Abstract
The zebrafish perplexed mutation disrupts cell proliferation and differentiation during retinal development. In addition, growth and morphogenesis of the tectum, jaw, and pectoral fins are also affected. Positional cloning was used to identify a mutation in the carbamoyl-phosphate synthetase2-aspartate transcarbamylase-dihydroorotase (cad) gene as possibly causative of the perplexed mutation and this was confirmed by gene knockdown and pyrimidine rescue experiments. CAD is required for de novo biosynthesis of pyrimidines that are required for DNA, RNA, and UDP-dependent protein glycosylation. Developmental studies of several vertebrate species showed high levels of cad expression in tissues where mutant phenotypes were observed. Confocal time-lapse analysis of perplexed retinal cells in vivo showed a near doubling of the cell cycle period length. We also compared the perplexed mutation with mutations that affect either DNA synthesis or UDP-dependent protein glycosylation. Cumulatively, our results suggest an essential role for CAD in facilitating proliferation and differentiation events in a tissue-specific manner during vertebrate development. Both de novo DNA synthesis and UDP-dependent protein glycosylation are important for the perplexed phenotypes.
WE have used a forward genetic approach in zebrafish to identify and study genes essential for retinal development. The neural retina of zebrafish, as in other vertebrates, arises from neuroepithelial cells that line the optic cup. All of these elongated cells rapidly divide through multiple rounds of mitosis before the first group of progenitor cells leaves the cell cycle. At the time of cell cycle exit, retinal progenitor cells undergo cell type fate decisions, initiate postmitotic cell migration, and begin to differentiate into one of the seven major cell types found in vertebrate retinas (reviewed in Ohnuma et al. 2001; Levine and Green 2004). In fish and other ectothermic vertebrates, the retina continues to grow throughout larval development from a population of slowly proliferating stem cells found at the outer margin of the neural retina. Fundamental questions in retinal development, and developmental biology in general, center on the coordination of cell proliferation, cell cycle exit, and differentiation of progenitor cells.
The perplexed mutation was identified in a genetic screen for ethylnitrosourea (ENU)-induced mutants that showed disrupted retinal lamination in the absence of gross embryological defects (Link et al. 2001). Embryos homozygous for the perplexed mutation have small eyes and a reduced tectum and die between 8 and 12 days postfertilization (dpf). Our previous analyses indicated that the perplexed gene is essential for the transition from a proliferative to a postmitotic state within the neural retina. Elevated cell death was observed at the time when retinal progenitor cells normally begin to withdraw from the cell cycle. In addition, retinal differentiation and morphogenesis were dramatically diminished in surviving cells within perplexed retinas. Genetic mosaic analysis indicated that the mutation is non-cell autonomous for both survival and differentiation. As part of this study, we have used positional cloning and other experiments to identify and confirm that a mutation in the gene encoding the trifunctional enzyme carbamoyl-phosphate synthetase2-aspartate transcarbamylase-dihydroorotase (CAD) is responsible for the perplexed phenotypes.
CAD is the rate-limiting enzyme for de novo biosynthesis of pyrimidine-based nucleotides and catalyzes the first three steps in de novo pyrimidine biosynthesis (reviewed in Jones 1980; Figure 1). Utilizing ATP, CAD converts glutamine and bicarbonate to dihydroorotate for the production of orotate. Orotate is then rapidly converted to uridine-5′-diphosphate (UDP), which is a precursor for production of cytosine- and thymine-based nucleotides and ultimately RNA and DNA synthesis. UDP is also a precursor of UDP-sugar intermediates, which are required for post-translational modification of many proteins. The function of CAD, therefore, is important for both de novo RNA and DNA synthesis and for specific types of protein glycosylation. In most cells, UDP and other nucleotides can also be provided by the salvage pathways. Several cultured cell lines lack functional CAD, but can be maintained in a pyrimidine-supplemented medium (Davidson and Patterson 1979; Patterson et al. 1992; Qiu and Davidson 1998). The utilization of de novo vs. salvage pathways for pyrimidine biosynthesis in vivo has been proposed to be dependent on tissue and cell type, as well as on the mitotic state of a cell (Anderson and Parkinson 1997).
Figure 1.—
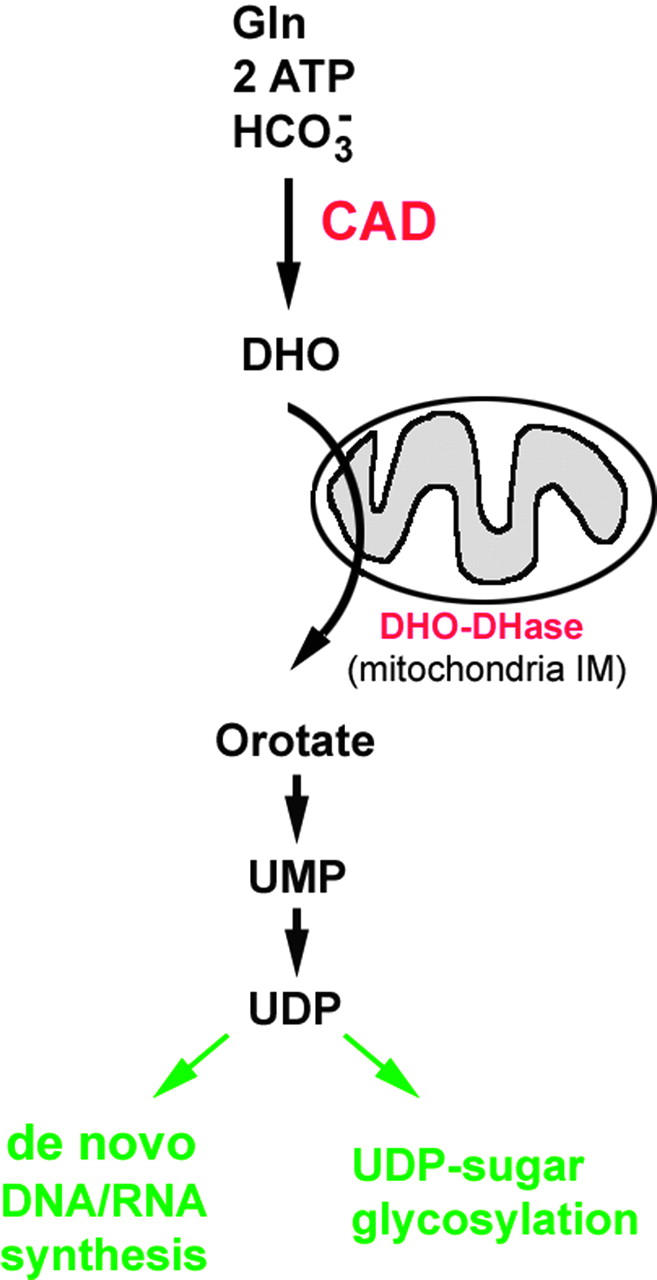
Illustration of the role of the carbamoyl-phosphate synthetase2-aspartate transcarbamylase-dihydroorotase enzyme (CAD) in de novo biosynthesis of dihydroorotate (DHO) and its rapid conversion to uridine diphosphate (UDP). CAD utilizes glutamine (Gln), adenosine triphosphate (ATP), and bicarbonate HCO−3 to synthesize DHO. All reactions take place in the cell cytoplasm except for that of dihydroorotate dehydrogenase (DHO-DHase), which occurs at the mitochondria inner membrane (IM). Loss of CAD activity can affect DNA/RNA nucleotide biosynthesis as well as UDP-dependent protein glycosylation events.
In Drosophila, a mutation in CAD—rudimentary—was among the first described by Thomas Hunt Morgan and was used for discovering meiotic recombination (Morgan 1913; Sturtevant 1913). Flies with the rudimentary mutation show malformed and reduced wings as well as female sterility (Morgan 1911, 1915). The mutation is lethal only when larvae are grown on pyrimidine-free medium. While these results show that CAD has tissue-specific functions during invertebrate development, the role of CAD in vertebrate development has not been investigated.
In this study, we have used positional cloning, gene knockdown, and pyrimidine rescue experiments to identify and confirm a mutation in the zebrafish cad gene as causative for the perplexed mutation. To address tissue specificity we have investigated cad gene expression during development in several vertebrate species. Finally, we have further analyzed the retinal and nonretinal phenotypes of perplexed by comparing cad mutants to embryos with mutations in other genes required for either nucleotide synthesis or UDP glycosylation. Cumulatively, our results suggest an essential and complex role for CAD in facilitating proliferation and differentiation events in a tissue-specific manner during vertebrate development. Our observations are consistent with the hypothesis that basic metabolic pathways are key targets of regulatory signals essential for coordinating cellular proliferation and differentiation during development.
MATERIALS AND METHODS
Mutant alleles:
The following mutant alleles were used in this study (the gene affected is in brackets and the original citation is in parentheses): perplexeda52 [cad] (Link et al. 2001); hi688 [ribonucleotide reductase R2] and hi954 [UDP-glucuronic acid decarboxylase] (Golling et al. 2002); and hi2694 [cad], hi3378 [UDP-glucuronic acid/UDP-N-acetylgalactosamine dual transporter], and hi3510 [thymidylate synthase] (Amsterdam et al. 2004).
Positional cloning:
Bulked segregant linkage analysis:
The recessive perplexeda52 mutation was outcrossed with wild-type AB fish and the mutant line was propagated by repeated AB outcrossings (Link et al. 2001). Since meiotic recombination rates are lower in male zebrafish compared to those in females, the use of male meiosis is favorable for bulked segregant analysis to identify on which chromosome the mutation is located (Singer et al. 2002). A mapping panel to maximize the information content of the male parent was generated by outcrossing an AB +/perplexed fish with a TL +/+ fish and then backcrossing a resulting TL +/perplexed heterozygous male with an AB +/perplexed female. Genomic DNA was isolated from homozygous perplexed mutant embryos and wild-type siblings and two pools of 20 mutant and 20 wild-type embryos were used for bulked segregant analysis. Simple sequence-length polymorphism markers (Knapik et al. 1998; Shimoda et al. 1999; http://zebrafish.mgh.harvard.edu/zebrafish/index.htm) spaced ∼20 cM apart across the length of the genome were amplified by polymerase chain reaction (PCR) and the products were analyzed on 3% agarose gels. Once linkage was detected, 96 individual mutants were genotyped to confirm linkage and to refine the critical interval.
High-resolution linkage mapping:
Given that increased rates of recombination are more advantageous for high-resolution linkage mapping, new mapping panels informative for recombination in the female parents were generated. AB +/perplexed fish were outcrossed to WIK +/+ fish and resulting WIK +/perplexed were mated to AB +/perplexed to generate homozygous perplexed mutants for fine mapping. Fluorescent primers (Proligo, Boulder, CO) were designed for markers z8150 and z9794 and 530 mutant embryos were genotyped on a CEQ 8000 genetic analysis system (Beckman Coulter, Fullerton, CA) to identify recombinant embryos. BLAST searches were used to identify finished BAC clones from the NCBI database (http://www.ncbi.nlm.nih.gov/genome/seq/DrBlast.html) and scaffolds from the zebrafish assembly version 4 (http://www.ensembl.org/Danio_rerio/) that were located in the critical region. SeqMan II software (DNASTAR, Madison, WI) was used to assemble the BAC clones and scaffolds into a single contig that spanned the entire length of the critical interval containing the perplexed mutation. Single-nucleotide polymorphisms (SNPs) producing restriction fragment length polymorphisms (RFLPs) were identified by PCR amplifying, cloning, and sequencing fragments across the region from the female parent. These new informative markers were used to refine the critical interval.
Candidate gene search:
Candidate genes within the perplexed critical region were identified using GENSCAN (http://genes.mit.edu/GENSCAN.html). Total RNA was isolated from pools of 100 perplexed and wild-type embryos using a VERSAGENE RNA purification kit (Gentra Systems, Minneapolis) and cDNA synthesized using oligo(dT) and SuperScript II as described by the manufacturer (Invitrogen, Carlsbad, CA). The full-length ORF representing the cad gene was amplified using primers (forward, 5′-AGGGACAGCATGCTATTTGG-3′; reverse, 5′-TGGTCCCAATTGGATAGGAA-3′) and AccuPrime Taq polymerase as described by the manufacturer (Invitrogen). Amplified products were cloned, sequenced, and analyzed for potential mutations. The identified T-to-G transversion introduces an AvaII restriction enzyme site, which was subsequently used to confirm that the mutation was present in genomic DNA from perplexed embryos.
Orotic acid and uridine rescue:
The sodium salts of orotic acid (no. O3000) and uridine (no. U0750) were obtained from Sigma (St. Louis). Stock solutions made in embryo medium (Westerfield 1995) and 50 nl of various concentrations (1 μm–10 mm) were injected into the yolk sack of developing wild-type and perplexed embryos at 20 hr postfertilization (hpf). Partial rescue of eye, jaw, and fin development was observed at a minimal concentration of 1 μm for orotic acid and 100 μm for uridine. Concentrations >1000 μm resulted in early developmental lethality. To evaluate retinal histology, embryos were fixed in 4% paraformaldehyde at 72 hpf and processed for cryosectioning. Sections were mounted on glass slides and nuclei were stained with Hoechst 33258 (0.5 μg/ml in PBS).
Morpholino antisense knockdown:
We designed three independent morpholino (MO) antisense oligonucleotides (Gene Tools, Philomath, OR) to the zebrafish cad gene. CADMO1 (TTAATTTTCACTTACCAACTTCACC) overlapped exon 1 donor sequence and was used at 40 μm. CADMO2 (AAACAAAAATAAACCTTGCTGAGTC) overlapped exon 2 donor sequence and was used at 30 μm. CADMO3 (TAAAGATGCCATTTTCAGCGACATG) overlapped the ATG start site and was used at 200 μm. These oligonucleotides were diluted in sterile water with 0.02% phenol red for visualization purposes and 15 nl was injected into one- or two-cell stage wild-type embryos. As controls, standard control morpholino (GeneTools) was injected at equivalent concentrations. To confirm efficacy of splicing inhibition, a subset of embryos injected with MO1 and MO2 were taken for reverse transcription-polymerase chain reaction (RT-PCR) analysis (Draper et al. 2001). For MO1 and MO2, 30 control and 30 CADMO1- or CADMO2-injected embryos were collected at 32 or 48 hpf for RT-PCR analysis. A forward primer specific to exon 1 (CADEX1F1 GTCTGTTCGGTGCCGTATCT) and a reverse primer specific to exon 3 (CADEX3R1 GCTCTCTGAGCCACTCATCC) were used to amplify the cDNA pools. Analysis indicated that both MOs induce alternative splicing events that disrupt gene function.
Histology:
Semithin retinal sections were obtained after fixing embryos overnight at 4° in 2.5% glutaraldehyde/1% paraformaldehyde/phosphate-buffered sucrose, pH 7.4. Embryos were dehydrated and infiltrated with Epon/Araldite. Transverse sections, 1–2 μm, were heat mounted and stained with 1% methylene blue in 1% borax.
Alcian blue cartilage staining:
PTU-treated embryos (∼15) were placed in 1.5-ml plastic centrifuge tubes and fixed in 1 ml of 3.7% neutral buffered formaldehyde at room temperature for 2 hr. Embryos were then rinsed in PBS and transferred to Alcian blue stain (0.1% Alcian blue in 80% ethanol/20% glacial acetic acid) for overnight incubation at room temperature. The next day, embryos were rinsed in 100% ethanol and gradually rehydrated in PBS. Further washes were carried out in 1% KOH in PBS. Embryos were mounted in 1% low melting agarose for photography.
Riboprobes and in situ hybridization:
Localization of mRNA in zebrafish was performed on whole embryos (Jowett and Lettice 1994). For chicken and mouse, in situ hydridization was performed on paraffin-sectioned embryos as described in Lee et al. (2005). Digoxegenin-labeled cRNA probes corresponding to the following regions of cDNA were used: zebrafish, 6365–7000 bp, accession no. AY880246; chicken, 43–3043 bp, accession no. XM_426217; and mouse, 6139–6930 bp, accession no. NM_023525.
Transgenesis and time-lapse microscopy:
Wild-type or perplexed mutant embryos were injected at the one- to four-cell stage with ∼15 nl of 50 ng/μl circular plasmid DNA encoding histoneH2B::green fluorescent protein (GFP) fusion protein (Köster and Fraser 2001). Embryos were grown in PTU to block pigment synthesis. At 32–34 hpf, embryos were anesthetized with 0.05% Tricane and embedded in 1.0% low-melt agarose. Fish were oriented on their side within a 35-mm2 culture dish (P35G-1.5-10-C; MatTek, Ashland, MA). Cells labeled with the transgene were then imaged with a Nikon C1 confocal microscope for 15–20 hr. Z-sections (40 μm total depth) were collected every 12 min. This time was determined empirically to be sufficient to capture M-phase for each cell. Temperature was maintained throughout all experiments at 28.5° using a stage incubator.
Cell cycle analysis:
Image planes from confocal time-lapse microscopy were converted from IDS to ND format using Metamorph Imaging software (Universal Imaging, Philadelphia). These data were then arrayed by time and z-plane using the multidimensional analysis tool suite. Individual cells were followed from M-phase to M-phase. M-phase was easily viewed by the condensed and elongated nature of the chromatin. The time required for this was recorded as the total cell cycle period. If a cell underwent apoptosis, as evident by DNA fragmentation, the latency from the last M-phase was recorded. In all, we quantified the cell cycle period or latency to cell death for n = 26 perplexed retinoblasts (four time-lapse experiments) and n = 27 wild-type retinoblasts (three time-lapse experiments).
RESULTS
Zebrafish embryos with the perplexed mutation show reduced eye size and lack retinal cell morphogenesis (Link et al. 2001). Increased apoptosis, as compared to that in wild-type siblings, is apparent by histology at 40 hpf. These phenotypes suggested that the perplexed gene was critical for normal proliferation and differentiation in the retina. Linkage mapping with microsatellite markers localized perplexed to chromosome 20, between z8150 and z9794. Recombinant mutant embryos were then used in conjunction with additional microsatellite markers and newly developed SNPs. Markers z4394 and z536 showed no recombinants out of 530 embryos genotyped. BACs and scaffolds (assembly version 4) were identified that spanned the entire critical region between z13672 and z8164 (Figure 2A). Genscan analysis of this region revealed two potential candidate genes, a selective LIM-binding factor homolog and cad. Cloning and sequencing of the cad open reading frame from perplexed and wild-type embryos revealed a mutation at nucleotide position 3848. This T-to-G transversion produces a nonconservative methionine to arginine substitution at position 1283 of the protein. Comparison of sequence surrounding M1283 in the carbamoyl-phosphate synthetase 2 domain of cad revealed that this methionine is conserved from humans to Caenorhabditis elegans (Figure 2, B and C).
Figure 2.—
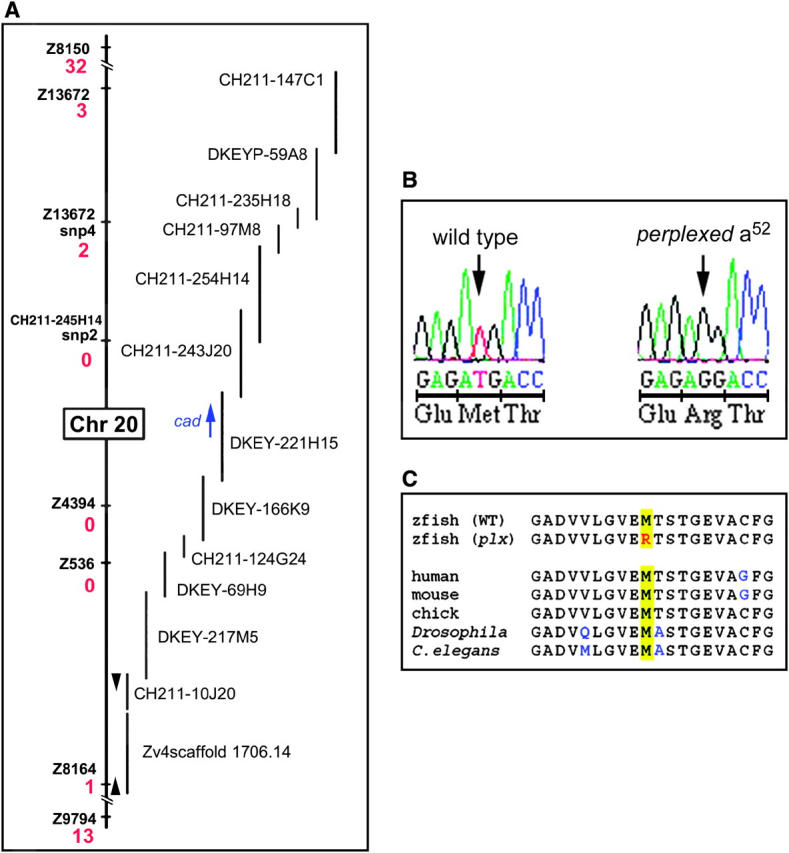
Positional cloning of the perplexed mutation. (A) Genetic and physical map of the critical interval for the perplexed locus of chromosome 20. Informative markers are listed with the associated number of recombination events per 1060 meioses (red). BAC and genomic scaffolds are to the right of the genetic map. The cad open reading frame is represented as a blue arrow. (B) Sequence analysis and predicted protein composition of wild type and the perplexed mutation at amino acid 1283. (C) Sequence comparisons among diverse phyla show absolute conservation for M1283 (yellow highlight) and near perfect similarity for surrounding residues. Amino acids different from zebrafish are indicated in blue. The accession number for the complete coding sequence of zebrafish cad is AY880246.
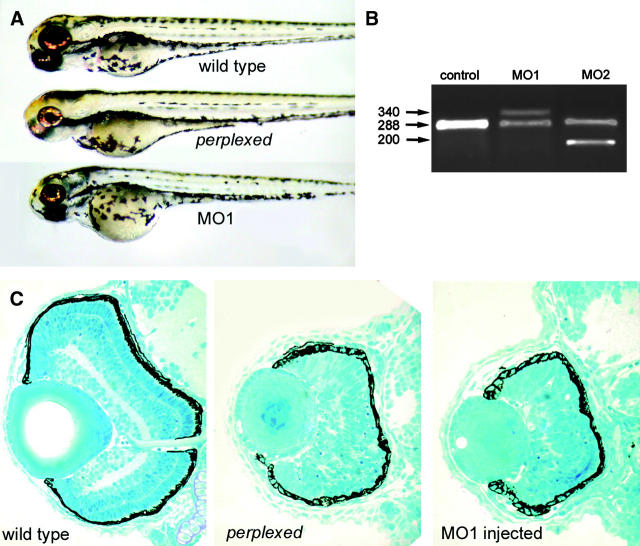
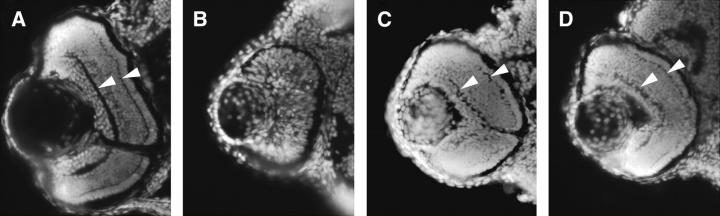
To confirm that the M1283R mutation in cad was responsible for the perplexed phenotype, we designed antisense oligonucleotides (“morpholinos”) to either disrupt pre-mRNA splicing or block translation (Nasevicius and Ekker 2000; Draper et al. 2001). Wild-type embryos injected with any of these morpholinos phenocopied the perplexed mutation (Figure 3). Whole-embryo morphology and retinal histology show the similarities between perplexed and CAD morphant embryos. To further test whether the mutation in cad is responsible for the perplexed phenotype, we attempted to rescue the phenotype by providing pyrimidine nucleotide precursors that are in the de novo biosynthetic pathway, but downstream of CAD. Injection of either orotic acid or uridine into 24-hpf mutant embryos was able to rescue the retinal defects of the perplexed mutation as judged by eye size and plexiform layer formation (Figure 4). Partial rescue of jaw and fin morphogenesis was also noted. Neither of the compounds had an effect on the development of wild-type embryos (data not shown).
Figure 3.—
CAD knockdown phenocopies the perplexed mutation. (A) Whole-embryo morphologies of 84-hpf wild-type, perplexed mutant, and CAD MO1 morphant embryos. (B) RT-PCR analysis of pre-mRNA splice-disrupting morpholinos indicates altered mRNA processing. Control morpholino-injected embryos produced the expected 288-bp product, while the splice-disrupting morpholinos produced alternate splice forms. (C) Histological analysis of wild-type, perplexed, and CAD MO1 morphant eyes. Indistinguishable phenotypes were observed in perplexed and CAD morphant embryos.
Figure 4.—
Rescue of perplexed retinal phenotype by CAD products. Retinal histology is shown of (A) wild-type embryos injected with buffer, (B) perplexed embryos injected with buffer, (C) perplexed embryos injected with 100 μm uridine, and (D) perplexed embryos injected with 1 μm orotic acid. Embryos were injected at 20 hpf and analyzed at 72 hpf by using Hoechst to stain nuclei in cryosections. Note the partial rescue of both eye size and lamination in C and D. Arrowheads indicate inner and outer plexiform lamination in A, C, and D.
Finally, a recently reported insertional mutagenesis screen in zebrafish described a retroviral insertion into the cad locus (Amsterdam et al. 2004). We obtained this line, hi2694, for phenotype analysis and complementation tests. Embryos homozygous for the hi2694 allele showed a phenotype indistinguishable from the perplexed embryos and pairwise crosses between perplexed and hi2694 heterozygotes were noncomplementing for the mutant phenotype. Cumulatively, these data demonstrate that a loss-of-function mutation in cad is responsible for the perplexed phenotype. The ability of supplied orotic acid or uridine to rescue perplexed mutant phenotypes also explains the non-cell-autonomous nature of the perplexed mutation. When mutant cells are placed within a wild-type environment, the compounds downstream of CAD provide substrates for UDP synthesis within the perplexed cells. Orotic acid and uridine can be taken up by adjacent cells through free diffusion, by specific transporters, or via gap junctions depending on cell type and local concentration (Anderson and Parkinson 1997).
In addition to showing reduced eye and tectal size, perplexed embryos also show dismorphic and reduced fins as well as malformed jaw structures. However, other aspects of embryogenesis appear normal. To explore the tissue specificity of the perplexed phenotype, we investigated the developmental expression of cad mRNA. We found that cad transcripts were provided to the egg maternally (Figure 5). When zygotic transcription begins in zebrafish (∼512-cell stage), cad expression is not spatially restricted. However, by the 18-somite stage, cad mRNA expression is downregulated in the posterior region of the embryo and upregulated in the CNS with particularly high expression levels in the retina and tectum. This trend continues until ∼36 hpf. At this time, expression within the eyes and tectum becomes restricted to cells within the proliferative germinal zones. By 48 hpf, transcripts for cad become prominent within the branchial arch regions, which will give rise to jaw structures, and within the developing fin buds. Moderate levels of cad expression are found within the liver, pancreas, and intestine at 3–4 dpf, a time when cad expression in the tectum, retina, and branchial arches has been downregulated. Overall, high levels of cad expression correspond to cells undergoing rapid proliferation or initiating differentiation, consistent with the dismorphic phenotypes of perplexed mutants.
Figure 5.—

In situ mRNA analysis of zebrafish cad. (A) Maternal stores of cad are found in embryos prior to zygotic transcription. (B) Eighteen somite stage embryos begin to show restricted high-level mRNA expression in the eyes and anterior CNS. (C) Sagittal view of a 24-hpf embryo shows cad expression has become further enhanced in the eyes and tectum. (D) Dorsal view of a 24-hpf embryo shows high cad expression within the retina, tectum, and proliferative zone of the hindbrain. (E) Sagittal and (F) dorsal views of 48-hpf embryos show expression in retinal and tectal proliferative zones, condensing jaw cartilage, and fin buds.
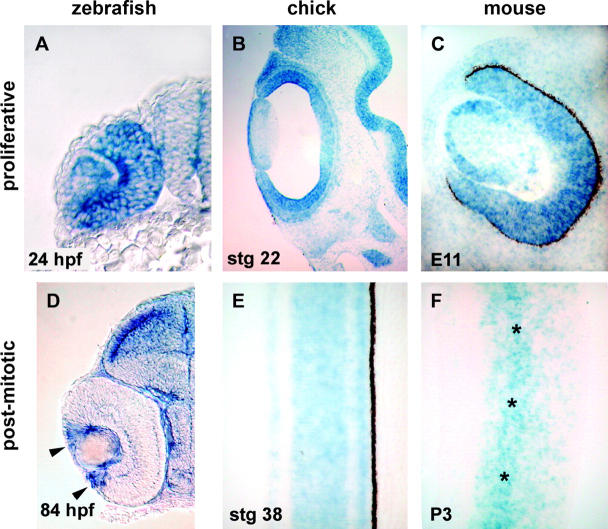
To address whether the cad expression pattern in zebrafish is conserved in other vertebrates, we examined localization of cad transcripts by in situ hybridization in chicken and mouse embryo sections. Similar to zebrafish, the retina and tectum of chicken and mouse embryos showed high levels of expression during proliferative stages, with subsequent decreases following cell cycle exit. At the early developmental times, before cell cycle exit has commenced in zebrafish, chick, or mouse, cad expression was uniform across the neural retina (Figure 6, A–C). In the 84-hpf zebrafish retina, cad expression was maintained in the marginal zone where retinoblasts continue to proliferate (Figure 6D). In the stage 38 chick retina, when lamination has been established, cad expression has been downregulated uniformly (Figure 6E). However, in the P3 mouse retina, increased cad expression in still observed in the inner nuclear layer where late proliferating bipolar and Müller cells reside (Figure 6F).
Figure 6.—
High levels of cad expression in zebrafish, chick, and mouse retinas. Proliferative stages of retinal development were analyzed for cad expression in (A) zebrafish, 24 hpf; (B) chick, stage 22; and (C) albino mouse, embryonic day 11. Postmitotic stages of retinal development were also analyzed for cad expression in (D) zebrafish, 84 hpf (note the high level of expression in the proliferative retinal marginal zone, arrowheads); (E) chick, stage 38; and (F) mouse, postnatal day 3. Asterisks denote a high level of expression in the inner nuclear layer at this time. Basal surface is to the right and apical is to the left. Reduced pigmentation in D was due to use of phenylthiourea, which blocks pigment synthesis in zebrafish. Reduced pigmentation facilitated assessment of gene expression in the retinal pigment epithelium.
Several studies in cell culture have suggested an important role for pyrimidine nucleotides in modulating the rate of cell proliferation (Huisman et al. 1979; Colquhoun and Newsholme 1997; Sigoillot et al. 2004). In addition, the activity of CAD is modulated throughout the cell cycle with the highest activities found in S-phase (Morford et al. 1994; Sigoillot et al. 2002, 2003). Our previous studies with the perplexed mutation showed that the proportion of retinal cells in S-phase was dramatically increased at a time when retinal cells in wild-type embryos were postmitotic. This result could be due to either an increased cell cycle period or a delay in cell cycle exit of retinal progenitors. To address this, we used a time-lapse imaging technique to directly measure cell cycle parameters in living embryos. To label cells for imaging, wild-type or mutant embryos were injected at the one- to four-cell stage with a plasmid encoding a fusion protein of histone H2B and GFP (Köster and Fraser 2001). This manipulation allows for mosaic expression of the transgene, such that individual cells can be followed from mitosis to mitosis via confocal time-lapse microscopy. Figure 7 shows a time series of either a wild-type (Figure 7A) or a perplexed (Figure 7B) retinal neuroepithelial cell completing one full cell cycle. In vertebrate neuroepithelial cells, the nucleus moves from the apical to basal surface—a behavior known as interkinetic nuclear migration. Our time-lapse analyses demonstrate that the average cell cycle period of perplexed retinal cells was twice as long as that of wild-type retinal cells (Table 1). Interkinetic nuclear migration slowed proportionately to the cell cycle delay in perplexed, but no differences in M-phase kinetics were noted. We also calculated the proportion of dying cells and the latency from M-phase to nucleus fragmentation. No cell death was observed in GFP-labeled wild-type cells, consistent with the low levels of normal apoptosis previously described for the zebrafish retina (Biehlmaier et al. 2001). In contrast, 25% of the retinal cells labeled in the perplexed retina underwent apoptosis and this typically occurred within 2 hr from the last cell division. These observations provide in vivo and genetic evidence that CAD function is essential for normal cell cycle progression.
Figure 7.—
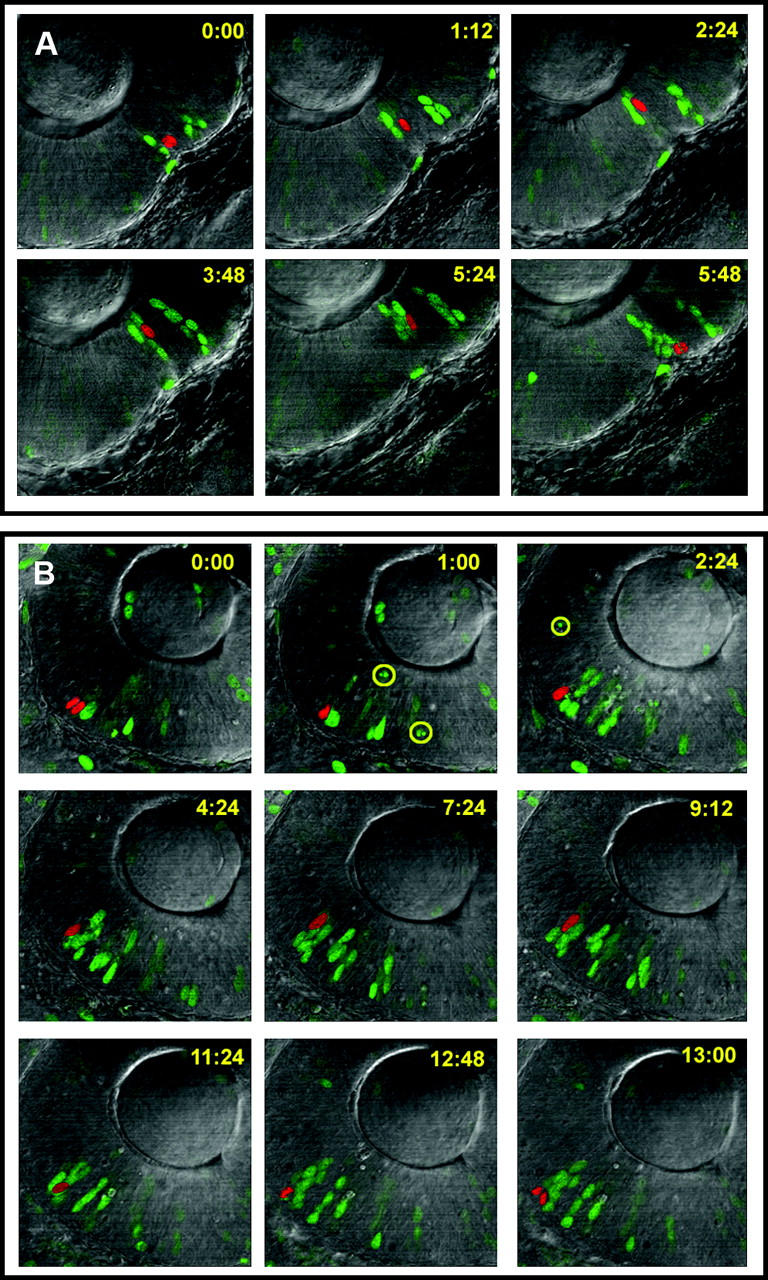
Confocal time-lapse analysis of retinal cell cycle dynamics. (A) Wild-type and (B) perplexed retinal progenitors are shown. The red pseudo-colored cell expressed histone H2B:::GFP and can be seen to enter mitosis in the top left (time 0:00). Representative images are shown throughout the cell cycle. Green cells, also labeled with histoneH2B:::GFP, were followed by separate analyses. Yellow numbers show time in minutes from the first imaged M-phase. The lens is located in the top half of each image and the ventricular zone at the retinal pigment epithelium is toward the bottom (pigmentation was inhibited with PTU). Apoptosis can be seen occurring in some labeled cells in the perplexed retina (highlighted by yellow circles). Nonlabeled cells dying are also visible by their pyknotic profiles.
TABLE 1.
Cell cycle parameters of retinal progenitors
| Parameter | Wild type | perplexed |
|---|---|---|
| Cell cycle (hr) | 6.3 ± 1.3 | 12.1 ± 1.6 |
| Apoptosis: latency from M-phase (hr) |
NA | 1.6 ± 1.3 |
| Apoptotic cells | 0 | 9 |
| Total cells imaged | 26 | 27 |
CAD activity is required for de novo UDP biosynthesis, which is required for both DNA synthesis and post-translational modification. To address whether disruption of one or the other of these pathways was more or less responsible for the perplexed phenotype we analyzed several additional zebrafish mutants, all of which had been produced by retroviral insertion (Table 2) (Golling et al. 2002; Amsterdam et al. 2004). The mutant hi688 has an insertion in ribonucleotide reductase R2 (Golling et al. 2002). The other mutant, hi3510, has an insertion in the thymidylate synthase gene (Amsterdam et al. 2004). Ribonucleotide reductases are composed of subunits R1 and R2. This enzyme catalyzes the synthesis of deoxyribonucleotides from their corresponding ribonucleotides. Therefore, ribonucleotide reductase provides the only source of precursor nucleotides—from either de novo or salvage pathways—for DNA synthesis (Jordan and Reichard 1998). Thymidylate synthase catalyzes the methylation of dUMP to dTMP and is essential for de novo synthesis of thymidine, which is also essential for DNA synthesis.
TABLE 2.
Mutant comparison
| Allele | Gene | Metabolic function | Phenotype |
|---|---|---|---|
| plx a52 | cad |
De novo synthesis of pyrimidines (DNA synthesis and UDP glycosylation) |
Retinal proliferation Retinal differentiation Jaw differentiation |
| hi688 | ribonucleotide reductase R2 | Synthesis of dNTP's (DNA synthesis) | Early lethal, pan-degeneration |
| hi3510 | thymidylate synthase | De novo synthesis of thymidine (DNA synthesis) | Retinal proliferation |
| hi3378 | UDP-glucuronic acid/UDP-N-acetylgalactosamine dual transporter | Transport of nucleotide sugar into Golgi apparatus (UDP glycosylation) |
Jaw differentiation |
| hi954 | UDP-glucuronic acid decarboxylase | Synthesis of UDP xylose (UDP glycosylation) | Jaw differentiation |
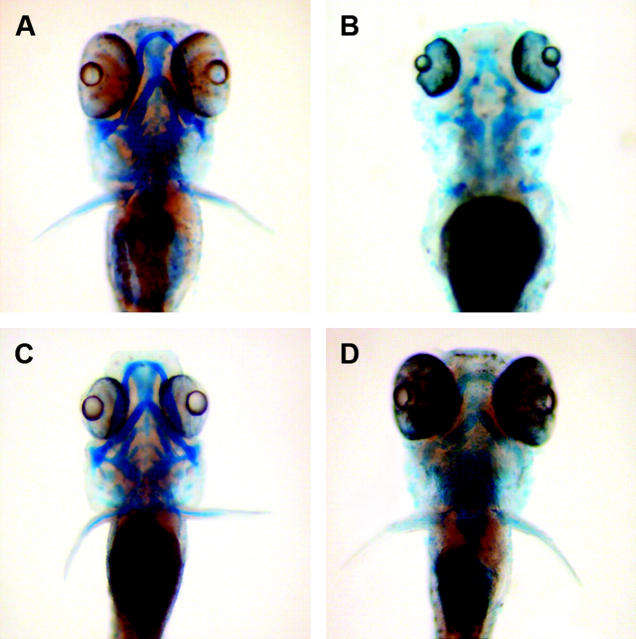
To examine if the lack of nucleotides for DNA synthesis was likely to be the main cause of the perplexed phenotypes, particularly given the extent to which the cell cycle in retinal cells was affected, we examined development in these mutants. The ribonucleotide reductase R2 mutant showed very severe deficits and early embryonic lethality and could not be analyzed further. Thymidylate synthase mutants, which affect de novo DNA synthesis, but not UDP-dependent glycosylation, show reduced eye size. Interestingly, retinal lamination and differentiation appear normal as compared to that in perplexed retinas (Figure 8). To assay jaw and fin formation and differentiation, Alcian blue cartilage staining and histological inspections were conducted (Figure 9). Thymidylate synthase mutants showed normal jaw structures and fin morphogenesis (Figure 9C). In contrast, perplexed embryos showed severe dismorphogenesis of the jaws and fins (Figure 9D). The overall staining intensity of Alcian blue was reduced in perplexed and notably absent were Meckel's, cleithrum, and hyosymplectic cartilage.
Figure 8.—
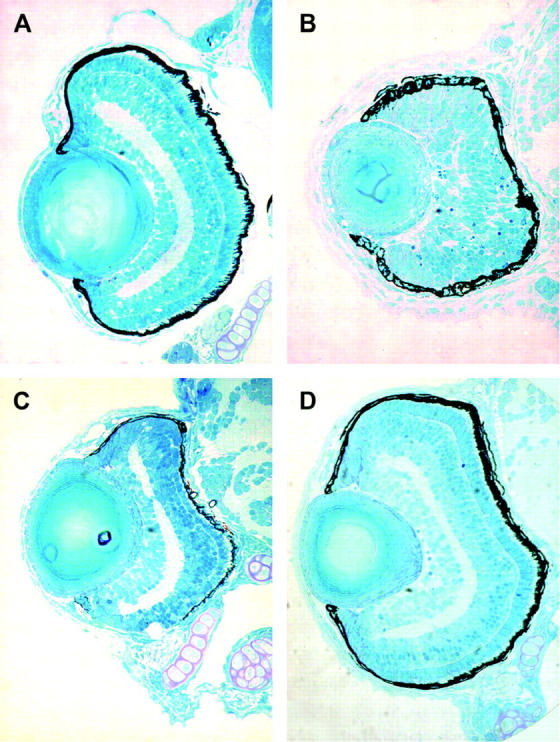
Retinal histology of CAD pathway mutants. (A) Wild type; (B) perplexed; (C) thymidylate synthase mutant (3510); (D) UDP-glucuronic acid/UDP-N-acetylgalactosamine dual transporter mutant (3378). Note the small eye, but normal differentiation in the thymidylate synthase mutant. No ocular defects were observed in the UDP-glucuronic acid/UDP-N-acetylgalactosamine dual transporter mutant.
Figure 9.—
Jaw and fin phenotypes of CAD pathway mutants. (A) Wild type; (B) perplexed (CAD); (C) thymidylate synthase mutant (3510); (D) UDP-glucuronic acid/UDP-N-acetylgalactosamine dual transporter mutant (3378). Alcian blue staining was used to label differentiating cartilage. Note the reduced and dismorphic jaw cartilage and fins in perplexed. Reduced cartilage, but normal fins were observed in the UDP-glucuronic acid/UDP-N-acetylgalactosamine dual transporter mutant. Neither jaw nor fin defects were observed in the thymidylate synthase mutants, although the eyes are small.
To examine the role of UDP-dependent glycosylation we analyzed the hi3378 mutant, which lacks the UDP-glucuronic acid/UDP-N-acetylgalactosamine dual transporter. This transporter is part of a large family of nucleotide sugar transporters, where each member has specificity for a particular nucleotide-sugar compound (Bulter and Elling 1999). In humans there are five UDP-sugar transporters (Ishida and Kawakita 2004). Mutations in these transporters prevent the movement of the nucleotide-sugar intermediates from the cytoplasm to the Golgi apparatus and therefore block a subset of UDP-dependent glycosylation events. We also investigated hi954, which has a mutation in the UDP-glucuronic acid decarboxylase gene. This enzyme is required for the conversion of UDP-glucuronic acid to UDP xylose, which is used for synthesis of numerous glycoconjugates abundant in the extracellular matrix and on cell surfaces. Embryos homozygous for a retroviral insertion in the UDP-glucuronic acid/UDP-N-acetylgalactosamine dual transporter had normal eye size and retinal differentiation (Figure 8D). However, cartilage differentiation and jaw morphogenesis was disrupted. In particular, Alcian blue staining was severely reduced and Meckel's cartilage and the ethmoid plate appeared absent (Figure 9D). Fin development was not affected by mutations in this nucleotide-sugar transporter. Normal eye and fin morphology, but similarly disrupted jaw development, was observed in mutants for UDP-glucuronic acid decarboxylase.
Together these data suggest that defects in both de novo nucleotide synthesis and UDP-dependent protein glycosylation contribute to the perplexed phenotypes. Specifically, decreased retinal precursor cell proliferation is likely due to low levels of nucleotides required for DNA synthesis as both cad and thymidylate synthase mutants show reduced retinal size. The retinal lamination and differentiation phenotypes of perplexed, however, are more likely to be caused by pathways other than DNA synthesis because thymidylate synthase mutants show normal retinal lamination and cellular differentiation. The jaw defects in perplexed can be attributed to UDP-dependent protein glycosylation, as mutants that affect subsets of this post-translational modification pathway show defects in jaw development. Cumulatively, our comparison of multiple mutants suggests that the perplexed phenotype is complex and not simply due to a generalized decrease in de novo pyrimidine biosynthesis. Instead, the defects of perplexed are due to the misregulation of UDP, which impinges on several critical pathways that have tissue-specific consequences.
DISCUSSION
The perplexed mutation in zebrafish affects cell proliferation and differentiation in a tissue-restricted manner. Specifically, retinal, tectal, jaw, and fin morphogenesis is affected. Using positional cloning techniques we have identified a missense mutation within the carbamoyl phosphate synthetase 2 domain of the cad gene in perplexed embryos. Targeted gene knockdown and pyrimidine rescue experiments confirmed this mutation as causative for the perplexed phenotypes. Similar phenotypes between cad morphants (embryos in which cad was inhibited by antisense oligonucleotides) and hi2694 (a retroviral insertional mutant in the first exon of cad) strongly suggest that the perplexed mutation results in loss-of-function for CAD activity. The tissue specificity of the perplexed mutation can be explained by the high levels of gene expression in the tissues that display dismorphic phenotypes. The Drosophila cad mutation and our analysis of cad expression in other vertebrates suggest that a tissue-enhanced developmental expression of this gene is conserved through evolution.
Our analyses also suggest that the utilization of products of de novo pyrimidine biosynthesis may differ between different cell types. Comparative analysis of mutations that differentially affect either de novo nucleotide synthesis or UDP-dependent glycosylation suggests that retinal development requires de novo nucleotide synthesis for proliferation and UDP-dependent glycosylation for differentiation. Jaw development does not appear to require de novo nucleotide synthesis, but is dependent on the role of CAD in facilitating glycosylation. Although DNA/RNA synthesis and UDP-dependent glycosylation are the principal downstream pathways affected by CAD, additional uncharacterized metabolic products of CAD may also be important for the phenotypes associated with the perplexed mutation. In particular, phenotypes such as retinal differentiation, where we were unable to find a similar defect in other downstream metabolic mutants, may be due to alternative pathways. Our comparative analysis has been limited to currently identified mutations.
The imaging experiments to directly measure the cell cycle period of retinal progenitor cells are consistent with an important role for CAD in cell proliferation. Retinoblasts with the perplexed mutation required twice as long to complete one cell cycle and also showed increased cell death. One possibility for the increase in cell death might be due to a lack of pyrimidine nucleotides required for S-phase and a subsequent induction of a checkpoint arrest, which activates apoptosis. Alternatively, activation of apoptotic pathways may be more direct as recent studies have indicated that CAD is a target for caspase-mediated degradation during cell death and loss of CAD activity, therefore, facilitates apoptosis (Huang et al. 2002).
Observations with perplexed described here are consistent with previous studies that have implicated CAD as a key target of signaling pathways that regulate cell proliferation and differentiation (reviewed in Huang and Graves 2003). For example, in cell culture CAD was found to be a direct target of mitogen-activated protein (MAP) kinases. The MAP kinase Erk2 was found to phosphorylate CAD and increase its enzymatic activity (Graves et al. 2000). Direct phosphorylation of CAD by protein kinase A (PKA) was also found to correlate with increased activity (Carrey et al. 1985; Sigoillot et al. 2002). Interestingly, PKA phosphorylation also rendered CAD more susceptible to degradation, suggesting negative feedback regulation (Carrey 1986). Finally, an important role for CAD in proliferation is supported by the observation that CAD activity is upregulated in multiple cancer cell lines (Kizaki et al. 1980; Aoki and Weber 1981; Sigoillot et al. 2004).
In addition to post-translational regulation, CAD is also regulated transcriptionally and in a manner consistent with growth and differentiation. For example, when proliferation of ts13 cells is inhibited by serum starvation, cad mRNA synthesis and stability is decreased (Rao and Davidson 1988). The opposite effects on cad mRNA are observed with serum stimulation of quiescent cells. Likewise, when cells of the HL-60 myeloid line are induced to undergo terminal differentiation, cad mRNA expression is nearly extinguished (Rao et al. 1987). Compelling evidence now implicates a direct Myc-dependent mechanism for activating the cad promoter during proliferation (Miltenberger et al. 1995; Boyd and Farnham 1997; Bush et al. 1998). Further analysis of the cad promoter has indicated that the Brg1 complex, a SWI/SNF-type chromatin remodeling complex, associates with Myc to induce cad gene expression (Pal et al. 2003). In the absence of high levels of Myc, the Brg1 complex associates with transcriptional repressor proteins and cad transcription levels fall. Interestingly, the young mutation in zebrafish, which shows delayed cell cycle exit and blocked retinal cell differentiation, is caused by a null mutation in brg1 (Link et al. 2000; Gregg et al. 2003). Furthermore, this mutation inhibits a wave of MAP kinase acitivity that normally associates with retinal cell differentiation (Gregg et al. 2003).
Overall, our analysis of the perplexed mutation indicates that the pyrimidine enzyme CAD has a central, but tissue-specific role in coordinating cell proliferation and differentiation, supporting a model that was proposed on the basis of numerous cell culture findings (Huang and Graves 2003). The comparative analysis of perplexed to zebrafish mutants that affect DNA nucleotide precursors or UDP-dependent glycosylation suggests that the role of CAD is complex and may differ in function depending on cell type. For example, within the retina, CAD appears to be required during proliferative phases to provide sufficient quantities of DNA nucleotides for genome replication. Without CAD, the time needed to complete one cell cycle of a retinal neuroepithelial cell is extended twofold. Retinal cell differentiation is also disrupted in perplexed and this phenotype sets perplexed/CAD mutants apart from other mutants that affect de novo synthesis of DNA nucleotides. We suggest that the role of CAD in UDP-dependent glycosylation is the reason for the retinal differentiation phenotype. Future studies to disrupt specific UDP-dependent glycoproteins will be required to identify the key substrates for retinal cell differentiation.
Acknowledgments
We thank Adam Amsterdam and Jeff Gross for sharing data and mutant lines prior to publication. We also gratefully acknowledge the technical assistance from Michael Cliff and Melissa Reske. This project was funded by American Heart Association grant 0225071Y (V.L.), National Institutes of Health grant RO1EY01467 (B.L.), and a March of Dimes Basil O'Connor Fellowship (B.L.).
References
- Amsterdam, A., R. M. Nissen, Z. Sun, E. C. Swindell, S. Farrington et al., 2004. Identification of 315 genes essential for early zebrafish development. Proc. Natl. Acad. Sci. USA 101: 12792–12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, C. M., and F. E. Parkinson, 1997. Potential signalling roles for UTP and UDP: sources, regulation and release of uracil nucleotides. Trends Pharmacol. Sci. 18: 387–392. [DOI] [PubMed] [Google Scholar]
- Aoki, T., and G. Weber, 1981. Carbamoyl phosphate synthetase (glutamine-hydrolyzing): increased activity in cancer cells. Science 212: 463–465. [DOI] [PubMed] [Google Scholar]
- Biehlmaier, O., S. C. Neuhauss and K. Kohler, 2001. Onset and time course of apoptosis in the developing zebrafish retina. Cell Tissue Res. 306: 199–207. [DOI] [PubMed] [Google Scholar]
- Boyd, K. E., and P. J. Farnham, 1997. Myc versus USF: discrimination at the cad gene is determined by core promoter elements. Mol. Cell. Biol. 17: 2529–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulter, T., and L. Elling, 1999. Enzymatic synthesis of nucleotide sugars. Glycoconj. J. 16: 147–159. [DOI] [PubMed] [Google Scholar]
- Bush, A., M. Mateyak, K. Dugan, A. Obaya, S. Adachi et al., 1998. c-myc null cells misregulate cad and gadd45 but not other proposed c-Myc targets. Genes Dev. 12: 3797–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrey, E. A., 1986. Nucleotide ligands protect the inter-domain regions of the multifunctional polypeptide CAD against limited proteolysis, and also stabilize the thermolabile part-reactions of the carbamoyl-phosphate synthase II domains within the CAD polypeptide. Biochem. J. 236: 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrey, E. A., D. G. Campbell and D. G. Hardie, 1985. Phosphorylation and activation of hamster carbamyl phosphate synthetase II by cAMP-dependent protein kinase: a novel mechanism for regulation of pyrimidine nucleotide biosynthesis. EMBO J. 4: 3735–3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun, A., and E. A. Newsholme, 1997. Aspects of glutamine metabolism in human tumour cells. Biochem. Mol. Biol. Int. 41: 583–596. [DOI] [PubMed] [Google Scholar]
- Davidson, J. N., and D. Patterson, 1979. Alteration in structure of multifunctional protein from Chinese hamster ovary cells defective in pyrimidine biosynthesis. Proc. Natl. Acad. Sci. USA 76: 1731–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper, B. W., P. A. Morcos and C. B. Kimmel, 2001. Inhibition of zebrafish fgf8 pre-mRNA splicing with morpholino oligos: a quantifiable method for gene knockdown. Genesis 30: 154–156. [DOI] [PubMed] [Google Scholar]
- Golling, G., A. Amsterdam, Z. Sun, M. Antonelli, E. Maldonado et al., 2002. Insertional mutagenesis in zebrafish rapidly identifies genes essential for early vertebrate development. Nat. Genet. 31: 135–140. [DOI] [PubMed] [Google Scholar]
- Graves, L. M., H. I. Guy, P. Kozlowski, M. Huang, E. Lazarowski et al., 2000. Regulation of carbamoyl phosphate synthetase by MAP kinase. Nature 403: 328–332. [DOI] [PubMed] [Google Scholar]
- Gregg, R. G., G. B. Willer, J. M. Fadool, J. E. Dowling and B. A. Link, 2003. Positional cloning of the young mutation identifies an essential role for the Brahma chromatin remodeling complex in mediating retinal cell differentiation. Proc. Natl. Acad. Sci. USA 100: 6535–6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, M., and L. M. Graves, 2003. De novo synthesis of pyrimidine nucleotides; emerging interfaces with signal transduction pathways. Cell Mol. Life Sci. 60: 321–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, M., P. Kozlowski, M. Collins, Y. Wang, T. A. Haystead et al., 2002. Caspase-dependent cleavage of carbamoyl phosphate synthetase II during apoptosis. Mol. Pharmacol. 61: 569–577. [DOI] [PubMed] [Google Scholar]
- Huisman, W. H., K. O. Raivio and M. A. Becker, 1979. Simultaneous estimation of rates of pyrimidine and purine nucleotide synthesis de novo in cultured human cells. J. Biol. Chem. 254: 12595–12602. [PubMed] [Google Scholar]
- Ishida, N., and M. Kawakita, 2004. Molecular physiology and pathology of the nucleotide sugar transporter family (SLC35). Pflugers Arch. 447: 768–775. [DOI] [PubMed] [Google Scholar]
- Jones, M. E., 1980. Pyrimidine nucleotide biosynthesis in animals: genes, enzymes, and regulation of UMP biosynthesis. Annu. Rev. Biochem. 49: 253–279. [DOI] [PubMed] [Google Scholar]
- Jordan, A., and P. Reichard, 1998. Ribonucleotide reductases. Annu. Rev. Biochem. 67: 71–98. [DOI] [PubMed] [Google Scholar]
- Jowett, T., and L. Lettice, 1994. Whole-mount in situ hybridizations on zebrafish embryos using a mixture of digoxigenin- and fluorescein-labelled probes. Trends Genet. 10: 73–74. [DOI] [PubMed] [Google Scholar]
- Kizaki, H., J. C. Williams, H. P. Morris and G. Weber, 1980. Increased cytidine 5′-triphosphate synthetase activity in rat and human tumors. Cancer Res. 40: 3921–3927. [PubMed] [Google Scholar]
- Knapik, E., A. Goodman, M. Ekker, M. Chevrette, J. Delgado et al., 1998. A microsatellite genetic linkage map for zebrafish (Danio rerio). Nat. Genet. 18: 338–343. [DOI] [PubMed] [Google Scholar]
- Köster, R., and S. Fraser, 2001. Tracing transgene expression in living zebrafish embryos. Dev. Biol. 233: 329–346. [DOI] [PubMed] [Google Scholar]
- Lee, V. M., M. Bronner-Fraser and C. V. H. Baker, 2005. Restricted response of mesencephalic neural crest to sympathetic differentiation signals in the trunk. Dev. Biol. 278: 175–192. [DOI] [PubMed] [Google Scholar]
- Levine, E. M., and E. S. Green, 2004. Cell-intrinsic regulators of proliferation in vertebrate retinal progenitors. Semin. Cell Dev. Biol. 15: 63–74. [DOI] [PubMed] [Google Scholar]
- Link, B., P. Kainz, T. Ryou and J. Dowling, 2001. The perplexed and confused mutations affect distinct stages during the transition from proliferating to post-mitotic cells within the zebrafish retina. Dev. Biol. 15: 436–453. [DOI] [PubMed] [Google Scholar]
- Link, B. A., J. M. Fadool, J. Malicki and J. E. Dowling, 2000. The zebrafish young mutation acts non-cell-autonomously to uncouple differentiation from specification for all retinal cells. Development 127: 2177–2188. [DOI] [PubMed] [Google Scholar]
- Miltenberger, R. J., K. A. Sukow and P. J. Farnham, 1995. An E-box-mediated increase in cad transcription at the G1/S-phase boundary is suppressed by inhibitory c-Myc mutants. Mol. Cell. Biol. 15: 2527–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morford, G., J. N. Davidson and E. C. Snow, 1994. Appearance of CAD activity, the rate-limiting enzyme for pyrimidine biosynthesis, as B cells progress into and through the G1 stage of the cell cycle. Cell Immunol. 158: 96–104. [DOI] [PubMed] [Google Scholar]
- Morgan, T. H., 1911. The origin of nine wing mutations in Drosophila. Science 33: 384. [DOI] [PubMed] [Google Scholar]
- Morgan, T. H., 1913. Factors and unit characters in Mendelian heredity. Am. Nat. 47: 5–16. [Google Scholar]
- Morgan, T. H., 1915. The infertility of rudimentary mutants of Drosophila ampelophila. Am. Nat. 49: 240–250. [Google Scholar]
- Nasevicius, A., and S. C. Ekker, 2000. Effective targeted gene ‘knockdown’ in zebrafish. Nat. Genet. 26: 216–220. [DOI] [PubMed] [Google Scholar]
- Ohnuma, S., A. Philpott and W. A. Harris, 2001. Cell cycle and cell fate in the nervous system. Curr. Opin. Neurobiol. 11: 66–73. [DOI] [PubMed] [Google Scholar]
- Pal, S., R. Yun, A. Datta, L. Lacomis, H. Erdjument-Bromage et al., 2003. mSin3A/histone deacetylase 2- and PRMT5-containing Brg1 complex is involved in transcriptional repression of the Myc target gene cad. Mol. Cell. Biol. 23: 7475–7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, D., R. Berger, J. Bleskan, D. Vannais and J. Davidson, 1992. A single base change at a splice acceptor site leads to a truncated CAD protein in Urd-A mutant Chinese hamster ovary cells. Somat. Cell Mol. Genet. 18: 65–75. [DOI] [PubMed] [Google Scholar]
- Qiu, Y., and J. N. Davidson, 1998. CAD overexpression in mammalian cells. Adv. Exp. Med. Biol. 431: 481–485. [DOI] [PubMed] [Google Scholar]
- Rao, G. N., and J. N. Davidson, 1988. CAD gene expression in serum-starved and serum-stimulated hamster cells. DNA 7: 423–432. [DOI] [PubMed] [Google Scholar]
- Rao, G. N., E. S. Buford and J. N. Davidson, 1987. Transcriptional regulation of the human CAD gene during myeloid differentiation. Mol. Cell. Biol. 7: 1961–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda, N., E. W. Knapik, J. Ziniti, C. Sim, E. Yamada et al., 1999. Zebrafish genetic map with 2000 microsatellite markers. Genomics 58: 219–232. [DOI] [PubMed] [Google Scholar]
- Sigoillot, F. D., D. R. Evans and H. I. Guy, 2002. Growth-dependent regulation of mammalian pyrimidine biosynthesis by the protein kinase A and MAPK signaling cascades. J. Biol. Chem. 277: 15745–15751. [DOI] [PubMed] [Google Scholar]
- Sigoillot, F. D., J. A. Berkowski, S. M. Sigoillot, D. H. Kotsis and H. I. Guy, 2003. Cell cycle-dependent regulation of pyrimidine biosynthesis. J. Biol. Chem. 278: 3403–3409. [DOI] [PubMed] [Google Scholar]
- Sigoillot, F. D., S. M. Sigoillot and H. I. Guy, 2004. Breakdown of the regulatory control of pyrimidine biosynthesis in human breast cancer cells. Int. J. Cancer 109: 491–498. [DOI] [PubMed] [Google Scholar]
- Singer, A., H. Perlman, Y. Yan, C. Walker, G. Corley-Smith et al., 2002. Sex-specific recombination rates in zebrafish (Danio rerio). Genetics 160: 649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant, A. H., 1913. The linear arrangement of six sex-linked factors in Drosophila as shown by their mode of association. J. Exp. Zool. 14: 43–59. [Google Scholar]
- Westerfield, M., 1995 The Zebrafish Book. University of Oregon Press, Eugene, OR.