Abstract
Objective
Symptoms of borderline personality disorder (BPD) may reflect distinct breakdowns in the integration of posterior and frontal brain networks. We used a high temporal resolution measure (40-Hz gamma phase synchrony) of brain activity to examine the connectivity of brain function in BPD.
Methods
Unmedicated patients with BPD (n = 15) and age-and sex-matched healthy control subjects (n = 15) undertook a task requiring discrimination of salient from background tones. In response to salient stimuli, the magnitude and latency of peak gamma phase synchrony for early (0–150 ms post stimulus) and late (250–500 ms post stimulus) phases were calculated for frontal and posterior regions and for left and right hemispheres. We recorded skin conductance responses (SCRs) and reaction time (RT) simultaneously to examine the contribution of arousal and performance.
Results
Compared with controls, patients with BPD had a significant delay in early posterior gamma synchrony and a reduction in right hemisphere late gamma synchrony in response to salient stimuli. Both SCR onset and RT were also delayed in BPD, but independently from differences in synchrony. The delay in posterior synchrony was associated with cognitive symptoms, and reduced right hemisphere synchrony was associated with impulsivity.
Conclusions
These findings suggest that distinct impairments in the functional connectivity of neural systems for orienting to salient input underlie core dimensions of cognitive disturbance and poor impulse control in BPD.
Medical subject headings: brain, borderline personality disorder, cognition, electroencephalography, gamma synchrony
Abstract
Objectif
Des symptômes de trouble limite de la personnalité (TLP) peuvent refléter des ruptures distinctes de l'intégration des réseaux cérébraux postérieur et frontal. Nous avons utilisé une mesure à haute résolution temporale (synchronisme de phase gamma de 40 Hz) de l'activité cérébrale pour étudier la connectivité des fonctions cérébrales dans des cas de TLP.
Méthodes
Des patients non médicamentés et ayant un TLP (n = 15), jumelés selon l'âge et le sexe avec des sujets témoins en bonne santé (n = 15), ont entrepris une tâche exigeant la discrimination des tons de premier plan de ceux de l'arrière-plan. En réponse aux stimuli de premier plan, on a calculé l'ordre de grandeur et la latence du synchronisme de pointe en phase gamma des phases précoce (0–150 ms après le stimulus) et tardive (250–500 ms après le stimulus) pour les régions frontale et postérieure et les hémisphères gauche et droit. Nous avons consigné simultanément des réponses de la conduction cutanée (RCC) et le temps de réaction (TR) pour analyser la contribution de l'éveil et le rendement.
Résultats
Comparativement aux sujets témoins, les patients qui avaient un TLP présentaient un retard important du synchronisme gamma postérieur précoce et une réduction du synchronisme gamma tardif de l'hémisphère droit en réponse à des stimuli de premier plan. L'apparition des RCC et le TR étaient aussi retardés chez les sujets qui avaient un TLP, mais indépendamment des différences au niveau du synchronisme. On a établi un lien entre le retard du synchronisme postérieur et des symptômes cognitifs, ainsi qu'entre une réduction du synchronisme de l'hémisphère droit et l'impulsivité.
Conclusions
Ces résultats indiquent que des déficits distincts de la connectivité fonctionnelle des systèmes neuraux en ce qui a trait à l'orientation des intrants de premier plan sous-tendent des dimensions fondamentales de troubles de la cognition et un mauvais contrôle des impulsions dans des cas de TLP.
Introduction
Borderline personality disorder (BPD) is characterized by a pervasive pattern of instability in interpersonal relationships, self-image and affect, together with marked impulsivity. It has a debilitating impact on the individual, leading to suicidal and parasuicidal behaviours, severe emotional disturbances and admission to hospital.1 Up to 70% of patients with BPD have experienced early trauma, including long-term childhood sexual and physical abuse, which acts as a major vulnerability factor for this disorder.2,3
Although BPD has been described comprehensively in the psychoanalytical literature,4,5 it has received less attention with regard to cognitive behavioural approaches.6 Given its complex phenotype and lack of obvious structural brain changes, BPD presents a particular challenge for the emerging focus on integrating psychiatry and neuroscience7,8 and the corresponding identification of links between behaviour and brain function. In terms of behaviour, the clinical symptoms of BPD reflect a core breakdown of affective regulation and impulse control and cognitive distortion.
Prefrontal brain systems have received the most attention as the likely site of affect dysregulation and associated interpersonal disturbances in BPD.5,9–12 Drawing on Hughlings Jackson's theory of mind, Meares et al4,13 proposed an integrative model of BPD, in which the experience of trauma may cause subtle arrests in the development of prefrontal function, which produce a breakdown in the regulation of affect and interpersonal functions. The prefrontal cortex (particularly the medial portion) provides the top-down feedback necessary for intention and motivated behaviour by generating multiple options about the outcomes of particular choices and the fine-tuning required for the subtlety of more abstract or “secondary” emotional states.14 Consistent with this view, lesions of the medial prefrontal cortex (MPFC) in childhood have been found to impair the regulation and interpretation of emotion necessary for the “higher level” operations of empathy, pro-social behaviour and interpersonal function in adulthood.10 The peak period of maturation in both the prefrontal cortex and behavioural regulation is over childhood and adolescence, when the abusive events related to BPD also typically occur. Indeed, adults with BPD have been found to show abnormal MPFC-generated neural activity, which is more characteristic of younger individuals.15 Patients with BPD also show disturbances in the resting metabolism of frontal networks.16
In a complementary conceptualization of BPD, Posner et al12,17 focused on the role of the prefrontal cortex (particularly the anterior cingulate of the MPFC) in executive attention. Using cognitive testing, patients with BPD were found to have an impairment in executive control, which was associated with high levels of negative emotionality.12 The link between prefrontal control (or regulation) and negative emotion may reflect the strong structural and functional connections between the MPFC and the limbic amygdala, which has been implicated in negative emotion processing and arousal.18 Related evidence for amygdala hyperfunction in BPD19 is consistent with a lack of top-down prefrontal regulation.
It is important to note that these previous studies of prefrontal function in BPD have revealed associations between prefrontal impairments and emotional disturbances in BPD using cognitive tasks that are not designed to elicit emotional responses. The findings, therefore, add weight to the view that breakdowns in higher-level integration and regulation of information in BPD may have emotion-related consequences. Another line of research suggests that an additional deficit in BPD may involve lack of integration within posterior parietal networks.20 Parietal abnormalities in BPD have been revealed using functional neuroimaging.21 Neuropsychological studies have also reported subtle visuospatial perception and learning deficits in BPD, associated with the parietal lobe and reflecting an inability to distinguish relevant from extraneous information.22,23 Similarly, in electrophysiology studies, patients with BPD have been found to show abnormally slowed and reduced responses over the parietal cortex in a task requiring discrimination of task-relevant from irrelevant information.24–26 Abnormalities were observed for the N200 and P300 ERP components elicited by this discrimination.27 Parietal-dependent deficits in BPD have been related to the presence of behavioural dyscontrol, suggesting that parietal disturbances may contribute to symptoms of cognitive disturbance and impulsivity (rather than affect regulation).
In this study, we examined the view that the core neural basis of BPD may be a breakdown in the effective functional connectivity of cortical networks, but that different symptoms may be produced by lack of connectivity in frontal as opposed to posterior cortical networks. We examined high temporal resolution functional connectivity occurring at the millisecond timescale of cognition. Connectivity was elicited in response to an oddball task, which requires a selective response to salient stimuli (infrequent high-pitched tones presented among frequently occurring low-pitched tones).
Two new issues in relation to BPD were examined in the study. First, we employed a neurophysiological measure (40-Hz gamma phase synchrony) that examines functional brain connections and disconnections on a rapid timescale. Second, we examined connectivity (or synchrony) within posterior as well as frontal brain regions, with regard to the core symptoms of BPD. Whereas previous studies have investigated the relation between BPD symptoms and neurophysiological techniques (such as event-related potentials [ERPs]), the gamma measure of synchronous brain activity has not been used.
Gamma phase synchrony refers to activity within the high-frequency gamma electroencephalography (EEG) band (cycling at 40 times per second), which occurs synchronously and in phase across multiple brain sites. It was first demonstrated using depth recording in animals that synchronization, rather than a simple increase in firing rate, is the critical factor in achieving a coherent perception of salient stimuli and the selection of appropriate responses.28 We have developed a mathematical method for extracting and analyzing the synchronicity of gamma activity across multiple sites using scalp-recorded EEG in human subjects.29 Task-relevant stimuli in the oddball task have been found to elicit 2 peaks of gamma synchronization: an early “gamma 1” peak (that occurs around stimulus onset) and a late “gamma 2” peak (250–500 ms post stimulus). Previous studies of clinical groups, such as subjects with schizophrenia, have highlighted the utility of gamma phase synchrony in differentiating specific disturbances in functional connectivity.30,31 The late peak in gamma synchrony has been found to be related to both behavioural performance and changes in phasic arousal, in both patient and healthy comparison groups.32 Moreover, patients with BPD have been found to show reduced skin conductance arousal.11 For these reasons, we recorded skin conductance responses (SCRs) and behavioural data (accuracy and reaction time [RT]) simultaneously with gamma synchrony data to address the possible contribution of variation in performance and arousal to differences between patients with BPD and healthy controls.7
It was predicted that, relative to matched healthy control subjects, patients with BPD would show a lack of frontal and posterior integration, indexed by gamma synchrony. We expected frontal and parietal disconnections to distinguish core symptoms of BPD, but to be independent of variations in performance or arousal.
Methods
Fifteen patients with a diagnosis of borderline personality disorder (4 males, 11 females; mean age 28.8 yr) were recruited from the Borderline Personality Disorder Treatment program at Westmead Hospital, Sydney, Australia, and 15 age-and sex-matched healthy comparison subjects were recruited from the general Western Sydney community. This study was conducted at the Brain Dynamics Centre, Westmead Hospital and University of Sydney. Diagnosis of Borderline Personality Disorder was confirmed according to the International Statistical Classification of Diseases and Related Health Problems (ICD-10)33 criteria by 2 independent psychiatrists, blind to the purpose of the study. All subjects with BPD were unmedicated at the time of testing and were not receiving any other form of treatment. They had in each case entered a psychotherapy program at Westmead Hospital but participated in this study before starting psychotherapeutic treatment. Psychotherapy typically commenced soon after testing. Control subjects were screened for history of psychiatric illness (themselves or first-degree relative) and treatment with psychiatric medication using the Composite International Diagnostic Interview (CIDI)34 and the Westmead Hospital Clinical Information Base questionnaire (WHCIB).35 Exclusion criteria for subjects with BPD and healthy subjects were recent history of substance abuse, significant head injury, epilepsy, other neurological abnormalities and developmental disability (assessed using Section M from the CIDI and the WHCIB). Subjects in both groups were within the normal range for intelligence (90–110) as assessed by the NART (New Adult Reading Test).36
The Revised Diagnostic Interview for Borderlines (DIB-R37) was used by the BPD treatment program clinicians to quantify the core features of this disorder: affective disturbance (or dysregulation), impulsive behaviour, relationship disturbance and cognitive disturbance. Patients with BPD had a mean score of 7.9 (standard deviation [SD] 2.0) on this scale, reflecting a high level of symptoms.
All subjects were asked to refrain from smoking or drinking caffeinated beverages for at least 2 hours before the testing. There was no difference between groups with regard to nicotine dependence. After a complete description of the study was provided to all subjects, written, informed consent was obtained in accordance with the National Health and Medical Research Council ethical guidelines. Ethics approval was provided by the Western Sydney Area Health Service Human Research Ethics Committee.
Behavioural task
We used a conventional auditory oddball paradigm with 40 target (1500 Hz) and 225 background (1000 Hz) tones. Target tones represented 15% of the total stimuli and background tones, 85%. The tones were presented binaurally through headphones for 50 ms, with a 10-ms rise and fall time at 80 db above the hearing threshold, determined individually before the presentation of the oddball stimuli. The interstimulus interval was 1.3 s. All tones were presented pseudorandomly with the constraint that no successive target tones were presented. Testing was undertaken in a sound-and light-attenuated room. Subjects were instructed to ignore background tones and to press a reaction time button with the middle finger of both hands (to counterbalance motor effects) in response to a target tone. In instructions to subjects, the importance of speed and accuracy were emphasized equally. To limit EOG (electro-oculography) contamination, subjects were instructed to look at a small dot on a computer monitor placed 60 cm away from the subject's face during the auditory oddball task.
Gamma data acquisition
EEG activity was recorded from 19 scalp electrode sites according to the International 10–20 EEG system, using an electrode cap. Linked earlobes served as reference. Horizontal eye movement potentials were recorded using 2 electrodes, placed 1 cm lateral to the outer canthus of each eye. Vertical eye movement potentials were recorded using 2 electrodes placed on the middle of the supraorbital and infraorbital regions of the left eye. All electrode impedances were less than or equal to 5 kΩ. All potentials were amplified 200 times and acquired on a DC system at a sampling rate of 250 Hz. EOG was corrected offline using the procedure described by Gratton et al.38 Only correctly identified target epochs for which a button-press response was obtained within 1 s of the target tone were analyzed. All subjects responded accurately to at least 35 target tones.
Behavioural data acquisition
Accuracy and RT to each target stimulus were recorded via a button press. Subjects were instructed to identify targets as quickly and as accurately as possible. RT was recorded as the first registered pressing of the button.
SCR data acquisition
Skin conductance was recorded via a pair of silver–silver chloride electrodes with 0.05 mol/L sodium chloride gel placed on the digits II and III of the left (nondominant) hand. The electrode pairs were supplied by a constant voltage, and the current change representing conductance was recorded with the use of a DC amplifier.
Data reduction
Gamma data
Gamma activity was examined for the correctly identified target stimuli. Narrow gamma-band signals (37–41 Hz) were extracted, based on evidence that key aspects of information processing during the oddball paradigm are contained within this frequency range.29,39 Data were detrended by subtracting the line of best fit over 512 samples to remove possible electromyography contaminants. For each single trial epoch (with a sample defined by –500 ms before stimulus to 700 ms post stimulus), from each recording site, a 64-sample Welch window was then moved along sample by sample, and a fast Fourier transform (FFT) was used to compute the phase of the gamma frequency component at each sample position. This procedure produced a time series of gamma-phase activity from each electrode site.
Gamma synchrony was calculated by means of circular variance using the phase of the given electrode sites.29 This yielded a singular estimate of the degree of phase locking at each time point for each recording site. The single-trial waveforms were then averaged over correct target–response trials. Within the averaged gamma synchrony responses, phase locking peaked within 2 latency windows: an early (gamma 1) synchrony peak at –150 ms to 150 ms, which was maximal around stimulus onset, and a later (gamma 2) synchrony peak at 250–500 ms. Within these windows, the peak amplitude and latency for gamma 1 and gamma 2 was scored for global synchrony (across all 19 sites) and for frontal (Fp1/2, F3/4, F7/8 and Fz) and posterior (T5/6, P3/4, Pz and O1/2) regions. To determine the contribution of laterality, we also examined synchrony for the left (Fp1, F3, F7, T3, T5, C3, P3 and O1) and right (Fp2, F4, F8, T4, T6, C4, P4 and O2) hemispheres. The definition of these regions according to multiple sites (rather than pairs) provides spatial contiguity, such that only robust and reliable variations in topographical synchrony are represented in these values.
Behavioural data
Accuracy was indexed by the number of target stimuli correctly identified, and RT was measured in milliseconds for each pressing of the button in response to target stimuli.
SCR data
The presence of phasic SCR was determined by a sigmoid-exponent mathematical model designed for short interstimulus interval paradigms.40 This model allows for overlapping composite signals to be broken down into phasic SCR and tonic SCR components. SCRs were evaluated as an unambiguous increase (> 0.05 μS) in conductance with respect to each pre-target stimulus baseline.41 All epochs containing an SCR within 1–3 s after each of the 40 target tones in each subject were evaluated.
Statistical analysis
Peak amplitude and latency for gamma 1 and gamma 2 (for global, frontal and posterior regions, and left and right hemispheres) were analyzed using analysis of variance (ANOVA) with the between-subjects variable of group (BPD v. healthy controls). Given evidence that gamma 1 and 2 reflect distinct processes, that amplitude is not determined by latency, and that the method for calculating regional gamma synchrony differentiates distinctive topographical changes, a simple Bonferroni correction for multiple comparisons was not considered appropriate. In light of these factors, previous gamma studies comparing clinical and control subjects have relied on an uncorrected α of 0.05. However, in this study, we used a nonparametric permutation model to verify the significant differences revealed by ANOVA. In this model, a random permutation of the data from all subjects determines the probability that differences between groups were obtained purely by chance.
RT and SCR indices were first analyzed using independent group t tests. Analyses of covariance were then conducted to ascertain the degree of variance in between-groups gamma synchrony analyses accounted for by behavioural (accuracy, RT) and SCR onset indices.
Pearson correlation analyses were used to examine the relations between gamma, SCR and behavioural variables, which show a significant difference between groups, and symptom scores on the DIB-R. Given the exploratory nature of these analyses, we used a p value of 0.05, and results were interpreted with appropriate caution. However, selection of the data was undertaken according to reliance on variables that were significant in the initial between-group comparisons.
Results
Gamma data
No significant differences between BPD and control groups were found for global synchrony for either gamma 1 (F1,28 = 0.07, p = 0.79) or gamma 2 (F1,28 = 0.001, p = 0.98).
With regard to regional synchrony, the BPD group showed a significant delay in posterior gamma 1 synchrony compared with healthy controls (F1,28 = 6.094, p = 0.020). The permutation model confirmed this difference, showing that the probability of having incorrectly rejected the null hypothesis was 0.016.
The group difference in posterior synchrony is shown in Figure 1.
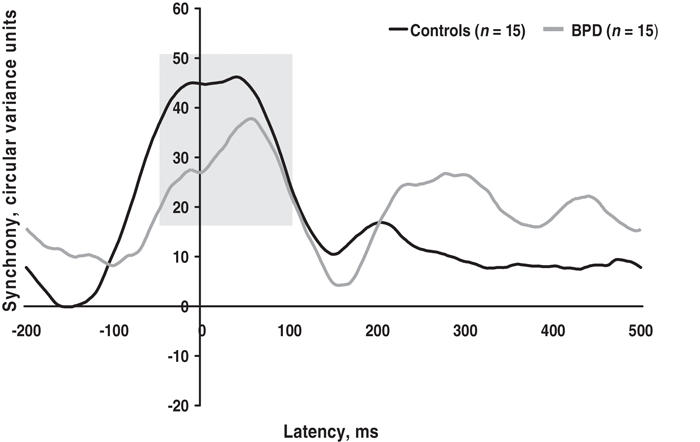
Fig. 1: Patients with borderline personality disorder (BPD) had a significant delay in early gamma synchrony (peaking around stimulus onset, indicated by shading) in the posterior region, relative to healthy control subjects.
The magnitude of gamma 2 synchrony was also found to be significantly reduced in the right hemisphere for BPD compared with healthy control subjects (F1,28 = 6.234, p = 0.019). This difference was also confirmed by the permutation model, which showed that the probability of having incorrectly rejected the null hypothesis was 0.021. This difference is shown in Figure 2.
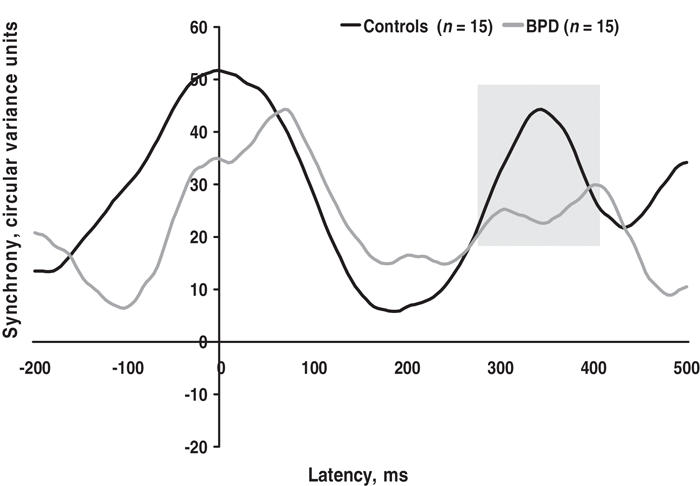
Fig. 2: Patients with borderline personality disorder (BPD) had a significant reduction in late gamma synchrony (peaking 250–500 ms post stimulus, indicated by shading) in the right hemisphere, relative to healthy control subjects.
Contrary to expectations, there were no group differences in frontal synchrony for gamma 1 or gamma 2.
Behavioural data
The mean accuracy for subjects with BPD was 39.9 (SD 0.5) of 40 target tones and for control subjects was 39.9 (SD 0.4). There was no difference in group accuracy.
Mean RT for BPD was 406 (SD 120) ms and for controls, 313 (SD 50) ms. Subjects with BDP had a significantly delayed RT compared with controls (t1,28 = –2.77, p = 0.010).
SCR data
The mean SCR amplitude for subjects with BPD was 1.2 (SD 0.04) μS and for controls was 1.3 (SD 0.03) μS. There were no significant differences in SCR amplitude (t1,28 = –0.594, p = 0.53).
Mean SCR onset for patients with BPD was 1785 (SD 25) ms and for healthy controls, 1595 (SD 22) ms. Subjects with BPD were found to have a significantly delayed SCR onset compared with controls (t1,28 = –2.18, p = 0.038).
Gamma, behavioural and SCR data relations
For gamma 1, neither controls nor patients with BPD showed a correlation between posterior synchrony and RT. However, patients with BPD showed a significant and positive correlation between the latency of posterior gamma 1 synchrony and the latency of SCR onset (r14 = 0.543, p = 0.039), which was not present in controls (r14 = 0.149, p = 0.62). However, the strength of the significant correlation in patients with BPD was not significantly greater than the strength of the nonsignificant correlation in controls (p = 0.27).
For gamma 2, healthy controls showed a trend toward a positive association between right hemisphere gamma 2 synchrony and RT (controls: r14 = 0.481, p = 0.07; BPD: r14 = –0.18, p = 0.51), which was absent in patients with BPD. There was also a positive association between right hemisphere gamma 2 and the latency of SCR onset for healthy controls (r14 = 0.612, p = 0.015), whereas there was a negative correlation between these variables for patients with BPD (r14 = –0.558, p = 0.030). In this case, the positive associations present in control subjects differed from the negative correlations observed in BPD, at trend level for gamma 2 and RT (p = 0.07) and significantly for gamma 2 and SCR onset (p < 0.001).
Analysis of covariance showed that significant group differences in RT and SCR onset did not account for the significant difference between BPD and control groups in gamma 1 and 2 synchrony. RT did not covary linearly with differences in either posterior gamma 1 latency (F1,28 = 0.570, p = 0.46) or the magnitude of right hemisphere gamma 2 synchrony (F1,28 = 0.003, p = 0.95). Similarly, the latency of SCR onset was not a significant linear covariate for posterior gamma 1 (F1,28 = 3.391, p = 0.77) or right hemisphere gamma 2 (F1,28 = 0.011, p = 0.98) group differences.
Gamma and symptom relations
We found a significant positive correlation between the latency of posterior gamma 1 synchrony and cognitive disturbances in patients with BPD (r14 = 0.521, p = 0.048), suggesting that slower posterior gamma 1 is associated with a more pronounced cognitive distortion. By contrast, the magnitude of right hemisphere gamma 2 synchrony was significantly negatively related to impulsivity (r14 = –0.532, p = 0.045), indicating a greater reduction in magnitude with more pronounced loss of impulse control. There was also a weak trend toward an association between the delay in SCR onset and impulsivity (r14 = –0.454, p = 0.09), reflecting slower latency with less severe loss of impulse control. The direction of this association suggests that the slowing of SCR orienting reflects an attempt to gain control of impulses. Although we found no significant associations between affect dysregulation and gamma disturbances, these null findings may be the result of a lack of intersubject variation (i.e., subjects showed a consistently high level of affect dysregulation, equivalent to a “ceiling effect”).
Discussion
In this study, we used a high temporal resolution index, 40-Hz gamma phase synchrony, to identify breakdowns in neural synchronization in unmedicated patients with BPD, and relations between these breakdowns and core BPD symptoms. Behavioural and skin conductance data were recorded concurrently to determine whether performance or arousal variation contribute to the loss of synchrony in BPD. In response to task-relevant target stimuli in an oddball task, patients with BPD exhibited a delay in posterior gamma synchrony that occurred around stimulus onset and a subsequent reduction in right hemisphere gamma synchrony that occurred 250–500 ms post stimulus. Delayed posterior synchrony was related to cognitive symptoms, whereas reduced right hemisphere synchrony was associated with impulsivity. RT and the onset of SCRs in response to targets were also delayed in patients with BPD, but these delays did not account for the group differences in synchrony.
The early peak in gamma synchrony (gamma 1), occurring around stimulus onset, has been interpreted as an index of the preparatory processing and initial integration of sensory stimulation, given that the oddball task has a fixed inter-stimulus interval.29 Studies of human attentional processing suggest that posterior attentional networks are engaged in the initial orienting to salient sensory input.12 The delay in posterior gamma 1 in BPD suggests that these patients may have an impairment in the engagement of these posterior attentional networks and a consequent delay in the integration of sensory features. This proposal is consistent with functional neuroimaging evidence for posterior parietal deficits in BPD and cognitive tests that reveal parietal-dependent impairments in extracting relevant from extraneous information.21–23 The association of delayed posterior synchrony with cognitive distortions in BPD suggests that a lack of initial sensory integration may be a distinct deficit underlying difficulties with higher-order cognition in this disorder. Nevertheless, confirmation of this association in independent samples is warranted, given the marginally significant nature of the finding.
Gamma 2, on the other hand, which is a late peak in gamma synchrony occurring around 250–500 ms post stimulus, corresponds to the time frame of the P300 ERP component and is thought to provide an index of contextual integration involved in the selection of task-relevant from task-irrelevant information.42 Our observation that gamma 2 synchrony was significantly decreased in patients with BPD may reflect a breakdown in context evaluation in this group, consistent with findings of an abnormal P300,24–26 and possibly arising from the initial delay in sensory discrimination. The right hemisphere locus of the gamma 2 reduction is consistent with the laterality of the posterior orienting network.12 The association between the loss of right gamma synchrony and impulsivity in patients with BPD is also consistent with the role of the right hemisphere in emotion processing,42 although this association again requires verification in independent samples. Indeed, it has been proposed that early psychological trauma affects the development of the right brain, leading to deficits in the ability to modulate responses to stress.43 In patients with BPD with greater variation in affective dysregulation, we would hypothesize a further association between these symptoms and right hemisphere breakdowns in gamma integration.
Our observation that patients with BPD also had a delay in both behavioural responses (RT) and SCR onset is consistent with a breakdown in orienting to salient (target) stimuli. Moreover, patients with BPD had an abnormal pattern of relations between synchrony and RT and SCR onset, consistent with our finding that RT and SCR disturbances did not covary in a linear manner with group differences in gamma synchrony. Whereas healthy subjects showed a positive relation between late gamma synchrony and both RT and SCR onset expected from previous evidence,39 patients with BPD had a dissociation between late gamma synchrony and performance, and a reverse relation between late synchrony and SCR onset in the right hemisphere. Although selective responding is normally associated with late synchrony, delayed RT in patients with BPD was instead related to the delay in early posterior synchrony. These findings are consistent with a dysregulation in central, autonomic and behavioural responses to task-relevant information in BPD.
Taken together, the disturbances in posterior and right hemisphere gamma synchrony in BPD point to a breakdown in dynamic integration of neural systems for task-relevant information, which may produce the associated loss of cognitive function and impulse control. Previous studies have focused on frontal brain networks as a primary site of disturbance underlying symptoms of BPD. Although we did not reveal localized frontal disturbances in this study, it remains possible that posterior and right-sided disturbances in synchrony involve a breakdown in the integration of these networks with frontal executive systems. Indeed, it has been proposed that the failure to integrate parietal with other cortical networks represents a distinct deficit in BPD,20 and our findings suggest this deficit may underlie symptoms of impulsivity in particular. Similarly, we have also found a discoordination between the frontal early P300 and later parietal P300 subcomponents in patients with BPD.15 This proposal would also accord with the concept of multiple attention systems, in which effective processing of salient stimuli relies on the cooperation of posterior and frontal systems.12 It is not likely that the oddball task has simply been unable to reveal frontal deficits, given that schizophrenia has been associated with a loss of frontal gamma synchrony on this task,31 and even normal aging shows frontal changes in synchrony.44 Of course, it would nonetheless be valuable to investigate the generality of gamma synchrony disturbances to BPD in a different behavioural task.
Several methodological issues might be considered in the interpretation of the results of this study. A methodological strength was the inclusion of unmedicated subjects with BPD, so that results were not confounded by the effects of medication. Whereas unmedicated status might typically produce a less unwell sample, the symptom ratings of subjects in this study would suggest that they are in fact at the high end of clinical severity. With regard to the oddball task, the use of a fixed interstimulus interval may have played some role in producing such an early gamma 1 response, reflecting the contribution of stimulus anticipation. However, this factor is unlikely to account for delayed gamma 1 responses in BPD given that the interstimulus interval was standardized across all subjects.
The findings of this study provide evidence that BPD is characterized by specific disturbances in neural synchrony related to core symptoms of cognitive impairment and impulsivity. These findings are consistent with the description of the borderline syndrome as a fragmentation of the self, a lack of wholeness and an inability to integrate the positive and negative aspects of the self and the external world, which contribute to the distress experienced by affected individuals.5,43,45 To further examine the regional localization of integrative disturbances in BPD, future studies might also examine gamma phase synchrony using tasks designed to emphasize frontal executive and regulation processes. Functional neuroimaging, with analysis of functional connectivity, would also provide important convergent information on the breakdown of integrative processing in BPD across both cortical and subcortical networks.
Acknowledgments
Dr. Williams is a Pfizer senior research fellow.
Footnotes
Contributors: Drs. Williams, Gordon and Meares contributed to the conception of the study. Dr. Sidis acquired the data; Drs. Sidis and Williams analyzed the data. All the authors wrote the article, critically revised the article and gave final approval for its publication.
Competing interests: None declared for Drs. Williams, Sidis and Meares. Dr. Gordon is the current CEO of the Brain Resource Company; no company products were used in this study.
Correspondence to: Dr. Leanne M. Williams, Brain Dynamics Centre, Acacia House, Westmead Hospital, Westmead, Sydney, NSW 2145 Australia; fax 61 2 9635 7734; lea@psych.usyd.edu.au
References
- 1.Wilkinson-Ryan T, Westen D. Identity disturbance in borderline personality disorder: an empirical investigation. Am J Psychiatry 2000;157:528-41. [DOI] [PubMed]
- 2.Brodsky BS, Cloitre M, Dulit RA. Relationship of dissociation to self-mutilation and childhood abuse in borderline personality disorder. Am J Psychiatry 1995;152:1788-92. [DOI] [PubMed]
- 3.Goldman SJ, D'Angelo EJ, DeMaso DR. Psychopathology in the families of children and adolescents with borderline personality disorder. Am J Psychiatry 1993;150:1832-5. [DOI] [PubMed]
- 4.Meares R, Stevenson J, Gordon E. A Jacksonian and biopsychosocial hypothesis concerning borderline and related phenomena. Aust N Z J Psychiatry 1999;33:831-40. [DOI] [PubMed]
- 5.Meares R. The contribution of Hughlings Jackson to an understanding of dissociation. Am J Psychiatry 1999;156:1850-5. [DOI] [PubMed]
- 6.Kern RS, Kuehnel TG, Teuber J, et al. Multimodal cognitive-behavior therapy for borderline personality disorder with self-injurious behavior. Psychiatr Serv 1997;48:1131-3. [DOI] [PubMed]
- 7.Gordon E. Integrative neuroscience: bringing together biological, psychological and clinical models of the human brain. London: Harwood Academic Press; 2000.
- 8.Kandel ER. Biology and the future of psychoanalysis: a new intellectual framework for psychiatry revisited. Am J Psychiatry 1999;156: 505-24. [DOI] [PubMed]
- 9.Bohus M, Schahl C, Lieb K. New developments in the neurobiology of borderline personality disorder. Curr Psychiatry Rep 2004;6:43-50. [DOI] [PubMed]
- 10.Anderson SW, Damasio H, Tranel D, et al. Long-term sequelae of prefrontal cortex damage acquired in early childhood. Dev Neuropsychol 2000;18:281-96. [DOI] [PubMed]
- 11.Herpertz SC, Schwenger UB, Kunert HJ, et al. Emotional responses in patients with borderline as compared with avoidant personality disorder. J Personal Disord 2000;14:339-51. [DOI] [PubMed]
- 12.Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci 1990;13:25-42. [DOI] [PubMed]
- 13.Meares RA. The metaphor of play: disruption and restoration in the borderline experience. Rev. ed. London: Routledge; 2005.
- 14.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci 1996;351:1413-20. [DOI] [PubMed]
- 15.Meares RA, Melkonian D, Gordon E, et al. Distinct pattern of P3a event-related potential in borderline personality disorder. Neuroreport. 2005;16:289-93. [DOI] [PubMed]
- 16.Juengling FD, Schmahl C, Hesslinger B, et al. Positron emission tomography in female patients with borderline personality disorder. J Psychiatr Res 2003;37:109-15. [DOI] [PubMed]
- 17.Posner MI, Rothbart MK, Vizueta N, et al. Attentional mechanisms of borderline personality disorder. Proc Natl Acad Sci U S A 2002;99:16366-70. [DOI] [PMC free article] [PubMed]
- 18.Williams LM, Phillips ML, Brammer MJ, et al. Arousal dissociates amygdala and hippocampal fear responses: evidence from simultaneous fMRI and skin conductance recording. Neuroimage 2001;14:1070-9. [DOI] [PubMed]
- 19.Herpertz SC, Dietrich TM, Wenning T, et al. Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biol Psychiatry 2001;50:292-8. [DOI] [PubMed]
- 20.Arehart-Treichel J. Fine line separates personality quirks from personality disorder. Psychiatr News 2002;37:20.
- 21.Lepping P, Swinton M. Borderline personality disorder associated with psychotic symptoms and parietal lobe abnormalities. Psychiatr Prax 2004;31:96-9. [DOI] [PubMed]
- 22.O'Leary KM. Neuropsychological testing results. Psychiatr Clin North Am 2000;23:41-60. [DOI] [PubMed]
- 23.O'Leary KM, Brouwers P, Gardner DL, et al. Neuropsychological testing of patients with borderline personality disorder. Am J Psychiatry 1991;148:106-11. [DOI] [PubMed]
- 24.Blackwood DH, St Clair DM, Kutcher SP. P300 event-related potential abnormalities in borderline personality disorder. Biol Psychiatry 1986;21:560-4. [DOI] [PubMed]
- 25.Drake ME, Phillips BB, Pakalnis A. Auditory evoked potentials in borderline personality disorder. Clin Electroencephalogr 1991;22: 188-92. [DOI] [PubMed]
- 26.Kutcher SP, Blackwood DHR, Gaskell DF, et al. Auditory P300 does not differentiate borderline personality disorder from schizotypal personality disorder. Biol Psychiatry 1989;26:766-74. [DOI] [PubMed]
- 27.Roberts LE, Harald R, Lutzenberger W, et al. Mapping P300 waves onto inhibition: Go/No-Go discrimination. Electroencephalogr Clin Neurophysiol 1994;92:44-55. [DOI] [PubMed]
- 28.Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci 1995;18:555-86. [DOI] [PubMed]
- 29.Haig AR, Gordon E, Wright JJ, et al. Synchronous cortical Gamma-band activity in task-relevant cognition. Neuroreport 2000;11:669-75. [DOI] [PubMed]
- 30.Haig AR, Gordon E, De Pascalis V, et al. Gamma activity in schizophrenia: Evidence of impaired network binding? Clin Neurophysiol 2000;111:1461-8. [DOI] [PubMed]
- 31.Symond M, Harris AWF, Gordon E, et al. Gamma synchrony in first episode schizophrenia: a disturbance of high-temporal resolution functional connectivity. Am J Psychiatry 2005;162:459-65. [DOI] [PubMed]
- 32.Lee KH, Williams LM, Breakspear M, et al. Synchronous Gamma activity: a review and contribution to an integrative neuroscience model of schizophrenia. Brain Res Brain Res Rev 2003;41:57-78. [DOI] [PubMed]
- 33.World Health Organization. International statistical classification of diseases and related health problems. 10th rev. Geneva: WHO; 1992.
- 34.Robins LN, Wing J, Wittchen HU, et al. The Composite International Diagnostic Interview. An epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry 1988;45:1069-77. [DOI] [PubMed]
- 35.Williams LM, Gordon E, Bahramali H, et al. Late component ERPs are associated with distinct schizophrenic syndromes. Int J Neurosci 2000;105:37-52. [DOI] [PubMed]
- 36.Nelson HE. National Adult Reading Test. Windsor (UK): NFER-Nelson Publishing Company; 1982.
- 37.Zanarini MC, Gunderson JG, Frankenburg FR, et al. The revised diagnostic interview for borderlines: discriminating BPD from other axis II disorders. J Personal Disord 1989;3:10-8.
- 38.Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol 1983;55:468-84. [DOI] [PubMed]
- 39.Haig AR, Gordon E, De Pascalis V. Peak gamma latency correlated with reaction time in a conventional oddball paradigm. Clin Neurophysiol 1999;110:158-65. [DOI] [PubMed]
- 40.Lim CL, Gordon E, Rennie C, et al. Dynamics of SCR, EEG and ERP activity in an oddball paradigm with short interstimulus intervals. Psychophysiology 1999;36:543-51. [PubMed]
- 41.Boucsein W. Electrodermal activity. New York: Plenum Press; 1992.
- 42.Schore AN. Dysregulation of the right brain: a fundamental mechanism of traumatic attachment and the psychopathogenesis of posttraumatic stress disorder. Aust N Z J Psychiatry 2002;36:9-30. [DOI] [PubMed]
- 43.Gunderson JG. The borderline patient's intolerance of aloneness: insecure attachments and therapist availability. Am J Psychiatry 1996;153:752-8. [DOI] [PubMed]
- 44.Paul RH, Clark CR, Lawrence J, et al. Age-dependent change in executive function and gamma 40 Hz phase synchrony. J Integr Neurosci 2005;4:63-76. [DOI] [PubMed]
- 45.Wildgoose A, Waller G, Clarke S, et al. Psychiatric symptomatology in borderline and other personality disorders: dissociation and fragmentation as mediators. J Nerv Ment Dis 2000;188:757-63. [DOI] [PubMed]


