Abstract
OBJECTIVE
Neurocognitive dysfunction has been shown to occur in roughly 25% of patients undergoing carotid endarterectomy (CEA). Despite this, little is known about the mechanism of this injury. Recently, several groups have shown that new diffusion weighted imaging (DWI)-positive lesions are seen in 20% of patients undergoing CEA. We investigated to what degree neurocognitive dysfunction was associated with new DWI lesions.
METHODS
Thirty-four consecutive patients undergoing CEA were subjected to preand postoperative cognitive evaluation with a battery of neuropsychological tests. Postoperative magnetic resonance imaging was performed in all patients within 24 hours of surgery. Lesions that showed high signal on DWI and restricted diffusion on apparent diffusion coefficient maps but no abnormal high signal on the fluid-attenuated inversion recovery images were considered hyperacute.
RESULTS
Cognitive dysfunction was seen in eight (24%) patients. New hyperacute DWI lesions were seen in three (9%). Only one (13%) of the patients with cognitive dysfunction had a new DWI lesion. Two thirds of the new DWI lesions occurred in the absence of cognitive deterioration. Patients with cognitive dysfunction had significantly longer carotid cross-clamp times.
CONCLUSION
Neurocognitive dysfunction after CEA does not seem to be associated with new DWI positive lesions.
Keywords: Apparent diffusion coefficient, Carotid endarterectomy, Diffusion weighted imaging, Neuropsychological testing, Outcome magnetic resonance imaging
Carotid endarterectomy (CEA) is an effective means of preventing future stroke (1, 6, 11, 14). The low occurrence of perioperative complications such as stroke makes this a very safe procedure. However, subtle cognitive decline occurs in 28% of patients undergoing CEA when they are evaluated by a battery of neuropsychometric (NP) tests 1 day after surgery (5). This functional change in performance reflects a significant alteration in the brain because patients who have cognitive dysfunction also have a statistically significant increase in S100B, a glial protein serum marker of brain injury, at 24 and 48 hours after surgery (2).
Cognitive dysfunction may arise because of multiple reasons. The two most evident relate to ischemic events caused by emboli or global hypoperfusion. Gaunt et al. (4) have shown that the majority of patients having CEA have an average of five emboli per procedure. Furthermore, these emboli, at least when generated during preclamp dissection, may lead to diffusion weighted imaging (DWI) or T1-weighted contrast-enhanced magnetic resonance imaging (MRI) lesions of cerebral infarct (17). On the other hand, upon clamping the carotid artery, there may be a decrease in cerebral blood flow determined by xenon, transcranial Doppler ultrasonography, and electroencephalography (EEG). In fact, there can be as much as a 50% decline in cerebral blood flow before significant changes become evident on the electroencephalogram (15).
Therefore, we wanted to determine whether patients having CEA and neurocognitive changes also have acute ischemic lesions on MRI associated with the surgical procedure. To identify these lesions and determine when they were produced, we acquired MRIs using DWI and calculated apparent diffusion coefficient (ADC) maps, which were correlated with fluid-attenuated inversion recovery (FLAIR) images, 12 to 24 hours after CEA. Assessment of the acuity of cerebral ischemia was based on previously published guidelines (7). Lesions that showed high signal on DWI and restricted diffusion on ADC maps but no abnormal high signal on the FLAIR images were considered hyperacute, less than 24 hours old. Those lesions with high signal on DWI and FLAIR but with restricted diffusion were considered acute, approximately 1 to 5 days old. Lesions with high signal on DWI and FLAIR, but normal to prolonged diffusion on ADC maps (pseudonormalization), were considered subacute, more than 5 days old. Lesions with high signal on DWI and FLAIR but ADC values greater than 120% of normal were considered chronic (>3 wk old). Our hypothesis was that cognitive decline is caused by focal cerebral ischemia that would be reflected in structural cerebral changes seen by DWI (3, 16).
METHODS
Subjects
Thirty-four consecutive patients, with stenosis 70% or more on the operative side undergoing elective CEA were recruited to participate in this institutional review board approved study. The mean age of these patients was 69.1 years (standard deviation 8.2 yr); 73.5% were male. Of the 34 patients, 41.2% were symptomatic. After written consent was obtained, patients were assessed with a battery of NP tests at two time points, before surgery and 1 day after surgery, as described previously (5). We included all patients who were able to perform the NP tests in English and excluded all patients with a postoperative stroke. Postoperative DWI was performed within 24 hours of surgery on all patients in the study.
Anesthesia
No patients were premedicated. All patients received general anesthesia, with routine hemodynamic and temperature monitoring as described previously, as well as a radial arterial catheter for measuring blood pressure continuously and an 8 channel EEG (Neurotrac II; Moberg Medical, Inc., Ambler, PA) (5). Sedation before induction consisted of fentanyl and midazolam. Shunting across the surgical site was performed selectively only if there was evidence of cerebral ischemia by EEG upon clamping the carotid artery. This was defined as a 50% or greater decrease in amplitude in the alpha or beta frequencies and a similar increase in the delta or theta frequencies.
Surgery
All CEAs were performed by members of either the neurovascular service or the vascular service. Surgery consisted of positioning the patient supine with the head in an extended midline position. An incision was made along a skin crease from just below the angle of the mandible to near the midline through skin, subcutaneous tissue, and platysma. The common, internal, and external carotid arteries were exposed and controlled. All patients undergoing CEA received either 5000 or 6000 U of heparin as a bolus. A saphenous vein patch was used in 16 patients by two surgeons. In 18 patients, the artery was closed with a single suture line. All patients were extubated in the operating room and stayed in an intensive care environment overnight. All patients remained in the hospital for 1 to 3 days for postoperative pain scoring and NP testing.
Neuropsychometric Evaluation
Patients were assessed with a battery of NP tests. All examinations were administered by the same research assistant trained to administer and score these NP tests under the supervision of a neuropsychologist. Five raw scores were generated from the battery of NP tests, which were chosen to represent a limited range of cognitive domains. Halstead-Reitan Trails parts A and B evaluated visual conceptual and visuomotor tracking by timing how long it took a subject to connect consecutively numbered circles with a single line (part A) and then connect the same number of consecutively numbered and lettered circles by alternating between the two sequences (part B). The Controlled Oral Word Association Test evaluated verbal fluency and provided information on the function of the dominant hemisphere (“left”). Patients were asked to generate as many words as they could, beginning with three target letters (C, F, and L) in 1 minute. Their raw score was the sum of the words produced for all three letters. A different set of target letters was provided at follow-up. The copy portion of the Rey Complex Figure test was administered to assess perceptual and visuospatial organization and provided information on the function of the nondominant hemisphere (“right”). Patients were asked to copy the Rey Complex Figure to the best of their ability (9). A standardized scoring system was used to evaluate the presence of specific design features and the accuracy of their location (10). The Boston Naming Test was administered as a measure of any deficits in recognition and naming of common objects. The test consists of 60 large ink drawings of objects. Patients were asked to give the name of each object. Stimulus cues were given to patients who could not recognize the item at all and phonemic cues were given to patients who recognized the objects but could not name them. The score was generated based on both the number of successful attempts at naming the objects and the number of cues needed. The Boston Naming Test is recommended to elicit any left hemisphere damage. Although we would have preferred a more extensive battery, our population of older patients better tolerated this test because of its short administration time.
To detect cognitive changes, each of the preoperative and postoperative test components was individually scored. The change in each test score was calculated and was then scaled to test scores of a frequency matched control group of spine patients to generate z-scores. The z-scores for each test at each time point were then summed to give a total score for cognitive change. A patient was said to have significant cognitive decline if his or her total score was 2 or more standard deviations from the mean of the total scores of patients in the control group (5).
Magnetic Resonance Imaging
DWI studies were obtained using a 1.5 T commercial MRI system (Signa Echospeed, G.E. Medical Systems, Milwaukee, WI) with echo-planar imaging using a spin-echo pulse sequence modeled on the Stejskal-Tanner method (13). Images are acquired using a single-shot echo-planar imaging spin-echo pulse sequence with TE 97 ms, TR 10,000 ms, 1 NEX, FOV 30 × 19 cm, matrix 128 × 128, and separately applied orthogonal x, y, and z axis diffusion gradients with b = 0 and b = 1000 seconds/mm2. Scan time is 40 seconds for acquisition of 20 5 mm thick sections with no gap from the vertex through the midbrain including the cerebellum. Composite DW images are computed as the average signal of the x, y, and z gradient images.
ADC trace maps are calculated as the average of the orthogonal ADC values (mm2/s) derived from the slope of a two-point semilog fit of signal change from b = 0 to b = 1000. (b = ′672 ′20′64′32 ′20g2 (′44 - ′64/3) where ′67 = the proton gyromagnetic ratio, ′64 = the diffusion gradient duration, g = the diffusion gradient amplitude, and ′44′20′3d = the diffusion gradient separation (onset of 1st gradient to onset of 2nd gradient). Apparent diffusion coefficient (ADC) = −(1/b)ln(S/S0). Isotropic ADC = (ADCx + ADCy + ADCz)/3. Diffusion MRI at Columbia University Medical Center is used for the detection of early cerebral infarction, which appears as high signal on DW images and low signal on ADC maps, indicating restricted diffusion less than 80% of the presumed normal contralateral comparable anatomic region (12). Other methods of diffusion imaging such as “navigated spin-echo,” “radial K-space mapping,” and “line scanning” are not commonly used at Columbia University Medical Center for stroke diagnosis.
In addition, we used the FLAIR algorithm to obtain nine contiguous 5 mm thick sections, TR 11,000 ms, TE 145 ms, TI 2600 ms, ETL 16, 256 × 192 matrix, 22 cm FOV, 1 NEX, three interleaved three-section acquisitions in the axial plane through periventricular, and centrum semiovale white matter (scan time 8:48 min). The long inversion time of the FLAIR sequence approximately matches the T1 relaxation time of cerebrospinal fluid (CSF), resulting null signal suppression of CSF, whereas the long TE produces very heavy T2-weighting. This method produces superior contrast between lesions and adjacent normal tissue and between lesions and CSF compared with conventional T2-weighted spin echo images.
DWI produces images in which the contrast between tissues is dependent on differences in microscopic water diffusion. Water diffusion rates are lowest in white matter, higher in gray matter, and highest in CSF. In pathological tissue, the lowest diffusion rates are seen in acute infarction (restricted intracellular diffusion), higher rates in chronic infarcts and reactive interstitial edema, and highest rates in CSF-filled cystic collections such as surgical cavities or arachnoid cysts. Changes in the DWI signal intensity occur within minutes of the onset of ischemic injury because of a reduction in the ADC of water arising from cytotoxic edema, and these last at least 24 hours (8). In addition, the quantitative ADC maps can be used to estimate the time of onset of ischemia and infarction (7). When the ADC values are less than 80% of normal, and the DWI scan shows a lesion that is absent on the FLAIR image, the lesion is hyperacute (<24 h old). If the ADC values are less than 80% of normal, but both the DWI and FLAIR images show a lesion, the lesion is acute (1–4 d old). However, when the ADC values are 80% or greater, and both the DWI and FLAIR images show a lesion, the infarct is subacute (1–2 wk old). The ADC map is said to have “pseudonormalized.” When the ADC values are greater than 120% of normal, and both the DWI and FLAIR images show a lesion, the infarct is chronic, typically more than 3 to 4 weeks old. Because all the patients in this study were scanned within 24 hours of surgery, the ADC can be used to indicate whether an MRI lesion was caused during CEA.
The purpose of using MRI techniques was threefold: 1) to prove that injury had occurred, 2) to provide evidence as to the etiology of this injury, and 3) to determine whether the lesions occurred during the course of performing CEA. The analysis of the images was performed by consensus of two neuroradiologists who were both blinded to whether the patients were injured.
RESULTS
Demographic and intraoperative variables of noninjured and injured patients are shown in Table 1. As was shown previously, there is no significant difference between symptomatic and asymptomatic patient in terms of cognitive performance at baseline (not shown). No patients had postoperative strokes (5). However, a total of eight patients (24%) had cognitive decline (Fig. 1). As a group, injured patients had significantly longer carotid artery cross-clamp times, 44.2 ± 16.2 minutes versus 63.1 ± 16.9 minutes (P = 0.008) (Table 1). With use of group analysis, significant differences were seen 1 day after surgery with the following NP tests: Controlled Oral Word, Rey Copy, and Trails B (Table 2). Seven of these eight (87%) cognitively injured patients had no evidence of procedural induced infarction on immediate postoperative imaging (Fig. 1). A total of four patients were found to harbor DWI positive lesions after surgery. One of these lesions, however, lacked restricted diffusion (ADC) and was therefore determined to have occurred before CEA in a patient with preoperative transient ischemic attacks. Therefore, there were three patients in whom DWI lesions were believed to be causally related to the endarterectomy itself (3/34; 9%). Only one of these patients had evidence of significant cognitive decline. This patient had a left CEA and showed high signal foci by DWI and restricted diffusion on ADC maps representing multiple small acute infarcts along the ipsilateral anterior cerebral artery and middle cerebral artery terminal per-fusion zone (Fig. 2A). The two remaining patients with acute lesions by DWI scans and restricted ADC did not suffer cognitive dysfunction (Fig. 2, B and C). Both infarcts were very small. One of these infarcts was seen in the right anterior/middle/posterior cerebral border zone in a patient who had undergone a right CEA (Fig. 2B). The other was seen in the right head of caudate nucleus in a patient who had also undergone a right CEA (Fig. 2C).
TABLE 1.
Demographic and operative summary of patients included in the studya
| Variable | All patients (n = 34) (SD) | Uninjured (n = 26) (SD) | Injured (n = 8) (SD) | Significant difference |
|---|---|---|---|---|
| Age, yr | 69.1 (8.2) | 68.9 (8.5) | 69.8 (7.6) | No |
| Sex, male/female | 25/9 | 19/7 | 6/2 | No |
| Handedness, right/left | 31/3 | 23/3 | 8/0 | No |
| Height, cm | 172.2 (10.2) | 172.77 (11.1) | 170.34 (6.7) | No |
| Weight, kg | 77.42 (11.9) | 77.11 (12.8) | 78.47 (9.4) | No |
| Education, yr | 15.2 (3.3) | 15.6 (3.1) | 14.0 (3.55) | No |
| Hypertension | 28 | 23 | 5 | No |
| Diabetes mellitus | 4 | 4 | 0 | No |
| Previous cerebrovascular accident or transient ischemic attack | 14 | 12 | 2 | No |
| Previous myocardial infarction | 11 | 7 | 4 | No |
| Previous carotid endarterectomy | 6 | 4 | 2 | No |
| Duration of surgery, min | 183.9 (53.5) | 174.8 (45.5) | 213.4 (69.3) | No (P=0.07) |
| Duration of cross-clamp, min | 47.5 (16.7) | 44.2 (16.2) | 63.1 (16.9) | Yes (P=0.008) |
| Fentanyl, mg/kg | 2.30 (0.94) | 2.38 (0.92) | 2.04 (1.04) | No |
| Midazolam, mg/kg | 0.03 (0.002) | 0.03 (0.002) | 0.03 (0.01) | No |
SD, standard deviation.
FIGURE 1.
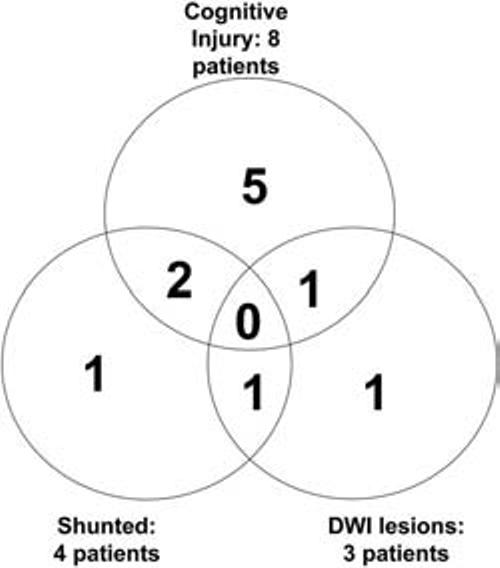
Venn diagram of results showing overlap of cognitive dysfunction (8 patients), new lesions based on DWI (3 patients), and need for shunting (4 patients).
TABLE 2.
Group comparison of individual test performance in uninjured and injured patientsa
| Neuropsychometric test | Uninjured (n = 26) (SD) | Injured (n = 8) (SD) | Difference (P) |
|---|---|---|---|
| Boston Naming: baseline (BL) | 54.5 (4.4) | 53.8 (4.0) | 0.69 |
| 1 day postoperative | 56.9 (3.7) | 55.4 (3.9) | 0.37 |
| Controlled Oral Word (BL) | 46.0 (11.6) | 40.4 (11.9) | 0.24 |
| 1 day postoperative | 51.8 (11.4) | 39.1 (12.2) | 0.01 |
| Rey Copy (BL) | 29.0 (4.3) | 29.0 (5.0) | 0.99 |
| 1 day postoperative | 51.8 (11.4) | 39.1 (12.2) | 0.02 |
| Trails A (BL) | 47.0 (16.5) | 43.8 (15.9) | 0.63 |
| 1 day postoperative | 41.6 (14.0) | 51.0 (18.6) | 0.13 |
| Trails B (BL) | 101.8 (36.3) | 91.25 (37.3) | 0.48 |
| 1 day postoperative | 89.0 (34.8) | 187.8 (153.8) | <0.001 |
SD, standard deviation. Increasing values demonstrate improved cognitive performance for Boston Naming, Controlled Oral Word, and Rey Copy. These test scores are unitless. However, decreasing values in seconds show improved cognitive performance for Trails A and Trails B.
FIGURE 2.
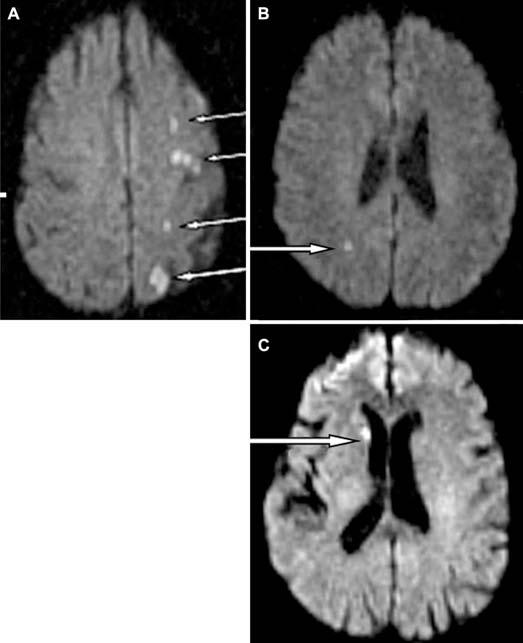
DWI scans from three patients (A–C) with significant DWI lesions. Only one patient (A) had cognitive dysfunction; the others (B and C) did not. Of note, even though scans of the brain from the vertex through the brain stem including the cerebellum were analyzed, the images shown are for those parts of the brain that show the most prominent lesions. Lesions indicated by white arrows.
Thus, although somewhat underpowered, statistical analysis showed no significant correlation between cognitive decline and the existence of new diffusion-weighted abnormalities on immediate postoperative MRI (P = 1.0 by Fisher's exact test). Nevertheless, additional analyses revealed that four (12%) patients required insertion of a shunt during surgery for signs of ischemia on the basis of EEG. Only one of these patients had evidence of a new cerebral lesion by DWI but did not show evidence of NP injury; 50% (2/4) demonstrated cognitive decline compared with only 20% (6/30) of those not requiring shunting.
Finally, 11 patients had evidence of cortical and subcortical FLAIR positive, DWI negative lesions consistent with chronic ischemic changes. Of these patients, three (27%) showed cognitive decline. This was similar to the incidence witnessed in those without FLAIR lesions (5/23; 22%, P = not significant).
DISCUSSION
Thirty-four patients were evaluated using NP tests before and 1 day after CEA. DWI scans of the brain were performed on these patients 1 day after surgery. Eight (24%) patients showed significant cognitive deficits on the first postoperative day. Of those eight, only one (13%) patient was found to have a DWI lesion referable to the endarterectomy. The remaining two patients with acute DWI lesions did not have NP decline.
Although the possible reasons for cognitive dysfunction after CEA have been discussed previously, it is worth reiterating that regional cerebral hypoperfusion and microembolism from the surgical site are considered the likeliest culprits. Wolf et al. (17) showed that the latter is particularly common during CEA, with 32 of 33 patients showing emboli generation via transcranial Doppler ultrasonography high intensity transients (HITs) analysis. Not only were these emboli associated with dissection of the carotid artery before its clamping (45.5%) and at declamping (97%), but the number of embolic signals generated during preclamp dissection of the artery were highly correlated with postoperative evidence of new DWI lesions (P = 0.027). This link between particulate micro-emboli and new DWI lesions led us to wonder whether significant microembolism as evidenced by new DWI abnormalities was responsible for cognitive deficits after CEA. Although we did not measure whether emboli were present during CEA in this series of patients, we were unable to tightly correlate cognitive changes with diffusion-weighted imaging changes to support the hypothesis that emboli were responsible. In fact, the vast majority of cognitive injury in our study is completely DWI negative, and in the one case in which a DWI abnormality was found, the injury seemed to be more consistent with regional hypoperfusion of the distal watershed rather than a shower of emboli (Fig. 2A). Conversely, when DWI abnormalities were seen without cognitive injury, these lesions appeared to be somewhat more embolic (Fig. 2, B and C). Although the exact etiology of these small lesions is not entirely clear, one reason that these lesions are cognitively occult may be that the NP tests used are measures of global cerebral functioning, which are not sensitive enough to detect such small lesions. Nevertheless, it seems likely, at least in this patient cohort, that cerebral hypoperfusion that is most often DWI negative plays a more significant role in cognitive injury than microembolization. Further evidence for this comes from the fact that patients who were selectively shunted for significant EEG changes at the time of carotid cross-clamping were more likely to develop cognitive change than those who did not develop EEG changes. Consistent with the hypothesis of hypoperfusion is the significantly prolonged carotid artery cross-clamp times in the cognitively injured group (Table 1). However, in a larger sample of 139 patients, we were unable to confirm cognitive injury with prolonged carotid artery cross-clamp time. Our results in this study may result from limited sample size.
To further our understanding of the nature of cognitive deterioration after CEA, future studies will need to directly compare the rate of embolization in those with and without cognitive injury. Furthermore, additional studies will need to examine whether those unshunted patients who developed injury have minor EEG changes that may signal borderline perfusion. In addition, because this study used an 80% threshold for ADC restriction, future work should examine whether lesser ADC declines are more predictive of cognitive change. Finally, the statistical power of this study was such that even if the incidence of cognitive dysfunction were 50%, there was only 17% power to detect a difference with α = 0.05. Thus, more patients are needed to confirm these preliminary findings.
Nevertheless, it seems that the presence of acute ischemic lesions on DWI is not tightly correlated with cognitive dysfunction after CEA. Additional work is needed to determine whether lesion size and location, the insensitivity of the NP battery, or both are responsible for this low correlation.
Acknowledgments
Dr. Eric Heyer is supported in part by a grant from the National Institutes of Health (RO1 AG17604). Some patients were admitted to the Herbert and Florence Irving Clinical Research Center supported by a grant from the National Institutes of Health (RR 00 645).
Contributor Information
Eric J. Heyer, Departments of Anesthesiology and Neurology, Columbia University, New York, New York
Robert DeLaPaz, Department of Radiology, Columbia University, New York, New York
Robert Sciacca, Department of Medicine, Columbia University, New York, New York
E. Sander Connolly, Jr., Department of Neurological Surgery and Neurology, Columbia University, New York, New York
REFERENCES
- 1.Collaborators NASCET Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 2.Connolly E, Winfree C, Rampersad A, Sharma R, Mack W, Mocco J, Solomon R, Todd G, Quest D, Stern Y, Heyer E. Serum S100B protein levels are correlated with subclinical neurocognitive declines after carotid endarterectomy. Neurosurgery. 2001;49:1076–1083. doi: 10.1097/00006123-200111000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiebach JB, Schellinger PD, Jansen O, Meyer M, Wilde P, Bender J, Schramm P, Juttler E, Oehler J, Hartmann M, Hahnel S, Knauth M, Hacke W, Sartor K. CT and diffusion-weighted MR imaging in randomized order: Diffusion-weighted imaging results in higher accuracy and lower interrater variability in the diagnosis of hyperacute ischemic stroke. Stroke. 2002;33:2206–2210. doi: 10.1161/01.str.0000026864.20339.cb. [DOI] [PubMed] [Google Scholar]
- 4.Gaunt ME, Martin PJ, Smith JL, Rimmer T, Cherryman G, Ratliff DA, Bell PR, Naylor AR. Clinical relevance of intraoperative embolization detected by transcranial Doppler ultrasonography during carotid endarterectomy: A prospective study of 100 patients. Br J Surg. 1994;81:1435–1439. doi: 10.1002/bjs.1800811009. [DOI] [PubMed] [Google Scholar]
- 5.Heyer EJ, Sharma R, Rampersad A, Winfree CJ, Mack WJ, Solomon RA, Todd GJ, McCormick PC, McMurtry JG, Quest DO, Stern Y, Lazar RM, Connolly ES., Jr A controlled prospective study of neuropsychological dysfunction following carotid endarterectomy. Arch Neurol. 2002;59:217–222. doi: 10.1001/archneur.59.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hobson RW, 2nd, Weiss DG, Fields WS, Goldstone J, Moore WS, Towne JB, Wright CB. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med. 1993;328:221–227. doi: 10.1056/NEJM199301283280401. [DOI] [PubMed] [Google Scholar]
- 7.Lansberg MG, Thijs VN, O'Brien MW, Ali JO, de Crespigny AJ, Tong DC, Moseley ME, Albers GW. Evolution of apparent diffusion coefficient, diffusion-weighted, and T2-weighted signal intensity of acute stroke. AJNR Am J Neuroradiol. 2001;22:637–644. [PMC free article] [PubMed] [Google Scholar]
- 8.Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: Application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401–407. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- 9.Lezak MD. Neuropsychological Assessment. Oxford University Press; New York: 1983. [Google Scholar]
- 10.Meyers J, Meyers K. Rey Complex Figure Test and Recognition Trial Professional Manual. Psychological Assessment Resources, Inc.; Odessa, FL: 1995. [Google Scholar]
- 11.MRC European Carotid Surgery Trial Interim results for symptomatic patients with severe (70-99%) or with mild (0-29%) carotid stenosis. European Carotid Surgery Trialists' Colaborative Group. Lancet. 1991;337:221–227. [PubMed] [Google Scholar]
- 12.Sorensen AG, Buonanno FS, Gonzalez RG, Schwamm LH, Lev MH, Huang-Hellinger FR, Reese TG, Weisskoff RM, Davis TL, Suwanwela N, Can U, Moreira JA, Copen WA, Look RB, Finklestein SP, Rosen BR, Koroshetz WJ. Hyperacute stroke: Evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology. 1996;199:391–401. doi: 10.1148/radiology.199.2.8668784. [DOI] [PubMed] [Google Scholar]
- 13.Stejskal E, Tanner J. Spin diffusion measurements: Spin echoes in the presence of time-dependent field gradients. J Chem Phys. 1965;42:288–291. [Google Scholar]
- 14.Study Executive Committee for the Asymptomatic Carotid Atherosclerosis Study Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 15.Sundt TM, Jr, Sharbrough FW, Piepgras DG, Kearns TP, Messick J, Jr, O'Fallon WM. Correlation of cerebral blood flow and electroencephalographic changes during carotid endarterectomy: With results of surgery and hemodynamics of cerebral ischemia. Mayo Clinic Proc. 1981;56:533–543. [PubMed] [Google Scholar]
- 16.van Everdingen K, van der Grond J, Kappelle L, Ramos L, Mali W. Diffusion-weighted magnetic resonance imaging in acute stroke. Stroke. 1998;29:1783–1790. doi: 10.1161/01.str.29.9.1783. [DOI] [PubMed] [Google Scholar]
- 17.Wolf O, Heider P, Heinz M, Poppert H, Sander D, Greil O, Weiss W, Hanke M, Eckstein H. Microembolic signals detected by transcranial Doppler sonography during carotid endarterectomy and correlation with serial diffusion-weighted imaging. Stroke. 2004;35:e373–e375. doi: 10.1161/01.STR.0000143184.69343.ec. [DOI] [PubMed] [Google Scholar]


