Abstract
The chromosomes of the macronuclear (expressed) genome of Tetrahymena thermophila are generated by developmental fragmentation of the five micronuclear (germline) chromosomes. This fragmentation is site specific and directed by a conserved 15-bp chromosome breakage sequence (Cbs element). This article reports the construction of a library enriched for chromosome breakage junctions and the development of a successful scheme for the genome-wide isolation and characterization of functional Cbs junctions. Twenty-three new Cbs junctions were characterized and each was assigned to a specific micronuclear chromosome or chromosome arm. Two distinct previously unreported variant chromosome breakage sequences were found, each in two or more functional Cbs elements. Analysis of natural Cbs junctions confirmed that microheterogeneity in the macronuclear telomere addition site is associated with chromosome fragmentation. The physical and genetic characterization of these functional chromosome breakage junctions is reported in the accompanying article in this issue. The whole-genome shotgun sequencing and auto-assembly phase of the Tetrahymena Genome Initiative has recently been completed at The Institute for Genome Research (TIGR). By providing unique sequence from the natural ends of macronuclear chromosomes, Cbs junctions characterized in the work reported here will serve as useful sequence tags for relating macro- and micronuclear genetic, physical, and sequence maps.
AS is typical of ciliates, Tetrahymena thermophila displays nuclear dimorphism. Every cell contains two nuclei with distinct but closely related genomes (reviewed by Prescott 1994). The micronucleus (MIC) contains the germline genome, the store of genetic information for the sexual progeny. It is diploid, contains five pairs of chromosomes, and divides mitotically. No known genes are expressed in the MIC.
The macronucleus (MAC) is the somatic nucleus. It is actively expressed during vegetative multiplication and thus solely determines the cell's phenotype. The MAC genome, although derived from the MIC genome during development, has a strikingly different chromosomal organization and ploidy. It consists of ∼250–300 acentromeric chromosomes, varying in size from 21 kb to at least 3.3 Mb (average ∼700 kb; Altschuler and Yao 1985; Conover and Brunk 1986). The smallest known MAC chromosome, the rDNA, codes for the major ribosomal RNAs. Each MAC chromosome that has been investigated is amplified and maintained at ∼45 copies per MAC (Doerder et al. 1992); the only known exception is the 9000-ploid rDNA. The MAC divides amitotically, resulting in a random distribution of chromosome copies (reviewed by Orias 1998). Every MAC chromosome contains at each end a readily identifiable telomere, an ∼200- to 400-bp-long tract of tandem repeats of the hexanucleotide GGGGTT/CCCCAA (Blackburn and Gall 1978; Yao et al. 1981; Yao 1989).
The MAC chromosomes are generated by fragmentation of the germline (MIC) chromosomes in the developing macronuclear anlagen during conjugation, but the molecular mechanism remains to be elucidated. Yu et al. (1990) showed that telomerase expressed by the parental macronucleus is responsible for healing the ends created by chromosome breakage and Fan and Yao (1996) proposed that telomerase acts in concert with the cleavage reaction. Two proteins that accumulate exclusively during conjugation, PDD2p and the piwi homolog TWI1p, are required for site-specific breakage and DNA elimination in the Tetrahymena MAC anlage and are essential for survival of the progeny (Nikiforov et al. 1999; Mochizuki et al. 2002). TWI1p is required for the accumulation of a 26-nucleotide class of “scan RNAs,” proposed to have a novel function in DNA elimination (Mochizuki et al. 2002) through a mechanism akin to RNA silencing (Zamore 2001).
Fragmentation occurs at a specific 15-bp sequence present exclusively in the MIC, the chromosome breakage sequence (Cbs element). The first Cbs elements were identified as conserved segments of micronuclear sequence flanking the rDNA (Yao et al. 1985). Yao et al. (1987) cloned five additional Cbs junctions by hybridization to an oligonucleotide containing the conserved Cbs. All the inserts contained the 15-bp element (TAAACCAACCTCTTT), identical in sequence to those immediately flanking the rDNA. The sequences surrounding the Cbs elements were all unique and had higher-than-average A + T content (∼90%). Yao et al. (1990) showed that Cbs is a necessary and sufficient cis-acting element for this fragmentation cleavage. During fragmentation the Cbs element and only 4–31 bp on either side are deleted (Fan and Yao 1996) in conjunction with de novo telomere addition. Thus chromosome breakage is very precise in Tetrahymena and the individual Cbs sites reliably mark micronuclear-specific locations where cleavage occurs during the genesis of the macronuclear chromosomes.
The ultimate goal of this work is to construct a physical scaffold of the MIC genome. The approach is to clone Cbs sites and their adjacent MAC-destined DNA to sequence tag the ends of adjacent MAC chromosomes and determine their size. Clones from adjacent Cbs will tag the two ends of a single MAC chromosome, thus allowing the concatenation of consecutive Cbs junctions into a physical MIC scaffold.
The objective of this study was to investigate the feasibility of cloning and physically characterizing every Cbs junction. In this article, the construction of DNA libraries enriched for Cbs-containing inserts is reported and a set of efficient and reliable tests to determine whether or not a particular insert carries a functional Cbs is validated. Twenty-three new Cbs junctions were characterized and the existence of functional variants in Cbs was confirmed and extended. All identified Cbs junctions were mapped to MIC chromosomes (arms). The accompanying article by Cassidy-Hanley et al. (2005)(this issue) identifies the physical sizes of the MAC chromosomes derived from each Cbs junction and reports the genetic mapping of roughly a third of these junctions.
MATERIALS AND METHODS
Strains:
DNA was derived from inbred strains B and C3 of T. thermophila, strains that have been used extensively for making genetic maps. The inbred strain B DNA was obtained from strains SB1969 and SB210 (Table 1). To obtain B DNA for making the library and for PCR analysis, the genetic and preparative procedures described in detail in Hamilton and Orias (2000) were used. SB1969 was crossed to SB210. Progeny from >100,000 pairs were mass selected for cycloheximide (cy) and 2-deoxy-d-galactose resistance. DNA was prepared from this culture, which contained cells estimated to be between 20 and 30 fissions old. DNA from this mixture of independently differentiated macronuclei was used to “average out” developmental heterogeneity introduced during MAC differentiation (see results). C3 DNA was obtained in an analogous way from C3 strains SB3539 and SB3546 (Table 1); progeny were mass selected for cy and paromomycin resistance prior to DNA preparation. Whole-cell DNA was isolated from CU526, a unisomic 1 derivative of inbred strain B, by the method described in detail in Hamilton and Orias (2000). CU526 contains exclusively chromosome 1 DNA in its MIC. For assigning Cbs junctions to a chromosome (or chromosome arm), a previously described panel of nullisomic strains derived from inbred strain B (Hamilton and Orias 2000) was used.
TABLE 1.
Strain list
| Strain | Genotype | Phenotype | Mating type |
Reference |
|---|---|---|---|---|
| CU428.2 | mpr1-1/mpr1-1 | mp-s | VII | Cole and Bruns (1992) |
| CU526 | Contains multiple copies of chromosome 1 | pm-r | VII | D. Cassidy-Hanley (unpublished results) |
| SB 1969 | chx1-1/chx1-1 | cy-s | II | Allen et al. (1996) |
| SB 210 | gal1-1/gal1-1 | gal-s | VI | Sanford and Orias (1981) |
| SB 3539 | chx1-1[C3]/chx1-1[C3] | cy-s | I | Hamilton and Orias (2000) |
| SB 3546 | pmr1-1[C3]/pmr1-1[C3] | pm-s | VI | Hamilton and Orias (2000) |
Phenotypes: mp-s, sensitive to 15 μg/ml 6-methyl-purine; cy-s, sensitive to 15 μg/ml cycloheximide; gal-s, sensitive to 0.25% 2-deoxygalactose; pm-s, sensitive to 100 μg/ml paromomycin; pm-r, resistant to 100 μg/ml paromomycin.
Cbs-related nomenclature:
Every functional Cbs was assigned a name, of the form Cbs XY-Z, where X represents the MIC chromosome number, Y is the chromosome arm (L or R; note that there are no published nullisomics that allow chromosome 5 arm assignments), and Z is a serial isolation number. Cbs junctions previously identified by Yao et al. (1987) were assigned the lowest serial numbers for their corresponding chromosome arms.
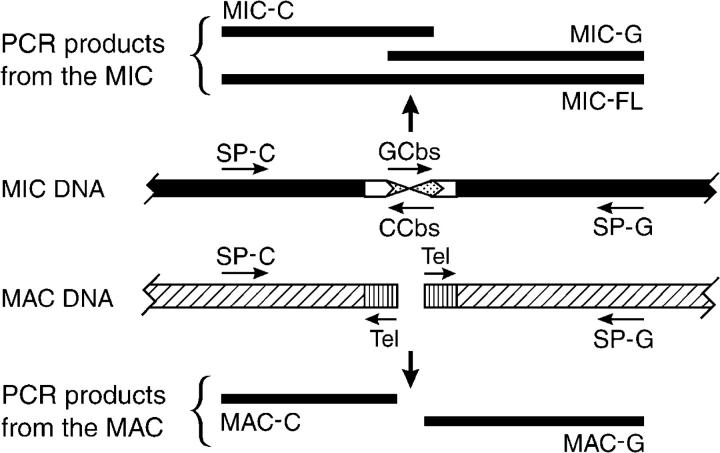
Convergent specific primers used to PCR amplify a Cbs junction were named with the Cbs insert name followed by a G or C, depending on whether the primer was downstream from the 3′-end of the G-rich or the C-rich strand of the Cbs element, respectively. The G-specific primer is used with either the GCbs primer or the Tel primer to amplify the “G” side of the junction, while the C-specific primer is used with either the CCbs or the Tel primer to amplify the “C” side of the junction (Figure 1).
Figure 1.—
Specific PCR amplification products templated by MIC or MAC DNA in a Cbs region. SP-C and SP-G are specific PCR primers designed from the DNA sequence of a Cbs insert. GCbs and CCbs are the single strands of the canonical Cbs element 5′-AAAGAGGTTGGTTTA-3′ (GCbs) and its inverse complement (CCbs). Tel is an inwardly oriented PCR primer derived from the telomere sequence 5′-CCCCAACCCCAACC-3′. (Top) PCR amplification products templated exclusively by MIC DNA: MIC-FL is the full-length product, while MIC-C and MIC-G are the two “half-products.” (Bottom) PCR amplification “half-products” templated exclusively by MAC DNA.
Construction of canonical-Cbs-enriched libraries:
Micronuclear DNA from T. thermophila SB210 was isolated by a modification of the protocol of Chau and Orias (1996). Micronuclear DNA was sheared for cloning by sonicating for 5 sec on ice (with a microtip probe on a Sonifier cell disruptor at a setting of 1, model W185D, Branson Sonic Power). The ends of the sonicated DNA were repaired with T4 DNA polymerase. Blunt adaptors were made by annealing equimolar amounts of two primers: CP1 (CTGACTACTGAGCTCGAGAC) and 5′ phosphorylated PHIL1 (GTCTCGAGCTCAGTAGTCAGGG). The adaptor was ligated overnight at 8° to the sonicated, repaired DNA. Additional libraries were constructed by digesting either MIC DNA or whole-cell unisomic 1 DNA to completion with Sau3A. Adaptors with a Sau3A-compatible overhang were constructed by annealing two primers (CP1 and 5′ phosphorylated AC1B: GATCGTCTCGAGCTCAGTAGTCAG) and ligated to the digested DNA as above.
A modification of the magnetic bead capture protocol of Shepard and Rae (1997) was used to enrich for inserts containing a Cbs element. A total of 400 ng of adaptor-ligated micronuclear DNA was mixed with 10 pmol of biotinylated Cbs oligonucleotide, AAAGAGGTTGGTTTANGGAAGGX (where X is TEG biotin; Operon Technologies, Alameda, CA) in a total volume of 9 μl in a 1.5-ml microfuge tube. The micronuclear DNA was denatured with 1 μl of 1 n NaOH (0.1 n final) for 5 min at room temperature. The denaturation reaction was neutralized by the addition of 100 μl of prewarmed Tris-hybridization solution (6× SSPE [0.27 m NaCl, 15 mm NaH2PO4, 1.5 mm Na2EDTA (pH 7.4)], 0.1% Tween 20, 50 mm Tris-HCl, pH 7.4) and maintained at 37° for 1 hr. During this period, streptavidin-coated magnetic beads (Streptavidin MagneSphere paramagnetic particles, Promega, Madison, WI) were prepared by transferring 200 μl of beads to a 1.5-ml microfuge tube, washing three times with an equal volume of TE buffer (10 mm Tris HCl, pH 8.0, 1 mm EDTA), and resuspending in 20 μl of Tris-hybridization solution. After hybridization of the biotinylated oligo to the DNA, 20 μl of washed magnetic beads were added to the 110 μl in hybridization mix. The beads were kept in suspension for 30 min by shaking at 400 rpm on a microfuge tube shaker at room temperature. The beads were collected with a magnet (MagneSphere Technology magnetic separation stand, Promega) and washed twice with room temperature 2× SSPE, 37° 2× SSPE, and 37° 0.5× SSPE and once with 65° 0.5× TE. The beads were resuspended at each step by repeated pipetting. Fifteen seconds were allowed for bead capture by the magnet. To thermally release captured micronuclear DNA from the bead-bound biotinylated-Cbs oligonucleotide, the beads were resuspended in 10 μl of 0.5× TE and incubated for 3 min at 80°. The beads were immobilized on the magnet for a few seconds and the supernatant was transferred to a fresh tube. Five microliters of the 80° eluate were subjected to a second cycle of denaturation, hybridization, washing, and elution.
The eluted single-stranded DNA was next PCR amplified. A total of 3.5 μl of the second-cycle eluate was used as template in a 30-μl PCR reaction that also contained 3 μl of 10× PCR buffer (500 mm KCl, 100 mm Tris-HCl pH 8.3, 15 mm MgCl2, 0.01% gelatin), 3 μl of 10 mm MgCl2, 7.2 μl of dNTPs (dissolved in water at 1.25 mm each), 2.5 μl of 20 μm CP1 primer, and 0.15 μl of AmpliTaq DNA polymerase (Perkin-Elmer, Norwalk, CT) at 5 units/μl. Temperature cycling conditions were: 5 min at 90°, followed by 30 cycles of 30 sec at 90°, 30 sec at 58°, and 3 min at 68°, followed by a terminal extension period of 20 min at 68°. The insertion of this PCR step raised concerns about differential amplification that are addressed later.
PCR reaction products were cloned into the plasmid pCR2.1-TOPO (Invitrogen, San Diego) and transformed into chemically competent TOP10 cells according to the supplier's instructions. Positive clones were identified as white colonies, arrayed in 6 × 8 patterns corresponding to half of a 96-well plate on Luria-Bertani ampicillin agar plates and screened for insert size by colony PCR.
Colony PCR:
Each 25-μl PCR reaction contained 2.5 μl of 10× PCR buffer, 2.5 μl of 10 mm MgCl2, 4 μl of dNTPs (dissolved in water at 1.25 mm each), 2.5 μl of 4 μm CP1 primer, 13.4 μl of H2O, and 0.125 μl of AmpliTaq DNA polymerase at 5 units/μl. Pipette tips on a multichannel pipetter were used to “scratch” and transfer a sample of each arrayed colony to the wells of a 96-well PCR plate. The temperature cycling conditions were: 15 min at 94°, followed by 35 cycles of 1 min at 94°, 1 min at 50°, and 2 min at 72°, followed by a terminal extension period of 7 min at 72°. Inserts were sized by electrophoresis of 10 μl of the PCR reaction in a 1.5% agarose gel. The remaining PCR product was dot blotted.
Dot blotting and hybridization:
A total of 200 μl of 6× SSC (225 mm NaCl, 25.5 mm sodium citrate, pH 7.4) was added to the leftover colony-PCR product in the 96-well plate. The products were heated to 99° for 5 min, placed on ice, and transferred to a nylon membrane (Nytran supercharge, Schleicher and Schuell, Keene, NH) using a microsample filtration manifold (Minifold, Schleicher & Schuell). The DNA was crosslinked to the membrane in a GS Gene Linker UV chamber (Bio-Rad, Hercules, CA). The G-rich Cbs oligonucleotide (AAAGAGGTTGGTTTA) was end labeled with T4 polynucleotide kinase (New England BioLabs, Beverly, MA) and [γ-32P]ATP and the probe was purified with a “push column” (Stratagene, La Jolla, CA). The membranes were prehybridized for 2 hr at 65° in 6× SSC, 5× Denhardt's reagent (Sambrook et al. 1989), 20 mm Tris, pH 8.0, 0.125% SDS, 2 mm EDTA, and 27 μg/ml salmon sperm DNA. Probe at 1 × 106 cpm/ml was added; hybridization occurred overnight at room temperature. The membrane was washed twice with 4× SSC at 37° and twice with 2× SSC at 37°. X-ray film was exposed to the membrane, usually for 1 hr.
Plasmid DNA and DNA sequencing:
Plasmid DNA was isolated using a QIAGEN (Chatsworth, CA) kit. Plasmid DNA was sequenced using the Big Dye Terminator Cycle-Sequencing-Ready Reaction kit (PE Applied Biosystems). Nucleotide sequences were determined using an ABI 310 Genetic Analyzer. Sequences were aligned using the BLAST 2 sequences website at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov).
PCR characterization of cloned inserts:
Additional PCR reactions were done to further characterize all potential Cbs-positive inserts. The first PCR amplification used the adaptor primer (CP1) alone to more precisely determine the length of the insert. The other two amplifications used the CP1-6 primer (CTGACTACTGAGCT) in combination with either the G-rich (GCbs: AAAGAGGTTGGTTTA) or the C-rich (CCbs: TAAACCAACCTCTTTN) Cbs primers. Each 25-μl PCR reaction contained 5 μl of plasmid DNA (5 ng/μl), 2.5 μl of 10× PCR buffer (see recipe above), 2.5 μl of 10 mm MgCl2, 4 μl of dNTPs (dissolved in water at 1.25 mm each), 1.25 μl of 4 μm CP1, 1.25 μl of 4 μm Cbs primer, 8.4 μl of H2O, and 0.125 μl of AmpliTaq (5 units/μl). The temperature cycling conditions used were: 5 min at 94°, followed by 35 cycles of 1 min at 94°, 1 min at 50°, and 2 min at 72°, followed by a terminal extension period of 7 min at 72°. When successful, the latter two PCR tests confirmed the presence of a Cbs-related sequence and gave its distance to each end of the insert. The shortened version of the CP1 primer (CP1-6), with a Tm closer to that of the Cbs primers, increased the success rate for amplification. Occasionally, the C-rich Cbs primer, containing just the 15 bases of the Cbs element, failed to give amplification products in combination with specific primers. Addition of degeneracy at the 3′-end increased the success rate for these reactions, but even so the use of this primer required more fastidious amplification conditions.
Specific primers and PCR conditions:
Specific primers flanking each Cbs site were designed with the aid of the 3′ primer program (http://www.genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi; Rozen and Skaletsky 2000). Primer Tm's were targeted for 53°. PCR conditions were optimized using a temperature-gradient PCR machine (PCR Express, Hybaid), which varied the annealing temperature from 45° to 60°. Annealing temperatures that gave a single band of the expected size were chosen for further use. A list of specific PCR primers and annealing conditions is given in Table 2. Each 25-μl PCR reaction contained 5 μl of genomic DNA (5 ng/μl), 2.5 μl of 10× PCR buffer (see recipe above), 2.5 μl of 10 mm MgCl2, 4 μl of dNTPs (dissolved in water at 1.25 mm each), 2.5 μl of 4 μm CP1, 8.4 μl of H2O, and 0.125 μl of AmpliTaq (5 units/μl). B and C3 DNAs were alternated as template across the temperature gradient so that Cbs products with size polymorphisms could be easily detected. PCR conditions were similarly optimized for the combinations of each specific primer with a telomere primer (Tel primer: CCCCAACCCCAACC).
TABLE 2.
Cloned Cbs junctions: source and conditions for PCR amplification
| Cbs name |
Source library |
Cbs insert size (kb) |
Specific primers: SP-G and SP-Cb | Temperaturec | Band sized |
|---|---|---|---|---|---|
| 1L-1 | 5′ rDNA | NA | G: TAAAACTAACAACAAAAAGCAA | 55° | 1.2 |
| C: AACTCAGATTTCATTTTTCAAG | |||||
| 1L-2 | 3′ rDNA | NA | G: GGGTTTTAACTTATTTTTAA | 55° | 0.5 |
| C: ATAGCAAATTGTTATATAGA | |||||
| 1L-3 | Sau3A | 1.1 | G: TATAATTCATGAATAAAAGC | 55° | 1.0 |
| C: AATACTTCTAACTATGAAAG | |||||
| 1L-4 | Uni1 | 1.2 | G: ATCCAATATTAGGAACTGAAGC | 55° | 1.1 |
| C: TTTAATCATCTTAAAACTGAAGC | |||||
| 1L-5a | Uni1 | 0.6 | G: TAAGTGTCATAGCTCCCAAG | 60° | 0.5 (−) |
| C: AATTAAGTCTATTATATTAGCAAAAAG | |||||
| 1L-6 | Sau3A | 1.0 | G: TTGATGAAACTTTAACGAGTAA | 55° | 0.55 (<) |
| C: TGATTTTAGTTATCAGAATTCATTT | |||||
| 1R-1 | Sheared | 1.6 | G: CAAAAATTCAAAAAGTAGGT | 55° | 1.55 (<) |
| (Tt826) | C: GATCAACTTCTGTATGATGTT | ||||
| 1R-2 | Sheared | 0.85 | G: ATAAACCGCTTTTTAACTTTAG | 55° | 0.7 (>) |
| C: TCTATTATGTTATTTATGATGTAACG | |||||
| 1R-3a | Sheared | 0.3 | G: TTTATTATTCTTATTTTTGTAAATTG | 55° | 0.15 |
| C: AAATATTTTTAAGAGGTTGGTTTe | |||||
| 1R-4 | Uni1 | 1.3 | G: AATCTCTTATATATTTTCAACCTTAG | 55° | 0.9 (<) |
| C: ACCACAAAAGTAAGTTAAGTACG | |||||
| 1R-5 | Uni1 | 0.8 | G: TTTCTTAAGAGTAGGGCTTTAG | 55° | 0.7 (−) |
| C: AAATCTTCTTTCTTCCATCTATC | |||||
| 1R-6 | Sheared | 0.8 | G: TGAAATTTGTTAAAATTAAAGGA | 55° | 0.6 |
| C: TGGATAATTTATAGGTCGTTTC | |||||
| 1R-7 | Sheared | 0.85 | G: TCACTTTTGAACTTACAAGCAA | 50° | 0.45 |
| C: TTTAATAATAAAAAATATGTTATAAGe | |||||
| 1R-8 | Sau3A | 0.6 | G: AAGCTTAAAAGAAAATTAGTTAAAAA | 50° | 0.5 |
| C: AGCTACTATTTTATGAGATTACCA | |||||
| 2R-1 | Tt814, Tt816 | NA | G: CTACATTAAAAATGATAAAA | 50° | 0.35 |
| C: CATGCATTTTTAATTTTGAG | |||||
| 3L-1 | Sau3A | 1.15 | G: CCTAAGTTAATAGATATTTTG | 50° | 1.05 (>) |
| (Tt701) | C: AATAAACTGCACATAAAACT | ||||
| 3L-2 | Sheared | 0.55 | G: AGATTAAACATAAGGATTCAAAC | 55° | 0.4 |
| (Tt819) | C: TTGGTTATCTTTTAGTAAAGTTTG | ||||
| 3L-3 | Sheared | 0.8 | G: TCAAAATTGGCTTTTAATTTC | 50° | 0.6 |
| C: AAAATAATTCAAACTCTTCTCAAC | |||||
| 3L-4 | Sheared | 0.6 | G: TTCTGTTATATTATGAATGTGGA | 50° | 0.45 (−) |
| C: TCAAGTTTTATCAAACAAAATG | |||||
| 3R-1 | Sau3A | 0.8 | G: GAATTTATATTATTTTAAGTTTCAACCC | 55° | 0.65 |
| C: AAATTATCTTTTTCTTTTGCTATC | |||||
| 4L-1 | Tt835, Tt829 | NA | G: TTTATCTATTATTTAAAAAC | 45° | 0.25 |
| C: CTAACAGTTTAATTAATAAG | |||||
| 4L-2 | Sheared | 1.3 | G: AATAGAATAGCGACCATTAG | 55° | 1.2 |
| C: ATACTGATTTTTGCAACAAC | |||||
| 4L-3 | Sheared | 0.9 | G: TTTATTCCTAAAAAATTAAAAGTTC | 50° | 0.7 (>) |
| C: AAACAAAATTTTAGCATAAAAAG | |||||
| 5-1 | Sau3A | 0.95 | G: CAATAATTTCAAAAAAATGG | 55° | 0.9 (<) |
| C: TAAAAAAGCAGGATTACAAT | |||||
| 5-2 | Sau3A | 1.55 | G: GATCCAACTATTAATTATTTCTT | 50° | 1.3 |
| C: CTTTCTTATTCTGCTTTTCC | |||||
| 5-3 | Sheared | 0.75 | G: TACTAATCTTATGGTTCTTCACAC | 60° | 0.6 (−) |
| C: CATGATAAAATGTTATTGTTGTTG | |||||
| 5-4a | Sheared | 1.4 | G: TTCCTTTTTCATTTAAAGTTTG | 60° | 1.2 |
| C: TATTATTGATGCTAAAAATTGC | |||||
| 5-5a | Sheared | 0.55 | G: TGAATAAGCATAGCACCTATTT | 55° | 0.45 |
| C: AAATTTTGCCTTAGTGTTTCTA | |||||
| 5-6a | Sau3A | 0.7 | G: AGAAAGTAGCTAACTTCATAAAA | 55° | 0.65 |
| C: AAAGTAACTAAAAATAATCTCCTTG | |||||
| 5-7 | Sau3A | 1.8 | G: GATCAATAGTTTGACAAATAAAAA | 55° | 1.7 |
| C: ATTCATAAAAGTATGCACAATG |
Sau3A, Sau3A-digested MIC DNA; Sheared, sheared MIC DNA; Uni1, unisomic 1 Sau3A-digested whole-cell DNA; rDNA, 5′ and 3′ flanks (Yao et al. 1985); Tt-prefixed Cbs, originally characterized by Yao et al. (1987); those reisolated from the Cbs library are in parentheses.
A functional variant Cbs element sequence: 1A substitution for Cbs 1L-6, 5-5, and 5-6, and 15A substitution for Cbs 1R-3 and 5-4.
Sequences are listed 5′–3′; see materials and methods for explanation of G and C designation.
PCR annealing temperature; all other Cbs amplification parameters are as described in materials and methods.
Band size is the size of the PCR product (in kilobases) from B DNA. For B-C3 polymorphic bands, “>,” “<,” and “−” in parentheses indicate that the C3 band is larger, smaller, or missing, respectively, compared to B.
The primer overlaps the Cbs or lies in the MIC limited sequence immediately adjacent to the Cbs.
RESULTS
Isolation of new Cbs-containing inserts:
Libraries of Cbs-containing DNA inserts were made using an enrichment method based on binding a biotinylated GCbs oligonucleotide to MIC DNA fragments, as described in detail in materials and methods. Key steps of this procedure were: (1) random shearing or Sau3A digestion of MIC DNA and ligation of an adaptor; (2) hybridization of the denatured products to a biotinylated Cbs oligonucleotide and capture of the hybrids using paramagnetic streptavidin beads; (3) high-stringency washes followed by elution of the captured fragments; (4) a second cycle of hybridization, capture, washing, and elution; and (5) PCR amplification and cloning of eluted fragments.
More than 600 clones from the Sau3A library and ∼1300 from the sheared DNA library were characterized. The Sau3A digest produced a range of fragments from >5 kb to <100 bp; maximum intensity was seen at ∼1.2 kb. Cbs junctions lacking nearby Sau3A sites would reside on large fragments, which are less likely to be cloned; therefore, a sheared DNA library was also constructed. The DNA was not size selected after shearing. Sheared fragments ranged from 2 kb to <100 bp; maximum intensity on an ethidium-bromide-stained gel was seen at ∼1 kb.
Library members likely to have a functional Cbs insert were identified next. Ideally, the inserts should contain at least 300 bp of DNA on each side of the Cbs element since this would increase the odds of detecting both MAC chromosomes when probing Southern blots of pulsed-field gels. Every transformant was screened by colony PCR using the CP1 adaptor primer. This identified the clones that contained adaptor-flanked inserts, allowed an estimate of the insert size, and provided DNA to dot blot. Table 3 shows the percentage of adaptor-flanked insert-plus colonies and the average insert size for each library. To identify potential Cbs-containing clones, the dot blot was hybridized to an end-labeled GCbs oligonucleotide and autoradiographed, as described in materials and methods. Plasmid DNA was prepared from colonies that resulted in even the faintest hybridization signal; these clones were further characterized. The sheared library yielded a higher fraction of putative Cbs-positive clones (Table 3).
TABLE 3.
Cbs library statistics
| Library | Insert size range |
Average insert size |
% insert-positive clonesa |
% Cbs-positive clonesb |
% additive PCR productsc |
|---|---|---|---|---|---|
| Sau3A | 2.0–0.2 kb | 0.9 kb | 75 | 27 | 45 |
| Sheared | 2.0–0.2 kb | 0.75 kb | 79 | 44 | 46 |
Percentage of clones in which the CP1 adaptor primer amplified a PCR product in colony PCR screening.
Percentage of insert-positive clones that gave a positive hybridization signal on a dot blot probed with the GCbs oligo.
Percentage of Cbs-positive clones in which the length of the products primed with CP1 and CCbs and CP1 and GCbs added up to the full-length insert (MIC-C + MIC-G = MIC-FL; see Figure 1).
Three separate PCR amplifications were done to confirm the size of the insert and locate the putative Cbs element within it. The first used the CP1 (adaptor) primer alone; this confirmed the presence and length of the insert. The other two amplifications used the CP1-6 primer in combination with either the GCbs or the CCbs primers. Inserts with the following additional characteristics were given highest priority for further characterization: (a) amplification products were produced with all three primer combinations; (b) the sum of the lengths of the products primed with the two Cbs oligonucleotides was approximately equal to the full length of the insert; (c) each of the Cbs-oligonucleotide-primed products was at least 300 bp long; and (d) the three PCR product length combinations seemed unique (to minimize the chance of characterizing repeat isolations). Some inserts failed to give amplification products primed with the CCbs oligonucleotide. In these, the corresponding distance was calculated by subtraction of product lengths in the two PCR reactions that worked (using CP1 primers alone and CP1-6 with the GCbs primer). About 45% of the putative Cbs-plus clones passed this PCR product addition test (MIC-FL = MIC-C + MIC-G; see Figure 1 and Table 3). Of these clones, only half had at least 300 bp on each side of the Cbs element.
To identify repeated incidences of each characterized Cbs insert, the library dot blots were stripped and reprobed with individual Cbs inserts or with pools of up to nine inserts at a time. For example, when both libraries were screened with the first nine Cbs junctions, the Sau3A library had 9% repeat clones while the sheared library had about twice that fraction (21%). Repeats were excluded from subsequent work.
High-priority inserts were then sequenced from both ends; virtually all of them had a Cbs element with the canonical chromosome breakage sequence. (The few exceptions are addressed below.) The sequence information was used to design specific primers flanking the putative Cbs in each insert (Table 2). These primers were used to determine, by several independent approaches, whether or not an insert that had passed all the previous tests carried a functional Cbs site.
Testing putative Cbs-containing inserts for chromosome breakage function:
MIC-specific amplification across a chromosome breakage site:
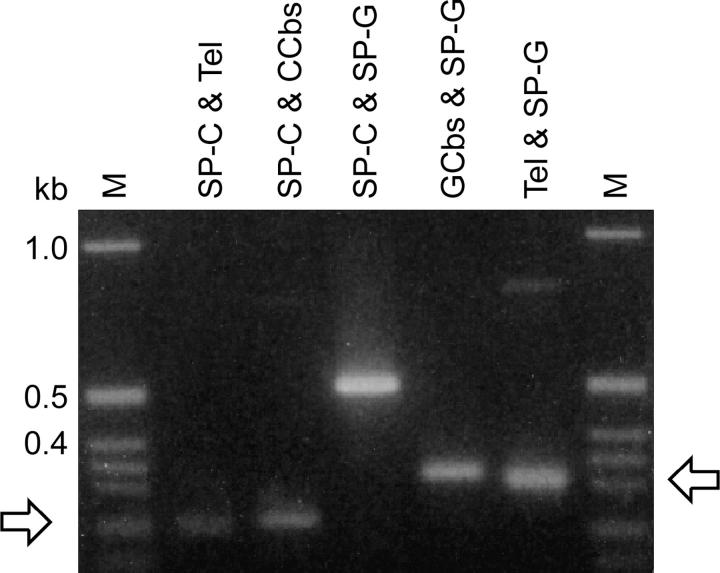
Using the pair of specific primers flanking the Cbs, each Cbs junction was assigned to a MIC chromosome arm by the nullisomic-based PCR test of Cassidy-Hanley et al. (1994). A key element of this approach is that PCR amplification across a Cbs site is templated exclusively by MIC DNA (see Figure 1). Although the primers can also bind to MAC DNA, no PCR product is obtained because each primer binds to a different MAC chromosome. Whole-cell genomic DNA from a panel of nullisomic strains was used as template for the PCR amplification (Hamilton and Orias 2000). Such strains are missing both copies of entire chromosomes and chromosome arms in their MIC, but grow normally because they have wild-type MACs. These tests allow assignment of the Cbs junction to a chromosome arm because the PCR product is generated, unless the DNA template comes from a strain missing the chromosome arm that bears the Cbs. The results of one such test are illustrated in Figure 2. Using this approach, Cbs junctions have been mapped to all five MIC chromosomes.
Figure 2.—
Nullisomic mapping of the Cbs 1L-5 junction. DNA from inbred strain B and C3 (controls) and from nullisomic strains missing both copies of the indicated chromosomes or chromosome arms was PCR amplified using the Cbs 1L-5G and 1L-5C primers. The amplification pattern of the 0.6-kb band (arrow) allows assignment of this junction to the left arm of MIC chromosome 1 (1L).
The nullisomic mapping results also provide strong evidence for the functionality of each Cbs junction. This method depends upon the PCR product being templated from MIC DNA, as expected when primers flank a functional Cbs. When primer binding sites flank a nonfunctional Cbs-like element, they are not separated by chromosome breakage and every nullisomic strain allows amplification of the specific PCR product (our unpublished observations).
MAC-specific amplification of the telomere adjacent region:
If the Cbs is indeed functional, then chromosome breakage should have led to telomere addition at predictable distances from the specific primers that flank the Cbs. This prediction was tested in separate PCR amplifications using each specific primer in combination with the inwardly directed Tel primer (see Figures 1 and 3). Bands with the predicted lengths were observed at high primer-annealing stringency for every insert listed in Table 2 (data not shown). These results also indicate that the specific primers were located within a MAC-destined segment and suggest that there are no internally eliminated sequences in the junction regions of these Cbs elements. In contrast, when the insert carries a nonfunctional Cbs-related sequence, no neighboring telomere and no PCR product are observed (data not shown). Since the generation of telomeres is the hallmark of chromosome breakage, this test provides the most definitive evidence of whether or not an insert carries a functional Cbs element.
Figure 3.—
Specific PCR amplification of MIC-limited or MAC-destined segments flanking a functional Cbs element results in products of similar length. Amplification products from the Cbs 1R-1 junction of inbred strain B are illustrated here. Primer nomenclature and sequences are as in Figure 1 and Table 2, respectively. Arrows point to pairs of related PCR products from either side of the junction.
The results of the two functionality tests (the nullisomic mapping test and the Tel and specific primer-dependent PCR) agreed in every case. Only one insert that passed the dot-blot hybridization test failed the functionality tests; it had a variant Cbs element with a 5A substitution.
Two observations are noteworthy regarding Tel-primed amplification:
Even though the telomeres provide ∼250–400 bp of repeats capable of binding the Tel primer, the PCR products have nearly the same size as the corresponding products using the Cbs oligonucleotide as primer (Figure 3 and data for other Cbs junctions, not shown). This result is consistent with previous findings that the final PCR product results from the binding of the Tel primer to the innermost telomere repeat (Orias et al. 1991). This size similarity, observed in all the natural junctions reported here, confirms previous experimental findings that the total amount of MIC-limited DNA associated with a Cbs element is <75 bp (Fan and Yao 1996).
The Tel-primed PCR products show some dispersion in comparison to the corresponding Cbs oligonucleotide-primed products. This dispersion can be attributed to a combination of two factors: the microheterogeneity with respect to the telomere addition sites described by Fan and Yao (1996) and the use of template DNA preparations from young progeny (<30 fissions old) of a mass cross (see materials and methods), which contain a mixture of independently processed MAC chromosomes. This interpretation is supported in a later section.
A Cbs library targeted to chromosome 1:
To determine if Cbs inserts located in a predetermined MIC chromosome could be isolated, a Cbs-enriched library from a uni-1 clone (CU526, also derived from inbred strain B) was made. This clone contains exclusively chromosome 1 DNA in its MIC (P. Bruns, D. Cassidy-Hanley and H. Smith, personal communication). The library was made from restriction-digested whole-cell DNA (rather than from purified MIC DNA), as described in materials and methods. A small number (178) of insert-plus clones were screened and 38 putative Cbs-containing (hybridization-positive) inserts were further characterized. Four clones contained new Cbs elements that proved to be functional by the tests described above: two map to the left arm of MIC chromosome 1 and the other two to the right arm (Table 2). The remaining clones were not unique (13 repeats distributed among five previously identified chromosome 1 Cbs junctions), were too small, or the Cbs was located too close to the insert end. Thus the use of unisomic strains allows the MIC chromosome-targeted isolation of Cbs junctions. This success also means that the method is able to enrich for MIC Cbs inserts even in the presence of a huge excess of MAC DNA.
Functional Cbs elements with variant sequence:
Two observations suggested that the Cbs libraries were unlikely to be complete:
When the Cbs libraries were probed with labeled DNA from the first nine functional Cbs inserts, ∼20% of the inserts hybridized with the pooled probe. Only 3% repeats would be expected if all ∼300 potential junctions were present in the libraries.
Three of the first nine inserts isolated (Cbs 1R-1, 3L-1, and 3L-2) were independent reisolations of junctions originally described by Yao et al. (1987). With 300 Cbs junctions to isolate, it seemed statistically unlikely that three of the seven previously identified junctions would be found so quickly.
The incompleteness of the libraries could be explained by the existence of Cbs sequence variants that were missed by this hybridization-based enrichment procedure.
To pursue the sequence variant hypothesis, five inserts that had hybridized weakly to the canonical Cbs oligonucleotide were characterized. Two of them passed all the functionality tests and had Cbs elements with a variant sequence: A-for-T substitutions at positions 1 or 15 in the C-rich strand. (The remaining three inserts failed the functionality tests and their sequenced regions contained no sequences closely related to the Cbs element.) Altogether, five Cbs with terminal A-for-T substitutions were isolated from the Cbs libraries (Table 2), suggesting that the ability to hybridize to the Cbs oligonucleotide during the enrichment procedure is not severely affected by these substitutions. Thus, every canonical Cbs element found so far is functional, but not every functional Cbs element has the canonical sequence.
Genetic and developmental DNA polymorphisms associated with Cbs junctions:
By alternating B and C3 DNA as template while optimizing PCR conditions for specific primers (see materials and methods), chromosome-breakage-site-associated polymorphisms (CBSPs) between these two inbred strains were detected. Every Cbs junction, including those described by Yao et al. (1987), was screened for CBSPs. PCR products from both sides of each junction were generated using the Tel primer in combination with the appropriate specific primer (see Figure 1) to determine on which side of the Cbs element the CBSP resides. Altogether, CBSPs were detected in 11 of the 26 inserts characterized. Most of the CBSPs result in PCR band-size differences, while in some CBSPs the band is present in B (the strain from which the specific primer sequence was obtained) and absent in C3 (Table 2). CBSPs were genetically mapped to linkage and coassortment groups (see accompanying article by Cassidy-Hanley et al. 2005).
Cbs 5-1 displays an unusual type of size polymorphism. It consists of a 50-bp segment, which includes the Cbs element, present in single copy in inbred strain C3 and as a tandem repeat in inbred strain B. This tandem repeat is reminiscent of the cluster of three Cbs elements on the 5′ side of the rDNA minichromosome (Yao et al. 1985).
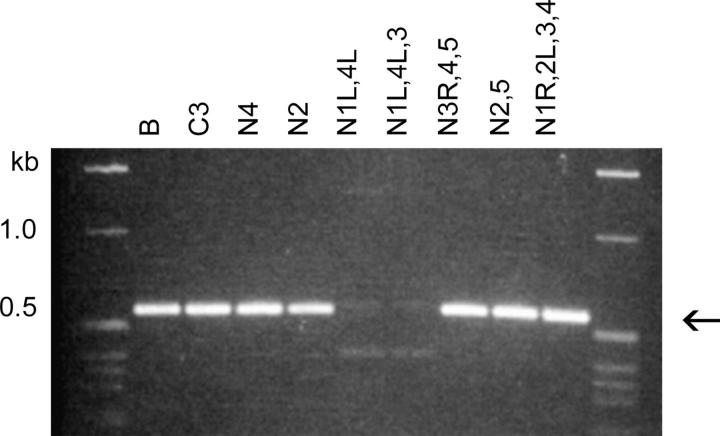
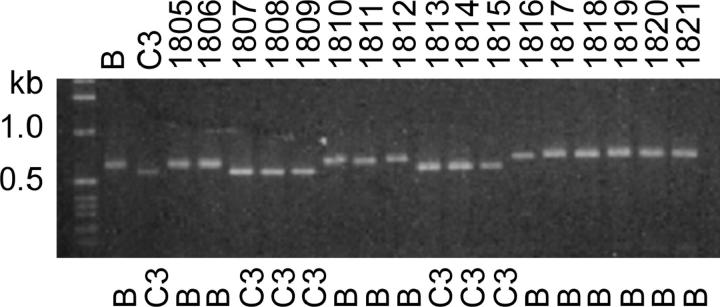
A different sort of DNA polymorphism was detected as a small variation in the Tel-primed PCR product size when screening a panel of B/C3 heterozygous cell lines subcloned after undergoing >500 fissions (panel of terminal assortants, described in the accompanying article by Cassidy-Hanley et al. 2005). This variation was observed for every junction tested and is superimposed on that caused by any genetic B-C3 size polymorphism, e.g., Cbs 1R-4, illustrated in Figure 4. This small size variability can be attributed to developmental microheterogeneity in the in vivo telomere addition site, as previously detected by Fan and Yao (1996) using an in vitro-constructed plasmid model system. To confirm this interpretation, Tel/Cbs3L-2 specific primer PCR products from members of this panel were sequenced; a range of telomere addition sites lying 14 to 34 bp from the edge of the Cbs element were found (Figure 5). It can be concluded that assortment genetically resolved the multiplicity of very similar MAC products resulting from the microheterogeneity in the site of telomere addition.
Figure 4.—
Genetic and developmental DNA polymorphisms associated with Cbs 1R-4G. DNAs from mass cultures of young B and C3 homozygotes (<30 fissions old) and from B/C3 heterozygotes passaged for at least 500 fissions (panel of terminal assortants; see materials and methods) were PCR amplified using the Tel and Cbs 1R-4G primers. The SB prefix has been omitted from the names of the terminal assortants (1805–1821). The major size difference (compare B and C3 lanes) is determined by the germline-encoded allele difference; the allele assorted to purity is indicated under each lane. The minor, within-allele size variation (e.g., compare assortants 1810–1812) is developmental and results from telomere addition site microheterogeneity associated with chromosome breakage during MAC differentiation (see text).
Figure 5.—
Sequence analysis of developmental microheterogeneity in the macronuclear telomere addition site at the 3L-2 Cbs junction. The telomere-adjacent regions of 11 terminal assortants were PCR amplified using the Tel and 3L-2G primers. The sequences of the products are shown in 5′–3′ orientation. Telomeric repeats are shown in boldface type, beginning at the first position where the MAC and MIC sequence (bottom line) diverge. Sequences corresponding to the Tel primer are underlined. When a partial telomere repeat unit in the MIC (italicized) just precedes the site of MIC/MAC sequence divergence, it is unclear which was the first nucleotide added by telomerase. PCR products templated from whole-cell DNA from mass cultures of inbred B and C3 progeny (analogous to lanes B and C3 in Figure 4) showed a major sequence component identical in size to that of assortant SB1806. No genetic polymorphism is associated with this Cbs.
DISCUSSION
Functional variants of the Cbs element:
The vast majority of the Cbs elements found in this work have the sequence reported by Yao et al. (1987). This is not surprising because the Cbs-containing inserts were isolated after screening with the canonical Cbs oligonucleotide, as were most of the inserts isolated by Yao et al. (1987). Unexpected was the incompleteness of the Cbs-enriched libraries, as well as the immediate reisolation of three of the seven distinct Cbs junctions described by Yao et al. (1987). As a result, the libraries were searched for possible variants, identified as inserts that hybridized weakly to the canonical Cbs oligonucleotide. In total, five functional Cbs elements representing two previously unreported, naturally occurring variant sequences, the 1A and the 15A substitutions (taking the C-rich strand as reference), were found. The 15A substitution, created by in vitro mutagenesis in a plasmid model and tested in vivo (Fan and Yao 2000), behaved as a fully functional Cbs variant. The 1A substitution was not tested in that system.
Only five T. thermophila Cbs junctions had been previously isolated in an unbiased way, i.e., by looking in the MIC for a likely chromosome breakage element in the neighborhood of a randomly chosen, subtelomeric MAC segment. Two Cbs elements with a variant sequence, the 13A substitution, were previously reported. The first is one of three clustered Cbs elements on the 5′ side of the rDNA MAC minichromosome (Yao et al. 1985). Because of the clustering, it was not known if the 13A element was functional. Its second occurrence is as the single Cbs element in a functional junction reported by Yu et al. (1990) in the context of a chromosome healing study. A study of the Cbs elements flanking the micronuclear rDNA locus in several other tetrahymenine ciliates (Coyne and Yao 1996) showed nearly complete evolutionary conservation of the chromosome breakage sequence except for a number of single base differences (15A, 15G, or 14C). Because of possible interspecific differences, the relevance of these observations to T. thermophila was unclear.
The evidence from this work suggests that a much larger fraction of the functional Cbs elements than previously suspected has a variant sequence. On the basis of the single-position substitutions reported here and by Yu et al. (1990) the sequence of functional Cbs elements can be summarized as WAAACCAACCTCWTW, where W stands for A or T. A more extensive, unbiased survey of the Cbs elements, searching the MIC sequence adjacent to randomly cloned inserts containing telomere addition sites, is underway and will provide information on the relative frequencies of canonical vs. variant Cbs. Several additional distinct, functional variants have been identified in that survey (our unpublished observations), which will be separately reported upon its completion.
All the Cbs elements that passed the functionality tests, including those with variant sequence (1A and 15A substitutions), appear to have full fragmentation activity. Cbs elements that fail to mediate complete fragmentation would not be expected to pass the nullisomic mapping test: they should generate PCR products with every nullisomic strain, as PCR should sensitively detect junction DNA molecules that escaped fragmentation. Conversely, the variant that failed the functionality tests (the 5A substitution, untested by Fan and Yao 2000) gave no evidence of “leaky” fragmentation activity, as the Tel and specific primer-dependent PCR test should be a sensitive measure of such activity. Thus, fragmentation in natural Cbs junctions seems to be an all-or-none activity.
Efficiency of the Cbs-isolation method:
Since the Cbs is necessary and sufficient for fragmentation (Yao et al. 1990), 250–300 Cbs elements should be present in the MIC. Current MAC genome assemblies contain 104 Mb (http://www.tigr.org/tdb/e2k1/ttg/). Since ∼15% of the MIC genome sequence complexity is eliminated from the developing MAC (Yao et al. 1984), the minimum estimated size of the MIC genome is ∼120 Mb. Thus the Cbs junctions should be represented in the MIC at a frequency of ∼1 for every 400 kb. The method presented in this study results in enrichment for these sequences. To find a single Cbs-containing clone in a nonenriched MIC library (with an average insert size of 1 kb), 400 clones would have to be screened. With this enrichment method ∼1500 insert-plus clones have so far yielded 36 unique Cbs junctions. This represents a frequency of ∼1 in 42 and an ∼10-fold enrichment. (Other unique Cbs-clones are almost certainly present in these libraries but have not been characterized because they reside on small inserts and/or are located near an insert end.) Additional evidence for the usefulness of this method is the successful cloning of Cbs junctions from whole-cell DNA (from a unisomic-1 strain), in spite of an ∼100-fold excess of MAC over MIC DNA.
Clones obtained by the Cbs enrichment method have been used to establish and validate a valuable approach to MIC:MAC relational mapping, but the surprising incompleteness of the Cbs libraries reveals inadequacies of the method for identifying every Cbs junction in the MIC genome. This drawback is due in part to the previously undiscovered range of degeneracy present in functional chromosome breakage sequences (discussed above) and in part from the extensive PCR amplification required after the enrichment process. In particular, sequenced repeat clones were found to be identical in every way (clone boundaries and cloned PCR errors) to the original isolate. In hindsight, differential PCR amplification could be partially alleviated by using hydrodynamically sheared, sized DNA fragments. This shearing method is more random and size selection would minimize the preferential PCR amplification of smaller products. In the end, the closure of the MAC genome sequence should circumvent the need to clone MAC telomere-adjacent sequence and greatly facilitate the isolation of all the Cbs junctions in the genome.
Prospects for the use of sequenced Cbs junctions in the genome project:
This study has uncovered features and has provided methodology and reagents that are very useful for whole-genome mapping and for relating genetic, physical, and sequence maps.
A powerful method for enriching MIC libraries for Cbs-containing inserts has been developed.
A combination of PCR tests that rigorously identify functional Cbs and accomplish their deletion mapping to chromosome arms has been validated.
As reported in the accompanying article (Cassidy-Hanley et al. 2005, this issue), the Cbs-flanking sequences allow the identification of the specific macronuclear chromosomes that flank each Cbs by providing unique sequence tags and a source of specific probe for determining chromosome size.
A number of chromosome-breakage-site-associated polymorphisms have been identified and genetically mapped (Cassidy-Hanley et al. 2005, this issue). This mapping, in combination with the deletion mapping of every junction to a chromosome arm, reported here, will facilitate anchoring physical and whole-genome sequence maps to the genetic maps of the MIC and MAC.
Acknowledgments
We thank Glenn Herrick and anonymous reviewers for many improvements resulting from their critical reading of an earlier version of this manuscript. We gratefully acknowledge the support of the National Institutes of Health (grant RR-02391 from the National Center for Research Resources) to E.O. and the National Science Foundation (grant 9817121) to P.J.B.
Sequence data from this article have been deposited with the EMBL/GenBank Data Libraries under accession nos. AY653004, AY653005, AY653006, AY653007, AY653008, AY653009, AY653010, AY653011, AY653012, AY653013, AY653014, AY653015, AY653016, AY653017, AY653018, AY653019, AY653020, AY653021, AY653022, AY653023, AY653024, AY653025, AY653026, AY653027, AY653028, AY653029.
References
- Allen, S. L., D. Zeilinger and E. Orias, 1996. Mapping three classical isozyme loci in Tetrahymena: meiotic linkage of EstA to the ChxA linkage group. Genetics 144: 1489–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler, M. I., and M. C. Yao, 1985. Macronuclear DNA of Tetrahymena thermophila exists as defined subchromosomal-sized molecules. Nucleic Acids Res. 13: 5817–5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn, E. H., and J. C. Gall, 1978. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J. Mol. Biol. 120: 33–53. [DOI] [PubMed] [Google Scholar]
- Cassidy-Hanley, D., M. C. Yao and P. J. Bruns, 1994. A method for mapping germ line sequences in Tetrahymena thermophila using the polymerase chain reaction. Genetics 137: 95–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy-Hanley, D., Y. Bisharyan, V. Fridman, J. Gerber, C. Lin et al., 2005. Genome-wide characterization of Tetrahymena thermophila chromosome breakage sites. II. Physical and genetic mapping. Genetics 170: 1623–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau, M. F., and E. Orias, 1996. An improved method to obtain high molecular weight DNA from purified micro- and macronuclei of Tetrahymena thermophila. J. Eukaryot. Microbiol. 43: 198–202. [DOI] [PubMed] [Google Scholar]
- Cole, E. S., and P. J. Bruns, 1992. Uniparental cytogamy: a novel method for bringing micronuclear mutations of Tetrahymena into homozygous macronuclear expression with precocious sexual maturity. Genetics 132: 1017–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover, R. K., and C. F. Brunk, 1986. Macronuclear DNA molecules of Tetrahymena thermophila. Mol. Cell. Biol. 6: 900–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne, R. S., and M.-C. Yao, 1996. Evolutionary conservation of sequences directing chromosome breakage and rDNA palindrome formation in tetrahymenine ciliates. Genetics 144: 1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerder, F. P., J. C. Deak and J. H. Lief, 1992. Rate of phenotypic assortment in Tetrahymena thermophila. Dev. Genet. 13: 126–132. [DOI] [PubMed] [Google Scholar]
- Fan, Q., and M. Yao, 1996. New telomere formation coupled with site-specific chromosome breakage in Tetrahymena thermophila. Mol. Cell. Biol. 16: 1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, Q., and M. C. Yao, 2000. A long stringent sequence signal for programmed chromosome breakage in Tetrahymena thermophila. Nucleic Acids Res. 28: 895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton, E. P., and E. Orias, 2000 Genetically mapping new mutants and cloned genes, pp. 263–278 in Tetrahymena thermophila, edited by D. J. Asai and J. D. Forney. Academic Press, New York. [DOI] [PubMed]
- Mochizuki, K., N. A. Fine, T. Fujisawa and M. A. Gorovsky, 2002. Analysis of a piwi-related gene implicates small RNAs in genome rearrangement in Tetrahymena. Cell 110: 689–699. [DOI] [PubMed] [Google Scholar]
- Nikiforov, M. A., J. F. Smothers, M. A. Gorovsky and C. D. Allis, 1999. Excision of micronuclear-specific DNA requires parental expression of pdd2p and occurs independently from DNA replication in Tetrahymena thermophila. Genes Dev. 13: 2852–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orias, E., 1998. Mapping the germ-line and somatic genomes of a ciliated protozoan, Tetrahymena thermophila. Genome Res. 8: 91–99. [DOI] [PubMed] [Google Scholar]
- Orias, E., N. Hashimoto, M. F. Chau and T. Higashinakagawa, 1991. PCR amplification of Tetrahymena rDNA segments starting with individual cells. J. Protozool. 38: 306–311. [DOI] [PubMed] [Google Scholar]
- Prescott, D. M., 1994. The DNA of ciliated protozoa. Microbiol. Rev. 58: 233–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen, S., and H. J. Skaletsky, 2000 Primer3 on the WWW for general users and for biologist programmers, pp 365–386 in Bioinformatics Methods and Protocols: Methods in Molecular Biology, edited by S. Krawetz and S. Misener. Humana Press, Totowa, NJ. [DOI] [PubMed]
- Sambrook, J., E. F. Fritsch and T. Maniatis, 1989 Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Sanford, Y. M., and E. Orias, 1981. Phenylketonuric Tetrahymena: phenylalanine hydroxylase mutants and other tyrosine auxotrophs. Proc. Natl. Acad. Sci. USA 78: 7614–7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard, A. R., and J. L. Rae, 1997. Magnetic bead capture of cDNAs from double-stranded plasmid cDNA libraries. Nucleic Acids Res. 25: 3183–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, M.-C, 1989 Site-specific chromosome breakage and DNA deletion in ciliates, pp. 715–734 in Mobile DNA, edited by D. E. Berg and M. M. Howe. American Society for Microbiology, Washington, DC.
- Yao, M.-C, E. H. Blackburn and J. Gall, 1981. Tandemly repeated C–C-C-C-A-A hexanucleotide of Tetrahymena rDNA is present elsewhere in the genome and may be related to the alteration of the somatic genome. J. Cell Biol. 90: 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, M.-C, S. J. Choi, S. Yokoyama, C. F. Austerberry and C. H. Yao, 1984. DNA elimination in Tetrahymena: a developmental process involving extensive breakage and rejoining of DNA at defined sites. Cell 36: 433–440. [DOI] [PubMed] [Google Scholar]
- Yao, M.-C, S. G. Zhu and C. H. Yao, 1985. Gene amplification in Tetrahymena thermophila: formation of extrachromosomal palindromic genes coding for rRNA. Mol. Cell. Biol. 5: 1260–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao, M.-C, K. Zheng and C. H. Yao, 1987. A conserved nucleotide sequence at the sites of developmentally regulated chromosomal breakage in Tetrahymena. Cell 48: 779–788. [DOI] [PubMed] [Google Scholar]
- Yao, M.-C, C. H. Yao and B. Monks, 1990. The controlling sequence for site-specific chromosome breakage in Tetrahymena. Cell 63: 763–772. [DOI] [PubMed] [Google Scholar]
- Yu, G. L., J. D. Bradley, L. D. Attardi and E. H. Blackburn, 1990. In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature 344: 126–132. [DOI] [PubMed] [Google Scholar]
- Zamore, P. D., 2001. RNA interference: listening to the sound of silence. Nat. Struct. Biol. 8: 746–750. [DOI] [PubMed] [Google Scholar]