Abstract
Objectives. We examined the relationships among maternal smoking in pregnancy, fetal development, and the risk of asthma in childhood.
Methods. We conducted a population-based cohort study, where all 58 841 singleton births were followed for 7 years using nationwide registries.
Results. Maternal smoking increased the risk of asthma (adjusted odds ratio = 1.35; 95% confidence interval = 1.13, 1.62 for high exposure). Low birthweight and preterm delivery increased the risk of asthma at the age of 7, whereas being small for gestational age did not.
Conclusions. Maternal smoking in pregnancy increases the risk of asthma during the first 7 years of life, and only a small fraction of the effect seems to be mediated through fetal growth.
Smoking during pregnancy is a well-established determinant of fetal growth and risk of low birthweight.1 Maternal smoking in pregnancy may influence the development of the fetal respiratory system, as suggested by findings of a relation between maternal smoking in pregnancy and lung function impairment in newborns.2–7 A recent study provided suggestive evidence that low birthweight is a predictor of subsequent childhood asthma.8 There is also some evidence that maternal smoking increases the risk of childhood asthma.9–15
However, little is known about the causal pathway between maternal smoking and the risk of childhood asthma. We therefore assessed the effects of maternal smoking in pregnancy on fetal development and the risk of asthma during the first 7 years of life in a cohort of Finnish children born in 1987. In particular, we examined whether the effect of smoking in pregnancy on the risk of childhood asthma is mediated mainly through reduced fetal growth and duration of pregnancy.
METHODS
Data Sources and Study Population
The source population comprised all children born in Finland in 1987 (n = 60 254). Of these, we focused on the 58 841 singleton births, following them by means of registries for their first 7 years of life.16,17 Childhood health data were gathered from 5 national administrative health registries for the years 1987 through 1994.16
Information on each child’s birthweight and gestational age as well as maternal smoking habits during pregnancy was obtained from the Finnish Medical Birth Registry, which was established in 1987 and is run by the National Research and Development Centre for Welfare and Health. Information on maternal smoking was categorical: nonsmoking, < 10 cigarettes per day, and > 10 cigarettes per day.
Health Outcomes
The primary outcome of interest was asthma. Fetal growth and preterm delivery were used as pregnancy outcomes for the effects of maternal smoking, and determinants and intermediate outcomes for the relation between maternal smoking and asthma. We used 3 different measures of fetal growth: birthweight in g, low birthweight (< 2500 g), and small for gestational age (SGA). Preterm delivery was defined as length of gestation being less than 37 weeks (gestational age is practically always verified at maternity care clinics by ultrasound examination during the 18th week of gestation). SGA was defined according to the Finnish population-based growth curves.18 Presence of asthma was defined on the basis of at least 1 hospitalization due to asthma (ICD-9 code 49319), at least 1 entitlement to free medication due to asthma (ICD-9 code 49319), or at least 1 entitlement to special care support (which can be granted for families with a disabled child or a child who has a long-term illness needing continuous help or surveillance) due to asthma before the age of 7 years.
Relations of Interest
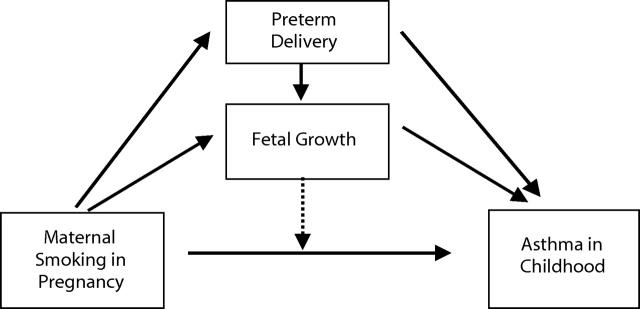
Figure 1 ▶ illustrates our hypothesized causal model, which we studied empirically by estimating the relations of interest. The relation between maternal smoking and the risk of asthma was of primary interest. Maternal smoking was proposed to reduce fetal growth and duration of pregnancy, and low birthweight and preterm delivery were expected to be determinants of childhood asthma. The effect of maternal smoking was expected to be partially mediated by both reduced fetal growth and preterm delivery; they also were proposed to have independent effects. Further, low birthweight and preterm delivery were possible effect modifiers of the relation between maternal smoking and the risk of asthma in the child.
FIGURE 1—
The hypothesized causal model of the effect of maternal smoking in pregnancy on childhood risk of asthma. Solid arrows indicate a causal relationship; the broken arrow indicates effect modification.
Statistical Methods
We estimated the prevalences of the reproductive outcomes with 95% confidence intervals (CIs) based on the binomial distribution, and the mean of birthweight with CIs based on the t distribution. For the dichotomous outcomes, odds ratio (OR) was the measure of effect. We used logistic regression analysis to estimate adjusted ORs for the relations between maternal smoking, pregnancy outcomes, and risk of asthma. The basic adjustment was made using the following core covariates: gender, birth order, maternal age, marital status, and maternal occupation as an indicator of socioeconomic status. Additional adjustment was made for low birthweight and preterm delivery when studying the relations between maternal smoking and asthma, and for maternal smoking when studying the relations between pregnancy outcomes and asthma. Furthermore, we used a separate category for missing information on maternal smoking, which was included in the logistic regression models (results not shown). We studied the relation between maternal smoking and asthma separately among low- and normal-birthweight children.
RESULTS
Table 1 ▶ presents the study population according to maternal smoking in pregnancy. Smoking was related to young age, not being married, and low education. The follow-up rate was 99.9 %, which eliminated the possibility of selection bias.
TABLE 1—
Characteristics of the Study Population, by Maternal Smoking Level During Pregnancy: The Finnish 1987 Birth Cohort
| Nonsmoking (n = 47 848), % | < 10 Cigarettes per Day (n = 5729), % | ≥ 10 Cigarettes per Day (n = 3055), % | Total (n = 58 841a), % | |
| Child’s gender | ||||
| Male | 51.1 | 51.5 | 52.7 | 51.2 |
| Maternal age, y | ||||
| ≤ 19 | 2.4 | 8.1 | 7.1 | 3.2 |
| 20–25 | 19.1 | 30.0 | 27.5 | 20.7 |
| 26–30 | 37.5 | 32.0 | 29.9 | 36.5 |
| 31–35 | 27.1 | 20.9 | 23.0 | 26.2 |
| 36–40 | 11.3 | 7.6 | 10.4 | 10.9 |
| > 40 | 2.5 | 1.4 | 2.1 | 2.4 |
| Parity | ||||
| Nulliparous | 38.8 | 46.6 | 41.1 | 39.7 |
| 1 | 36.2 | 33.4 | 33.7 | 34.9 |
| 2 | 16.6 | 14.2 | 16.1 | 15.9 |
| 3 | 4.8 | 4.1 | 6.1 | 4.7 |
| ≥ 4 | 3.6 | 1.6 | 2.8 | 3.3 |
| Marital status | ||||
| Married | 85.6 | 62.2 | 54.5 | 79.8 |
| Cohabiting | 10.0 | 24.3 | 28.0 | 12.3 |
| Single | 4.3 | 13.3 | 17.3 | 5.9 |
| Maternal occupation | ||||
| Entrepreneur or farmer | 1.7 | 1.7 | 2.0 | 1.7 |
| Upper white collar | 14.9 | 5.4 | 4.4 | 13.5 |
| Lower white collar | 50.5 | 44.1 | 38.7 | 49.1 |
| Blue collar | 18.7 | 33.1 | 38.2 | 21.1 |
| Student | 3.2 | 3.5 | 2.5 | 3.3 |
| Homemaker | 7.4 | 8.9 | 9.7 | 7.6 |
| Other or unknown | 3.6 | 3.3 | 4.5 | 3.7 |
aIncludes 2209 subjects with missing information on maternal smoking.
The risk of low birthweight was 0.031 (95% CI = 0.030, 0.032), the risk of SGA was 0.020 (95% CI = 0.019, 0.021), and the risk of preterm delivery was 0.048 (95% CI = 0.046, 0.049) (Table 2 ▶). A total of 3.4% (95% CI = 3.3, 3.5) of the children were diagnosed with asthma by age 7 years.
TABLE 2—
Mean Birthweight and Risks of Adverse Pregnancy Outcomes, by Maternal Smoking Level During Pregnancy
| Exposure Category | n | Mean Birthweight g (95% CI) | Low Birthweight (< 2500 g) Risk (95% CI) | Small for Gestational Age Risk (95% CI) | Preterm Delivery (< 37 Weeks) Risk (95% CI) |
| No smoking | 47 848 | 3605 (3600, 3610) | 0.027 (0.025, 0.029) | 0.015 (0.014, 0.016) | 0.045 (0.043, 0.048) |
| < 10 Cigarettes per day | 5729 | 3431 (3417, 3446) | 0.045 (0.039, 0.051) | 0.040 (0.034, 0.045) | 0.052 (0.046, 0.058) |
| > 10 Cigarettes per day | 3055 | 3344 (3323, 3365) | 0.065 (0.054, 0.073) | 0.058 (0.047, 0.066) | 0.070 (0.059, 0.079) |
| Total | 58 841 | 3570 (3560, 3580) | 0.031 (0.030, 0.032) | 0.020 (0.019, 0.021) | 0.048 (0.046, 0.049) |
Note. CI = confidence interval.
All the indexes of fetal growth and preterm delivery were related to maternal smoking with a clear exposure–response pattern (Table 2 ▶). The average birthweight was 250 g lower among the newborns of mothers who smoked more than 10 cigarettes per day compared with newborns of nonsmoking mothers. The risk of low birthweight was more than 2-fold, that of preterm delivery approximately 1.5-fold, and that of SGA more than 3-fold in newborns of heavy smokers. These risk estimates were reduced only modestly after adjustment for known determinants of these pregnancy outcomes (Table 3 ▶).
TABLE 3—
Crude and Adjusted Odds Ratios (ORs) for Adverse Pregnancy Outcomes, by Maternal Smoking Level During Pregnancy
| Low Birthweight (< 2500 g) | Small for Gestational Age | Preterm Delivery (< 37 Weeks) | ||||
| Exposure Category | Crude OR (95% CI) | Adjusted OR (95% CI) | Crude OR (95% CI) | Adjusted ORa (95% CI) | Crude OR (95% CI) | Adjusted ORa (95% CI) |
| No smoking | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| < 10 cigarettes per day | 1.68 (1.46, 1.92) | 1.64 (1.43, 1.88) | 2.71 (2.33, 3.15) | 2.59 (2.22, 3.01) | 1.13 (1.00, 1.29) | 1.12 (0.98, 1.26) |
| > 10 cigarettes per day | 2.39 (2.05, 2.79) | 2.23 (1.91, 2.61) | 3.90 (3.30, 4.62) | 3.74 (3.15, 4.43) | 1.55 (1.34, 1.79) | 1.45 (1.26, 1.68) |
Note. CI = confidence interval.
aLogistic regression analysis: adjusted for the core covariates including gender, birth order, maternal age, marital status, and index of socioeconomic status.
Low birthweight and preterm delivery increased the risk of asthma, whereas SGA did not (Table 4 ▶). Adjustment for core covariates slightly increased the effect estimate for low birthweight, whereas additional adjustment for maternal smoking had no influence. The effect estimate for preterm delivery was stable in both types of adjustment. The relation between SGA and the risk of asthma was close to unity.
TABLE 4—
Risks and Crude and Adjusted Odds Ratios (ORs) for Diagnosis of Asthma by the Age of 7, by Adverse Pregnancy Outcome
| Determinants | Risk | Crude OR (95% CI) | Core Covariatesa Adjusted OR (95% CI) | Core Covariates Plus Maternal Smokinga Adjusted OR (95% CI) |
| Low birthweight (< 2500 g) | ||||
| No | 0.033 | 1.00 | 1.00 | 1.00 |
| Yes | 0.058 | 1.69 (1.38, 2.06) | 1.83 (1.50, 2.24) | 1.83 (1.50, 2.24) |
| Small for gestational age | ||||
| No | 0.034 | 1.00 | 1.00 | 1.00 |
| Yes | 0.030 | 0.89 (0.63, 1.24) | 0.93 (0.66, 1.30) | 0.92 (0.66, 1.30) |
| Preterm delivery (< 37 Weeks) | ||||
| No | 0.033 | 1.00 | 1.00 | 1.00 |
| Yes | 0.054 | 1.63 (1.38, 1.94) | 1.64 (1.38, 1.95) | 1.64 (1.38, 1.95) |
Note. CI = confidence interval.
aLogistic regression analysis: adjusted for the core covariates including gender, birth order, maternal age, marital status, and index of socioeconomic status.
The risk of developing asthma during the first 7 years of life was related to maternal smoking (Table 5 ▶). The OR adjusted for gender, birth order, maternal age, and an index of socioeconomic status was 1.23 (95% CI = 1.07, 1.42) for light maternal smoking and 1.35 (95% CI = 1.13, 1.62) for heavy maternal smoking during pregnancy. Further adjustment for birthweight and gestational age decreased the risk estimates only slightly, indicating that the majority of the influence was not due to these pregnancy outcomes. The adjusted OR for any maternal smoking was 1.27 (95% CI = 1.13, 1.43). We further elaborated the relations between maternal smoking and asthma separately in normal- and low-birthweight children. The adjusted OR in normal-birthweight children was 1.24 (95% CI = 1.07, 1.43) for light maternal smoking and 1.34 (95% CI = 1.11, 1.62) for heavy maternal smoking. In low-birthweight children, the corresponding ORs, 1.13 (95% CI = 0.63, 2.04) and 0.95 (95% CI = 0.50, 1.78), indicated no association.
TABLE 5—
Risks and Crude and Adjusted Odds Ratios (ORs) for Diagnosis of Asthma by the Age of 7, by Maternal Smoking Level During Pregnancy
| Exposure Category | Risk (95% CI) | Crude OR (95% CI) | Core Covariatesa Adjusted OR (95% CI) | Core Covariates Plus Birthweighta Adjusted OR (95% CI) | Core Covariates Plus Birthweight Plus Gestational Agea Adjusted OR (95% CI) |
| No smoking | 0.033 | 1.00 | 1.00 | 1.00 | 1.00 |
| < 10 Cigarettes per day | 0.041 (0.036, 0.047) | 1.25 (1.09, 1.44) | 1.23 (1.07, 1.42) | 1.19 (1.03, 1.37) | 1.20 (1.04, 1.38) |
| > 10 Cigarettes per day | 0.045 (0.037, 0.054) | 1.36 (1.14, 1.63) | 1.35 (1.13, 1.62) | 1.29 (1.08, 1.54) | 1.31 (1.09, 1.58) |
Note. CI = confidence interval.
aLogistic regression analysis: adjusted for the core covariates including gender, birth order, maternal age, marital status, and index of socioeconomic status.
DISCUSSION
In our large cohort study, the risks of low birthweight, SGA, and preterm delivery were strongly related to maternal smoking in pregnancy, with a distinct exposure–response pattern. Low birthweight and preterm delivery increased the risk of asthma by 83% and 64%, respectively. The risk of developing asthma during the first 7 years was 25% higher in children whose mother smoked less than 10 cigarettes per day during pregnancy and 36% higher in children whose mothers smoked more than 10 cigarettes per day. The effect of maternal smoking was only slightly reduced when taking into account fetal growth and preterm delivery, indicating that only a small fraction of the effect is mediated through these pregnancy outcomes.
Validity of Results
The source population comprised all children registered as born in Finland in 1987. For the purposes of this study, we focused on singleton births. The Finnish Birth Registry receives information on practically all the children born in Finland.17
We expected to identify almost all diagnosed cases of asthma through the use of registries recording subsidized drug and other treatment. The National Social Insurance Institute covers all residents of Finland and provides 75% reimbursement for asthma medications for those with asthma who fulfill the institute’s diagnostic criteria. This reimbursement right was given for lifetime until the early-mid 1990s, when the system changed so that, for new cases, use of medication is required for 6 months before the patient gets the reimbursement right, and this right can be for only a limited time period. The reimbursement right for asthma is indicated with a number on the patient’s Social Insurance card. Registry information on special support and hospital discharge registration served as complementary information on persons not receiving drug treatment.
The only potential source of selection bias was the lack of information on smoking in pregnancy. This information was missing for only 3.8% of the mothers. Therefore, even in the worst-case scenario, the magnitude of bias would be small. Information on smoking in pregnancy and other relevant factors was collected before the onset of asthma, minimizing the possibility of information bias. We cannot fully exclude the possibility that information on maternal smoking may have influenced the diagnosis made by the physicians, but in our opinion, this should be of minor concern.
We were able to control for a relatively large number of potential confounders. However, due to registry-based data collection, we did not have information on family smoking habits after the birth. Maternal smoking in pregnancy and postnatal exposure to environmental tobacco smoke (ETS) from maternal smoking are strongly correlated. Therefore, effect estimates calculated for smoking in pregnancy probably also include a partial effect of ETS exposure during childhood. The targets for the prevention of asthma include preventing young women from starting to smoke and helping them to quit before or during pregnancy. The smoking of the father and other family members is also to some extent related to maternal smoking in pregnancy and thus to fetal and childhood exposure to ETS.
Synthesis With Existing Knowledge
The relation between parental smoking and childhood asthma has mainly been studied with a focus on postnatal exposure, either combining or separating smoking of both parents, whereas fewer studies have addressed the role of smoking in pregnancy.12,13 Cross-sectional studies focusing on current parental smoking and the presence of asthma are likely to underestimate the studied relation, because parents are likely to reduce smoking at least in the presence of the child after the beginning of early symptoms and signs or diagnosis of asthma in the child. Strachan and Cook12 reported results of a meta-analysis of 8 longitudinal studies of asthma and wheezing, assessing the incidence rather than prevalence of asthma as the outcome. The incidence of asthma or wheezing was related to maternal smoking, but the effect was stronger in the first 5 to 7 years of age (for 4 of the studies, summary OR = 1.31 [95% CI = 1.22, 1.41]) than during the school years (summary OR = 1.13 [95% CI = 1.04, 1.22]). The effect estimate of maternal smoking in the present study, 1.27 (95% CI = 1.13, 1.43), is similar to the summary estimate from the meta-analysis, although the present study did not take into account postnatal exposure or the mother’s exposure to ETS during pregnancy.
Two longitudinal studies and a cross-sectional study of asthma and asthma-like symptoms published after this meta-analysis elaborated the role of in utero exposure.14,15 In a 2-year cohort study of 3754 children born in Oslo, the risk of bronchial obstruction increased in children exposed to ETS compared with unexposed children, with an adjusted OR of 1.6 (95% CI = 1.3, 2.1).14 A similar effect was seen in relation to both maternal and paternal smoking alone. Parental smoking at the time of the child’s birth was used as the measure of exposure to eliminate the selective reduction of exposure due to early symptoms and signs. Results from a cohort study of 499 children of asthmatic or allergic parents from Boston suggest that maternal smoking in pregnancy predominantly determines the development of early symptoms of asthma.15 The risk of repeated wheezing episodes during the first 12 months was related to maternal smoking in pregnancy, with a relative risk of 1.83 (95% CI = 1.12, 3.00). The addition of paternal smoking did not add to the predictive power of maternal smoking.
Evidence of the relative importance of prenatal exposure was provided by a recent cross-sectional study of 5762 California schoolchildren with a retrospective recording of in utero, previous, and current exposure.11 In utero exposure to maternal smoking without subsequent postnatal exposure to ETS was related to the presence of asthma in 4th-, 7th-, and 10th-grade children, with an adjusted OR of 1.8 (95% CI = 1.1, 2.9). In contrast, current or previous exposure to ETS was not associated with asthma risk, but the risk of lifetime wheezing was increased, with an OR of 1.3 (95% CI = 1.1, 1.5).
Our study was, to our knowledge, the first attempt to assess whether fetal growth and preterm delivery lie in the causal pathway between maternal smoking in pregnancy and development of asthma in childhood. As we expected from previous literature,1 maternal smoking was a strong determinant of the studied pregnancy outcomes. Further, all 3 pregnancy outcomes were determinants of asthma. Similar findings were reported recently in a Danish study of 4795 male conscripts,8 in which the adjusted OR of asthma related to low birthweight (< 2500 g) was 1.5 (95% CI = 0.7, 3.1), compared with conscripts with a birthweight of 3001 to 3500 g. However, the adjusted OR related to preterm delivery (< 37 gestational weeks) was 0.8 (95% CI = 0.3, 2.0), compared with conscripts born at term.
Concluding Remarks
Consistent with our prior hypothesis, the risk of asthma in the children we studied was related to maternal smoking in pregnancy. Adjustment for birthweight and gestational age reduced the relation between maternal smoking only slightly, which meant that most of the effect was not mediated through fetal growth or preterm delivery. The fraction explained by fetal growth and preterm delivery would probably be higher when taking into account postnatal maternal smoking and paternal smoking, which are likely to be related to prenatal maternal smoking.
The present findings summarize the harmful effects of maternal smoking both on fetal development and for development of asthma in children. Maternal smoking in pregnancy increases the risk of asthma during the first 7 years of life. Low birthweight and preterm delivery are independent determinants of asthma, but only a small fraction of the effect of maternal smoking on asthma seems to be mediated through fetal growth.
Contributors J. Jaakkola conceived the hypothesis of the study, planned the analyses, participated in interpretation of data, and wrote the article. M Gissler. established the cohort by conducting the registry linkages, performed statistical analyses, and contributed to the planning of the analyses and writing of the article.
Human Participant Protection The data linkage was approved by the National Data Protection Authority and by all the register-keeping organizations.
Peer Reviewed
References
- 1.Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 2.Stick SM, Burton PR, Gurrin L, Sly PD, LeSouef PN. Effects of maternal smoking during pregnancy and a family history of asthma on respiratory function in newborn infants. Lancet. 1996;348:1060–1064. [DOI] [PubMed] [Google Scholar]
- 3.Lødrup Carlsen KC, Jaakkola JJ, Nafstad P, Carlsen KH. In utero exposure to cigarette smoking influences lung function at birth. Eur Respir J. 1997;10:1774–1779. [DOI] [PubMed] [Google Scholar]
- 4.Hoo AF, Henschen M, Dezateux C, Costeloe K, Stocks J. Respiratory function among preterm infants whose mother smoked during pregnancy. Am J Respir Crit Care Med. 1998;158:700–705. [DOI] [PubMed] [Google Scholar]
- 5.Milner AD, Marsh MJ, Ingram DM, Fox GF, Susiva C. Effects of smoking in pregnancy and neonatal lung function. Arch Dis Child Fetal Neonatal Ed. 1999;80:F8–F14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanrahan JP, Tager IB, Segal MR, Tosteson TD, Castile RCT, Van Vunakis H, et al. The effect of maternal smoking during pregnancy on early infant lung function. Am Rev Respir Dis. 1992;145:1129–1135. [DOI] [PubMed] [Google Scholar]
- 7.Young S, Le Souef PN, Geelhoed GC, Stick SM, Turner KJ, Landau LI. The influence of a family history of asthma and parental smoking on airway responsiveness in early infancy. N Engl J Med. 1991;324:1168–1173. [DOI] [PubMed] [Google Scholar]
- 8.Steffensen FH, Sorensen HT, Gillman MW, Rothman KJ, Sabroe S, Fischer P, et al. Low birth weight and preterm delivery as risk factors for asthma and atopic dermatitis in young adult males. Epidemiology. 2000;11:185–188. [DOI] [PubMed] [Google Scholar]
- 9.Infante-Rivard C, Gautrin D, Malo JL, Suissa S. Maternal smoking and childhood asthma. Am J Epidemiol. 1999;150:528–531. [DOI] [PubMed] [Google Scholar]
- 10.Gilliland FD, Li YF, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med. 2001;163:429–436. [DOI] [PubMed] [Google Scholar]
- 11.London SJ, James Gauderman W, Avol E, Rappaport EB, Peters JM. Family history and the risk of early-onset persistent, early-onset transient, and late-onset asthma. Epidemiology. 2001;12:577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strachan DP, Cook DG. Parental smoking and childhood asthma: longitudinal and case-control studies. Thorax. 1998;53:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jaakkola JJ, Jaakkola MS. Effects of environmental tobacco smoke on the respiratory health of children. Scand J Work Environ Health. 2002;28(suppl 2):71–83. [PubMed] [Google Scholar]
- 14.Nafstad P, Kongerud J, Botten G, Hagen JA, Jaakkola JJ. The role of passive smoking in the development of bronchial obstruction during the first 2 years of life. Epidemiology. 1997;8:293–297. [DOI] [PubMed] [Google Scholar]
- 15.Gold DR, Burge HA, Carey V, Milton DK, Platts-Mills T, Weiss ST. Predictors of repeated wheeze in the first year of life: the relative roles of cockroach, birth weight, acute lower respiratory illness, and maternal smoking. Am J Respir Crit Care Med. 1999;160:227–236. [DOI] [PubMed] [Google Scholar]
- 16.Gissler M, Hemminki E, Louhiala P, Järvelin M. Health registers as a feasible means of measuring health status in childhood—a seven-year follow-up of the 1987 Finnish birth cohort. Paediatr Perinat Epidemiol. 1998;12:437–455. [DOI] [PubMed] [Google Scholar]
- 17.Gissler M, Teperi J, Hemminki E, Meriläinen J. Data quality after restructuring a nationwide medical birth registry. Scand J Soc Med. 1995;23:75–80. [DOI] [PubMed] [Google Scholar]
- 18.Pihkala J, Hakala T, Voutilainen P, Raivio K. Characteristics of recent fetal growth curves in Finland [in Finnish]. Duodecim. 1989;105:1540–1546. [PubMed] [Google Scholar]
- 19.International Classification of Diseases, Ninth Revision. Geneva, Switzerland: World Health Organization; 1980.