Abstract
Myocyte enhancer factor-2 (MEF2) is a transcription factor that is necessary for embryonic muscle development in Drosophila and vertebrates; however, whether this factor is required during later muscle development remains largely unknown. Using heteroallelic combinations of different Mef2 mutant alleles, we isolated and characterized a temperature-sensitive combination. Through temperature-shift experiments, we obtained adult animals that were lacking proper MEF2 function. Many of these individuals died as mature pupae, and those that eclosed showed poor locomotion and an inability to fly. Histological analysis of these animals revealed a requirement for MEF2 in skeletal muscle patterning, although these animals had strikingly normal amounts of muscle tissue. Using quantitative polymerase chain reaction, we determined that expression of the MEF2-regulated actin gene Act57B was severely reduced in these animals. By contrast myofibrillar actin genes unique to the adult stage were only mildly affected. Since MEF2 mutant adults were still capable of forming muscle tissue, we conclude that MEF2 is required for the expression of only a subset of muscle structural genes in the adult. These results indicate that additional muscle-specific factors function to control the myogenesis of complex and diverse muscle in the adult.
THE myocyte enhancer factor-2 (MEF2) family of transcription factors play a critical role in the development of skeletal, smooth, and cardiac muscle. Four MEF2 factors, MEF2 A–D, exist in mammals and a single MEF2 has been identified in Drosophila. These factors all show homology within a conserved MADS [MCM1, Agamous, Deficiens, serum response factor (SRF)] domain as well as an adjacent domain known as the MEF2 domain (reviewed in Black and Olson 1998). MEF2 proteins bind as dimers to the consensus sequence YTAWWWWTAR (Y, C/T; W, A/T; R, A/G) found in control regions of numerous muscle-specific genes (Andres et al. 1995). Additionally, the vertebrate MEF2 family has been shown to cooperate in skeletal muscle differentiation with the myogenic basic helix-loop-helix (bHLH) transcription factors Myf5, MyoD, MRF4, and myogenin (Li and Capetanaki 1994; Black et al. 1995; Molkentin et al. 1995; Naidu et al. 1995).
In vertebrates the first mef2 gene to be expressed during embryogenesis is mef2-c, in the myocardial precursors. This is followed by expression of the other mef2 genes in those cells. High levels of mef2 expression can also be detected in the developing skeletal and smooth muscle cell lineages as well as in the developing central nervous system (reviewed in Black and Olson 1998). Gene knockout experiments in mice have shown differing requirements for each of the mef2 genes. Animals deficient for mef2-c die early in development (Lin et al. 1997a), whereas animals deficient for mef2-a suffer from cardiac sudden death shortly after birth (Naya et al. 2002). Consistent with the presence of MEF2-binding sites in the promoters of muscle-specific genes, a number of genes such as α-actin and α-myosin heavy chain are downregulated in mef2-c- and mef2-a-deficient animals.
Similarly, expression of the single Drosophila Mef2 gene is first detected in the mesodermal precursor cells prior to gastrulation and continues to be expressed in the unspecified mesoderm thereafter. Expression of Mef2 persists in precursors and differentiated cells of the somatic and visceral musculature, as well as the dorsal vessel (Lilly et al. 1994; Nguyen et al. 1994). Drosophila embryos deficient for MEF2 die during embryogenesis and display a complete lack of differentiated muscle fibers (Bour et al. 1995; Lilly et al. 1995; Ranganayakulu et al. 1995). The observation that a large number of muscle structural genes contain functional MEF2-binding sites in their promoters, or rely heavily upon Mef2 function for their expression, supports a direct role for MEF2 in controlling myogenesis and provides an explanation for the profound muscle defects observed in MEF2-null animals (Lin et al. 1996; Damm et al. 1998; Stronach et al. 1999; Bagni et al. 2002; Kelly et al. 2002).
Despite the requirement of Mef2 for proper embryonic muscle development in Drosophila, there is still more to be learned concerning the expression and function of this gene in adult muscle development. Adult Drosophila possess an array of physiologically diverse skeletal muscle fibers, which are adapted to their individual functions (reviewed in Bernstein et al. 1993). These muscles arise de novo from pools of twist-expressing myoblasts segregated during embryogenesis and that proliferate during larval development (Crossley 1978; Bate et al. 1991). The thorax contains large fibrillar muscles termed the indirect flight muscles (IFMs) consisting of two muscle groups termed the dorsal longitudinal muscles (DLMs) and dorsoventral muscles (DVMs), which provide the power for flight (Vigoreaux 2001). The thorax also contains direct flight muscles (DFMs), which control wing angle during flight (Miller 1950), and muscles of the leg, which are required for jumping and locomotion. The DFMs and leg muscles are termed tubular, due to the arrangement of a number of myofibrils around a central lumen in each fiber. These muscles comprise greater than one fiber per muscle (Soler et al. 2004). The adult abdomen contains a third class of muscle fibers that are also tubular in organization but that comprise a single fiber per muscle.
Previous studies of Mef2 expression have indicated potentially important roles for MEF2 in adult muscle development: MEF2 protein is present in the adepithelial cells of the wing imaginal disc (Ranganayakulu et al., 1995), which give rise to the adult thoracic musculature (Poodry and Schneidermann, 1970; Reed et al., 1975; Fernandes et al. 1991); Mef2 expression is upregulated in the whole animal at 12 hr after puparium formation (White et al. 1999), a time at which important muscle patterning events are occurring (Fernandes et al. 1991); Northern blot analysis also indicates Mef2 expression in the whole animal persists throughout pupal development (Nguyen et al. 1994).
There is also functional evidence of a role for MEF2 in adult myogenesis, since the thoracic musculature in hypomorphic Mef2 mutant adults show subtle patterning defects (Ranganayakulu et al. 1995; Cripps and Olson 1998; Nguyen et al. 2002). However, since requirements for MEF2 in adult development have utilized hypomorphic alleles, which retain significant MEF2 function, it has not yet been determined whether the requirement of MEF2 for muscle formation in the adult is as profound as that in embryos.
In this article we define the role of Mef2 in adult muscle development in Drosophila. We report the isolation and characterization of a temperature-sensitive combination of Mef2 mutant alleles and, using this combination, we further investigate the importance of Mef2 in adult muscle specification. Our results indicate that strikingly normal amounts of muscle tissue form in these Mef2 mutant adults. However, these animals did display reduced viability and poor locomotion, which arise from muscle patterning defects and reductions in the expression levels of a subset of muscle structural genes.
MATERIALS AND METHODS
Fly stocks and temperature-shift experiments:
All crosses were carried out in plastic bottles or vials containing standard medium (Carpenter 1950). y w were used as controls. Mef2 mutant alleles used in this study were generously provided by Elliot Goldstein (Arizona State University; Goldstein et al. 2001). Act57B-lacZ lines were described previously (Kelly et al. 2002).
For complementation analyses and for isolation of live heteroallelic mutants, alleles were balanced over a CyO chromosome carrying a Kr-GFP transgene (CyO, GFP; Casso et al. 1999). Genotyping was carried out using an Olympus fluorescence-dissecting microscope. For crosses to Mef222-21 to confirm allelism, at least 180 flies were screened for each cross. For other crosses 90–541 flies were screened for each cross. For immunohistochemical analysis of mutant embryos, the alleles were balanced over a CyO chromosome carrying a wg-lacZ enhancer trap (CyO, wg-lacZ; Heemskerk and Dinardo 1994).
For the complementation analyses crosses were of the format CyO, GFP / Mef2x × CyO, GFP / Mef2y. Crosses were set up at 18° (permissive temperature), 25°, and 29° (restrictive temperature). The progeny from each cross were collected and scored daily until the vials produced no more flies. Genotypes were assigned on the basis of the presence or absence of the Cy and GFP markers. In the absence of any lethality the balancer heterozygotes were predicted to occur at twice the frequency of the heteroallelic adults. Therefore the mutant viability was calculated by dividing the number of heteroallelic escapers by half the number of balancer heterozygotes.
For larval temperature-shift experiments, flies were allowed to lay eggs at 18° on agar mixed with grape juice, and Mef2 mutant larvae were collected at the L1 stage. Larvae were collected into well-yeasted vials for subsequent culture. Of the 400 larvae initially collected, 200 were moved to an incubator set at the restrictive temperature and allowed to develop. The remaining 200 were retained at the permissive temperature. All pupae and adults were checked for the absence of Cy and GFP markers.
Embryo immunohistochemistry:
For analysis of embryonic muscle development, mutants balanced over a CyO, wg-lacZ balancer were crossed en masse in cages and allowed to lay eggs at 18° and 29° on agar mixed with grape juice. Embryos were collected over a time period to allow development to stage 16 (32 hr at 18°, 18 hr at 25°, 13 hr and 20 min at 29°).
Embryos were stained with polyclonal rabbit anti-myosin heavy chain (Kiehart and Feghali 1986) at 1:1000 dilution and with polyclonal mouse anti-β-galactosidase (Promega Life Science, Madison, WI) at 1:1000 dilution, as described in Patel (1994). Signal was generated using the Vectastain Elite staining kit and DAB substrate (Vector Labs, Burlingame, CA). Absence of β-galactosidase expression arising from the balancer chromosome indicated that animals were of the heteroallelic mutant genotype.
Adult histochemistry, immunohistochemistry, and in situ hybridization:
For paraffin embedding, pupae of the desired age and genotype were collected, and the anterior and posterior ends were removed using a scalpel. After fixation overnight in 4% (w/v) paraformaldehyde in PBS, samples were treated and embedded in paraffin (Tyco Healthcare) as described previously (Cripps et al. 1998). Sections of 7- to 10-μm thickness were cut using a microtome (Spencer 820, American Optical, Buffalo). After drying, sections were stained with haematoxylin and eosin using standard procedures, prepared for antibody staining as described in Cripps and Olson (1998) using Antigen Retrieval Citra (BioGenex, San Ramon, CA), or subjected to in situ hybridization according to Lovato et al. (2001).
For sections stained only with eosin and hematoxylin, samples were analyzed by bright field microscopy and dorsal longitudinal muscle fibers counted per half thorax. Total fibers per half thorax for each genotype were summed and the average taken. No left-right bias was observed.
For antibody staining, the primary antibody was polyclonal rabbit anti-MEF2 (Lilly et al. 1995) detected as described for embryos. Antibody-stained sections were counterstained with eosin.
For in situ hybridization, digoxigenin-labeled antisense and sense probes complementary to the Act57B 3′-untranslated region were used (Kelly et al. 2002). Hybridized probe was detected using anti-digoxigenin-AP (Roche Molecular Biologicals, Indianapolis) and an alkaline phosphatase detection kit (Vector Laboratories).
Pupae used for X-gal staining were collected at the desired time points and frozen in Tissue Tek (Sakura, CA) on a bed of dry ice and then stored in a −80° freezer overnight. Sections of 7- to 10-μm thickness were cut on a cryostat (Tissue-Tek II Cryomicrotome, Miles) and mounted on glass slides. Slides were fixed with 4% (w/v) formaldehyde and then incubated in X-gal-staining solution (Ashburner 1989). At least three transgenic lines for each construct were tested. After staining reactions, all sections were dehydrated through an ethanol series, washed with xylenes, and mounted in Cytoseal XYL (Richard-Allan Scientific).
Quantitative PCR:
Wild-type pupae were aged to 96 hr after puparium formation (APF) and mutant pupae were raised at 18° and 29° to equivalent stages. RNA was isolated using the Tri reagent according to the manufacturer's instructions (Molecular Research Center, Cincinnati). Total RNA was treated with DNase I for 10 min and precipitated with isopropanol and ammonium acetate. cDNA was then obtained from this RNA using the AMVRT kit provided by Sigma (St. Louis).
Quantitative PCR (QPCR) was performed on the ABI Prism 7700 SDS using primers designed within the 3′-UTR regions of Act57B, Act79B, Act88F, and the 18S rRNA genes. Primers used were: 18S 3′-UTR+, 5′-CAGCAGGCGCGTAAATTACC-3′; 18S 3′-UTR−, 5′-TCCTGTATTGTTATTTTTCGTCACTACCT-3′; 57B 3′-UTR+, 5′-TCTACTCACCTGTCTCCTGCTCAT-3′; 57B 3′-UTR−, 5′-CCAGCCGCCCACACA-3′; 79B 3′-UTR+, 5′-AGCGTAAGACATCCGACCAG-3′; 79B 3′-UTR−, 5′-TTCCGGTCTTTTCTCGTCTC-3′; 88F 3′-UTR+, 5′-GTTGTCGGTGCTCATCCTTC-3′; 88F 3′-UTR−, 5′-TGCTACTCGACATGGAGCAC-3′. QPCR was performed using the SYBR green kit obtained from Applied Biosciences (Foster City, CA) and reactions were prepared as described at http://www.appliedbiosystems.com. The resulting data were analyzed as described in Livak and Schmittgen (2001). The fold change for each gene from at least four QPCR assays were summed and the average and standard error of the mean were calculated. Statistical t-tests were performed on the results to determine the significance of the changes in expression from 18° to 29° (http://www.physics.csbsju.edu/cgi-bin/stats/t-test).
RESULTS
Mef2 is expressed in all skeletal muscle types during adult myogenesis:
We initially determined if Mef2 was expressed during the formation of all the major adult skeletal muscles and the time period during which it was detectable. We prepared paraffin sections of pupae aged to different timepoints and studied the expression of Mef2 in the forming skeletal muscles by antibody staining. These results are shown in Figure 1.
Figure 1.—
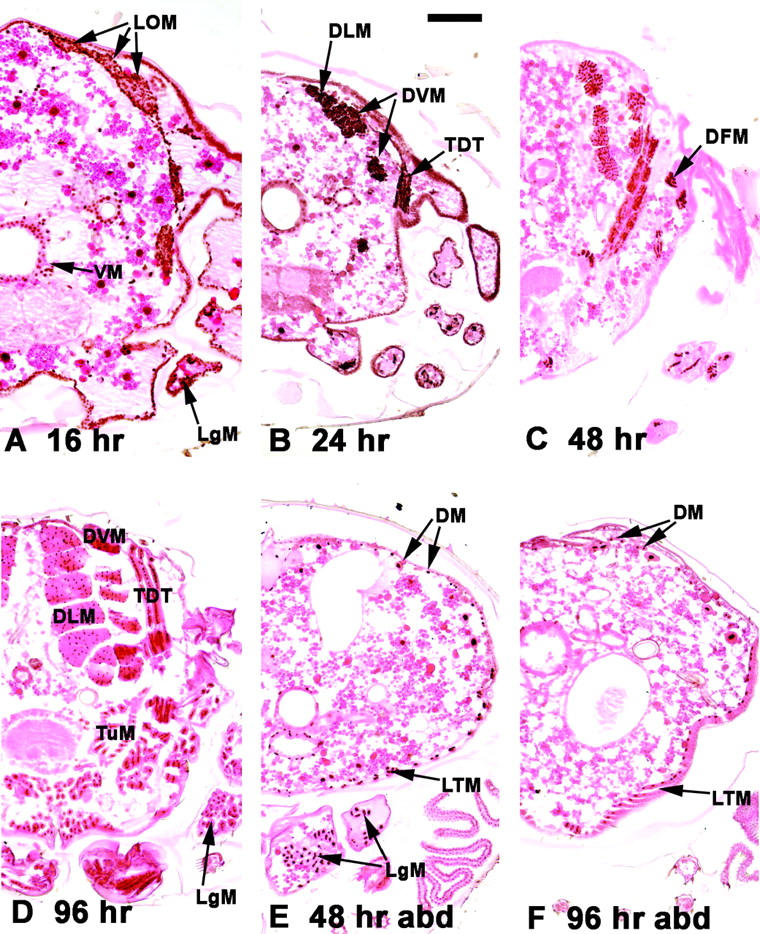
Expression of Mef2 during adult skeletal muscle development. Transverse paraffin sections (with dorsal side up) of aged pupae were reacted with a MEF2 antibody (brown stain) and counterstained with eosin. (A–D) Thoracic sections. (E and F) Abdominal sections (abd). Half-sections are shown for detail. (A) At 16 hr APF, myoblasts from the wing disc migrated dorsally to surround the LOM, which form the templates for the DLMs. Myoblasts also migrated ventrally. Myoblasts in the forming leg (LgM) were also observed. (B) At 24 hr APF MEF2-positive myoblasts had coalesced to form the major IFM fibers, the DLMs, and the DVMs. The developing tergal depressor of the trochanter (TDT) was also observed at this stage. (C) At 48 hr APF Mef2 expression persisted in the major fibers and was observed in direct flight muscles (DFMs) apposed to the base of the wing. (D) Mef2 expression persisted to the end of pupal development in all thoracic muscle types, including the fibrillar DLM and DVM and the TDT and other tubular muscles (TuM). (E) Mef2 expression was also detected in the developing abdominal muscles throughout pupal development, although this was more apparent at midpupal stages in the dorsal muscles (DM) and the lateral transverse muscles (LTM). (F) MEF2 was also detected in mature abdominal muscles. MEF2 was also detected in various muscles surrounding the gut (VM). Bar, 100 μm.
At 16 hr APF MEF2 was detected in a large number of skeletal myoblasts throughout the pupa. In the thorax (Figure 1A), MEF2 was detectable in the myoblasts below the everting wing disc, which are fated to form the large indirect flight muscles (IFMs). At this stage, clumps of MEF2-positive myoblasts were observed surrounding the three persistent larval oblique muscles, as well as MEF2-positive myoblasts migrating ventrally to form additional muscle fibers. MEF2 was also detected in the developing legs at this stage, presumably in forming muscle fibers.
At later stages of pupal development MEF2 accumulation persisted, where it was detected in both of the skeletal muscle types of the thorax: the fibrillar IFMs, including the dorsal longitudinal muscles (DLMs) and the dorsoventral muscles (DVMs), and the tubular muscles, including the large tergal depressor of the trochanter (TDT) and smaller direct flight muscles forming close to the base of the wing (Figure 1, B and C). MEF2 protein was also present in the mature muscles of 96-hr APF pupae (Figure 1D).
In the abdomen, MEF2 protein was detected in developing skeletal muscles throughout pupal development (Figure 1, E and F), including the ventrally located lateral transverse muscles (LTM) as well as the dorsal muscles (DM). In summary, Mef2 was detected at high levels in all of the three major classes of the adult skeletal musculature, throughout pupal development.
Isolation of a temperature-sensitive Mef2 heteroallelic combination:
Previous analyses of Mef2 function in adult muscle development have employed hypomorphic alleles, which still retain significant Mef2 activity (Ranganayakulu et al. 1995; Cripps and Olson 1998; Nguyen et al. 2002). Since twist temperature-sensitive mutants were identified as a heteroallelic combination of two distinct alleles (Thisse et al. 1987), we reasoned that Mef2 mutants might behave in a similar manner. Such mutants would allow us to more rigorously test the function of Mef2 in adult myogenesis.
To identify potentially temperature-sensitive combinations we therefore carried out a complementation analysis of the Mef2 alleles isolated by Goldstein et al. (2001). Each allele was initially crossed to the Mef2-null allele Mef222-21 (Bour et al. 1995) to confirm allelism to Mef2. In all cases, crossing to the 22-21 allele resulted in complete lethality (Table 1, first column). We then determined which heteroallelic combinations gave rise to adult escapers at 25°. This initial complementation analysis demonstrated that although the majority of crosses resulted in 0% survival, a number of crosses did produce Mef2 hypomorphic adults (Table 1). Preliminary histological analyses of these survivors showed muscle-patterning phenotypes similar to other hypomorphic combinations previously described (data not shown; Ranganayakulu et al. 1995; Nguyen et al. 2002).
TABLE 1.
Complementation analysis ofMef2 alleles at 25°
|
Mef2 parental alleles |
22-21 | 22-24 | 25-34 | 25-42 | 26-6 | 26-7 | 26-49 | 30-5 | 44-5 | 48-7 | 55-5 | 66-25 | 78-11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 22-21 | 0 | ||||||||||||
| 22-24 | 0 | 0 | |||||||||||
| 25-34 | 0 | 0 | 0 | ||||||||||
| 25-42 | 0 | 0 | 0 | 0 | |||||||||
| 26-6 | 0 | 0 | 0 | 0 | 0 | ||||||||
| 26-7 | 0 | 0 | 0 | 6 | 6 | 0 | |||||||
| 26-49 | 0 | 0 | 22 | 0 | 0 | 0 | 0 | ||||||
| 30-5 | 0 | 4 | 40 | 0 | 0 | 4 | 40 | 0 | |||||
| 44-5 | 0 | 0 | 0 | 0 | 0 | 0 | 24 | 24 | 0 | ||||
| 48-7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | |||
| 55-5 | 0 | 34 | 94 | 56 | 44 | 34 | 78 | 66 | 42 | 76 | 1 | ||
| 66-25 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 32 | 0 | 0 | 58 | 0 | |
| 78-11 | 0 | 0 | 6 | 0 | 0 | 0 | 18 | 16 | 0 | 0 | 36 | 0 | 0 |
The numbers are percentages and indicate the number of adult heteroallelic escapers recovered as a percentage of half the number of CyO adults resulting from the CyO, Cy GFP/Mef2x × Cyo, Cy GFP/Mef2y crosses. Total progeny from each cross was >94.
To determine if any of the combinations that allowed escapers at 25° might represent temperature-sensitive mutants, we then carried out complementation analyses at both 18° and 29° of a subset of combinations that show survival at 25° (Table 2). There was some variation in the percentage of heteroallelic survivors from each cross between temperatures. More importantly one combination, Mef230-5/Mef244-5, produced no escapers at 29°, vs. 58% survival of adult Mef2 hypomorphic animals at 18°. When stocks of either CyO, GFP/30-5 or CyO, GFP/44-5 alone were cultured at 18°, no escapers were observed (Table 2 and data not shown), indicating that the temperature-sensitive effect was specific to the heteroallelic combination.
TABLE 2.
Survival of viableMef2 combinations at different incubation temperatures
| Viability
|
||
|---|---|---|
| Mef2 allelic combination |
18° (%) | 29° (%) |
| 55-5/78-11 | 58 | 42 |
| 55-5/66-25 | 13 | 5 |
| 55-5/55-5 | 0 | 11 |
| 55-5/44-5 | 80 | 87 |
| 55-5/30-5 | 95 | 95 |
| 55-5/26-49 | 63 | 100 |
| 55-5/26-7 | 62 | 100 |
| 44-5/26-49 | 11 | 6 |
| 30-5/78-11 | 22 | 2 |
| 30-5/66-25 | 59 | 15 |
| 30-5/44-5 | 58 | 0 |
| 26-7/25-42 | 9 | 0 |
| 26-7/30-5 | 6 | 0 |
| 26-7/26-6 | 9 | 0 |
| 66-25/26-49 | 32 | 60 |
| 78-11/26-47 | 5 | 26 |
The numbers are percentages and were determined as in Table 1. Total progeny from each cross was >96.
To determine if the sensitivity to temperature displayed by the Mef230-5/Mef244-5 combination (hereafter referred to as 30-5/44-5, underlined in Table 2) resulted from a reduction in Mef2 activity, we determined if the phenotypes of mutant embryos raised at permissive and restrictive temperatures mimicked the phenotypes of canonical Mef2 mutants. Embryos from wild type, from heteroallelic crosses incubated at 18° and 29°, and from Mef222-21 mutants were stained with antibody to myosin heavy chain (MHC) to visualize differentiated muscle fibers (Kiehart and Feghali 1986).
Wild-type embryos at stage 16 displayed a complex array of skeletal body wall muscle fibers along the length of the animal (Figure 2A). Mutant embryos raised at 18° showed patterns of anti-MHC staining that were very similar to wild type, although there was some loss of fibers in the body wall muscles and the overall intensity of staining was reduced (Figure 2B). By contrast, there was a severe reduction in the number of MHC-positive muscle fibers in mutant embryos raised at 29°, indicating a major loss of terminally differentiated musculature (Figure 2C). Note that a small number of MHC-positive muscle fibers consistently formed in the 29° mutants, compared to the complete absence of fibers in the Mef2 nulls (Figure 2D).
Figure 2.—
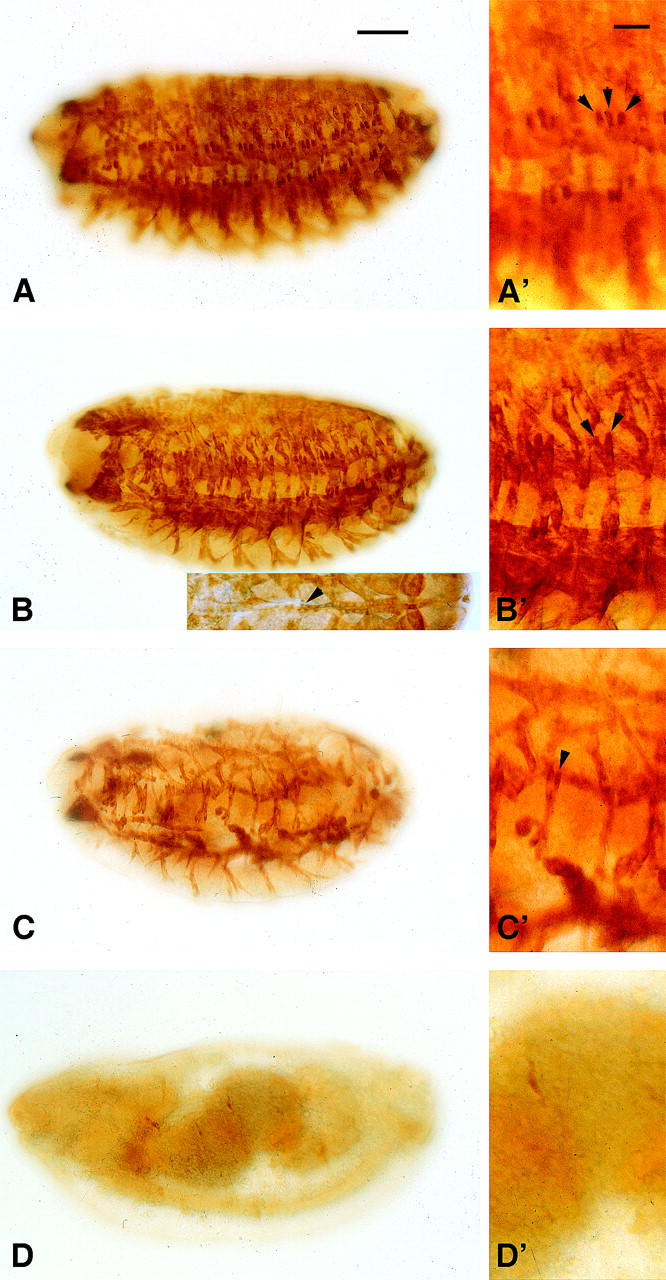
Muscle development in wild-type and Mef2 mutant embryos. Embryos at stage 16 were stained in parallel with an anti-myosin heavy-chain (MHC) antibody. (A) Wild-type animals showed a large number of MHC-positive body wall muscle fibers. (A′) The lateral transverse (LT) muscles 1–3 are indicated (arrowheads). (B) 30-5/44-5 mutants raised at 18° showed numerous muscle fibers although slightly reduced in number and with a reduced staining intensity. (Inset) The dorsal vessel formed in these mutants, but commonly showed organizational defects (arrowhead). (B′) The number of LT fibers was reduced. (C) 30-5/44-5 mutants raised at 29° showed a massively reduced number of muscle fibers. (C′) LT 1-3 were absent or reduced in number in all segments. (D) Mef222-21 mutant homozygotes showed a failure of muscle formation. The phenotypes of both wild-type and mutant genotypes were similar within each genotype stained. Anterior is to the left and dorsal is top. Inset in B is a dorsal view. Bar, 100 μm for A–D; 50 μm for A′–D′.
We also studied the dorsal vessel of 30-5/44-5 animals by anti-MHC staining, as it has been shown previously that this structure is particularly sensitive to differing levels of Mef2 expression (Gunthorpe et al. 1999). This revealed that some organizational defects were present in embryos raised at 18° (Figure 2B, inset) whereas the heart tube was not visible at all in anti-MHC-stained embryos raised at 29° (data not shown). The cardiac defects observed at 18° may explain the reduced survival of these animals to adulthood (58%).
Taken together, these results indicated that muscle development in 30-5/44-5 animals was sensitive to changes in developmental temperature. Since these phenotypes arose from mutants that had been defined by complementation as Mef2 mutants, and since the muscle development phenotypes were highly similar to those of Mef2 null and hypomorphic embryos previously described (Bour et al. 1995; Lilly et al. 1995; Ranganayakulu et al. 1995; Nguyen et al. 2002), we conclude that the 30-5/44-5 combination represents a temperature-sensitive Mef2 condition. This combination shows high levels of MEF2 activity at the permissive temperature and severely reduced function at the restrictive temperature.
A requirement for Mef2 in postembryonic myogenesis:
We next used the temperature-sensitive mutants to probe the function of MEF2 in adult myogenesis. We set up crosses of CyO, GFP/Mef230-5 × CyO, GFP/Mef244-5 at the permissive temperature of 18° and allowed embryos to incubate until hatching. We then selected newly hatched 30-5/44-5 L1 larvae on the basis of the absence of GFP expression. Of 400 larvae collected in this manner, 200 were immediately shifted to the restrictive temperature of 29° to reduce Mef2 function, while the remainder were incubated at 18° as a control (Figure 3).
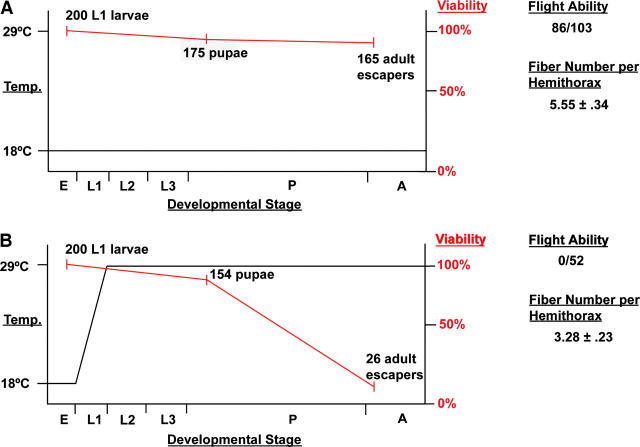
Figure 3.—
Summary of temperature-shift experiments and results obtained. The left axes denote temperature treatment of 30-5/44-5 mutants (black line). Viability of these mutants starting with 100% at L1 is represented by the red line and listed on the right axes. Indicated to the right of the graphs are the flight ability and the number of DLM fibers per hemithorax in adult escapers for each treatment. (A) Mutants raised at 18° throughout development. (B) Mutants raised at 18° during embryogenesis and at 29° thereafter. Developmental stages: (E) embryo, (L1–3) larval instars, (P) pupa, (A) adult.
Of the 200 larvae allowed to develop at 18°, 175 (87.5%) reached pupariation and of those, 165 (82.5%) reached adulthood (Figure 3A). Note that only mutant animals that had successfully completed embryogenesis and hatched from the egg were selected for this experiment. This resulted in a larger degree of survival to adulthood than was apparent in Table 2 from complementation crosses (only 58% survival) and indicated that there was ∼25% embryonic lethality at the permissive temperature.
Of the 200 larvae that were shifted to the restrictive temperature, 154 (77%) reached pupariation (Figure 3B). Thus, there was notable lethality (10%) of larvae lacking MEF2 function; however, a large proportion completed larval development.
Of the 154 pupae that formed at 29°, only 26 (13% of total) survived to adulthood (Figure 3B). Interestingly, many of the animals raised at 29° reached a late stage of pupal development and showed a darkening of the cuticle, but most failed to break free of the pupal case. Some animals did manage to partially eclose, but did not emerge fully. These data indicate an important requirement for MEF2 in pupal development.
Additional crosses were carried out to obtain enough samples for subsequent experiments, and similar percentages of survival were observed from these crosses in comparison with those described in the previous paragraph.
To initially determine if adult mutants had normal muscle function, we tested the flight ability of adult escapers that had been raised from L1 at each temperature. Of 103 animals raised at 18°, 86 (83.5%) were capable of normal flight when tapped gently from a vial. By contrast none of the 52 animals raised at 29° were capable of flying and simply dropped vertically. The poor ability of mutants to eclose when raised at 29°, and the poor flight ability of those that did eclose, indicated that these animals lacked proper muscle function.
Development of adult muscles in the absence of MEF2 function:
Since 30-5/44-5 animals raised at 29° were flightless and often failed to eclose, we next determined if there were any defects in the muscles of these animals. To analyze adult musculature we studied thoracic sections of wild-type animals, mutants raised at 18° throughout development, and mutants raised at 18° during embryogenesis and at 29° from L1.
Transverse sections of wild-type animals showed the regular arrangement of six DLMs per half thorax, which constitute a large proportion of the mass of the thorax (Miller 1950; Figure 4A, bracketed). Animals raised at 18° showed a slight reduction in DLM number, resulting in an average of 5.55 (n = 11) fibers per half thorax (Figure 3A), although a number of individuals showed normal fiber number (Figure 4B). Mutants raised at 18° frequently showed darker staining and smaller fibers in our sections, which we attributed to these animals being slightly dehydrated during their long development. Since the animals raised at this temperature often had nearly wild-type fiber counts, and since mutants raised at 29° did not show darker fibers, we concluded that the reduction in size of the fibers did not reflect a specific role for MEF2 under these conditions. Reductions in DLM fiber number were more severe at 29° as these animals had an average of 3.28 fibers (n = 18) per half thorax (Figures 3B and 4C). These results are consistent with the muscle pattern defects observed in Mef2 mutants by others (Ranganayakulu et al., 1995; Cripps and Olson 1998; Nguyen et al. 2002) and are likely a result of a defect in muscle template splitting as previously described (Cripps and Olson 1998).
Figure 4.—
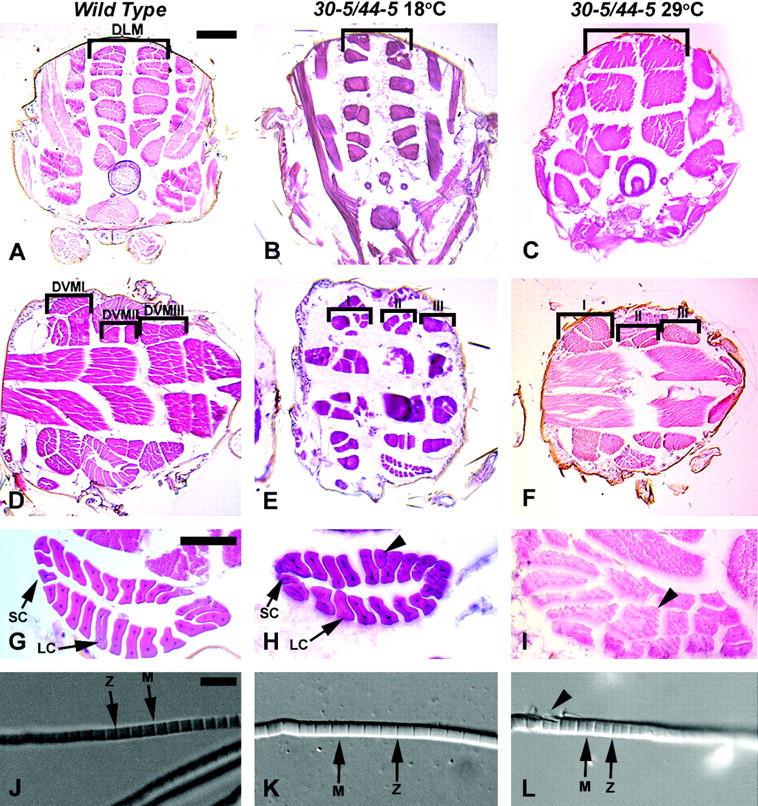
Histological analyses of wild-type and 30-5/44-5 mutants raised at the permissive and restrictive temperatures. Wild-type animals are 96-hr APF, and mutant animals raised at 18° and 29° were of equivalent stages. (A–C) Transverse thoracic sections to visualize DLMs (bracketed), dorsal to top. (D–F) Horizontal thoracic sections to visualize the DVM I-III fibers (bracketed), anterior to the left. (G–I) Horizontal view of TDT muscle, anterior to left. (J–L), Dissected myofibrils. Sections in A–I were stained with hematoxylin and eosin. (A) Wild type. (B) 30-5/44-5 mutant raised at 18°, where DLM fiber number and arrangement were similar to wild type. (C) 30-5/44-5 mutant raised at 29°, note the reduced number of DLM fibers. (D) Wild type. (E) 30-5/44-5 mutant raised at 18°. DVM fiber number remained similar to wild type but some patterning defects were seen. (F) 30-5/44-5 mutant raised at 29°, fiber number was not notably different from permissive temperature. (G) Wild-type TDT, indicating the small cells (SC) and the large cells (LC) of the muscle. (H) TDT of a 30-5/44-5 mutant raised at 18°, showing a nearly normal structure, but with a single abnormally shaped large cell (arrowhead). (I) TDT of a 30-5/44-5 mutant raised at 29°: the central lumen characteristic of this muscle was missing and many of the fibers were shaped oddly. (J) Myofibril isolated from the DLM of wild-type animal. Note the regular structure of the sarcomere and prominent Z-lines and M-lines (arrows). (K) Myofibril isolated from a DLMof 30-5/44-5 animal raised at 18° was apparently normal. (L) Myofibril isolated from a DLM of 30-5/44-5 animal raised at 29°; note the frayed appearance of the fiber at left (arrowhead). Bar for A–F, 100 μm; for G–I, 50 μm; for J–L, 5 μm.
Since previous studies focused solely on the development of the DLMs in Mef2 mutants, we extended our analyses of the muscle defects caused by the reduction of Mef2 activity. We prepared horizontal thoracic sections of adult animals raised at the restrictive and permissive temperatures. In wild-type animals there were seven distinct DVM fibers, organized into three muscles termed DVMI-III (Miller 1950; Figure 4D, bracketed). Within each muscle, the fibers were of similar sizes. Animals raised at 18° showed slight defects in the patterning of the DVM fibers compared to wild type, where occasionally additional fibers differed significantly in size within the same muscle (Figure 4E). Interestingly, the severity of patterning defects present in animals raised at 29° was not significantly different from that observed at the permissive temperature (Figure 4F).
We also visualized the TDT, a major tubular muscle that is physiologically distinct from the IFMs. Wild-type animals displayed a very regular set of TDT fibers that were arranged to form a tube with 4 small anterior fibers and ≥20 large posterior fibers (Figure 4G, arrows; O'Donnell et al. 1989; Peckham et al. 1990). In 30-5/44-5 animals raised at 18°, the TDT was almost indistinguishable from wild type in respect to the arrangement of fibers, although some fibers looked slightly irregular (Figure 4H, arrowhead). However, the TDT in 30-5/44-5 animals raised at 29° had fibers forming in the central lumen, and many of the fibers were oddly shaped (Figure 4I, arrowhead). The TDT in these animals was also mislocalized within the thorax as compared to wild-type animals or animals raised at the permissive temperature. Dissections of the abdomens of Mef2 mutants allowed us to observe relatively normal patterning of the supercontractile muscles of the abdomen in mutants compared to wild type (data not shown).
Myofibrils were also dissected from the DLMs of wild-type animals, 30-5/44-5 animals raised at 18° and 30-5/44-5 animals raised at 29°, to study the formation of muscle sarcomeres. Visualization of these fibers by differential interference contrast microscopy revealed no major defects in the myofibrils between wild type and 30-5/44-5 at either temperature treatment (Figure 4, J–L). We observed regular M lines and Z lines in the numerous myofibrils of all genotypes (Figure 4, J–L, arrows); however, we did see occasional fraying of the myofibrils in the mutants raised at 29° (Figure 4L, arrowhead). While this fraying could be attributed to mechanical damage during extraction of the myofibrils, fraying was not observed in either wild-type animals or animals raised at the permissive temperature, indicating that there were some structural defects in the myofibrils of restrictive mutants.
In summary our phenotypical analyses of muscle formation in Mef2 mutant adults indicated an important requirement for MEF2 function in the patterning of the muscle fibers. This requirement was apparent in the DLMs as well as in the TDT, a major tubular muscle of the adult, and our findings significantly extend earlier reports. Given the strict requirements of coordinated muscle function in the thorax for normal flight, we conclude that the patterning defects in the DLMs plus dysfunction of the tubular muscles due to fiber defects probably account for the poor flying ability of 30-5/44-5 mutant escapers at 29°. Mild ultrastructural defects in the IFM myofibrils might also compound this.
However, a more striking observation was that, in the almost-complete absence of MEF2 function, large amounts of adult muscle tissue were generated and sufficient muscle structural proteins were synthesized to form myofibrils. This result contrasted sharply with the requirement for Mef2 in embryonic muscle formation. Given the significant amount of muscle forming in the Mef2 mutants, this did not adequately explain the lethality that we observed.
Quantification of target gene expression in temperature-sensitive Mef2 animals:
Since eclosion requires the function of the abdominal supercontractile muscles (Miller 1950), we hypothesized that these muscles, although they formed normally, might be functionally defective in the Mef2 30-5/44-5 mutants. Previously, Fyrberg et al. (1983) showed using body-part dot blots that the Act57B actin gene was the predominant muscle actin gene expressed in the abdomen. This presumably represented expression in the abdominal skeletal muscles, although that was not demonstrated. By contrast, the actin genes Act79B and Act88F are strictly expressed in adult tubular and fibrillar muscles, respectively (Hiromi and Hotta 1985; Ball et al. 1987). Thus, among them, these three actin genes are expressed in the major adult muscle types. We therefore studied actin gene expression levels in mutants and controls by quantitative polymerase chain reaction (QPCR), to determine if Mef2 reduction had effects distinct between muscle types. Primer pairs within the 3′-UTR region of each of the target actin genes were generated. We also prepared primer pairs for the 18S ribosomal RNA gene to use as an internal control.
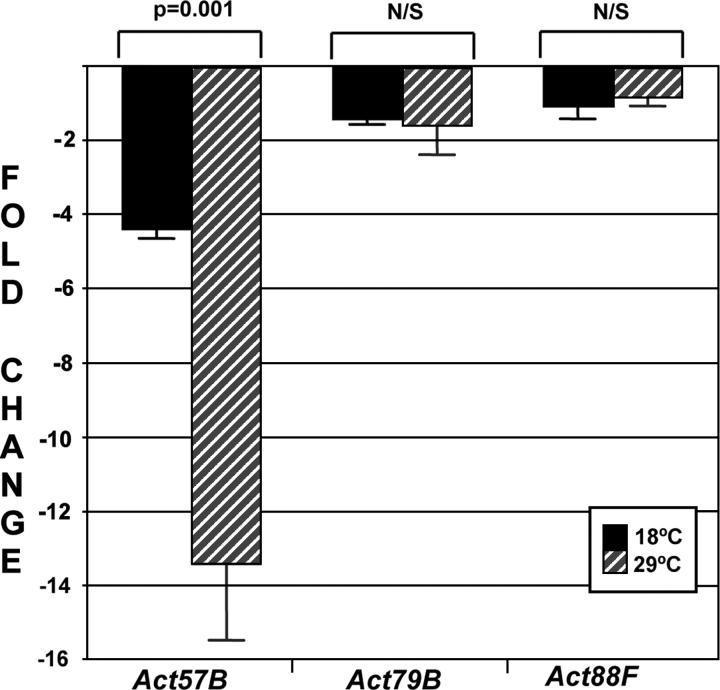
RNA was collected from wild-type pupae at 96 hr (APF) as well as from equivalent stage 30-5/44-5 animals raised at the permissive and restrictive temperatures. QPCRs were performed and the results analyzed as described in Livak and Schmittgen (2001). Our analysis showed that the expression of Act57B was decreased by 4.4-fold in 30-5/44-5 animals raised at 18° compared to wild type and by 13.46-fold in 30-5/44-5 animals raised at 29° compared to wild type. However, we saw very small decreases in the expression of Act79B and almost no difference in the expression of Act88F at either temperature (Figure 5). These results demonstrated a profound requirement for MEF2 function in the expression of the Act57B actin gene vs. the adult actin genes (Act79B and Act88F).
Figure 5.—
QPCR analysis of muscle gene expression in mutant adults. The transcript levels of three actin genes, Act57B, Act79B, and Act88F, were determined from total RNA of mature pupae. Fold expression changes were calculated for 30-5/44-5 at 18° (solid bars) and at 29° (shaded bars), compared to wild type. Values represent the average of four assays, and error bars represent the standard error of the mean. Act57B expression was dramatically reduced in the 30-5/44-5 mutant animals as compared to the other actin genes assayed. Student's t-test indicated that the differences in Act79B and Act88F transcript levels in permissive or restrictive mutants were not significant (N/S), but that the change in Act57B expression was highly significant.
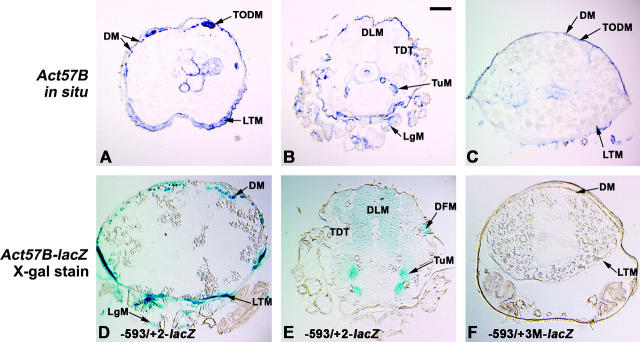
To confirm that the reduction in Act57B expression observed by QPCR was relevant to abdominal muscle function, we studied the expression of Act57B in transverse abdominal and thoracic sections of wild-type 96-hr APF animals by in situ hybridization. The results are presented in Figure 6. We found that indeed there were high levels of Act57B transcript detected in all of the ventral and dorsal abdominal muscles (Figure 6A). Act57B was also detected in a small number of tubular thoracic muscles, although we were unable to identify precisely which fibers these represented from our sections (Figure 6B). No reaction was observed using a sense probe (Figure 6C).
Figure 6.—
Expression and regulation of Act57B in adult Drosophila. (A–C) In situ hybridization to transverse paraffin sections (with dorsal side up) using either a 3′-UTR antisense Act57B probe (A and B) or a sense control probe (C). (A) In the abdomen, Act57B expression was detected in the DM, including the temporary oblique dorsal muscles (TODM) which are used for eclosion, as well as in the ventrally located LTM. (B) In the thorax, Act57B was detected in a small subset of TuM and in the leg (LgM) but not in the TDT or the IFMs. (C) No reproducible signal was observed with the control probe. (D–F) Transverse cryosections of Act57B-lacZ transgenic animals stained with X-gal. (D) The −593/+2 Act57B-lacZ transgene was active in the abdomen in the same muscle cells as the endogenous gene. (E) Transgene expression was also observed in the thoracic tubular muscles, although additionally at low levels in the IFMs. This may represent leakiness of the transgene or possibly a weak early expression of Act57B in these muscles. (F) The −593/+2 transgene in which the MEF2 site was mutated was inactive in adult sections. Bar, 100 μm.
Act57B expression in adults is directly regulated by MEF2:
We previously analyzed the function of the Act57B promoter in embryonic muscles and identified a region from –593/+2 that, when fused to a lacZ reporter, was active broadly in embryonic muscles. Within this promoter element, a conserved MEF2-binding site was required for high levels of muscle-specific expression (Kelly et al. 2002). To further explore the hypothesis that Act57B expression levels were affected by loss of MEF2 function, we determined if the –593/+2 Act57B-lacZ reporter construct was active in the adult muscles shown to express Act57B. We found that this reporter was strongly active in all of the abdominal skeletal muscles, as well as in some fibers in the thorax (Figure 6, D and E). These findings supported our in situ hybridization results described above and localized adult muscle enhancer activity.
To determine if the same MEF2 site that functioned in embryonic muscles also functioned in the adult, we studied the activity of a similar promoter-lacZ construct, in which the MEF2 site had been mutated. This construct was inactive in adult animals (Figure 6F), further supporting an important role for MEF2 in controlling Act57B expression and thus abdominal muscle function.
These results support the conclusions obtained by the QPCR assays and suggest that the misregulation of Act57B in animals lacking proper Mef2 function may be an important factor contributing to the lethality observed in our mutant animals. It may be possible that the lethality attributed to these animals may be exacerbated due to the lack of proper MEF2 function in other tissues besides muscle.
DISCUSSION
Skeletal muscles of animals compose a number of distinct fiber types, which are adapted to their particular function in the body (reviewed in Squire 1986). Much recent research has focused upon identifying both factors that are required for the formation of all skeletal muscles and factors that control subspecification within the skeletal muscle lineage. Prominent among the former group are members of the MEF2 family of muscle-enriched transcription factors. MEF2-encoding genes are expressed broadly in the musculature and have been shown to regulate a large number of muscle-specific genes (reviewed in Black and Olson 1998). MEF2 factors have also been implicated in contributing to adaptive changes in fiber type within a muscle (Chambers et al. 1994; Rao et al. 1996; Chin et al. 1998; O'Mahoney et al., 1998; Wu et al. 2000; Yan et al. 2001; Lin et al. 2002; Leszczynski and Esser 2003).
The model animal Drosophila undergoes an adult stage in which the skeletal muscles show similar examples of fiber specification to match function in the body. The adult skeletal muscles can be subdivided into thoracic fibrillar and tubular muscles and the simple tubular muscles of the abdomen. Each muscle type has a unique ultrastructure and expresses a partially overlapping suite of muscle-specific genes (reviewed in Bernstein et al. 1993), although individual Drosophila muscles generally compose a single fiber-type (Baylies et al. 1998).
The roles of specific transcription factors in the formation of adult muscles in Drosophila have been difficult to define, since many factors play important earlier roles in embryogenesis, such as twist (Bate et al. 1991; Baylies and Bate 1996). For this gene, temperature-sensitive mutants were used to address a role for adult muscle development. It was shown that twist functioned to activate Mef2 expression at the onset of pupal development (Cripps et al. 1998) and that in the absence of twist function the larval oblique muscle templates for the DLMs did not split to form the canonical six pairs of DLM fibers (Anant et al. 1998; Cripps and Olson 1998).
In this study we identified a temperature-sensitive combination of Mef2 alleles and used these mutants to demonstrate that MEF2 function was required for adult muscle patterning and expression of a subset of adult muscle protein genes. A striking observation was that much of adult myogenesis proceeded at the restrictive temperature, compared to very little embryonic myogenesis in the same mutants under the same conditions (compare Figure 2C with Figure 4C). These findings confirmed the important role that MEF2 plays in myogenesis, yet qualified its importance by pointing to the existence of additional factors that must contribute significantly to muscle development.
Why do our data indicate that there is a lesser emphasis upon MEF2 function in the adult vs. the embryo? It could be argued that there is still residual MEF2 function even at the restrictive temperature to allow adult MEF2-dependent myogenesis to proceed normally. Certainly it is true that residual fibers formed in embryos that developed at the restrictive temperature, indicating that a small amount of MEF2 function still remains. However, the vast difference between the amount of muscle in mutant embryos vs. mutant adults is difficult to discount and argues strongly for a reduced requirement for MEF2 in the adult. Furthermore, since it has been shown that Mef2 function is required for larval oblique muscles (LOM) splitting during formation of the DLM fibers (Cripps and Olson 1998), the theoretical null phenotype for this phenomenon would be three DLM fibers per hemithorax. In the restrictive mutants we see 3.3 fibers, only slightly greater than in the theoretical null phenotype, yet there are still significant quantities of muscle tissue.
Another possibility is that the MEF2 proteins in the heteroallelic mutants do not show temperature sensitivity at all stages of development. Numerous previous studies have identified both positively and negatively acting factors that interact with and collaborate with MEF2 to control myogenesis (see, for example, Molkentin et al. 1995; Spicer et al. 1996; Lin et al., 1997b; Yang et al. 1998; Johanson et al. 1999; Novitch et al. 1999; Postigo et al. 1999; Wilson-Rawls et al. 1999; McKinsey et al. 2000; Polly et al. 2003). It is therefore conceivable that the temperature-sensitive region of the mutant proteins used in this study maps to a part of the molecule that interacts with embryonic cofactors, but does not interact with adult cofactors. There is currently no direct evidence in Drosophila for the existence of such stage-specific MEF2 cofactors, yet the collaboration of MEF2 with myogenic basic helix-loop-helix factors in vertebrate skeletal muscle, but not in cardiac or smooth muscle, suggests that this could be a possibility. In defense of our findings, the phenotypes of our Mef2 mutants showing patterning defects in the adult DLMs yet still retaining substantial muscle tissue are similar to several other Mef2 mutant combinations (Ranganayakulu et al. 1995; Cripps and Olson 1998; Nguyen et al. 2002; our data not shown). Since these defects in the DLMs do not differ significantly from the effects of other Mef2 mutant combinations, it seems less likely that the phenotypes that we observed throughout the adult muscles arise from specific effects of individual mutations.
In further support of the argument that factors independent of MEF2 function in adult myogenesis is the existence of adult muscle gene regulatory elements that do not rely heavily upon MEF2 sites for their function. These include Paramyosin, where a MEF2 site in the promoter is essential for high levels of reporter gene expression in embryonic and larval muscles, but there is only a mild requirement for the integrity of the site in promoter function in adult muscles (Arredondo et al. 2001). Furthermore, an alternate promoter for Paramyosin, which controls the generation of a truncated isoform expressed at high levels in adult muscles only, does not contain a MEF2-binding site (Arredondo et al. 2001). In addition, Mas et al. (2004) described a promoter fragment of the Drosophila TpnT gene. A 1.4-kb fragment showed high levels of enhancer activity in tubular muscles of the thorax and abdomen (although not fibrillar muscles), yet this fragment lacked a conserved MEF2-binding site. By contrast, MEF2 sites are critical for the activities of TpnI (Marin et al. 2004) and Act57B (this study) regulatory elements in adult muscles. While it is possible for MEF2 to be recruited to enhancers by other factors in the absence of a canonical binding site (Molkentin et al. 1995), there is compelling evidence that MEF2 is critical to the expression of some, but not all, adult muscle structural genes in Drosophila.
This is also the case in other animals. Vertebrate mef2 gene knockouts retain significant muscle function and myofibrillar gene expression (Lin et al. 1997a; Naya et al. 2002), although it is reasonable to argue that this arises from redundancy with the remaining MEF2 factors. In the nematode Caenorhabditis elegans, deletion of the single MEF2-encoding gene CeMef2 has apparently no effect upon muscle development or function (Dichoso et al. 2000).
Given all of the above arguments it is more likely that the phenotypes that we observed in the adult musculature do represent a null (or nearly so) MEF2 phenotype. The phenotypes we observe are clearly more severe than those observed in other Mef2 allelic combinations tested (Ranganayakulu et al. 1995; Cripps and Olson 1998; Nguyen et al. 2002), since none of our mutants survived to adulthood when raised from early embryogenesis at the restrictive temperature throughout the life cycle, compared to the small numbers surviving in other studies. Furthermore the mutant phenotype, as reflected in the observed numbers of DLM fibers in adults, was more severe than the phenotypes of previously reported mutant combinations. For example, we observed an average of 3.3 DLM fibers per hemithorax of mutant animals raised at the restrictive temperature, as compared to previous observations of the Mef2 mutant allelic combination Mef265/Mef2113 in which the average DLM fiber number per hemithorax ranged from 3.6 to 4.1 (Cripps and Olson 1998). The mechanisms underlying temperature sensitivity in the 30-5/44-5 allelic combination should become more apparent with the further characterization of these alleles and their biochemical properties.
We conclude that additional factors must play important roles in adult muscle gene regulation and that these factors are likely to be particularly important to the formation of muscles specific to the adult stage. The differential requirement for MEF2 and other factors in adult muscle structural gene expression is exemplified by our observations of strikingly specific effects of loss of MEF2 function upon muscle actin gene expression. Actins whose expression is restricted to the adult stage were expressed at approximately normal levels in the Mef2 mutants. By contrast Act57B, which is expressed in muscles throughout development, was severely dependent upon MEF2, via direct binding of MEF2 to an active promoter element.
MEF2, while playing a diminished role in adult myogenesis, does play some part in the normal patterning of adult muscle tissue. The fact that the actin genes Act88F and Act79B, both adult-specific muscle actins, do not require MEF2 for activation demonstrates that there must be another pathway by which adult myogenesis is activated. However, because animals lacking proper MEF2 activity do not possess a wild-type physiology, there must be some pathways in which MEF2 remains key. The observation that MEF2 is required for Act57B regulation during adult myogenesis provides some insight into the phenotype and lethality of our temperature-sensitive animals and suggests that there are regulatory pathways involved in adult muscle formation that rely on MEF2 for proper function. Understanding how MEF2 functions in adults is important for understanding how muscle is properly formed; however, as it is not crucial to myogenesis, it is important to understand what MEF2-independent pathways are involved in this process.
What other factors might play roles in adult muscle formation? Previously it has been shown that, along with Mef2, vestigial (vg) is expressed in the wing disc adepithelial cells that later give rise to adult muscle (Ng et al. 1996). Moreover, Vg protein is also found in the myoblasts surrounding the forming DLMs and in some DLM nuclei (Sudarsan et al. 2001). Vg has been shown to interact with Scalloped (Sd), which has also been shown to be expressed in the adepithelial cells of the wing disc (Bernard et al. 2003). While it is not known whether the Vg-Sd complex directly activates muscle genes in Drosophila, vg mutants not only show a marked loss of muscle differentiation, but also lack expression of a key adult actin gene, Act88F (Bernard et al. 2003). It is therefore possible that the major myogenic roles of MEF2 have been superseded in the IFMs by factors like Vg and Sd.
A myogenic role for Sd in adult muscle development is particularly attractive given the roles of vertebrate homologs of Sd. Sd is a transcription enhancer factor (TEF) and a member of a larger family of proteins controlling muscle gene expression in other systems. TEF-1, the vertebrate homolog of Sd, has been shown to bind to the MEF2 consensus sequence in mammals (Karasseva et al. 2003) and has also been implicated in regulating muscle-specific genes such as cardiac Myosin Heavy Chain (Gupta et al. 1997) and cardiac Troponin T (Butler and Ordahl 1999). Further analysis of adult-muscle-specific promoters will very likely define the role of this important family of transcription factors in adult muscle development.
Acknowledgments
We are grateful to Elliot Goldstein for generously providing the fly stocks; the Bloomington Stock Center for other stocks; and Bruce Paterson for the anti-MEF2 antibody. This work was supported by a grant from the National Institutes of Health, NIH (GM61738), to R.M.C. P.W.B. was supported by an NIH supplemental training grant and a grant from NIH/Initiative for Minority Student Development (1-R25-GM60201). K.K.K.T. was supported by a predoctoral fellowship from the American Heart Association Desert/Mountain Affiliate. We also acknowledge technical support from the Department of Biology's Molecular Biology Facility, supported by NIH grant no. 1P20RR18754 from the Institute Development Award (IdeA) Program of the National Center for Research Resources.
References
- Anant, S., S. Roy and K. Vijayraghavan, 1998. Twist and Notch negatively regulate adult muscle differentiation in Drosophila. Development 125: 1361–1369. [DOI] [PubMed] [Google Scholar]
- Andres, V., M. Cervera and V. Mahdavi, 1995. Determination of the consensus binding site for MEF2 expressed in muscle and brain reveals tissue-specific sequence constraints. J. Biol. Chem. 270: 23246–23249. [DOI] [PubMed] [Google Scholar]
- Arredondo, J. J., R. M. Ferreres, M. Maroto, R. M. Cripps, R. Marco et al., 2001. Control of Drosophila Paramyosin/Miniparamyosin gene expression: differential regulatory mechanisms for muscle-specific transcription. J. Biol. Chem. 276: 8278–8287. [DOI] [PubMed] [Google Scholar]
- Ashburner, M., 1989 Drosophila: A Laboratory Handbook. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Bagni, C., S. Bray, J. A. Gogos, F. C. Kafatos and T. Hsu, 2002. The Drosophila zinc finger transcription factor CF2 is a myogenic marker downstream of MEF2 during muscle development. Mech. Dev. 117: 265–268. [DOI] [PubMed] [Google Scholar]
- Ball, E., C. C. Karlik, C. J. Beall, D. L. Saville, J. C. Sparrow et al., 1987. Arthrin, a myofibrillar component of insect flight muscle, is an actin-ubiquitin conjugate. Cell 51: 221–228. [DOI] [PubMed] [Google Scholar]
- Bate, M., E. Rushton and D. A. Currie, 1991. Cells with persistent twist expression are the embryonic precursors of adult muscles in Drosophila. Development 113: 79–89. [DOI] [PubMed] [Google Scholar]
- Baylies, M. K., and M. Bate, 1996. twist: a myogenic switch in Drosophila. Science 272: 1481–1484. [DOI] [PubMed] [Google Scholar]
- Baylies, M. K., M. Bate and M. R. Gomez, 1998. Myogenesis: a view from Drosophila. Cell 93: 921–927. [DOI] [PubMed] [Google Scholar]
- Bernard, F., A. Lalouette, M. Gullaud, A. Y. Jeantet, R. Cossard et al., 2003. Control of apterous by vestigial drives indirect flight muscle development in Drosophila. Dev. Biol. 260: 391–403. [DOI] [PubMed] [Google Scholar]
- Bernstein, S. I., P. T. O'Donnell and R. M. Cripps, 1993. Molecular genetic analysis of muscle development, structure, and function in Drosophila. Int. Rev. Cytol. 143: 63–152. [DOI] [PubMed] [Google Scholar]
- Black, B. L., and E. N. Olson, 1998. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Ann. Rev. Cell Dev. Biol. 14: 167–196. [DOI] [PubMed] [Google Scholar]
- Black, B. L., J. F. Martin and E. N. Olson, 1995. The mouse MRF4 promoter is trans-activated directly and indirectly by muscle-specific transcription factors. J. Biol. Chem. 270: 2889–2892. [DOI] [PubMed] [Google Scholar]
- Bour, B. A., M. A. O'Brien, W. L. Lockwood, E. S. Goldstein, R. Bodmer et al., 1995. Drosophila MEF2, a transcription factor that is essential for myogenesis. Genes Dev. 9: 730–741. [DOI] [PubMed] [Google Scholar]
- Butler, A. J., and C. P. Ordahl, 1999. Poly(ADP-ribose) polymerase binds with transcription enhancer factor 1 to MCAT1 elements to regulate muscle-specific transcription. Mol. Cell. Biol. 19: 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter, J. M., 1950. A new semisynthetic food medium for Drosophila. Dros. Inf. Serv. 24: 96–97. [Google Scholar]
- Casso, D., F.-A. Ramirez-Weber and T. B. Kornberg, 1999. GFP-tagged balancer chromosomes for Drosophila melanogaster. Mech. Dev. 88: 229–232. [DOI] [PubMed] [Google Scholar]
- Chambers, A. E., M. Logan, S. Kotecha, N. Towers, D. Sparrow et al., 1994. The RSRF/MEF2 protein SL1 regulates cardiac muscle-specific transcription of a myosin light-chain gene in Xenopus embryos. Genes Dev. 8: 1324–1334. [DOI] [PubMed] [Google Scholar]
- Chin, E., E. N. Olson, Q. Yang, J. Shelton, R. Bassel-Duby et al., 1998. A calcineurin dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 12: 2499–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps, R. M., and E. N. Olson, 1998. Twist is required for muscle template splitting during adult Drosophila myogenesis. Dev. Biol. 203: 106–115. [DOI] [PubMed] [Google Scholar]
- Cripps, R. M., B. L. Black, B. Zhao, C. L. Lien, R. A. Schulz et al., 1998. The myogenic regulatory gene Mef2 is a direct target for transcriptional activation by Twist during Drosophila myogenesis. Genes Dev. 12: 422–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley, A. C., 1978 The morphology and development of the Drosophila muscular system, pp. 499–560 in The Genetics and Biology of Drosophila, Vol. 2b, edited by M. Ashburner and T. R. F. Wright. Academic Press, New York.
- Damm, C., A. Wolk, D. Buttgereit, K. Loher, E. Wagner et al., 1998. Independent regulatory elements in the upstream region of the Drosophila beta 3 tubulin gene (beta Tub60D) guide expression in the dorsal vessel and the somatic muscles. Dev. Biol. 199: 138–149. [DOI] [PubMed] [Google Scholar]
- Dichoso, D., T. Brodigan, K. Y. Chwoe, J. S. Lee, R. Llacer et al., 2000. The MADS-box factor CeMEF2 is not essential for Caenorhabditis elegans myogenesis and development. Dev. Biol. 223: 431–440. [DOI] [PubMed] [Google Scholar]
- Fernandes, J., M. Bate and K. Vijayraghavan, 1991. Development of the indirect flight muscles of Drosophila. Development 113: 67–77. [DOI] [PubMed] [Google Scholar]
- Fyrberg, E. A., J. W. Mahaffey, B. J. Bond and N. Davidson, 1983. Transcripts of the six Drosophila actin genes accumulate in a stage and tissue-specific manner. Cell 33: 115–123. [DOI] [PubMed] [Google Scholar]
- Goldstein, E. S., S. L. Treadway, A. E. Stephenson, G. D. Gramstad, A. Keilty et al., 2001. A genetic analysis of the cytological region 46C-F containing the Drosophila melanogaster homolog of the jun proto-oncogene. Mol. Genet. Genomics 266: 695–700. [DOI] [PubMed] [Google Scholar]
- Gunthorpe, D., K. E. Beatty and M. V. Taylor, 1999. Different levels, but not different isoforms, of the Drosophila transcription factor DMEF2 affect distinct aspects of muscle differentiation. Dev. Biol. 215: 130–145. [DOI] [PubMed] [Google Scholar]
- Gupta, M. P., C. S. Amin, M. Gupta, N. Hay and R. Zak, 1997. Transcription enhancer factor 1 interacts with a basic helix-loop-helix zipper protein, max, for positive regulation of cardiac alpha-myosin heavy-chain gene expression. Mol. Cell. Biol. 17: 3924–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk, J., and S. Dinardo, 1994. Drosophila hedgehog acts as a morphogen in cellular patterning. Cell 76: 449–460. [DOI] [PubMed] [Google Scholar]
- Hiromi, Y., and Y. Hotta, 1985. Actin gene mutations in Drosophila: heat shock activation in the indirect flight muscles. EMBO J. 4: 1681–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson, M., H. Meents, K. Ragge, A. Buchberger, H. H. Arnold et al., 1999. Transcriptional activation of the myogenin gene by MEF2-mediated recruitment of Myf5 is inhibited by adenovirus E1A protein. Biochem. Biophys. Res. Commun. 265: 222–232. [DOI] [PubMed] [Google Scholar]
- Karasseva, N., G. Tsika, J. Ji, A. J. Zhang, X. Q. Mao et al., 2003. Transcription enhancer factor 1 binds multiple muscle MEF2 and A/T-rich elements during fast-to-slow skeletal muscle fiber type transitions. Mol. Cell. Biol. 23: 5143–5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, K. K., S. M. Meadows and R. M. Cripps, 2002. Drosophila MEF2 is a direct regulator of Actin57B transcription in cardiac, skeletal, and visceral muscle lineages. Mech. Dev. 110: 39–50. [DOI] [PubMed] [Google Scholar]
- Kiehart, D. P., and R. Feghali, 1986. Cytoplasmic myosin from Drosophila. J. Cell Biol. 103: 1517–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leszczynski, J., and K. Esser, 2003. The MEF2 site is necessary for induction of the myosin light chain 2 slow promoter in overloaded regenerating plantaris muscle. Life Sci. 73: 3265–3276. [DOI] [PubMed] [Google Scholar]
- Li, H., and Y. Capetanaki, 1994. An E box in the desmin promoter cooperates with the E box and MEF-2 sites of a distal enhancer to direct muscle-specific transcription. EMBO J. 13: 3580–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly, B., S. Galewsky, A. B. Firulli, R. A. Schulz and E. N. Olson, 1994. D-MEF2: a MADS box transcription factor expressed in differentiating mesoderm and muscle cell lineages during Drosophila embryogenesis. Proc. Natl. Acad. Sci. USA 91: 5662–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly, B., B. Zhao, G. Ranganayakulu, B. M. Paterson, R. A. Schulz et al., 1995. Requirement of MADS domain transcription factor D-MEF2 for muscle formation in Drosophila. Science 267: 688–693. [DOI] [PubMed] [Google Scholar]
- Lin, J., H. Wu, P. Tarr, C. Zhang, Z. Wu et al., 2002. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418: 797–801. [DOI] [PubMed] [Google Scholar]
- Lin, M.-H., H. T. Nguyen, C. Dybala and R. V. Storti, 1996. Myocyte-specific enhancer factor 2 acts cooperatively with a muscle activator region to regulate Drosophila tropomyosin gene muscle expression. Proc. Natl. Acad. Sci. USA 93: 4623–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, Q., J. Schwarz, C. Bucana and E. N. Olson, 1997. a Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science 276: 1404–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, S.-C., M.-H. Lin, P. Horvath, K. L. Reddy and R. V. Storti, 1997. b PDP1, a novel Drosophila PAR domain bZIP transcription factor expressed in developing mesoderm, endoderm and ectoderm, is a transcriptional regulator of somatic muscle genes. Development 124: 4685–4696. [DOI] [PubMed] [Google Scholar]
- Livak, K. J., and T. D. Schmittgen, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T) (-Delta Delta C) method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Lovato, T. L., S. M. Meadows, P. W. Baker, J. C. Sparrow and R. M. Cripps, 2001. Characterization of muscle actin genes in Drosophila virilis reveals significant molecular complexity in skeletal muscle types. Insect Mol. Biol. 10: 333–340. [DOI] [PubMed] [Google Scholar]
- Marin, M. C., J. R. Rodriguez and A. Ferrus, 2004. Transcription of Drosophila Troponin I gene is regulated by two conserved, functionally identical, synergistic elements. Mol. Biol. Cell 15: 1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mas, J. A., E. Garcia-Zaragoza and M. Cervera, 2004. Two functionally identical modular enhancers in Drosophila Troponin T gene establish the correct protein levels in different muscle types. Mol. Biol. Cell 15: 1931–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinsey, T. A., C. L. Zhang and E. N. Olson, 2000. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin dependent protein kinase-stimulated binding of 14–3-3 to histone deacetylase 5. Proc. Natl. Acad. Sci. USA 97: 14400–14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, A., 1950 The internal anatomy and histology of the imago of Drosophila melanogaster, pp. 420–534 in The Biology of Drosophila, edited by M. Demerec. Hafner Publishing, New York.
- Molkentin, J. D., B. L. Black, J. F. Martin and E. N. Olson, 1995. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell 83: 1125–1136. [DOI] [PubMed] [Google Scholar]
- Naidu, P. S., D. C. Ludolph, R. Q. To, T. J. Hinterberger and S. F. Konieczny, 1995. Myogenin and MEF2 function synergistically to activate the MRF4 promoter during myogenesis. Mol. Cell. Biol. 15: 2707–2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya, F. J., B. L. Black, H. Wu, R. Bassel-Duby, J. A. Richardson et al., 2002. Mitochondrial deficiency and cardiac sudden death in mice lacking the MEF2A transcription factor. Nat. Med. 8: 1303–1309. [DOI] [PubMed] [Google Scholar]
- Ng, M., F. J. Diazbenjumea, J. P. Vincent, J. Wu and S. M. Cohen, 1996. Specification of the wing by localized expression of wingless protein. Nature 381: 316–318. [DOI] [PubMed] [Google Scholar]
- Nguyen, H. T., R. Bodmer, S. M. Abmayr, J. C. Mcdermott and N. A. Spoerel, 1994. D-mef2: a Drosophila mesoderm-specific MADS box-containing gene with a biphasic expression profile during embryogenesis. Proc. Natl. Acad. Sci. USA 91: 7520–7524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, T., J. B. Wang and R. A. Schulz, 2002. Mutations within the conserved MADS box of the D-MEF2 muscle differentiation factor result in a loss of DNA binding ability and lethality in Drosophila. Differentiation 70: 438–446. [DOI] [PubMed] [Google Scholar]
- Novitch, B., D. Spicer, P. Kim, W. Cheung and A. Lassar, 1999. pRb is required for MEF2-dependent gene expression as well as cell-cycle arrest during skeletal muscle differentiation. Curr. Biol. 9: 449–459. [DOI] [PubMed] [Google Scholar]
- O'Donnell, P. T., V. L. Collier, K. Mogami and S. I. Bernstein, 1989. Ultrastructural and molecular analyses of homozygous-viable Drosophila melanogaster muscle mutants indicate there is a complex pattern of myosin heavy-chain isoform distribution. Genes Dev. 3: 1233–1246. [DOI] [PubMed] [Google Scholar]
- O'Mahoney, J., K. Guven, J. Lin, J. Joya, C. Robinson et al., 1998. Identification of a novel slow-muscle-fiber enhancer binding protein, MusTRD1. Mol. Cell. Biol. 18: 6641–6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, N. H., 1994 Imaging neuronal subsets and other cell types in whole-mount Drosophila embryos and larvae using antibody probes, pp. 445–487 in Drosophila melanogaster: Practical Uses in Cell Biology (Methods in Cell Biology, Vol. 44), edited by L. S. B. Goldstein and E. Fyrberg. Academic Press, New York. [DOI] [PubMed]
- Peckham, M., J. E. Molloy, J. C. Sparrow and D. C. S. White, 1990. Physiological properties of the dorsal longitudinal flight muscle and the tergal depressor of the trochanter muscle of Drosophila melanogaster. J. Muscle Res. Cell Motil. 11: 203–215. [DOI] [PubMed] [Google Scholar]
- Polly, P., L. Haddadi, L. Issa, N. Subramaniam, S. Palmer et al., 2003. hMusTRD1 alpha 1 represses MEF2 activation of the Troponin I slow enhancer. J. Biol. Chem. 278: 36603–36610. [DOI] [PubMed] [Google Scholar]
- Poodry, C. A., and H. A. Schneiderman, 1970. The ultrastructure of the developing leg of Drosophila melanogaster. Wilhelm Roux' Archiv. 166: 1–44. [DOI] [PubMed] [Google Scholar]
- Postigo, A., E. Ward, J. Skeath and D. Dean, 1999. zfh-1, the Drosophila homologue of ZEB, is a transcriptional repressor that regulates somatic myogenesis. Mol. Cell. Biol. 19: 7255–7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganayakulu, G., B. Zhao, A. Dokid, J. D. Molkentin, E. N. Olson et al., 1995. A series of mutations in the D-MEF2 transcription factor reveal multiple functions in larval and adult myogenesis in Drosophila. Dev. Biol. 171: 169–181. [DOI] [PubMed] [Google Scholar]
- Rao, M., M. Donoghue, J. Merlie and J. Sanes, 1996. Distinct regulatory elements control muscle-specific, fiber-type-selective, and axially graded expression of a myosin light-chain gene in transgenic mice. Mol. Cell. Biol. 16: 3909–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, C. T., C. Murphy and D. Fristrom, 1975. The ultrastructure of the developing pupal leg of Drosophila melanogaster. Wilhelm Roux' Archiv. 178: 285–302. [DOI] [PubMed] [Google Scholar]
- Soler, C., M. Daczewska, J. Da Ponte, B. Dastugue and K. Jagla, 2004. Coordinated development of muscles and tendons of the Drosophila leg. Development 131: 6041–6051. [DOI] [PubMed] [Google Scholar]
- Spicer, D., C. Rhee, W. Cheung and A. Lassar, 1996. Inhibition of myogenic bHLH and MEF2 transcription factors by the bHLH protein twist. Science 272: 1476–1480. [DOI] [PubMed] [Google Scholar]
- Squire, J. M., 1986 Muscle: Design, Diversity and Disease. Benjamin-Cummings, Menlo Park, CA.
- Stronach, B., P. Renfranz, B. Lilly and M. Beckerle, 1999. Muscle LIM proteins are associated with muscle sarcomeres and require dMEF2 for their expression during Drosophila myogenesis. Mol. Biol. Cell 10: 2329–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarsan, V., S. Anant, P. Guptan, K. Vijayraghavan and H. Skaer, 2001. Myoblast diversification and ectodermal signaling in Drosophila. Dev. Cell 1: 829–839. [DOI] [PubMed] [Google Scholar]
- Thisse, B., M. E. Messal and F. Perrin-Schmitt, 1987. The twist gene: isolation of a Drosophila zygotic gene necessary for the establishment of dorsoventral pattern. Nucleic Acids Res. 15: 3439–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigoreaux, J., 2001. Genetics of the Drosophila flight muscle myofibril: a window into the biology of complex systems. BioEssays 21: 1047–1063. [DOI] [PubMed] [Google Scholar]
- White, K. P., S. A. Rifkin, P. Hurban and D. S. Hogness, 1999. Microarray analysis of Drosophila development during metamorphosis. Science 286: 2179–2184. [DOI] [PubMed] [Google Scholar]
- Wilson-Rawls, J., J. Molkentin, B. Black and E. Olson, 1999. Activated notch inhibits myogenic activity of the MADS-box transcription factor myocyte enhancer factor 2C. Mol. Cell. Biol. 19: 2853–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, H., F. J. Naya, T. A. Mckinsey, B. Mercer, J. M. Shelton et al., 2000. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. EMBO J. 19: 1963–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, Z., A. Serrano, S. Schiaffino, R. Bassel-Duby and R. Williams, 2001. Regulatory elements governing transcription in specialized myofiber subtypes. J. Biol. Chem. 276: 17361–17366. [DOI] [PubMed] [Google Scholar]
- Yang, C. C., O. I. Ornatsky, J. C. Mcdermott, T. F. Cruz and C. A. Prody, 1998. Interaction of myocyte enhancer factor 2 (MEF2) with a mitogen-activated protein kinase, ERK5/BMK1. Nucleic Acids Res. 26: 4771–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]