Abstract
Objectives. We sought to determine the prevalence and predictors of unprotected anal intercourse (UAI) among HIV-positive men who have a single steady male partner with negative or unknown HIV serostatus.
Methods. We analyzed behavioral surveillance data from HIV-positive men who have sex with men (MSM) interviewed in 12 states between 1995 and 2000.
Results. Of 970 HIV-positive MSM who had a single steady male sex partner with negative or unknown serostatus, 278 (29%) reported UAI during the previous year. In a subset of 674 men who were aware of their infection, 144 (21%) had UAI. Among the men who were aware of their infection, factors found to be predictive of UAI in multivariate modeling were heterosexual self-identification, crack cocaine use, no education beyond high school, and a partner with unknown serostatus.
Conclusions. Even after learning of their infection, one fifth of HIV-positive MSM who had a single steady male partner with negative or unknown serostatus engaged in UAI, underscoring the need to expand HIV prevention interventions among these men.
In the past, HIV prevention programs in the United States focused primarily on HIV-negative persons at risk of acquiring HIV infection and concentrated less on the prevention needs of HIV-positive people.1,2 To effectively control the spread of HIV, it is essential that prevention programs expand the services available to HIV-positive people and develop interventions specifically for this population.3–8 In response to this critical need for prevention resources for the HIV-positive population, the Centers for Disease Control and Prevention (CDC) launched the Serostatus Approach to Fighting the Epidemic (SAFE) initiative.2 One of the principal goals of SAFE is to help HIV-positive people adopt and maintain HIV risk-reduction behavior. SAFE also targets the sex partners of HIV-positive people to encourage voluntary testing for those who are unaware of their HIV serostatus and to provide enhanced prevention services to those who are HIV negative.
Behavioral surveillance data from HIV-positive people and their partners will play a crucial role in the development of SAFE activities. These data are essential for designing prevention programs, identifying populations in greatest need of services, and evaluating the effectiveness of interventions.2,6,9 Accordingly, we examined behavioral surveillance data from a multisite interview project to describe unprotected anal intercourse (UAI) among HIV-positive men who have sex with men (MSM) who have a steady male sex partner with negative or unknown HIV serostatus.
MSM are of particular interest because of the disproportionate impact the HIV epidemic has had on this population. In the United States, it is estimated that the number of MSM living with HIV equals or exceeds the number of people living with HIV among all other risk groups combined.10 Furthermore, outbreaks of sexually transmitted diseases (STDs) among MSM who are predominantly HIV-positive in several US cities,11–14 as well as increasing rates of new STDs in these men,15 suggest that sexual risk behavior may be rising in this population. We chose to restrict our analysis to MSM who had a steady male partner. There is a tremendous need for HIV prevention programs to address sexual risk taking within male partnerships.16–19 Multiple studies have found that MSM are more likely to engage in UAI with a steady partner than with a casual one.16,17,20–22 Emotional intimacy within a steady male partnership is known to make consistent condom use extremely difficult,18,23 even for serodiscordant partners.24 As a result, the uninfected partner in these male couples may be at great risk of acquiring HIV infection.
METHODS
All 50 US states report cases of AIDS to the CDC, and 35 states also report cases of HIV infection. The Supplement to HIV/AIDS Surveillance (SHAS) project is an ongoing, cross-sectional interview study of individuals newly reported with HIV infection or AIDS in 11 states and 1 city. SHAS was designed to obtain information that is not collected through routine HIV and AIDS case reporting such as demographic and socioeconomic characteristics, sexual behaviors, substance use, medical care, and social services.
To participate in SHAS, individuals must be 18 years of age or older, speak English or Spanish, and be medically able to complete the 45-minute interview. Depending on the project site, trained interviewers recruit participants by 1 of 2 means. At facility-based sites (Colorado, Connecticut, Florida, Georgia, Michigan, and New Jersey), all individuals newly reported as having AIDS who receive care at selected medical facilities are eligible for interview, and at population-based sites (Arizona, Delaware, Los Angeles, New Mexico, South Carolina, and Washington), all individuals newly reported as having AIDS are eligible. In addition, at 7 of the sites (Arizona, Colorado, Florida, Michigan, New Jersey, New Mexico, and South Carolina), individuals newly reported as having HIV infection who have not developed AIDS are also eligible for interview. Because the proportion of people with AIDS reported to be MSM is very high in both Los Angeles and Washington, these population-based sites select for recruitment a 30% sample of MSM. Of the 21102 persons eligible to participate in SHAS during January 1995 to December 2000 (SHAS questionnaire version 5), 14031 (66%) completed interviews, 3227 (15%) refused, and 3844 (18%) could not be located.
Eligible HIV-positive people are invited to participate in SHAS and must provide informed consent to be interviewed. SHAS has been approved by local and CDC human subjects review boards, and each health department has procedures for ensuring patient confidentiality. Most health departments provide nominal financial reimbursement for participation. The SHAS project has been described in detail previously.9
We analyzed data collected with version 5 of the SHAS questionnaire and selected those men who had had oral or anal sex with another man during the year before interview. Our analysis was further restricted to men whose only sex partner in the past year was a steady male partner with negative or unknown serostatus. We stratified the data by the participant’s knowledge of his own serostatus during the 1-year period in which sexual behavior was examined (the year before interview). Strata were based on the number of months from HIV diagnosis to interview. Men interviewed 1–6 months after HIV diagnosis were aware of their infection for less than half of the year before interview; those interviewed 7–12 months after diagnosis were aware of their infection for most, but not all, of the year before interview; and those interviewed more than 12 months after diagnosis were aware of their infection for the entire year before interview. We focused our analysis on the latter group, because all the men in this group who had engaged in UAI with a partner of negative or unknown serostatus did so despite being aware of their own infection. Given a greater understanding of HIV-positive men who knowingly place their partners at risk, public health programs can more effectively develop prevention services for them.
Our outcome measure was any anal intercourse that was not protected with condoms (UAI) in the year before interview. Men who had anal intercourse but never used condoms during anal sex, or who only sometimes did, were classified as having had UAI. Using the χ2 or the Fisher exact test, we examined associations between UAI and the demographic characteristics listed in Table 1 ▶, as well as the closest CD4+ count within 6 months of interview, current antiretroviral therapy (ART), ART adherence, receipt of regular medical care, noninjection drug use, household income, size of the metropolitan area of residence, geographic region, type of recruitment (facility based or population based), and year of interview. All factors examined in bivariate analysis were then included as explanatory variables in a multiple logistic regression model. Variables with a χ2 P value of greater than .05 were excluded from the model with backward elimination. If we initially limited the explanatory variables in the regression model to factors significantly associated with UAI in bivariate analysis (P < .05), we obtained results that were identical to those of the original model. Because questions on ART and adherence were not added to version 5 of SHAS until March 1997, we could examine only the relationships between UAI and drug therapy and between UAI and drug adherence for men interviewed after this date.
TABLE 1—
Characteristics of 970 HIV-Positive MSM Who Had a Steady Sex Partner With Negative or Unknown HIV Serostatus: Supplement to HIV/AIDS Surveillance Project, 1995–2000
| Characteristic | No. (%) |
| Race/ethnicity | |
| White | 458 (47) |
| Black | 252 (26) |
| Hispanic | 218 (23) |
| AIAN | 13 (1) |
| API | 9 (1) |
| Multiracial | 9 (1) |
| Other | 11 (1) |
| Age, y | |
| 18–29 | 148 (15) |
| 30–39 | 492 (51) |
| 40–49 | 265 (27) |
| ≥50 | 65 (7) |
| Education | |
| < High school | 149 (15) |
| High school | 313 (32) |
| > High school | 503 (52) |
| Unknown | 5 (1) |
| Sexual self-identification | |
| Gay | 811 (84) |
| Bisexual | 118 (12) |
| Heterosexual | 12 (1) |
| Other | 18 (2) |
| Unknown | 11 (1) |
| AIDS diagnosis | |
| No | 164 (17) |
| Yes | 806 (83) |
| HIV serostatus of sex partner | |
| Negative | 720 (74) |
| Unknown | 250 (26) |
| Injection drug usea | |
| No | 957 (99) |
| Yes | 13 (1) |
| Crack cocaine usea | |
| No | 912 (94) |
| Yes | 58 (6) |
| Time from HIV diagnosis to interview, mo | |
| 0–6 | 191 (20) |
| 7–12 | 97 (10) |
| > 12 | 674 (69) |
| Unknown | 8 (1) |
Note. MSM = men who have sex with men; AIAN = American Indian/Alaska Native; API = Asian/Pacific Islander.
aDuring the past year.
RESULTS
From 1995 to 2000, 3939 HIV-positive men who were interviewed in SHAS reported having had oral or anal sex with another man in the past year; 1761 (45%) had 1 steady male sex partner. Of the MSM with 1 steady partner, 791 (45%) had an HIV-positive partner, 720 (41%) had an HIV-negative partner, and 250 (14%) had a partner with unknown HIV serostatus. An unknown serostatus may indicate that the partner had not disclosed his serostatus or that he had not been tested. In total, 970 MSM (55%) had a steady sex partner who was HIV negative or of unknown serostatus, and they were thus at risk of transmitting HIV to their partner.
The characteristics of the 970 HIV-positive MSM with a partner of negative or unknown serostatus are outlined in Table 1 ▶. Slightly more than half of these MSM were men of color, were aged 30–39 years, or had more than a high school education. Nearly all (96%) self-identified as gay or bisexual, and most (83%) had AIDS at the time of interview. About a quarter of the men did not know the HIV serostatus of their steady sex partner. Although a history of injection drug use was fairly common (11%), very few men (1%) had injected drugs in the past year. Similarly, whereas 17% of the men had ever used crack cocaine, only 6% had used it in the past year.
Among the 970 HIV-positive MSM, 765 (79%) had had anal intercourse in the year before interview, and 278 (29%) reported at least 1 episode of UAI. The proportion of men engaging in UAI varied greatly according to the participant’s knowledge of his HIV serostatus during the year before interview. The 191 men who were aware of their infection for less than half of the year before interview were significantly more likely to have engaged in UAI in the past year (52%) than were the 97 men who were aware of their infection for most of the year before interview (30%) or the 674 men who were aware of their infection for the entire year before interview (21%, P < .001).
We focused the remainder of our analysis on the 674 HIV-positive MSM who were aware of their infection for the entire year before interview. The characteristics of these men were nearly identical to those previously described for all 970 men in Table 1 ▶, with the exception that more (87%) had been diagnosed with AIDS and fewer (21%) had a partner with unknown serostatus. One hundred forty-four (21%) of the men reported engaging in UAI in the year before interview; 52 (36%) never used condoms during anal intercourse and 92 (64%) sometimes used condoms. Of the 144, 18 (13%) reported engaging exclusively in insertive UAI, 63 (44%) exclusively in receptive UAI, and 63 (44%) in both insertive and receptive UAI. Although receptive UAI was more common than insertive UAI, most (56%) of the 144 men who engaged in UAI reported at least some insertive UAI.
The proportion of MSM who engaged in UAI rose slightly over time, but this change was not statistically significant. Nineteen percent of the 297 men interviewed from 1995 to 1996 reported UAI during the past year, compared with 23% of the 239 men interviewed from 1997 to 1998 and 24% of the 138 men interviewed from 1999 to 2000 (P= .36). No significant associations were found between UAI and ART or ART adherence. Of 342 men interviewed from 1997 to 2000, 289 (85%) were receiving ART and 53 (15%) were not. The proportion of men receiving ART who reported engaging in UAI (22%) was similar to the proportion of men not receiving therapy who reported engaging in UAI (26%, P= .46). One hundred ninety-three (67%) of the 289 men on ART reported that they always took their medications as prescribed, whereas 96 (33%) reported that they only sometimes did. The proportion of men adhering to ART who reported UAI (21%) was about the same as the proportion of men not fully adhering to ART who reported UAI (24%, P= .53).
In both bivariate and multivariate analyses, 4 factors were found to be predictive of UAI in the HIV-positive MSM who were aware of their infection for the entire year before interview (Table 2 ▶). These factors were no education beyond high school, heterosexual self-identification, a steady sex partner with unknown HIV serostatus, and crack cocaine use during the past year. Although heterosexual self-identification was the strongest predictor of UAI (adjusted odds ratio = 8.3), the confidence limits around this risk estimate are extremely wide and overlap the risk estimates for the other predictive factors. Moreover, very few men identified as heterosexual. Just 5 (3%) of the 144 men who reported UAI identified as heterosexual, whereas 41 (28%) had a partner with unknown serostatus and 85 (59%) had no education beyond high school.
TABLE 2—
Bivariate Analysis and Logistic Regression Model of Factors Associated With UAI Among 664 HIV-Positive MSM Who Were Aware of Their HIV Infection for at Least 1 Year Before Interview
| Factor | No. (%) | Percentage engaging in UAI | OR (95% CI) | AOR (95% CI) |
| Race/ethnicity | ||||
| White | 337 (51) | 19 | 1.0 (reference) | — |
| Black | 166 (25) | 26 | 1.5 (0.9, 2.3) | — |
| Hispanic | 128 (19) | 20 | 1.1 (0.6, 1.8) | — |
| AIAN | 10 (2) | 30 | 1.8 (0.3, 8.1) | — |
| API | 8 (1) | 0 | — (0, 2.5) | — |
| Multiracial | 7 (1) | 43 | 3.1 (0.5, 19.0) | — |
| Other | 8 (1) | 13 | 0.6 (0, 4.8) | — |
| Age, y | ||||
| 18–29 | 87 (13) | 22 | 1.0 (reference) | — |
| 30–39 | 340 (51) | 23 | 1.1 (0.6, 2.0) | — |
| 40–49 | 192 (29) | 19 | 0.8 (0.4, 1.6) | — |
| ≥50 | 45 (7) | 18 | 0.8 (0.3, 2.1) | — |
| Education | ||||
| ≤High school | 300 (45) | 28 | 2.0 (1.4, 3.0) | 1.8 (1.2, 2.7) |
| > High school | 364 (55) | 16 | 1.0 (reference) | 1.0 (reference) |
| Heterosexual self-identification | ||||
| No | 657 (99) | 21 | 1.0 (reference) | 1.0 (reference) |
| Yes | 7 (1) | 71 | 9.6 (1.5, 101.1) | 8.3 (1.5, 47.5) |
| AIDS diagnosis | ||||
| No | 87 (13) | 29 | 1.0 (reference) | — |
| Yes | 577 (87) | 20 | 0.6 (0.4, 1.1) | — |
| HIV serostatus of sex partner | ||||
| Negative | 526 (79) | 19 | 1.0 (reference) | 1.0 (reference) |
| Unknown | 138 (21) | 30 | 1.8 (1.1, 2.8) | 1.8 (1.2, 2.9) |
| Injection drug usea | ||||
| No | 657 (99) | 21 | 1.0 (reference) | — |
| Yes | 7 (1) | 29 | 1.5 (0.1, 9.2) | — |
| Crack cocaine usea | ||||
| No | 627 (94) | 20 | 1.0 (reference) | 1.0 (reference) |
| Yes | 37 (6) | 46 | 3.5 (1.6, 7.2) | 3.1 (1.5, 6.3) |
| Time from HIV diagnosis to interview, y | ||||
| 1–4 | 265 (40) | 26 | 1.0 (reference) | 1.0 (reference) |
| ≥5 | 399 (60) | 18 | 0.7 (0.4, 1.0) | 0.6 (0.4, 0.9) |
Note. Ten HIV-positive MSM who were aware of their infection were excluded because the exact time from HIV diagnosis to interview could not be calculated. UAI = unprotected anal intercourse; MSM = men who have sex with men; OR = odds ratio; CI = confidence interval; AOR = adjusted odds ratio; AIAN = American Indian/Alaska Native; API = Asian/Pacific Islander.
aDuring the past year.
The regression model showed that the risk for UAI did not increase as the time since HIV diagnosis lengthened. Men who had been diagnosed with HIV infection 5 or more years before interview were significantly less likely to report engaging in UAI than were those diagnosed 1–4 years before interview. In addition, important factors not found to be significantly associated with UAI in multivariate analysis were race and ethnicity, an AIDS diagnosis before interview, and most recent CD4+ count within 6 months of interview.
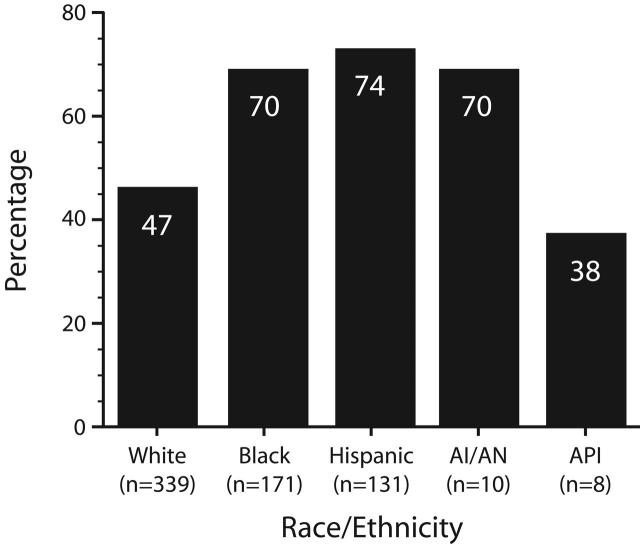
Although race and ethnicity were not independent predictors of UAI in the regression model, Black and Hispanic men were significantly more likely than White men to have 1 or more of the factors found to be predictive of UAI (P < .001, Figure 1 ▶). The proportion of American Indian and Alaska Native men who had at least 1 of the predictive factors (70%) was also greater than that of White men (47%), yet this difference did not quite achieve statistical significance (P = .13). By contrast, the proportion of Asian and Pacific Islander men who had 1 or more of the predictive factors (38%) was slightly less than that of White men, but this difference also was not statistically significant (P = .44).
FIGURE 1—
Proportion of HIV-positive MSM who had 1 or more of the factors (low educational level, heterosexual self-identification, partner with unknown serostatus, and crack use) identified in the regression model as predictive of UAI, by race and ethnicity.
Note. MSM = men who have sex with men; UAI = unprotected anal intercourse; AIAN = American Indian/Alaska Native; API = Asian/Pacific Islander.
DISCUSSION
We found a high prevalence of UAI among the 970 HIV-positive MSM included in our analysis. Even among the 674 men who knew they were infected for the entire year before interview, 21% had engaged in UAI with a steady sex partner of negative or unknown serostatus. Of particular concern, most of those who knew they were infected and engaged in UAI reported insertive UAI—the behavior with the greatest risk of transmitting HIV.25
Several factors, including no education beyond high school, heterosexual self-identification, and crack cocaine use, were predictive of UAI in our study population. MSM with lower educational levels may possess a decreased ability to access or comprehend HIV prevention information. They may also lack the communication skills needed to negotiate safe sex. Misperceptions about sexual risks and poor communication skills have been associated with unsafe sex in MSM.16,26,27 Similarly, less HIV prevention awareness may have contributed to the higher prevalence of UAI among the MSM who identified as heterosexual. These men may not be integrated into the gay community and thus would not benefit from the MSM-specific prevention programs available within this community.28 MSM who identify as heterosexual might also reject prevention messages directed toward MSM who identify as gay.29 Because high-risk sexual behavior in MSM has been repeatedly linked to drug use,30–32 and especially cocaine use,27,33–35 it is not surprising that crack cocaine use was one of the strongest predictors of UAI among the men in our study. Cocaine and other drugs can lower inhibitions and impair judgment regarding sexual risk.27,36 In addition, cocaine may serve as a marker of more severe drug abuse.34
Knowledge of HIV serostatus was a major modifier of sexual risk behavior among the MSM we interviewed. The prevalence of UAI was markedly lower among men who were aware of their infection for a greater proportion of the year before interview, suggesting that the HIV-positive men in our study had greatly reduced their sexual risk behavior after they learned of their infection. This finding is consistent with results from other studies that have shown that HIV-positive MSM decrease their risk behavior after learning that they are infected.37–40
Knowledge of their steady partner’s HIV serostatus also influenced the men’s sexual risk behavior. Compared with men whose partner’s serostatus was unknown, men who knew that their partner was HIV negative were significantly less likely to have engaged in UAI. Men who know that their partner is not infected with HIV may feel a greater personal responsibility to protect him from infection. HIV-positive MSM with higher levels of personal responsibility have been found to be less likely to engage in UAI.41 By contrast, HIV-positive men who do not know their partner’s serostatus or who have a partner who has not been tested might assume that he is already infected and consequently engage in UAI. Furthermore, because some HIV-positive MSM do not disclose their serostatus even to their steady partners,42,43 a man’s lack of knowledge of his partner’s serostatus could indicate a failure to discuss serostatus with him. Unprotected sex has been shown to be more prevalent among HIV-positive men who do not disclose their serostatus to their partners.44,45
Although HIV-positive MSM may modify their sexual risk behavior after learning of their infection, the possibility exists that, over time, they might eventually resume high-risk activities.46,47 However, we found no evidence of such a relapse. In fact, men who had known of their HIV infection for 5 or more years were less likely to have engaged in UAI than those who had known of their HIV infection for a shorter period. Because the probability of having at least 1 episode of UAI declines as the number of acts of anal intercourse falls,16 it is possible that the lower prevalence of UAI among men who had known of their HIV infection for 5 or more years resulted from a decrease in the frequency of sexual activity. Although data on the frequency of anal intercourse were not collected in the version of SHAS we used, we did control for factors that could account for a decline in the frequency of sex, such as older age, a lower CD4+ count, or an AIDS diagnosis, and still found that men who had known of their HIV infection for 5 or more years were significantly less likely to have reported UAI.
There is growing concern that sexual risk among MSM may have increased after 1996, when highly active antiretroviral therapy (HAART) became widely available.6,8 Some MSM may be less fearful of HIV infection as a result of treatment advances, or they may believe that HIV-positive men are unlikely to transmit HIV if they are receiving drug therapy.32,48–52 However, we did not find a substantial rise in UAI after 1996. Although a slightly higher proportion of the HIV-positive MSM interviewed in our study from 1997 to 2000 reported UAI than did those interviewed from 1995 to 1996, the difference was not statistically significant. Moreover, UAI was less prevalent among the men on ART and among those who adhered to treatment, not more prevalent, as one would expect if the men believed they were less likely to transmit HIV while undergoing therapy. If optimism about treatment benefits has truly led to an increase in UAI in the post-HAART era, the effect has probably been modest. Results from studies evaluating beliefs about HAART invariably show that only a very small proportion of MSM report UAI in association with treatment optimism.32,51,52
Some limitations apply to our results. The men included in our analysis may not be representative of all HIV-positive MSM in the United States. Nevertheless, SHAS is a multi-site project conducted throughout the country, and our study population was composed of men representing a broad range of age, racial/ethnic, and socioeconomic groups. As with all data collected through personal interview, our findings are subject to social desirability bias. Some HIV-positive men who engaged in UAI may have denied doing so when interviewed because they considered reporting UAI to be a socially undesirable response. Accordingly, our results may underestimate the true prevalence of UAI in our study population. Although our data included global measures of alcohol and drug use, we lacked information on substance use in conjunction with sexual activity. Substance use before or during sex has been closely linked to UAI in MSM31–35 and may be a major contributor to relapses of unsafe sex.53 This information would have been especially useful for examining predictors of UAI in men who used condoms inconsistently. Because version 5 of SHAS did not collect sexual risk behavior for each sex partner separately, we could not differentiate UAI that occurred with a steady partner from UAI that occurred with a nonsteady partner. Therefore, to measure UAI between pairs of steady partners, it was necessary for us to exclude men who had both steady and nonsteady partners from our analysis.
The high prevalence of UAI in this large, diverse sample of HIV-positive MSM with a steady sex partner of negative or unknown serostatus underscores the need to expand HIV prevention interventions among these men. Prevention activities should be incorporated into all health services that HIV-positive MSM routinely receive, such as primary care, case management, mental health counseling, and substance abuse treatment.1,33,54,55 A more holistic approach to the health care and prevention needs of HIV-positive men would address comorbid conditions, such as substance abuse and mental illness, that can impede effective behavioral change.31 Prevention programs should also help HIV-positive MSM develop the communication skills needed to disclose their HIV serostatus to their partner and to negotiate safe sex with him. In addition, interventions should emphasize the importance of counseling and voluntary HIV testing for an HIV-positive man’s steady sex partner. MSM who have lower educational levels or who identify as heterosexual require more intensive prevention outreach and intervention. This is true for some racial/ethnic minority MSM as well. Although race and ethnicity were not independent predictors of UAI in our study population, the factors found to be predictive of UAI were much more prevalent among Black, Hispanic, and American Indian/Alaska Native men.
As the CDC implements the SAFE program to enhance HIV prevention activities for HIV-positive individuals and their sex partners,2 it is critical that MSM not be neglected. A review of the CDC’s prevention funding for 2000 found that 15% of funds targeted MSM,56 despite the fact that MSM make up the majority of people living with HIV in the United States10 and for the largest proportion (> 40%) of new infections each year.6 Public health programs must renew their commitment to controlling the HIV epidemic in the MSM population and prioritize prevention efforts for these men, particularly MSM who are HIV positive and have male partners with negative or unknown serostatus.
Acknowledgments
We thank the project officers and interviewers of the Supplement to HIV/AIDS Surveillance (SHAS) Project Group. We also thank Danni Daniels, Lisa Lee, and Richard Wolitski for their insightful comments and Lynne Stockton for her editorial review.
Human Participant Protection The SHAS Project has been approved by local and Centers for Disease Control and Prevention (CDC) human subjects review boards.
Contributors P. Denning originated the analysis, analyzed the data, and led the writing. M. Campsmith supervised the study. Both authors helped to conceptualize ideas, interpret findings, and review drafts of the article.
Peer Reviewed
References
- 1.Kalichman SC, Kelly JA, Rompa D. Continued high-risk sex among HIV seropositive gay and bisexual men seeking HIV prevention services. Health Psychol. 1997;16:369–373. [DOI] [PubMed] [Google Scholar]
- 2.Janssen RS, Holtgrave DR, Valdiserri RO, Shepherd M, Gayle HD, De Cock KM. The serostatus approach to fighting the HIV epidemic: prevention strategies for infected individuals. Am J Public Health. 2001;91:1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Godin G, Savard J, Kok G, Fortin C, Boyer R. HIV seropositive gay men: understanding adoption of safe sexual practices. AIDS Educ Prev. 1996;8:529–545. [PubMed] [Google Scholar]
- 4.Marks G, Burris S, Peterman TA. Reducing sexual transmission of HIV from those who know they are infected: the need for personal and collective responsibility. AIDS. 1999;13:297–306. [DOI] [PubMed] [Google Scholar]
- 5.Semple SJ, Patterson TL, Grant I. Psychosocial predictors of unprotected anal intercourse in a sample of HIV positive gay men who volunteer for a sexual risk reduction intervention. AIDS Educ Prev. 2000;12: 416–430. [PubMed] [Google Scholar]
- 6.Wolitski RJ, Valdiserri RO, Denning PH, Levine WC. Are we headed for a resurgence of the HIV epidemic among men who have sex with men? Am J Public Health. 2001;91:883–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levi J. An HIV agenda for the new administration. Am J Public Health. 2001;91:1015–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stolte IG, Coutinho RA. Risk behaviour and sexually transmitted diseases are on the rise in gay men, but what is happening with HIV? Curr Opin Infect Dis. 2002;15:37–41. [DOI] [PubMed] [Google Scholar]
- 9.Buehler JW, Diaz T, Hersh BS, Chu SY. The Supplement to HIV/AIDS Surveillance Project: an approach for monitoring HIV risk behaviors. Public Health Rep. 1996;111:133–137. [PMC free article] [PubMed] [Google Scholar]
- 10.Karon JM, Rosenberg PS, McQuillan G, Khare M, Gwinn M, Petersen LR. Prevalence of HIV infection in the United States, 1984 to 1992. JAMA. 1996;276: 126–131. [PubMed] [Google Scholar]
- 11.Ashford DA, Townes JM, Castle J, et al. An outbreak of gonorrhea among gay men in Portland: risk factors for infection. International Conference on Emerging Infectious Diseases, Atlanta, Ga, March 8–11, 1998, abstract P-12.7.
- 12.Williams LA, Klausner JD, Whittington WLH, Handsfield HH, Celum C, Holmes KK. Elimination and reintroduction of primary and secondary syphilis. Am J Public Health. 1999;89:1093–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klausner JD, Wolf W, Fischer-Ponce L, Zolt I, Katz MH. Tracing a syphilis outbreak through cyber-space. JAMA. 2000;284:447–449. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Outbreak of syphilis among men who have sex with men—Southern California, 2000. MMWR Morb Mortal Wkly Rep. 2001;50:117–120. [PubMed] [Google Scholar]
- 15.Do AN, Hanson DL, Dworkin MS, Jones JL, Adult and Adolescent Spectrum of HIV Disease Project. Risk factors for and trends in gonorrhea incidence among persons infected with HIV in the United States. AIDS. 2001;15:1149–1155. [DOI] [PubMed] [Google Scholar]
- 16.Hays RB, Kegeles SM, Coates TJ. Unprotected sex and HIV risk taking among young gay men within boyfriend relationships. AIDS Educ Prev. 1997;9: 314–329. [PubMed] [Google Scholar]
- 17.Davidovich U, de Wit JBF, Stroebe W. Assessing sexual risk behaviour of young gay men in primary relationships: the incorporation of negotiated safety and negotiated safety compliance. AIDS. 2000;14:701–706. [DOI] [PubMed] [Google Scholar]
- 18.Seal DW, Kelly JA, Bloom FR, et al. HIV prevention with young men who have sex with men: what young men themselves say is needed. AIDS Care. 2000;12:5–26. [DOI] [PubMed] [Google Scholar]
- 19.Elford J, Bolding G, Maguire M, Sherr L. Gay men, risk and relationships. AIDS. 2001;15: 1053–1055. [DOI] [PubMed] [Google Scholar]
- 20.Dawson JM, Fitzpatrick RM, Reeves G, et al. Awareness of sexual partners’ HIV status as an influence upon high-risk sexual behaviour among gay men. AIDS. 1994;8:837–841. [PubMed] [Google Scholar]
- 21.Hoff CC, Coates TJ, Barrett DC, Collette L, Ekstrand M. Differences between gay men in primary relationships and single men: implications for prevention. AIDS Educ Prev. 1996;8:546–559. [PubMed] [Google Scholar]
- 22.Elford J, Bolding G, Maguire M, Sherr L. Sexual risk behaviour among gay men in a relationship. AIDS. 1999;13:1407–1411. [DOI] [PubMed] [Google Scholar]
- 23.McLean J, Boulton M, Brookes M, et al. Regular partners and risky behaviour: why do gay men have unprotected intercourse? AIDS Care. 1994;6:331–341. [DOI] [PubMed] [Google Scholar]
- 24.Remien RH, Carballo-Diéguez A, Wagner G. Intimacy and sexual risk behaviour in serodiscordant male couples. AIDS Care. 1995;7:429–438. [DOI] [PubMed] [Google Scholar]
- 25.Vittinghoff E, Douglas J, Judson F, McKirnan D, MacQueen K, Buchbinder SP. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am J Epidemiol. 1999;150: 306–311. [DOI] [PubMed] [Google Scholar]
- 26.Semple SJ, Patterson TL, Grant I. The sexual negotiation behavior of HIV-positive gay and bisexual men. J Consult Clin Psychol. 2000;68:934–937. [PubMed] [Google Scholar]
- 27.Molitor F, Facer M, Ruiz JD. Safer sex communication and unsafe sexual behavior among young men who have sex with men in California. Arch Sex Behav. 1999;28:335–343. [DOI] [PubMed] [Google Scholar]
- 28.Ratti R, Bakeman R, Peterson JL. Correlates of high-risk sexual behaviour among Canadian men of South Asian and European origin who have sex with men. AIDS Care. 2000;12:193–202. [DOI] [PubMed] [Google Scholar]
- 29.Whitehead TL. Urban low-income African American men, HIV/AIDS, and gender identity. Med Anthropol Q. 1997;11:411–447. [DOI] [PubMed] [Google Scholar]
- 30.Mattison AM, Ross MW, Wolfson T, Franklin D, HNRC Group. Circuit party attendance, club drug use, and unsafe sex in gay men. J Subst Abuse. 2001;13: 119–126. [DOI] [PubMed] [Google Scholar]
- 31.Stall RD, Paul JP, Barrett DC, Crosby GM, Bein E. An outcome evaluation to measure changes in sexual risk-taking among gay men undergoing substance use disorder treatment. J Stud Alcohol. 1999;60:837–845. [DOI] [PubMed] [Google Scholar]
- 32.Vanable PA, Ostrow DG, McKirnan DJ, Taywa-ditep KJ, Hope BA. Impact of combination therapies on HIV risk perceptions and sexual risk among HIV-positive and HIV-negative gay and bisexual men. Health Psychol. 2000;19:134–145. [DOI] [PubMed] [Google Scholar]
- 33.Benotsch EG, Kalichman SC, Kelly JA. Sexual compulsivity and substance use in HIV-seropositive men who have sex with men: prevalence and predictors of high-risk behaviors. Addict Behav. 1999;24: 857–868. [DOI] [PubMed] [Google Scholar]
- 34.McNall M, Remafedi G. Relationship of amphetamine and other substance use to unprotected intercourse among young men who have sex with men. Arch Pediatr Adolesc Med. 1999;153:1130–1135. [DOI] [PubMed] [Google Scholar]
- 35.Purcell DW, Parsons JT, Halkitis PN, Mizuno Y, Woods WJ. Substance use and sexual transmission risk behavior of HIV-positive men who have sex with men. J Subst Abuse. 2001;13:185–200. [DOI] [PubMed] [Google Scholar]
- 36.Wilson T, DeHovitz JA. STDs, HIV, and crack cocaine: a review. AIDS Patient Care STDS. 1997;11: 62–66. [DOI] [PubMed] [Google Scholar]
- 37.Fox R, Odaka NJ, Brookmeyer R, Polk BF. Effect of HIV antibody disclosure on subsequent sexual activity in homosexual men. AIDS. 1987;1:241–246. [PubMed] [Google Scholar]
- 38.McCusker J, Stoddard AM, Mayer KH, Zapka J, Morrison C, Saltzman SP. Effects of HIV antibody test knowledge on subsequent sexual behaviors in a cohort of homosexually active men. Am J Public Health. 1988; 78:462–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKusick L, Coates TJ, Morin SF, Pollack L, Hoff C. Longitudinal predictors of reductions in unprotected anal intercourse among gay men in San Francisco: the AIDS Behavioral Research Project. Am J Public Health. 1990;80:978–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higgins DL, Galavotti C, O’Reilly KR, et al. Evidence for the effects of HIV antibody counseling and testing on risk behaviors. JAMA. 1991;266: 2419–2429. [PubMed] [Google Scholar]
- 41.Wolitski R, Gomez CA, Parsons JT, Ambrose T, Remien RH. HIV-seropositive men’s perceived responsibility for preventing the transmission of HIV to others. XII World AIDS Conference, Geneva, Switzerland, June 28–July 3, 1998, abstract 23361.
- 42.Stempel RR, Moulton JM, Moss AR. Self-disclosure of HIV-1 antibody test results: the San Francisco General Hospital Cohort. AIDS Educ Prev. 1995;7: 116–123. [PubMed] [Google Scholar]
- 43.Wolitski RJ, Rietmeijer CAM, Goldbaum GM, Wilson RM. HIV serostatus disclosure among gay and bisexual men in four American cities: general patterns and relation to sexual practices. AIDS Care. 1998;10: 599–610. [DOI] [PubMed] [Google Scholar]
- 44.De Rosa CJ, Marks G. Preventive counseling of HIV-positive men and self-disclosure of serostatus to sex partners: new opportunities for prevention. Health Psychol. 1998;17:224–231. [DOI] [PubMed] [Google Scholar]
- 45.Kalichman SC, Nachimson D. Self-efficacy and disclosure of HIV-positive serostatus to sex partners. Health Psychol. 1999;18:281–287. [DOI] [PubMed] [Google Scholar]
- 46.Stall R, Ekstrand M, Pollack L, McKusick L, Coates TJ. Relapse from safer sex: the next challenge for AIDS prevention efforts. J Acquir Immune Defic Syndr. 1990; 3:1181–1187. [PubMed] [Google Scholar]
- 47.Adib SM, Joseph JG, Ostrow DG, Tal M, Schwartz SA. Relapse in sexual behavior among homosexual men: a 2-year follow-up from the Chicago MACS/CCS. AIDS. 1991;5:757–760. [PubMed] [Google Scholar]
- 48.Dilley JW, Woods WJ, McFarland W. Are advances in treatment changing views about high-risk sex? N Engl J Med. 1997;337:501–502. [DOI] [PubMed] [Google Scholar]
- 49.Kelly JA, Hoffmann RG, Rompa D, Gray M. Protease inhibitor combination therapies and perceptions of gay men regarding AIDS severity and the need to maintain safer sex. AIDS. 1998;12:F91–F95. [DOI] [PubMed] [Google Scholar]
- 50.Remien RH, Wagner G, Carballo-Diéguez A, Dolezal C. Who may be engaging in high-risk sex due to medical treatment advances? AIDS. 1998;12: 1560–1561. [DOI] [PubMed] [Google Scholar]
- 51.Van de Ven P, Kippax S, Knox S, Prestage G, Crawford J. HIV treatments optimism and sexual behaviour among gay men in Sydney and Melbourne. AIDS. 1999;13:2289–2294. [DOI] [PubMed] [Google Scholar]
- 52.Elford J, Bolding G, Maguire M, Sherr L. Combination therapies for HIV and sexual risk behavior among gay men. J Acquir Immune Defic Syndr. 2000; 23:266–271. [DOI] [PubMed] [Google Scholar]
- 53.Goodroad BK, Kirksey KM, Butensky E. Bareback sex and gay men: an HIV prevention failure. J Assoc Nurses AIDS Care. 2000;11:29–36. [DOI] [PubMed] [Google Scholar]
- 54.Marks G, Ruiz MS, Richardson JL, et al. Anal intercourse and disclosure of HIV infection among sero-positive gay and bisexual men. J Acquir Immune Defic Syndr. 1994;7:866–869. [PubMed] [Google Scholar]
- 55.Kalichman SC, Rompa D, Cage M, et al. Effectiveness of an intervention to reduce HIV transmission risks in HIV-positive people. Am J Prev Med. 2001;21: 84–92. [DOI] [PubMed] [Google Scholar]
- 56.Valdiserri RO, Ogden L, Janssen R, Onorato I. Aligning the US HIV prevention budget with national goals to reduce new infections by half. XIV International AIDS Conference, Barcelona, Spain, July 7–12, 2002, abstract WeOrG1369.