOI] [PubMed] [Google Scholar]
- Casso, D., F. Ramirez-Weber and T. B. Kornberg, 2000. GFP-tagged balancer chromosomes for Drosophila melanogaster. Mech. Dev. 91: 451–454. [DOI] [PubMed] [Google Scholar]
- Claros, M. G., and G. von Heijne, 1994. TopPred II: an improved software for membrane protein structure predictions. Comput. Appl. Biosci. 10: 685–686.7704669 [Google Scholar]
- Corpet, F., 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16: 10881–10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper, B., P. Saftig, K. Craessaerts, H. Vanderstichele, G. Guhde et al., 1998. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 391: 387–390. [DOI] [PubMed] [Google Scholar]
- De Strooper, B., W. Annaert, P. Cupers, P. Saftig, K. Craessaerts et al., 1999. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature 398: 518–522. [DOI] [PubMed] [Google Scholar]
- Epps, J. L., and S. Tanda, 1998. The Drosophila semushi mutation blocks nuclear import of bicoid during embryogenesis. Curr. Biol. 8: 1277–1280. [DOI] [PubMed] [Google Scholar]
- Epps, J. L., J. B. Jones and S. Tanda, 1997. oroshigane, a new segment polarity gene of Drosophila melanogaster, functions in hedgehog signal transduction. Genetics 145: 1041–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann, E., M. K. Lemberg, A. Weihofen, K. K. Dev, U. Dengler et al., 2004. Consensus analysis of signal peptide peptidase and homologous human aspartic proteases reveals opposite topology of catalytic domains compared with presenilins. J. Biol. Chem. 279: 50790–50798. [DOI] [PubMed] [Google Scholar]
- Grigorenko, A. P., Y. K. Moliaka, M. C. Soto, C. C. Mello and E. I. Rogaev, 2004. The Caenorhabditis elegans IMPAS gene, imp-2, is essential for development and is functionally distinct from related presenilins. Proc. Natl. Acad. Sci. USA 101: 14955–14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi, T., and S. S. Sisodia, 2003. The Notch ligands, Delta1 and Jagged2, are substrates for presenilin-dependent “gamma-secretase” cleavage. J. Biol. Chem. 278: 7751–7754. [DOI] [PubMed] [Google Scholar]
- Ishikawa, T., A. Matsumoto, T. Kato, Jr., S. Togashi, H. Ryo et al., 1999. DCRY is a Drosophila photoreceptor protein implicated in light entrainment of circadian rhythm. Genes Cells 4: 57–65. [DOI] [PubMed] [Google Scholar]
- Kimberly, W. T., J. B. Zheng, S. Y. Guenette and D. J. Selkoe, 2001. The intracellular domain of the beta-amyloid precursor protein is stabilized by Fe65 and translocates to the nucleus in a notch-like manner. J Biol. Chem. 276: 40288–40292. [DOI] [PubMed] [Google Scholar]
- Klemenz, R., U. Weber and W. J. Gehring, 1987. The white gene as a marker in a new P-element vector for gene transfer in Drosophila. Nucleic Acids Res. 15: 3947–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVoie, M. J., and D. J. Selkoe, 2003. The Notch ligands, Jagged and Delta, are sequentially processed by alpha-secretase and presenilin/gamma-secretase and release signaling fragments. J. Biol. Chem. 278: 34427–34437. [DOI] [PubMed] [Google Scholar]
- Lee, J. R., S. Urban, C. F. Garvey and M. Freeman, 2001. Regulated intracellular ligand transport and proteolysis control EGF signal activation in Drosophila. Cell 107: 161–171. [DOI] [PubMed] [Google Scholar]
- Lee, Y. S., and R. W. Carthew, 2003. Making a better RNAi vector for Drosophila: use of intron spacers. Methods 30: 322–329. [DOI] [PubMed] [Google Scholar]
- Lemberg, M. K., F. A. Bland, A. Weihofen, V. M. Braud and B. Martoglio, 2001. Intramembrane proteolysis of signal peptides: an essential step in the generation of HLA-E epitopes. J. Immunol. 167: 6441–6446. [DOI] [PubMed] [Google Scholar]
- Levitan, D., and I. Greenwald, 1995. Facilitation of lin-12-mediated signalling by sel-12, a Caenorhabditis elegans S182 Alzheimer's disease gene. Nature 377: 351–354. [DOI] [PubMed] [Google Scholar]
- Levy-Lahad, E., W. Wasco, P. Poorkaj, D. M. Romano, J. Oshima et al., 1995. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science 269: 973–977. [DOI] [PubMed] [Google Scholar]
- Lewis, E., 1968. Method of feeding ethyl methane sulfonat (EMS) to Drosophila males. Dros. Inf. Serv. 43: 193. [Google Scholar]
- Martoglio, B., R. Graf and B. Dobberstein, 1997. Signal peptide fragments of preprolactin and HIV-1 p-gp160 interact with calmodulin. EMBO J. 16: 6636–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumberg, D., R. Muller and M. Funk, 1995. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156: 119–122. [DOI] [PubMed] [Google Scholar]
- Nyborg, A. C., A. Y. Kornilova, K. Jansen, T. B. Ladd, M. S. Wolfe et al., 2004. Signal peptide peptidase forms a homodimer that is labeled by an active site-directed gamma-secretase inhibitor. J. Biol. Chem. 279: 15153–15160. [DOI] [PubMed] [Google Scholar]
- O'Neill, J. W., and E. Bier, 1994. Double-label in situ hybridization using biotin and digoxigenin-tagged RNA probes. Biotechniques 17: 870, 874–875. [PubMed] [Google Scholar]
- Ponting, C. P., M. Hutton, A. Nyborg, M. Baker, K. Jansen et al., 2002. Identification of a novel family of presenilin homologues. Hum. Mol. Genet. 11: 1037–1044. [DOI] [PubMed] [Google Scholar]
- Rawson, R. B., 2003. The SREBP pathway—insights from Insigs and insects. Nat. Rev. Mol. Cell. Biol. 4: 631–640. [DOI] [PubMed] [Google Scholar]
- Rogers, S. L., G. C. Rogers, D. J. Sharp and R. D. Vale, 2002. Drosophila EB1 is important for proper assembly, dynamics, and positioning of the mitotic spindle. J. Cell Biol. 158: 873–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner, D. Z., P. Fawcett and R. Losick, 1999. A family of membrane-embedded metalloproteases involved in regulated proteolysis of membrane-associated transcription factors. Proc. Natl. Acad. Sci. USA 96: 14765–14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena, M. T., E. H. Schroeter, J. S. Mumm and R. Kopan, 2001. Murine notch homologs (N1–4) undergo presenilin-dependent proteolysis. J. Biol. Chem. 276: 40268–40273. [DOI] [PubMed] [Google Scholar]
- Scheuner, D., C. Eckman, M. Jensen, X. Song, M. Citron et al., 1996. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat. Med. 2: 864–870. [DOI] [PubMed] [Google Scholar]
- Schneitz, K., P. Spielmann and M. Noll, 1993. Molecular genetics of aristaless, a prd-type homeo box gene involved in the morphogenesis of proximal and distal pattern elements in a subset of appendages in Drosophila. Genes Dev. 7: 114–129. [DOI] [PubMed] [Google Scholar]
- Sherrington, R., E. I. Rogaev, Y. Liang, E. A. Rogaeva, G. Levesque et al., 1995. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature 375: 754–760. [DOI] [PubMed] [Google Scholar]
- Spradling, A. C., 1986 P-element mediated transformation, pp. 175–197 in Drosophila: A Practical Approach, edited by D. B. Roberts. IRL Press, Oxford.
- Struhl, G., and I. Greenwald, 1999. Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature 398: 522–525. [DOI] [PubMed] [Google Scholar]
- Urban, S., J. R. Lee and M. Freeman, 2001. Drosophila rhomboid-1 defines a family of putative intramembrane serine proteases. Cell 107: 173–182. [DOI] [PubMed] [Google Scholar]
- Urban, S., J. R. Lee and M. Freeman, 2002. A family of Rhomboid intramembrane proteases activates all Drosophila membrane-tethered EGF ligands. EMBO J. 21: 4277–4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urny, J., I. Hermans-Borgmeyer, G. Gercken and H. C. Schaller, 2003. Expression of the presenilin-like signal peptide peptidase (SPP) in mouse adult brain and during development. Gene Expr. Patterns 3: 685–691. [DOI] [PubMed] [Google Scholar]
- Weihofen, A., and B. Martoglio, 2003. Intramembrane-cleaving proteases: controlled liberation of proteins and bioactive peptides. Trends Cell Biol. 13: 71–78. [DOI] [PubMed] [Google Scholar]
- Weihofen, A., M. K. Lemberg, H. L. Ploegh, M. Bogyo and B. Martoglio, 2000. Release of signal peptide fragments into the cytosol requires cleavage in the transmembrane region by a protease activity that is specifically blocked by a novel cysteine protease inhibitor. J. Biol. Chem. 275: 30951–30956. [DOI] [PubMed] [Google Scholar]
- Weihofen, A., K. Binns, M. K. Lemberg, K. Ashman and B. Martoglio, 2002. Identification of signal peptide peptidase, a presenilin-type aspartic protease. Science 296: 2215–2218. [DOI] [PubMed] [Google Scholar]
- Weihofen, A., M. K. Lemberg, E. Friedmann, H. Rueeger, A. Schmitz et al., 2003. Targeting presenilin-type aspartic protease signal peptide peptidase with gamma-secretase inhibitors. J. Biol. Chem. 278: 16528–16533. [DOI] [PubMed] [Google Scholar]
- Wolfe, M. S., W. Xia, B. L. Ostaszewski, T. S. Diehl, W. T. Kimberly et al., 1999. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature 398: 513–517. [DOI] [PubMed] [Google Scholar]
- Ye, Y., N. Lukinova and M. E. Fortini, 1999. Neurogenic phenotypes and altered Notch processing in Drosophila Presenilin mutants. Nature 398: 525–529. [DOI] [PubMed] [Google Scholar]
- Yoshida, H., Y. H. Inoue, F. Hirose, K. Sakaguchi, A. Matsukage et al., 2001. Over-expression of DREF in the Drosophila wing imaginal disc induces apoptosis and a notching wing phenotype. Genes Cells 6: 877–886. [DOI] [PubMed] [Google Scholar]