Abstract
Anticoagulant compounds, i.e., derivatives of either 4-hydroxycoumarin (e.g., warfarin, bromadiolone) or indane-1,3-dione (e.g., diphacinone, chlorophacinone), have been in worldwide use as rodenticides for >50 years. These compounds inhibit blood coagulation by repression of the vitamin K reductase reaction (VKOR). Anticoagulant-resistant rodent populations have been reported from many countries and pose a considerable problem for pest control. Resistance is transmitted as an autosomal dominant trait although, until recently, the basic genetic mutation was unknown. Here, we report on the identification of eight different mutations in the VKORC1 gene in resistant laboratory strains of brown rats and house mice and in wild-caught brown rats from various locations in Europe with five of these mutations affecting only two amino acids (Tyr139Cys, Tyr139Ser, Tyr139Phe and Leu128Gln, Leu128Ser). By recombinant expression of VKORC1 constructs in HEK293 cells we demonstrate that mutations at Tyr139 confer resistance to warfarin at variable degrees while the other mutations, in addition, dramatically reduce VKOR activity. Our data strongly argue for at least seven independent mutation events in brown rats and two in mice. They suggest that mutations in VKORC1 are the genetic basis of anticoagulant resistance in wild populations of rodents, although the mutations alone do not explain all aspects of resistance that have been reported. We hypothesize that these mutations, apart from generating structural changes in the VKORC1 protein, may induce compensatory mechanisms to maintain blood clotting. Our findings provide the basis for a DNA-based field monitoring of anticoagulant resistance in rodents.
THE introduction of warfarin and related anticoagulant compounds in the early 1950s produced a significant change for rodent control practice. The delayed action of such compounds, with mortality occurring days or even weeks after initial bait uptake makes them particularly suitable for the control of phobic species like brown rats (Rattus norvegicus). Today, control of commensal rodents, particularly of brown rats, relies predominantly on the use of anticoagulants.
During the same time period, coumarin derivatives have gained a leading role as oral anticoagulants for the treatment and prevention of thromboembolic events in humans. This class of anticoagulants target the vitamin K epoxide reductase (VKOR) complex, which fulfills a basic function in the recycling of vitamin K (Lowenthal and Macfarlane 1964). Vitamin K hydroquinone is an essential cofactor for the post-translational gamma-carboxylation of several blood coagulation factors (Nelsestuen et al. 1974; Stenflo et al. 1974; Sadowski et al. 1976; Cain et al. 1998) and other vitamin-K-dependent proteins (Presnell and Stafford 2002). During each carboxylation step, one molecule of vitamin K hydroquinone is oxidized to vitamin K 2,3 epoxide. The recycling of this micronutrient is achieved by the VKOR, and suppression of the VKOR by anticoagulants inhibits carboxylation of clotting factors and thus compromises the coagulation process.
In brown rats (R. norvegicus), resistance to the anticoagulants warfarin and diphacinone was first observed in 1958 (Boyle 1960), and warfarin resistance in the house mouse (Mus musculus/domesticus) was evident shortly after (Dodsworth 1961). The majority of reports came from Europe but resistance in commensal rodents was also documented in the United States, Canada, Japan, and Australia. An overview of the status of warfarin resistance is given in Table 1.
TABLE 1.
Current status of anticoagulant resistance in commensal rats and house mice
| Country | R. norvegicus | R. rattus | M. musculus domesticus | Reference |
|---|---|---|---|---|
| Belgium | + | + | Lund (1984); Baert (2003) | |
| Denmark | + | + | + | Myllymäki (1995); Lodal (2001) |
| Finland | + | Myllymäki (1995) | ||
| France | + | + | + | Myllymäki (1995) |
| Germany | + | + | + | Myllymäki (1995); Pelz (2001) |
| United Kingdom | + | + | + | Myllymäki (1995); Kerins et al. (2001) |
| Italy | + | Alessandroni et al. (1980) | ||
| The Netherlands | + | + | De Jonge (1994) | |
| Sweden | + | Lund (1984) | ||
| Switzerland | + | Muhr (1981) | ||
| Canada | + | + | Siddiqi and Blaine (1982) | |
| United States | + | + | + | Jackson and Ashton (1986) |
| Japan | + | Naganuma et al. (1981) | ||
| Australia | + | Saunders (1978) |
Since anticoagulant resistance caused significant reductions in the efficacy of control of rat and mouse populations, more potent anticoagulant compounds were developed during the 1970s and 1980s: first difenacoum and bromadiolone, followed later by the more potent “single-feed” compounds brodifacoum, flocoumafen, and difethialone. However, resistance to most of these compounds was reported soon after their introduction (Rowe et al. 1981; Greaves et al. 1982; MacNicoll and Gill 1987; Johnson 1988).
Breeding experiments have led to the conclusion that warfarin resistance is based on a single dominant autosomal gene (Rw) in brown rats and in house mice (War), which could be mapped to orthologous linkage groups on chromosomes 1 and 7, respectively (Greaves and Ayres 1967; Wallace and MacSwiney 1976; Kohn and Pelz 1999). In black rats, resistance was supposed to be multifactorial, judging from its unstable heredity (Greaves et al. 1976). It has been suggested that a second recessive gene was modifying the Rw gene to confer difenacoum resistance in some individuals of the Hampshire warfarin-resistant strain (Greaves and Cullen-Ayres 1988).
Recently, a first protein of the VKOR complex, named VKORC1 and found to be capable of reducing vitamin K-2,3-epoxide in a warfarin-sensitive manner, was identified (Li et al. 2004; Rost et al. 2004). Missense mutations at different positions of this protein have been detected in human warfarin-resistant patients and in the German resistant rat strain (Rost et al. 2004; Table 2). Upon recombinant expression in human HEK293 cells, mutated VKORC1 showed a reduced enzyme activity and a partial resistance toward warfarin inhibition.
TABLE 2.
VKORC1 mutations found inRw- andWar-resistant animals
| Strain/geographic area | No. of specimens |
Codon position |
Codon WT |
Codon Mut |
AA WT | AA Mut |
|---|---|---|---|---|---|---|
| R. norvegicus | ||||||
| UK, Hampshire resistant | 2 | 120 | CTG | CAG | Leu | Gln |
| UK, Berkshire resistant (Reading) | 2 | 120 | CTG | CAG | Leu | Gln |
| UK, Berkshire resistant (CSL) | 2 | 120 | CTG | CAG | Leu | Gln |
| UK, Scottish resistant (CSL) | 2 | 128 | CTG | CAG | Leu | Gln |
| UK, Welsh resistant (CSL) | 2 | 139 | TAT | TCT | Tyr | Ser |
| UK, Yorkshire, wild | 3 | 139 | TAT | TGT | Tyr | Cys |
| UK, Yorkshire, wild | 1a | 128 | CTG | CAG | Leu | Gln |
| Denmark, wild | 43 | 139 | TAT | TGT | Tyr | Cys |
| Belgium, wild | 14 | 139 | TAT | TTT | Tyr | Phe |
| France, wild | 6 | 139 | TAT | TTT | Tyr | Phe |
| France, wild | 1b | 35 | CGC | CCC | Arg | Pro |
| Germany, wild | 286 | 139 | TAT | TGT | Tyr | Cys |
| Germany, wild | 2 | 56 | TCC | CCC | Ser | Pro |
| M. musculus/domesticus | ||||||
| UK, CSL | 6 | 128 | TTA | TCA | Leu | Ser |
| UK, Reading | 4 | 139 | TAT | TGT | Tyr | Cys |
| Homo sapiens sapiens | ||||||
| Patient F (Rost et al. 2004) | 1 | 128 | CTC | CGC | Leu | Arg |
WT, wild-type sequence; Mut, mutated sequence; AA, amino acid.
Animal was compound heterozygous for Leu128Gln and Tyr139Cys.
Animal was compound heterozygous for Arg35Pro and Tyr139Phe.
This study compares different resistant laboratory rodent strains and wild-caught rats for mutations in the VKORC1 gene, which supposedly is the basic gene conferring anticoagulant resistance. We measure VKOR activities of the different mutations in a recombinant expression system and discuss the physiological, evolutionary, and practical implications of the mutations.
MATERIALS AND METHODS
Animals:
R. norvegicus:
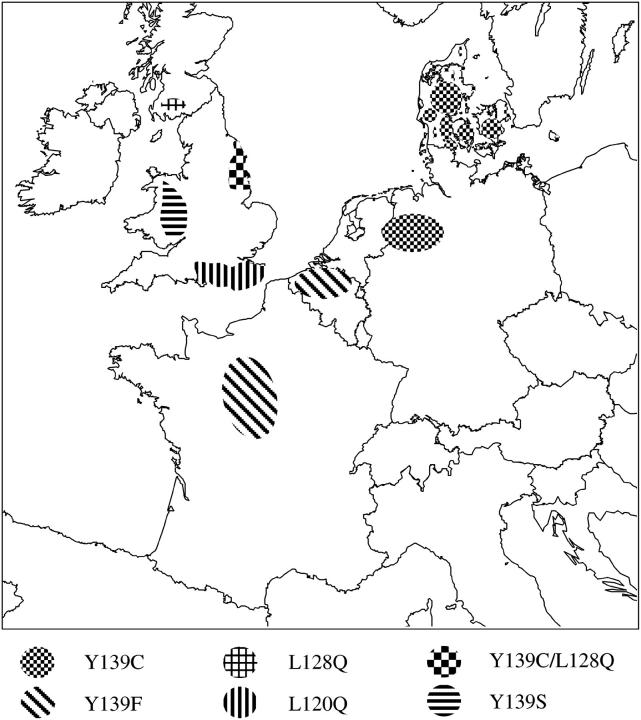
Rats of both sexes were analyzed. Animals of the laboratory outbred strains HW and HH were obtained from two independent laboratory stocks [Central Science Laboratory (CSL), York and Reading University], strains HS and HB1 were from the CSL, and strain HB2 from Reading. Four of these strains were derived from wild stock and were crossed with warfarin-susceptible Wistar-derived Tolworth albino susceptible to incorporate homozygous genes for Scottish (HS; Greaves and Ayres 1973), Welsh (HW; Greaves and Ayres 1969), Hampshire (HH; Greaves and Cullen-Ayres 1988), and Berkshire (HB1) resistance. The fifth strain was derived from wild Berkshire stock and crossed for four generations onto a warfarin-susceptible line of CD rats (obtained from Charles River UK) to incorporate homozygous genes for Berkshire resistance (HB2; Hussain 1998). A German strain was derived from wild stock and crossed with Wistar susceptibles to incorporate genes for at least warfarin resistance (Kohn and Pelz 1999). Wild rats were caught over the past years in the course of regional resistance monitoring programs and immediately analyzed for resistance by blood clotting response (BCR). They originated from the following geographic areas (Figure 1): Belgium—Flanders; Denmark—Bornholm, Fünen, Jutland, and Zealand; France—Yonne, Eure and Loire; Germany—Münsterland and Emsland; United Kingdom—Yorkshire.
Figure 1.—
Geographic origin of resistant rodent populations. Warfarin resistance areas in Europe are shown and locations where resistant rats were trapped in the wild are indicated. Different mutations are represented by different hatching.
M. musculus/domesticus:
The Reading susceptible mouse strain (MHS) was the Swiss mouse, an outbred strain obtained from Charles River UK. The Reading resistant mouse strain (MHR) was homozygous for a resistance gene that had been transferred through six generations onto the Reading susceptible mouse MHS background (C. V. Prescott, unpublished results). The mouse resistance gene was obtained from a wild population of house mice (M. musculus domesticus) originally trapped near Reading, United Kingdom (Prescott 1996).
At the CSL (York, UK) a homozygous warfarin-resistant mouse strain was derived by crossing mice of a warfarin-resistant PBI strain, which were direct descendants of the wild mice reported in 1976 (Wallace and MacSwiney 1976), with warfarin-susceptible mice of a LAC-gray strain. Offspring were selected for warfarin resistance and backcrossed to the LAC-gray strain for six generations. Warfarin-resistant mice of the sixth generation were mated with each other and homozygous warfarin-resistant individuals selected to produce a homozygous Cambridge resistant strain.
Blood clotting response test:
BCR resistance testing with warfarin was done as described (Martin et al. 1979) with modifications (MacNicoll and Gill 1993). BCR testing with bromadiolone was performed according to Gill et al. (1994)(1993). In Germany and Denmark, the test solution was administered by intraperitoneal injection instead of oral application, which had proved equally effective (H. J. Pelz, unpublished data) and is not confounded by any mechanism of reduced intestinal absorption. With each series of tests, the activity of the test solution was checked by including a Wistar albino rat as a known susceptible individual.
DNA sequence determination and mutation screening assays:
DNA was prepared from fresh or frozen tissue samples by standard procedures. The three exons and flanking intronic sequences of the VKORC1 gene were amplified by PCR and sequenced as described (Rost et al. 2004). For the mutation Tyr139Cys, an amplification refractory mutation system (ARMS)-PCR test was developed (Ye et al. 2001) using the outer primers F: ATC CTG AGT TCC CTG GTG TCT GTC GCT G and R: TCA GGG CTT TTT GAC CTT GTG TTC TGG C (2 pmol each) and the inner primers F: TGA TTT CTG CAT TGT TTG CAT CAC CAC ATG and R: CAA CAT CAG GCC CGC ATT GAT GGA AT (10 pmol each). PCR reactions were performed with Taq polymerase and 1× buffer (Invitrogen, San Diego) in the presence of 1.5 mm MgCl2 and 200 mm betaine for 3 min at 95° followed by 32 cycles of 20 sec at 95°, 20 sec at 62°, 10 sec at 70°, and a final 3 min at 70°. The outer primer pair generates a control band of 168 bp while the inner primers result in a band of 123 bp for the wild type and 101 bp for the mutated sequence, respectively.
The mutation Leu120Gln creates a novel StuI restriction site in exon 3. Digestion of the PCR product specific for exon 3 results in fragments of 195 and 135 bp in the presence of the mutation and a single band of 330 bp in the wild type (Rost et al. 2004). The mutation Leu128Gln generates a novel site for BsrI with fragments of 170 and 160 bp in mutant PCR products. The mutation Tyr139Ser can be detected by MnlI digestion as a fragment of 110 bp (plus several bands <50 bp) vs. 160 bp (plus bands <50 bp) in the wild type.
Expression studies and VKOR activity measurements:
VKOR activity was measured in transfected HEK293-EBNA cells (Invitrogen). For each experiment, 6 × 105 cells were plated onto 94-mm petri dishes. After 30 hr at 37° and 5% CO2, transfection (20 μg of DNA construct/dish) was done by the calcium phosphate method. After 40 hr at 35° and 3% CO2, transfected cells were washed in PBS, collected, and lyzed in 0.25 mm imidazole (pH 7.6) plus 0.5% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate hydrate (CHAPS). VKOR enzymatic activity was measured in whole-cell extracts as described (Wallin and Martin 1985). Mutations were introduced into the human cDNA cloned in pCEP4 (Invitrogen) by the QuikChange mutagenesis kit (Stratagene, Amsterdam). All transfection and activity assays were run in triplicate and are given as the mean.
RESULTS
Sequence analysis of resistant laboratory stocks:
R. norvegicus:
Scottish (HS), Welsh (HW), Hampshire (HH), and Berkshire (HB1 and HB2) homozygous resistant rats obtained from two independent laboratory stocks were analyzed for mutations in the VKORC1 gene. Three mutations were identified: the Hampshire and both Berkshire strains share a Leu120Gln mutation while resistant animals from the Scottish strain carry a Leu128Gln substitution. In the Welsh strain, tyrosine139 was found mutated to serine (Tyr139Ser). Mutations were absent from the anticoagulant-susceptible background strains.
M. musculus/domesticus:
Six mice from a colony established at the CSL in York were found to be homozygous for a Leu128Ser mutation. Four Reading homozygous resistant mice (MHR) carried the Tyr139Cys mutation observed before in rats. Neither sequence change was seen in the respective background strains. Sequencing results are summarized in Table 2.
Sequence analysis of wild-caught resistant brown rats:
Rats trapped in the wild in Belgium, Denmark, England, and Germany were tested for warfarin resistance by the blood clotting response test at the time of sampling. Subsequent analysis of the VKORC1 gene from frozen tissue specimens revealed mutations in all but three resistant animals (Table 2). Forty-three resistant wild rats from Denmark (Jütland, Zealand, Fünen, and Bornholm) showed the mutation Tyr139Cys, which had been previously identified in northwestern Germany (Rost et al. 2004). In 14 resistant rats caught in Flanders (Belgium), the same tyrosine139 was found mutated to phenylalanine (Tyr139Phe). Thirteen animals were heterozygous while one was homozygous for this amino acid substitution. None of the 13 susceptible rats caught in Flanders showed this mutation. Of six incidental catches from the regions of Burgundy and Centre in France, five carried this Tyr139Phe mutation homozygously while the sixth was compound heterozygous for Tyr139Phe and a novel variant at Arg35Pro.
Of three wild resistant rats from Yorkshire, two were heterozygous for Tyr139Cys/wild type and one was compound heterozygous for Leu128Gln/Tyr139Cys. The geographic origin of these wild-caught rats and of the resistance genes interbred into the laboratory stocks is depicted in Figure 1.
Since warfarin-resistant laboratory stocks were established from a single founder individual, we tested whether resistance in a wild population was genetically homogeneous or not. A total of 428 rats were trapped in 22 different locations of the resistance area of Münsterland/Emsland, Germany, and warfarin resistance was determined by BCR. Testing for the Tyr139Cys mutation by the ARMS-PCR screening assay (see materials and methods) revealed that 281 of 286 rats classified “resistant” by BCR testing carried this mutation while the mutation was absent from 137 of 142 individuals classified as susceptible by the BCR test. The full genotypes of the 10 discordant animals were determined by sequencing of the entire VKORC1 gene. Of the 5 rats testing positive by BCR (that is, resistant) but negative by ARMS-PCR (that is, wild type for Tyr139), three animals had no sequence variation in the entire VKORC1 coding region and thus may be regarded as false-positive BCR tests. In two animals, a sequence change was found in exon 1, leading to a Ser56Pro exchange. One animal each was homo- and heterozygous, respectively. Ser56Pro is, therefore, a second candidate mutation in the Münsterland resistance area. Five animals classified as “susceptible” by BCR were determined heterozygous by ARMS-PCR for the Tyr139Cys mutation. The mutation was confirmed by sequencing in all 5 animals. Thus, these may be seen as false-negative results of the BCR.
In Denmark, of 58 rats from 24 localities in four different regions/islands (Jütland, Zealand, Fünen, Bornholm), 15 were classified “susceptible” and 43 “resistant” according to BCR or feeding tests (World Health Organization 1982). All resistant individuals showed the Tyr139Cys mutation and all but one of the sensitive individuals were confirmed “wild type” by ARMS-PCR. Within this sample, 24 of 31 rats from two localities (Horsens, Jütland, and Vordingborg, Zealand) carried the mutation homozygously; the others showed heterozygous genotypes at position 139.
Recombinant expression and VKOR activity measurements:
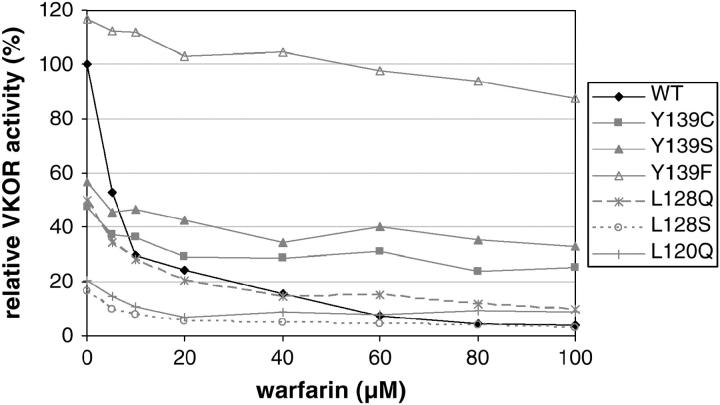
VKOR activity was measured in HEK293 cells transfected with a series of VKORC1 cDNA constructs and its inhibition by warfarin was determined (Figure 2). All experiments were run in triplicate to compensate for variation in transfection efficiency. Activity of the wild-type protein (0.74 ± 0.23 nmol/mg protein) was set to 100% and was progressively inhibited by warfarin to <4% at 100 μm. The mutation Tyr139Phe displayed an even higher basal activity (116%) and appeared largely insensitive to warfarin with 87% activity at 100 μm inhibitor. In comparison, the mutation Tyr139Ser showed 57% of wild-type activity in the absence of warfarin but still 33% residual activity at the highest inhibitor concentrations tested. Basal VKOR activity of mutation Tyr139Cys was ∼48% of wild type without warfarin and 25% at 100 μm. The two mutations Leu120Gln and Leu128Gln had initial activities of 50 and 20% of normal, respectively, and residual activities of 9% each at 100 μm warfarin. Substitution of Leu128 by serine caused the lowest VKOR activity (17%) and was least resistant to drug inhibition (3% at 100 μm). Both amino acid substitutions that were observed in individual wild rats, Arg35Pro and Ser56Pro, nearly abolished VKOR activity with <5% in the absence of warfarin. Upon inhibition the activity fell below our detection limit.
Figure 2.—
VKORC1 activity after recombinant expression in HEK293 cells. HEK293 cells were transfected by VKORC1 cDNA constructs and enzyme activity was measured as described in materials and methods. Activity of the wild-type construct in the absence of warfarin was set to 100%. All experiments were run in triplicate.
DISCUSSION
Previously, the VKORC1 gene that encodes an essential component of the vitamin K recycling complex was identified as the gene responsible for warfarin resistance and combined deficiency of vitamin-K-dependent clotting factors in humans (Rost et al. 2004). The chromosomal position of human VKORC1 is orthologous to the Rw locus in rats and the War locus in mice (Wallace and MacSwiney 1976; Kohn and Pelz 1999; Fregin et al. 2002). Thus, VKORC1 was the prime candidate gene for warfarin resistance in rodents as well. In the present study, we have identified VKORC1 mutations in all but three warfarin-resistant rodents tested.
Origin of mutations:
Apparently, codons 128 and 139 of the VKORC1 gene represent “hotspots” for mutations as two and three mutations, respectively, were identified in two species, rats and mice. Previously, leucine 128 had been found mutated to arginine in a human patient resistant to coumarin treatment for thrombosis (Rost et al. 2004). Interestingly, codon usage at these positions differs among species and none of the codons contains the mutation-prone CpG dinucleotide (Table 2). Thus, the gene sequence does not provide a straightforward explanation for the recurrent mutations.
Although all laboratory strains carrying Rw or War were established from single founder individuals, the recurrent and independent mutations in VKORC1 make it highly unlikely that these reflect neutral sequence variation among wild populations of rodents. This is reinforced by data from wild-caught animals, which display an even broader spectrum of resistance mutations with a Tyr139Phe mutation prevalent in Belgium and France, Arg35Pro in France, Ser56Pro as a second mutation in Münsterland, and proof of the Tyr139Cys mutation also occurring in England. In particular, the compound heterozygous rats from Yorkshire (Leu128Gln/Tyr139Cys) and Burgundy (Arg35Pro/Tyr139Phe) demonstrate that different mutations coexist in the same population. The resistant wild population in Germany (Münsterland, Emsland) appeared genetically rather homogeneous. In this largest field sample 10 discordant results between BCR and genotype as assessed by ARMS-PCR were observed. Upon subsequent sequencing of the entire VKORC1 coding region in the 10 discordant rats, a novel mutation, Ser56Pro, was found in two animals. Thus, of the 286 BCR-positive (resistant) rats, 3 had no mutations in VKORC1 and 5 of 147 BCR-negative animals were carriers of the Tyr139Cys mutation. Although it is possible that the 3 “false-positive” rats had developed resistance by another molecular mechanism, it appears more likely that they reflect failures of the BCR—a complex in vivo test, which has proven difficult to standardize. If so, our data allow for a reassessment of sensitivity and specificity of the BCR at 0.989 and 0.965, respectively.
Migration of rat clans and spread of resistance are well-established observations (Drummond 1966). It cannot be ruled out that resistant rats have migrated over considerable distances, e.g., from Denmark to Münsterland or vice versa. Alternatively, the present distribution of variability in VKORC1 sequence may reflect founder effects in species that are relatively new to Western Europe. Although R. rattus has been known in parts of Europe for ∼10,000 years, there is evidence that R. norvegicus may have been imported for the first time into Denmark from Russia in 1716 (Twigg 1975). Over the next 80 years they colonized the whole of Western Europe, but there is no information about whether this was a spread of rats from Denmark or whether other point importations from eastern countries occurred.
To date, mutations in the VKORC1 gene can be associated with warfarin resistance in three species, humans, rats, and mice. The four human resistant patients identified to date represent independent mutation events (Rost et al. 2004). The present data strongly argue for at least seven independent mutation events in R. norvegicus and two in mice. Taking into account the extensive use of anticoagulant compounds over >50 years, sequence changes appear to be rare spontaneous events. Apparently, they confer a tremendous selective advantage in the presence of anticoagulant baits.
Effect of mutations on protein function:
Upon recombinant expression in HEK293 cells, all but one of the amino acid replacements studied reduced VKORC1 activity and conferred warfarin resistance to varying degrees (Figure 2). The mutation Tyr139Cys showed 50% of wild-type activity in the absence of warfarin. VKOR activity has been measured in liver microsomes from a subset of 12 wild-caught resistant rats from Münsterland, which were shown to be Cys139 homozygotes. At 2 μm warfarin, microsomal VKOR activities varied between 72 and 97% of the uninhibited enzyme preparation (Thijssen and Pelz 2001). This compares well to our in vitro data, given that the HEK system may not comprise all the components present in liver (Note that much higher warfarin concentrations are required to inhibit the recombinant enzyme.) All three tyrosine 139 mutations retain higher than wild-type activities in the presence of warfarin even at high concentrations (Figure 2). In contrast, mutations at arginine 35, serine 56, leucine 120, and leucine 128, respectively, reduced VKORC1 activity to very low basal levels. In the presence of an inhibitor, activities of the Leu128, Arg35, and Ser56 mutations fell below our detection limit.
The VKORC1 protein is not fully characterized yet and its catalytic site(s) has not yet been determined experimentally. A multiple sequence alignment of 37 related genes from archaebacteria, eubacteria, and metazoa has identified three to four potential hydrophobic transmembrane domains, four cysteines and one serine/threonine to be fully conserved between species, and a CXXC motif known from thioredoxin oxidoreductases (Trx) as an electron donor in a variety of reduction reactions (see Figure 3; Goodstadt and Ponting 2004; Rost et al. 2004). None of these residues is involved in any of the known mutations, but serine 56 immediately precedes the conserved serine/threonine at position 57.
Figure 3.—
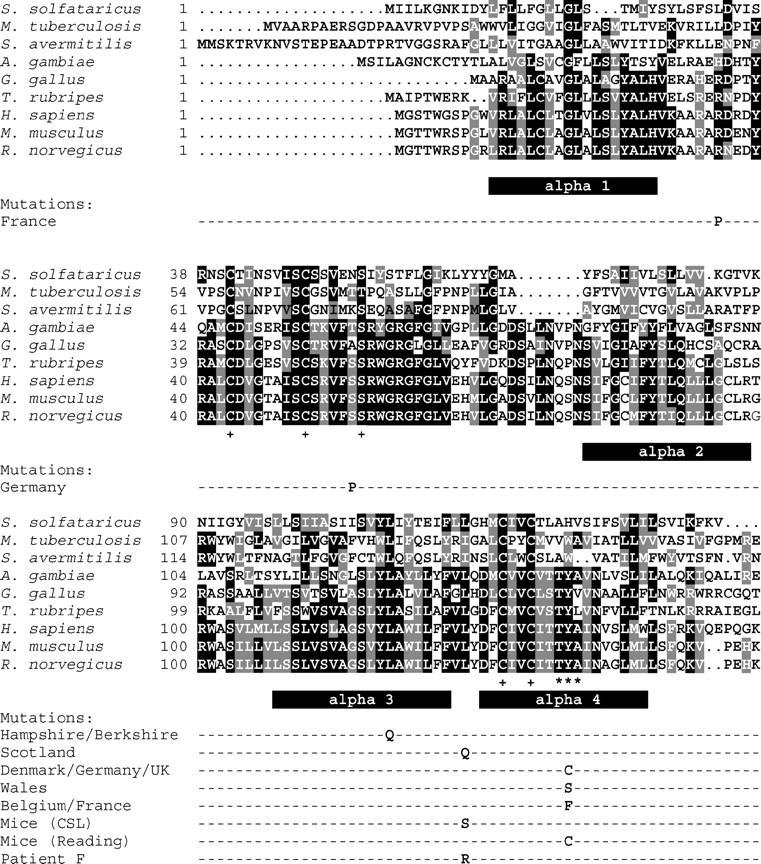
Sequence alignment of selected VKORC1 genes and related sequences. Sequence alignments were done by MultAlin (http://prodes.toulouse.inra.fr/multalin/multalin.html) and Boxshade (http://bioweb.pasteur.fr/seqanal/interfaces/boxshade.html). Predicted hydrophobic α-helical domains are represented by solid bars below the amino acid sequence. Fully conserved positions (as described by Goodstadt and Ponting 2004) are indicated by a + sign; the Thr-Tyr-Ala motif is marked by ***. Mutations observed in resistant animals are given by their single-letter amino acid code below the rat wild-type sequence.
The mutation hotspot at tyrosine139 is part of the hydrophobic sequence motif Thr-Tyr-Ala, which is also found in another dicoumarol and warfarin-sensitive quinone oxidoreductase (NQOR, E.C. 1.6.99.2, previously also known as DT-diaphorase; Hall et al. 1972). In NQOR, this tyrosine has been identified as the dicoumarol binding site by photoaffinity labeling and site-directed mutagenesis (Ma et al. 1992). Given its involvement in three mutation events conferring warfarin resistance, it is tempting to predict that Tyr139 may also be part of the warfarin-binding site in VKORC1.
In the HEK cell system, mutations affecting other amino acid residues have very low enzyme activities even in the absence of warfarin. If this were to reflect the situation in vivo, it is hard to conceive how animals could generate sufficient vitamin KH2 for effective gamma-carboxylation and clotting activity. Given the great selective pressure of warfarin poisoning, the functional elimination of VKORC1 by mutation may boost the upregulation of other pathways for the recruitment of vitamin KH2. Thus, warfarin resistance could be conferred by knocking down VKORC1 activity while switching to another warfarin-insensitive pathway would secure effective blood clotting for survival. That such compensatory mechanisms may be involved is suggested by the differences observed in dietary vitamin K requirements for some resistant laboratory strains (Bell and Caldwell 1973; MacNicoll 1985; Thijssen 1995). The two Berkshire strains (maintained at CSL, York, and Reading University, respectively) have the same mutation as the Hampshire strain (Leu120Gln), but only the Berkshire colony at Reading has an increased dietary vitamin K requirement that is comparable with that of the Hampshire strain. Furthermore, the Scottish (Leu128Gln) and the Münsterland strains (Tyr139Cys) require low substitution levels whereas the Welsh strain (Tyr139Ser) is dependent on high supplementation doses.
Effect of VKORC1 mutations on rodenticide resistance in vivo:
From the data presented here, it can be concluded that mutations in VKORC1 are the genetic basis of anticoagulant resistance in all laboratory strains and wild populations of rodents studied. However, at present these mutations alone cannot explain all aspects of resistance that have been reported in the past:
The marked difference in vitamin K requirement cannot be deduced from the underlying VKORC1 mutation alone. Further studies are needed to address this question.
Resistance to second generation anticoagulants has been reported for a proportion of resistant individuals (Rowe et al. 1981; Greaves et al. 1982; MacNicoll and Gill 1987; Johnson 1988) but this cannot be fully explained by variability in VKORC1 sequence data. It has been suggested that secondary or modifier genes confer resistance to second-generation anticoagulants in rats that carry the Rw gene (Greaves and Cullen-Ayres 1988; Kohn and Pelz 1999). However, so far all attempts to map the resistance gene(s) for difenacoum or bromadiolone by cross-breeding experiments have failed to identify a single Mendelian locus for the trait(s) (Greaves and Cullen-Ayres 1988; A. D. MacNicoll, unpublished data). Although in recombinant expression systems, VKORC1 alone is capable of reducing vitamin K-2,3-epoxide in a warfarin-sensitive manner (Figure 2; Li et al. 2004; Rost et al. 2004), it has long been postulated that the enzyme may be part of a larger complex in liver microsomes (Cain et al. 1997). In addition, variation in anticoagulant uptake, metabolism, and clearance may influence the efficacy of rodenticides in vivo. Further experiments will address the question whether VKORC1 is sensitive to second-generation anticoagulants.
Rodenticide resistance testing:
In this study, we have identified mutations in the VKORC1 gene sequence that correlate very well with the results from BCR tests for warfarin resistance but less well with resistance to second-generation anticoagulants. BCR tests require that rats or mice are trapped alive, transported to a central facility, and subjected to anticoagulant administration and blood sampling, which requires ethical approval and licensing in most countries. We have demonstrated that a PCR-based genetic test for mutations in the VKORC1 gene can successfully identify warfarin-resistant rats trapped in the field. This test could readily be applied to tissue from dead rodents and potentially to host genetic material sloughed off onto fecal pellets, providing a totally noninvasive sampling procedure. This genetic test will provide a simpler, and potentially cheaper, method for monitoring the distribution of warfarin-resistant rats and mice and for evaluating the impact of strategies to manage and overcome resistance.
Acknowledgments
The authors thank Dagmar Funck, Engelbert Kampling, Ralf Schlieper, and Pam Rummings for technical assistance. Gérard Grolleau greatly helped with his experience of resistance and field contacts to collect samples in France. This work was supported by grants to J.O. from the German National Genome Research Net Cardiovascular Diseases (BMBF-DLR-01GS0424) and from Baxter Germany and a grant from the Water Division of the Ministry of the Flemish Community (K.B.)
References
- Alessandroni, P., S. Marchini, A. Bernardo, F. Terranova and P. G. Turillazi, 1980. Valutazione della presenza di ratti resistenti al warfarin nella citta di Reggio Calabria. Ann. Ist. Super. Sanita 16: 271–286. [PubMed] [Google Scholar]
- Baert, K., 2003 Jaarrapport-Onderzoeksgroep Rattenbestijding, pp. 18–23. AMINAL afdeling Water, IBW, Geraardsbergen, Belgium.
- Bell, R. G., and P. T. Caldwell, 1973. Mechanism of warfarin resistance. Warfarin and the metabolism of vitamin K1. Biochemistry 12: 1759–1762. [DOI] [PubMed] [Google Scholar]
- Boyle, C. M., 1960. Case of apparent resistance of Rattus norvegicus Berkenhout to anticoagulant poisons. Nature 188: 517. [Google Scholar]
- Cain, D., S. M. Hutson and R. Wallin, 1997. Assembly of the warfarin-sensitive vitamin K 2,3-epoxide reductase enzyme complex in the endoplasmic reticulum membrane. J. Biol. Chem. 272: 29068–29075. [DOI] [PubMed] [Google Scholar]
- Cain, D., S. M. Hutson and R. Wallin, 1998. Warfarin resistance is associated with a protein component of the vitamin K 2,3-epoxide reductase enzyme complex in rat liver. Thromb. Haemost. 80: 128–133. [PubMed] [Google Scholar]
- De Jonge, J. T., 1994. Resistentieproblemen bij de bestrijding van knaagdieren in Nederland. Dierplagen en Milieu 42: 99–101. [Google Scholar]
- Dodsworth, E., 1961. Mice are spreading despite such poisons as warfarin. Minic. Engin. Lond. 3746: 1668. [Google Scholar]
- Drummond, D., 1966. Rats resistant to warfarin. New Sci. 968: 771–772. [Google Scholar]
- Fregin, A., S. Rost, W. Wolz, A. Krebsova, C. R. Muller et al., 2002. Homozygosity mapping of a second gene locus for hereditary combined deficiency of vitamin K-dependent clotting factors to the centromeric region of chromosome 16. Blood 100: 3229–3232. [DOI] [PubMed] [Google Scholar]
- Gill, J. E., G. M. Kerins and A. D. MacNicoll, 1993. Inheritance of low grade brodifacoum resistance in the Norway rat. J. Wildl. Manage. 56: 809–816. [Google Scholar]
- Gill, J. E., G. M. Kerins, S. D. Langton and A. D. MacNicoll, 1994. Blood-clotting response test for bromadiolone resistance in Norway rats. J. Wildl. Manage. 58: 454–461. [Google Scholar]
- Goodstadt, L., and C. P. Ponting, 2004. Vitamin K epoxide reductase: homology, active site and catalytic mechanism. Trends Biochem. Sci. 29: 289–292. [DOI] [PubMed] [Google Scholar]
- Greaves, J. H., and P. Ayres, 1967. Heritable resistance to warfarin in rats. Nature 215: 877–878. [DOI] [PubMed] [Google Scholar]
- Greaves, J. H., and P. Ayres, 1969. Linkages between genes for coat colour and resistance to warfarin in Rattus norvegicus. Nature 224: 284–285. [DOI] [PubMed] [Google Scholar]
- Greaves, J. H., and P. Ayres, 1973. Warfarin resistance and vitamin K requirement in the rat. Lab. Anim. 7: 141–148. [DOI] [PubMed] [Google Scholar]
- Greaves, J. H., and P. B. Cullen-Ayres, 1988 Genetics of difenacoum resistance in the rat, pp. 389–397 in Current Advances in Vitamin K Research, edited by J. W. Suttie. Elsevier, Amsterdam.
- Greaves, J. H., R. Redfern and B. Anasuya, 1976. Inheritance of resistance to warfarin in Rattus rattus L. J. Stored Prod. Res. 12: 65–70. [Google Scholar]
- Greaves, J. H., D. S. Sheperd and R. Quy, 1982. Field trials of second-generation anticoagulants against difenacoum-resistant Norway rat populations. J. Hyg. 89: 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, J. M., C. Lind, M. P. Golvano, B. Rase and L. Ernster, 1972 Structure and function of oxidation-reduction enzymes, pp. 433–443, edited by A. Akesson and A. Ehrenberg. Pergamon Press, Oxford.
- Hussain, I., 1998 Susceptibility to anticoagulants and the development of physiological resistance in Rattus norvegicus and Bandicota bengalensis. Ph.D. Thesis, University of Reading, Reading, UK.
- Jackson, W. B., and A. D. Ashton, 1986 Case histories of anticoagulant resistance, pp. 355–369 in Pesticide Resistance: Strategies and Tactics for Management, edited by National Research Council. National Academy Press, Washington, DC.
- Johnson, R. A., 1988. Performance studies with the new anticoagulant rodenticide, flocoumafen, against Mus domesticus and Rattus norvegicus. Eur. Plant Protect. Org. Bull. 18: 481–488. [Google Scholar]
- Kerins, G. M., N. Dennis, H. Atterby, J. E. Gill and A. D. MacNicoll, 2001 Distribution of resistance to anticoagulant rodenticides in the Norway rat (Rattus norvegicus Berk.) in England 1995–98, pp. 149–159 in Advances in Vertebrate Pest Management II, edited by H.-J. Pelz, D. P. Cowan and C. J. Feare. Filander Verlag, Fürth, Germany.
- Kohn, M. H., and H. J. Pelz, 1999. Genomic assignment of the warfarin resistance locus, Rw, in the rat. Mamm. Genome 10: 696–698. [DOI] [PubMed] [Google Scholar]
- Li, T., C. Y. Chang, D. Y. Jin, P. J. Lin, A. Khvorova et al., 2004. Identification of the gene for vitamin K epoxide reductase. Nature 427: 541–544. [DOI] [PubMed] [Google Scholar]
- Lodal, J., 2001 Distribution and levels of anticoagulant resistance in rats (Rattus norvegicus) in Denmark, pp. 139–148 in Advances in Vertebrate Pest Management II, edited by H.-J. Pelz, D. P. Cowan and C. J. Feare. Filander Verlag, Fürth, Germany.
- Lowenthal, J., and J. A. MacFarlane, 1964. The nature of the antagonism between vitamin K and indirect anticoagulants. J. Pharmacol. Exp. Ther. 143: 273–277. [PubMed] [Google Scholar]
- Lund, M., 1984. Resistance to the second-generation anticoagulant rodenticides. Proc. Vertebrate Pest Conf. 11: 89–94. [Google Scholar]
- Ma, Q., K. Cui, F. Xiao, A. Y. Lu and C. S. Yang, 1992. Identification of a glycine-rich sequence as an NAD(P)H-binding site and tyrosine 128 as a dicumarol-binding site in rat liver NAD(P)H:quinone oxidoreductase by site-directed mutagenesis. J. Biol. Chem. 267: 22298–22304. [PubMed] [Google Scholar]
- MacNicoll, A. D., 1985. A comparison of warfarin resistance and liver microsomal vitamin K epoxide reductase activity in rats. Biochim. Biophys. Acta 840: 13–20. [DOI] [PubMed] [Google Scholar]
- MacNicoll, A. D., and J. E. Gill, 1987 The occurrence and significance of rodenticide resistance in the UK, pp. 85–95 in British Crop Protection Council Monograph, No. 37. British Crop Protection Council, Thornton Heath, UK.
- MacNicoll, A. D., and J. E. Gill, 1993. Revised methodology for a blood clotting response test for identification of warfarin-resistant Norway rats (Rattus norvegicus). Eur. Plant Protect. Org. Bull. 23: 701–707. [Google Scholar]
- Martin, A. D., L. C. Steed, R. Redfern, J. E. Gill and L. W. Huson, 1979. Warfarin-resistance genotype determination in the Norway rat, Rattus norvegicus. Lab. Anim. 13: 209–214. [DOI] [PubMed] [Google Scholar]
- Muhr, A. C., 1981 Zur problematik der hausmausbekämpfung, resistenzprobleme und möglichkeiten ihrer überwindung, pp. 93–109 in Aktuelle Probleme der Bekämpfung und Abwehr von Ratten und Hausmäusen, edited by I. Iglisch. Pentagon Publishing, Frankfurt, Germany.
- Myllymäki, A., 1995. Anticoagulant resistance in Europe: appraisal of the data from the 1992 EPPO questionnaire. Pesticide Sci. 43: 69–72. [Google Scholar]
- Naganuma, K., A. Fujita, N. Taniguchi and S. Takada, 1981. Warfarin susceptibility in the roof rat, Rattus rattus, in some locations of Tokyo. Jap. J. Sanit. Zool. 32: 243–245. [Google Scholar]
- Nelsestuen, G. L., T. H. Zytkovicz and J. B. Howard, 1974. The mode of action of vitamin K. Identification of gamma-carboxyglutamic acid as a component of prothrombin. J. Biol. Chem. 249: 6347–6350. [PubMed] [Google Scholar]
- Pelz, H. J., 2001 Extensive distribution and high frequency of resistance to anticoagulant rodenticides in rat populations from north-western Germany, pp. 161–170 in Advances in Vertebrate Pest Management II, edited by H.-J. Pelz, D. P. Cowan and C. J. Feare. Filander Verlag, Fürth, Germany.
- Prescott, C. V., 1996 A preliminary study of the genetics of resistance in house mice, pp. 83–87 in Proceedings of the 17th Vertebrate Pest Conference, edited by R. M. Timm and A. C. Crabb. University of California, Davis, CA.
- Presnell, S. R., and D. W. Stafford, 2002. The vitamin K-dependent carboxylase. Thromb. Haemost. 87: 937–946. [PubMed] [Google Scholar]
- Rost, S., A. Fregin, V. Ivaskevicius, E. Conzelmann, K. Hoertnagel et al., 2004. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature 427: 537–541. [DOI] [PubMed] [Google Scholar]
- Rowe, F. P., C. J. Plant and A. Bradfield, 1981. Trials of the anticoagulant rodenticides bromadiolone and difenacoum against the house mouse (Mus musculus L.). J. Hyg. 87: 171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski, J. A., C. T. Esmon and J. W. Suttie, 1976. Vitamin K-dependent carboxylase. Requirements of the rat liver microsomal enzyme system. J. Biol. Chem. 251: 2770–2776. [PubMed] [Google Scholar]
- Saunders, G. R., 1978. Resistance to warfarin in the roof rat in Sydney, NSW. Search 9: 39–40. [Google Scholar]
- Siddiqi, Z., and W. D. Blaine, 1982. Anticoagulant resistance in house mice in Toronto, Canada. Environ. Health Rev. 32: 49–51. [Google Scholar]
- Stenflo, J., P. Fernlund, W. Egan and P. Roepstorff, 1974. Vitamin K dependent modifications of glutamic acid residues in prothrombin. Proc. Natl. Acad. Sci. USA 71: 2730–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen, H. H. W., 1995. Warfarin-based rodenticides: mode of action and mechanism of resistance. Pesticide Sci. 43: 73–78. [Google Scholar]
- Thijssen, H. H. W., and H.-J. Pelz, 2001 Sensitive and selective estimation of coumarin resistance by in vitro vitamin K epoxide reductase assay, pp. 181–192 in Advances in Vertebrate Pest Management II, edited by H.-J. Pelz, D. P. Cowan and C. J. Feare. Filander Verlag, Fürth, Germany.
- Twigg, G., 1975 The Brown Rat. David & Charles, Newton Abbot, UK.
- Wallace, M. E., and F. J. MacSwiney, 1976. A major gene controlling warfarin-resistance in the house mouse. J. Hyg. 76: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin, R., and L. F. Martin, 1985. Vitamin K-dependent carboxylation and vitamin K metabolism in liver. Effects of warfarin. J. Clin. Invest. 76: 1879–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 1982 Instructions for determining the susceptibility or resistance of rodents to anticoagulant rodenticides. WHO Vector Biology and Control Series 82, Vol. 843, p. 9.
- Ye, S., S. Dhillon, X. Ke, A. R. Collins and I. N. Day, 2001. An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res. 29: E88–88. [DOI] [PMC free article] [PubMed] [Google Scholar]