Abstract
Objective:
To assess the effects of preoperative systemic chemotherapy on remnant liver parenchyma, liver function, and morbidity after major liver resection for colorectal liver metastases.
Background:
Many patients operated upon for colorectal cancer liver metastases receive previous chemotherapy. Whether systemic chemotherapy alters liver parenchyma in such way that it increases the risks of liver resection remains unclear.
Patients and Methods:
Among 214 patients who received a liver resection for colorectal liver metastases between 1998 and 2002 in a single institution, 67 who underwent a major liver resection under total hepatic vascular exclusion form the basis of this report. Forty-five patients operated upon after systemic chemotherapy were compared with 22 who did not receive any chemotherapy in the 6 months prior to resection. Postoperative mortality, morbidity, liver function tests, and pathology of the resected liver in the two groups were compared.
Results:
There was no postoperative mortality. Values of liver function tests on days 1, 3, 5, and 10 were similar in both groups. Morbidity rate was higher in the chemotherapy group (38% versus 13.5%, P = 0.03). Postoperative morbidity was correlated with the number of cycles of chemotherapy administered before surgery but not to the type of chemotherapy. Preoperative chemotherapy was significantly associated with sinusoidal dilatation, atrophy of hepatocytes, and/or hepatocytic necrosis (49% versus 25%, P = 0.005).
Conclusion:
Prolonged neoadjuvant systemic chemotherapy alters liver parenchyma and increases morbidity after major resection under total hepatic vascular exclusion, but it does not increase operative mortality. This should be taken into consideration before deciding a major liver resection in patients who have received preoperative chemotherapy.
Prolonged neoadjuvant systemic chemotherapy alters liver parenchyma and increases morbidity after major liver resection but does not influence operative mortality.
Depending on the stage of the primary colorectal cancer, liver metastases occur in 20% to 70% of patients and represent the major cause of death in this disease.1,2 Surgical resection remains the only treatment that can, to date, ensure long-term survival in 25% to 40% of patients.3–8 It should be considered whenever liver metastases can be totally resected with clear margins and when there is no nonresectable extrahepatic disease. However, using the current indications for surgery, only 15% to 20% of patients with colorectal liver metastases are suitable for surgical resection.1,2 When liver metastases are nonresectable, palliative chemotherapy increases survival and enhances quality of life.9
Progresses including more efficient chemotherapy drugs such as oxaliplatin or irinotecan10,11 have opened new perspectives in the treatment of resectable and nonresectable liver metastases. These drugs can render resectable previously unresectable liver metastases.12–16 When liver metastases are resectable, the benefit of neoadjuvant chemotherapy on reducing the risks of recurrence after surgery is not yet demonstrated. However, in case of synchronous metastases, chemotherapy is often prescribed between the resection of the colorectal primary and the liver surgery.
The risks of liver resections have been reduced in recent years. Postoperative mortality is less than 5% in most reported series.5–7 Postoperative morbidity, including transient liver failure, hemorrhage, subphrenic abscesses, and biliary fistula, occur in 20% to 40% of patients.3–8 It is not clearly established whether systemic preoperative chemotherapy increases the risks of surgery due to injury of liver parenchyma. Recent data have suggested that prolonged systemic chemotherapy could induce steatosis and microvascular changes such as peliosis or sinusoidal congestion.17 Sclerosing cholangitis, dilatation, and fibrosis of the central veins and moderate hepatocellular necrosis or cholestasis in the centrilobular area have been reported with the intra-arterial use of fluorodeoxyuridine, a 5-fluorouracil (5-FU) metabolite.13,17 These pathologic changes can be responsible for an increased risk of bleeding during surgery as the parenchyma tends to be more congested and friable.13,15 Hepatic arterial chemotherapy prior to liver resection was associated with an increased postoperative morbidity in one series (57% versus 18%).13 The consequences of systemic chemotherapy on liver parenchyma and on postoperative course following resections have not been specifically analyzed in the reports that have studied resectability following preoperative chemotherapy. The populations studied were heterogeneous regarding the duration and the regimens of preoperative chemotherapy, the extent of liver resections performed and the clamping methods used.12,14,16,18–20 The pathologic changes induced by chemotherapy in these patients have not been reported.
The present study was designed to analyze the effects of preoperative systemic chemotherapy on remnant liver parenchyma and to assess the consequences on postoperative mortality, morbidity, and liver function tests. Among patients having received liver resection for metastases from colorectal cancer, we selected a homogeneous subgroup of patients who received a major liver resection under total vascular exclusion. In theory, the removal of more than three hepatic segments with continuous occlusion of hepatic inflow and outflow is at higher risk of inducing postoperative mortality and morbidity; therefore, this subgroup offers the better chances of showing an influence of preoperative chemotherapy.
PATIENTS AND METHODS
Patients
Between January 1998 and May 2002, 214 patients underwent an elective liver resection for colorectal cancer metastases in our institution. Sixty-seven of these patients met the following inclusion criteria: major liver resection (ie, removal of 3 liver segments or more), total hepatic vascular exclusion (TVE), no underlying chronic liver disease. Forty-five of these patients (67%) received systemic chemotherapy prior to liver resection with a delay between the end of the chemotherapy and the resection of less than 2 months. The other 22 patients did not receive any chemotherapy in the 6 months before the resection (control group).
Indication and Protocol of Systemic Chemotherapy
Chemotherapy regimens administered before hepatectomy were the following: continuous infusion of 5-FU and leucovorin (LV5FU2)21 in 14 patients, LV5FU2 + CPT-1111 in 12 patients, LV5FU2 + oxaliplatin (FOLFOX 4)10 in 38 patients, and 5-FU + irinotecan + oxaliplatin in 2 patients. Some patients received 2 lines of chemotherapy: LV5FU2 followed by FOLFOX (5 patients), LV5FU2 followed by FOLFIRI (1 patient), and FOLFOX followed by FOLFIRI (5 patients). The median number of cycles of chemotherapy per patients was 6 (range, 3–29).
In 24 of the 45 patients who received preoperative chemotherapy, the liver metastases were considered to be resectable with curative intent before chemotherapy administration. The remaining 21 patients were initially considered to have nonresectable liver metastases, which subsequently became resectable after chemotherapy.
Operative Procedure
All patients underwent preoperative assessment that included liver function tests, full blood count, coagulation tests, and measurement of serum urea, creatinine, and electrolytes. The resectability of hepatic lesions was assessed with liver ultrasound and chest and abdominal CT scan. Colonoscopy was undertaken to rule out local recurrence or metachronous colonic lesion. During operation, a complete examination of the liver was performed by palpation and intraoperative ultrasonography to accurately determine the location of the lesions, their relationship to the vascular system, and to rule out other lesions that could had been missed by preoperative investigations. TVE was indicated if one of the hepatic metastases was located near the termination of one hepatic vein or the vena cava. TVE included clamping of the hepatic pedicle and of the inferior vena cava below and above the liver.22 An initial test of vascular exclusion was undertaken after an adequate blood volume expansion with crystalloids to ensure that the procedure would be well tolerated.
Liver transsection was performed using a Kelly forceps or an ultrasonic dissector. Biliary and vascular pedicles were secured by sutures and clips, and hemostasis of the liver cut surface was completed with argon beam coagulation. Drainage of the subdiaphragmatic space was left in place for 24 to 48 hours.
Duration of warm ischemia, operative time, and blood transfusion requirements (packed red cell units) were recorded.
Postoperative Course
Patients were observed until the day of discharge from the hospital. Postoperative parameters of hepatocellular injury and recovery, including prothrombin time, bilirubin, transaminases levels (alanine aminotransferase, aspartate aminotransferase), γ-glutamyl transpeptidase, and alkaline phosphatase, were measured on days 1, 3, 5, and 10. Liver failure was defined by a prothrombin time of less than 50% of normal and/or serum bilirubin more than 50 μmol/L on postoperative day 5 or thereafter.23 Abdominal ultrasound was carried out in any patient with a suspected infected collection. The mean duration of hospital stay was recorded, and postoperative deaths and complications were assessed at a postoperative delay of 2 months.
Pathologic Examination
One sample from each liver specimen distant from the tumor was taken and retrospectively reviewed by two pathologists (M.A.-H., B.F.). Liver samples were fixed, paraffin-embedded, and stained with hematoxylin and eosin and Masson's trichrome. The presence of sinusoidal dilatation associated with atrophy of hepatocytes and/or hepatocytic necrosis was recorded. The steatosis was graded from 0 to 3: absent (grade 0), ≤20% of hepatocytes (grade 1), between 20% and 50% (grade 2), and >50% (grade 3). The lobular neutrophil infiltration was graded from 0 to 2: absent (grade 0), moderate (grade 1), important (grade 2). Fibrosis was estimated according to the Metavir score24: absent (F0), portal fibrosis without septa (F1), portal fibrosis with rare septa (F2), numerous septa without cirrhosis (F3), and cirrhosis (F4).
Statistical Analysis
Quantitative data were expressed as means ± SD. Comparisons between groups were analyzed by the χ2 test with Yates correction, the Mann-Whitney U test, or Student t tests for quantitative and qualitative variables as appropriate. Univariate Cox proportional hazard modeling was used to examine the relationship between the incidence of postoperative complication and the following continuous variables: age, sex, body mass index, duration of vascular clamping, duration of operation, number of liver segments resected, perioperative blood transfusion, preoperative chemotherapy, number of cycles of preoperative chemotherapy, type of preoperative chemotherapy, and associated gastrointestinal procedure. The predictive factors found to be significant by univariate analysis were subsequently analyzed using a Cox multivariate proportional hazard model. All statistical tests were performed at the 0.05 level. The computations were performed using the Stata Statistical software (Stata Corporation, College Station, TX).
RESULTS
Patients
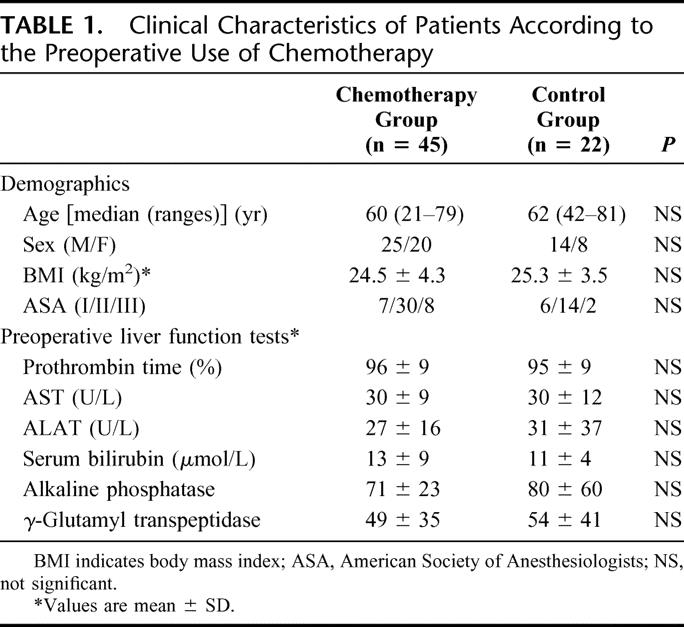
Sixty-seven patients were included in the study (39 men and 28 women). Median age was 61 years (range, 21–81 years). Primary tumor location was the colon in 45 patients (67%) and the rectum in 22 patients (33%). Liver metastases were synchronous in 31 patients (46%). The clinical characteristics of the 67 patients according to preoperative use of systemic chemotherapy (n = 45) or not (n = 22) are summarized in Table 1. There were no significant differences between the two groups in terms of age or gender, body mass index, ASA score, and preoperative liver function tests.
TABLE 1. Clinical Characteristics of Patients According to the Preoperative Use of Chemotherapy
Surgical Procedures
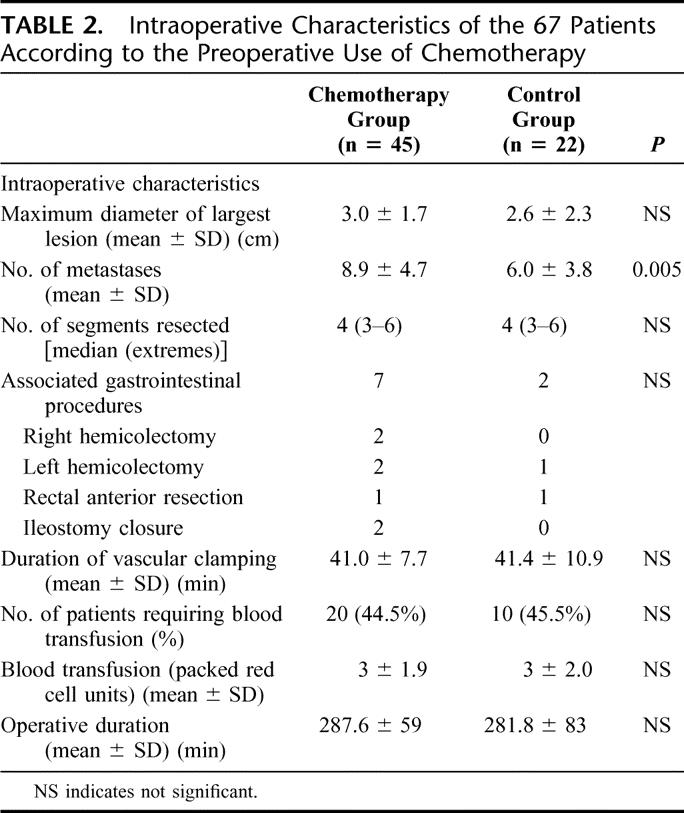
Major liver resections performed in this series were all performed with a curative intent and included 42 right lobectomies (segments 5, 6, 7, 8), 4 left lobectomies (segments 2, 3, 4), 15 right trisegmentectomies (segments 4, 5, 6, 7, 8 ± 1), 10 extended left trisegmentectomies (segments 2, 3, 4, 5, 8 ± 1), and 3 other trisegmentectomies (segments 4, 5, 6 in 2 cases and segments 6, 7, 8). In 11 cases (7 right lobectomies, 2 left lobectomies, and 2 right trisegmentectomies), a major liver resection was combined with one or several wedge resections. The intraoperative characteristics of the 67 patients are summarized in Table 2. The mean size of the largest lesion was not different between the two groups. The number of deposits (8.9 ± 4.7 versus 6.0 ± 3.8, P = 0.005) was significantly higher in the chemotherapy group, but the median number of liver segments resected was four in the two groups (Table 2). Portal vein embolization was performed in 2 patients (1 of each group) 1 month before liver resection to induce preoperative hypertrophy of the left lobe. For 9 patients, liver resection was combined with gastrointestinal procedures: right hemicolectomy (n = 2), left hemicolectomy (n = 3), anterior rectal resection with coloanal anastomosis (n = 2), and ileostomy closure (n = 2). There were no differences between the two groups in term of combined gastrointestinal procedures.
TABLE 2. Intraoperative Characteristics of the 67 Patients According to the Preoperative Use of Chemotherapy
Blood Transfusions, Duration of Vascular Clamping and Operative Time
The mean duration of vascular clamping was similar in the chemotherapy group and in the control group (41 ± 7 versus 41 ± 11 minutes). Twenty patients (44.5%) in the chemotherapy group and 10 (45.5%) in the control group required perioperative blood transfusions, and the mean number of packed red blood cells transfused was similar in the two groups (Table 2). The mean operative duration was similar in the two groups.
Postoperative Liver Function Tests
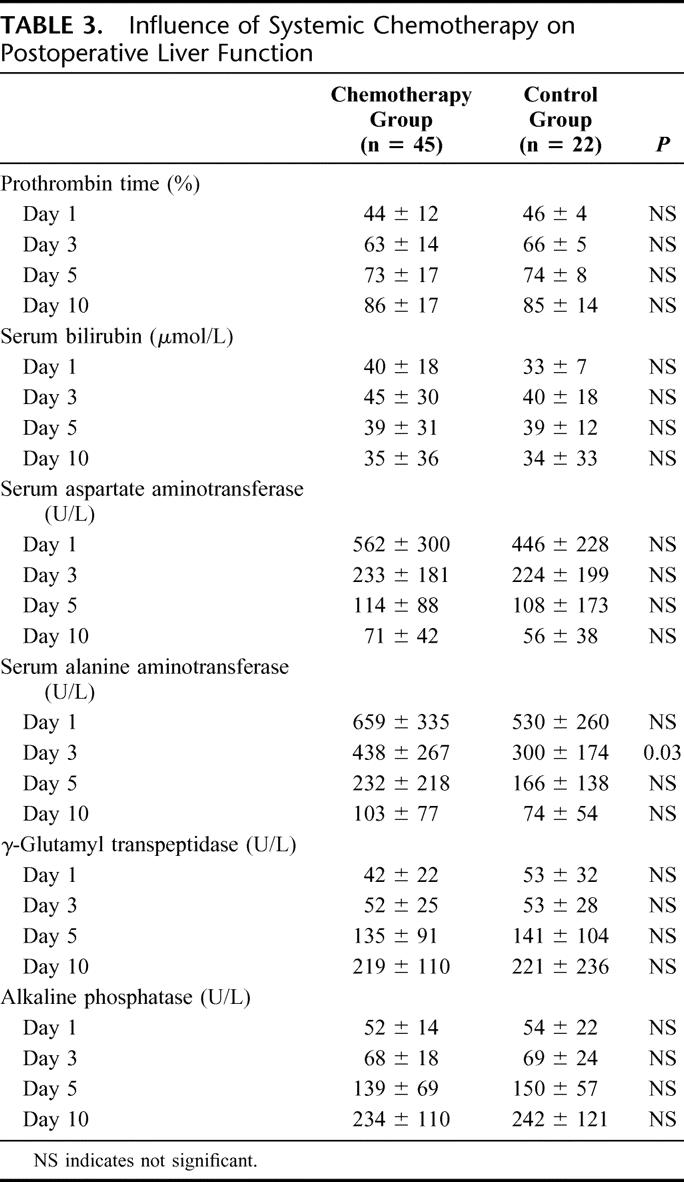
Liver function tests on postoperative days 1, 3, 5, and 10 are summarized in Table 3. As expected, we observed a moderate and transient decrease of prothrombin time level, a moderate increase of bilirubin level at day 1 and 3, an elevation of transaminase level until day 10, and a progressive elevation of γ-glutamyl transpeptidase and alkaline phosphatase correlated to postoperative liver regeneration. The differences were not significant between the two groups except for the serum ALT level at day 3, which was higher in the chemotherapy group.
TABLE 3. Influence of Systemic Chemotherapy on Postoperative Liver Function
Mortality and Morbidity
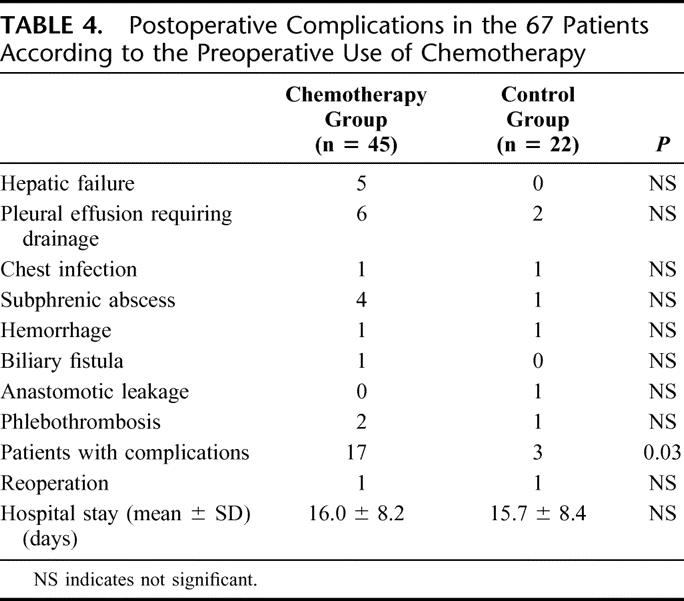
There was no postoperative death in either group. Postoperative complications occurred in 17 of 45 patients (37.8%) in the chemotherapy group and 3 of 22 patients (13.6%) in the control group (P = 0.03). Surgical and nonsurgical postoperative complications in each group are summarized in Table 4. Postoperative liver failure was never observed in the control group, whereas 5 patients in the chemotherapy group (11%) had a transient hepatic failure. These 5 patients had received a median number of 15 cycles of chemotherapy before surgery. The median number of segments removed and the duration of TVE were similar to those observed in the no chemotherapy group. Four of these 5 patients had steatosis involving more than 50% of the hepatocytes. Other complications were distributed equally between both groups. Seventeen patients in the chemotherapy group developed 20 postoperative complications. All subphrenic abscesses were managed nonoperatively, and one anastomotic leakage resolved spontaneously within 2 weeks. Two cases (1 in each group) of intraperitoneal hemorrhage by diffuse bleeding required reoperation on the second and the third postoperative days. The average duration of hospital stay was not different between the two groups.
TABLE 4. Postoperative Complications in the 67 Patients According to the Preoperative Use of Chemotherapy
In univariate analysis, age, sex, body mass index, duration of vascular exclusion of the liver, number of liver segments resected, and duration of operation were not associated with an increased morbidity rate. The risk of postoperative complication was significantly increased in patients with liver resection associated with a gastrointestinal procedure (7 of 9 patients, 77% versus 13 of 58, 22%, P = 0.002) and in patients receiving perioperative blood transfusion (14 of 30, 66% versus 6 of 37, 16%, P = 0.007). In the chemotherapy group, morbidity was increased among patients who received 6 or more cycles of chemotherapy (13 of 24 patients, 54%) compared with patients who received less than 6 cycles (4 of 21 patients, 19%, P = 0.047). Patients with uneventful postoperative course had received a median number of 5 (range, 3–29) cycles of CT preoperatively, whereas patients with postoperative morbidity had received a median number of 12 (range, 6–22) cycles. The risk of postoperative morbidity was correlated with the number of cycles of CT administered preoperatively (Fig. 1). Morbidity rate was similar in patients treated with 5-FU alone (3 of 8 patients, 37.5%) and patients treated with combination chemotherapy LV5FU2-CPT 11 or FOLFOX (14 of 37 patients, 37.8%).
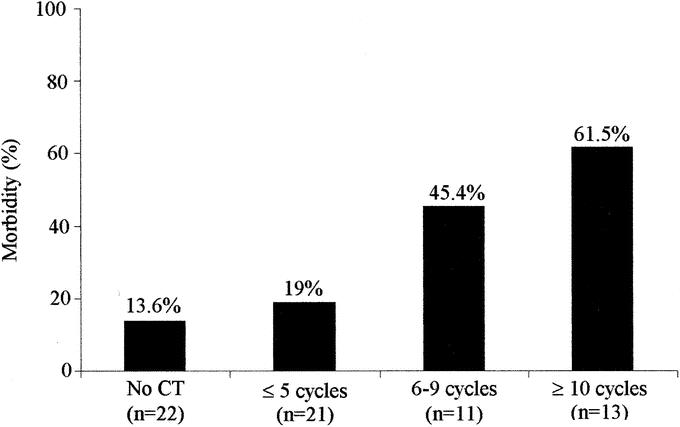
FIGURE 1. Influence of the number of cycles of CT on the percentage of postoperative morbidity.
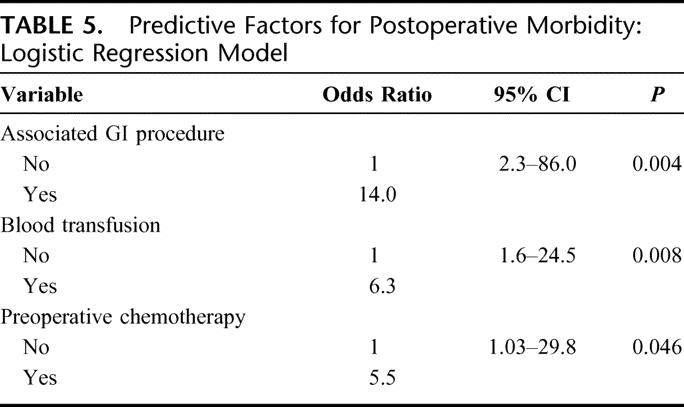
In multivariate analysis, three factors were independently associated with an increased risk of postoperative morbidity: an associated gastrointestinal procedure, a blood transfusion, and preoperative chemotherapy (Table 5).
TABLE 5. Predictive Factors for Postoperative Morbidity: Logistic Regression Model
Pathologic Changes
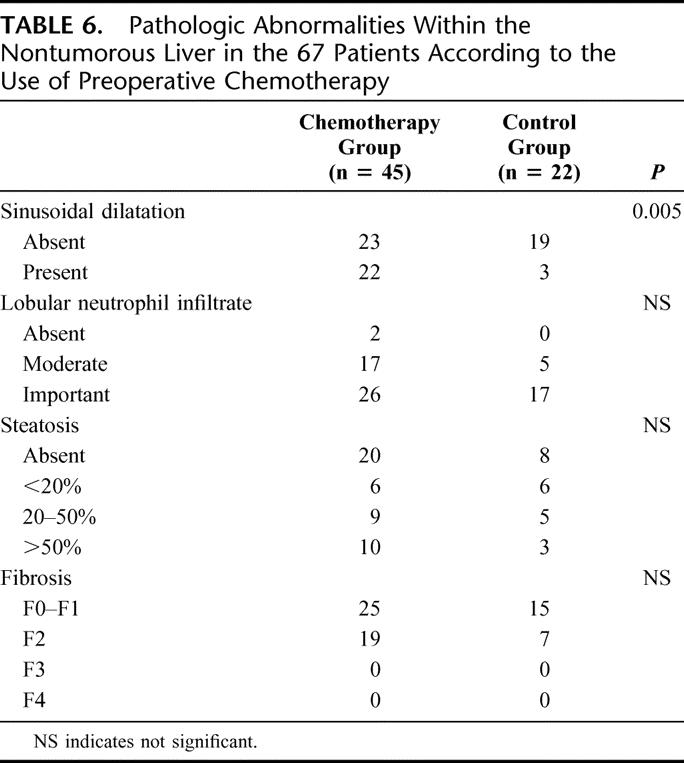
Pathologic examination was performed on liver parenchyma apart from metastases. Sinusoidal dilatation with atrophy of hepatocytes and/or hepatocytic necrosis was present in 49% of patients in the chemotherapy group, whereas it was observed in only 25% in the control group (P = 0.005). There was no difference between the two groups for steatosis (Table 6). Steatosis affecting more than 20% of hepatocytes was observed in 36% of patients who had not received preoperative chemotherapy, in 38% of patients who had received less than 5 cycles, in 45% of patients who had received 6 to 9 cycles, and in 46% of patients who had received more than 10 cycles of preoperative chemotherapy. Severe steatosis affecting more than 50% of the hepatocytes was observed in the same proportion of patients in the two groups and was also equally distributed among patients who had received preoperative chemotherapy according to the duration of the chemotherapy. There was no difference between the two groups for lobular neutrophil infiltration and fibrosis. Because patients with underlying chronic liver disease were excluded from this study, no grade F3 or F4 fibrosis was observed.
TABLE 6. Pathologic Abnormalities Within the Nontumorous Liver in the 67 Patients According to the Use of Preoperative Chemotherapy
DISCUSSION
The aim of this study was to determine if the risks of liver resection were increased by preoperative systemic chemotherapy in a group of patients undergoing a major liver resection for colorectal liver metastases. This study demonstrates that preoperative chemotherapy was not responsible for postoperative mortality but was associated with increased postoperative morbidity. Sinusoidal dilatation with atrophy of hepatocytes and/or hepatocytic necrosis was observed within the non-neoplastic liver parenchyma.
More and more patients currently undergoing liver resection for colorectal liver metastases have been previously treated with chemotherapy. In selected patients with unresectable metastases, disease may be down-staged by systemic or intra-arterial chemotherapy to the point that liver resection can be performed.12–16,20 Preoperative chemotherapy is already used in practice in many institutions prior to resection for both synchronous (during the delay between resections of the primary tumor and the metastases) and metachronous (as a “neoadjuvant” treatment) resectable liver metastases, although the principle of the method has not been, to date, formally validated.
Whether systemic preoperative chemotherapy alters liver parenchyma or increases the risk of liver resection has not been studied yet and appears important to determine. In patients whose metastases become resectable after chemotherapy, it could be helpful to know if liver resection should be performed as soon as possible or should be delayed, allowing a better shrinkage of tumor and thus less sacrifice of liver parenchyma. It can also be useful to decide which type of hepatectomy should be performed when the choice is possible between several wedge resections or a major liver resection removing at once all the deposits. Finally, the knowledge of pathologic changes induced by chemotherapy could influence the choice of the clamping method during the resection to prevent postoperative liver insufficiency.
Consequences of intra-arterial hepatic chemotherapy on both pathologic changes of nontumorous liver parenchyma and risks of subsequent liver resection have been well described.13,15,17 Following intra-arterial hepatic chemotherapy, sclerosing cholangitis, dilatation and fibrosis of the central veins, and moderate hepatocellular necrosis or cholestasis in the centrilobular area are frequently observed. These lesions appear to be both time- and dose-dependent. The hepatitis picture usually improves after interruption of chemotherapy, but the development of secondary sclerosing cholangitis is irreversible.17 These pathologic abnormalities may explain the higher rate of postoperative complications observed in patients who underwent liver resection after intra-arterial chemotherapy.13,15 Data concerning systemic chemotherapy are scarce. Many surgeons consider that livers submitted to prolonged systemic chemotherapy do not look as “healthy livers.” For this reason, when the estimated remnant liver volume is less than 30%, they tend to propose portal vein embolization or association of local ablative techniques with resection to avoid postoperative liver insufficiency.14,20 It has been suggested that prolonged systemic chemotherapy could induce steatosis, microvascular changes such as peliosis, or sinusoidal congestion.17,25 Whether the risk of postoperative liver insufficiency is increased in these conditions is still unclear. Some data suggest that preoperative systemic chemotherapy does not influence the risk of hepatic resection for colorectal and breast cancer liver metastases,18,19 and others have shown that high-dose chemotherapy, even when associated with autologous stem cell transplantation, did not increase the rate of postoperative complications in patients who underwent liver resection for germ cell tumors.26,27
It is hard to draw any conclusion from the few studies of liver resections following systemic preoperative chemotherapy because these series focused mainly on the long-term outcome of patients treated by this combined approach rather than on postoperative morbidity or liver parenchyma changes.14 Moreover, in these series, the origin of liver metastases, the chemotherapy regimens, the extent of liver resection, and the clamping method used were heterogeneous. To rule out the influence of these variables, our analysis was performed in a very selected population of patients undergoing major liver resection with a standardized technique for liver metastases from colorectal cancers. The choice of colorectal liver metastases was made to deal with homogeneous chemotherapy regimens. If systemic preoperative chemotherapy would impair liver function, the consequences would be more visible after large resection and small remnant liver combined with a prolonged warm ischemia. For these reasons, only major resections under TVE have been considered in the present study. It could be argued that liver resections can be performed without vascular clamping or with only a pedicular clamping that induces less hemodynamic consequences than TVE.28,29 However, in the cases selected, TVE was considered safer because of the location of one or several metastases close to the vena cava or the hepatic veins.
In this study, the two groups of patients are comparable according to the median number of liver segments resected and the volume of remnant liver parenchyma. The group receiving preoperative chemotherapy had more numerous tumors, but in theory the risk of liver failure after surgery is not related to the size of the resected tumor but to the percentage of functional liver parenchyma left in place. Thus, this imbalance should not interfere with the comparison between the two groups. The two groups were also similar for demographic data, preoperative liver function tests, association with gastrointestinal procedure, operative procedure, and perioperative blood transfusion. In the present study, pathologic examination of the nontumorous liver parenchyma confirmed that systemic chemotherapy did not induce fatty degeneration of the liver18 but was associated with microvascular changes, such as sinusoidal dilatation and atrophy of hepatocytes and/or hepatocytic necrosis. These changes did not increase bleeding during transsection since the need for blood transfusions and the mean number of packed red blood cells transfused were similar in both groups.
Our study also showed that biologic parameters of hepatocyte damage and recovery were not statistically different between the two groups in the postoperative period. The value and the evolution of these parameters are concordant with data reported in the literature for major hepatectomies on healthy liver parenchyma.22,28,30,31
Our results confirm that major liver resections can be performed under TVE without surgical mortality and with a postoperative morbidity similar to that reported in other large series.5–7,14,28 After preoperative chemotherapy, the incidence of postoperative complications was significantly increased, and this was mainly due to the higher incidence of transient postoperative insufficiency observed in the chemotherapy group. Pulmonary complications and subphrenic abscesses were the most frequent sources of morbidity in our series and were not statistically different between the two groups. They could be explained by the diaphragm dysfunction induced by TVE as previously reported.28
Transient postoperative liver failure was observed in 5 patients who had all received more than 10 cycles of preoperative chemotherapy. In these patients, the number of resected segments, the volume of remnant liver parenchyma, the duration of TVE, and the pathologic findings were similar to those of patients who were without postoperative liver insufficiency. Therefore, the explanation for this increased risk of transient postoperative liver insufficiency in patients who had received prolonged preoperative chemotherapy is unclear.
The type of chemotherapy regimen had no impact on postoperative morbidity. This latter point will need further assessment as new biologic agents such as cetuximab or bevacizumab may be widely used in the near future because they seem to improve the efficacy of chemotherapeutic agents but may increase the risks of bleeding. As previously reported,5,24 our data confirm that associated gastrointestinal procedures and perioperative blood transfusions are independent prognostic factors that have an impact on postoperative morbidity.
CONCLUSION
This study shows that prolonged systemic chemotherapy has induced pathologic changes in the nontumorous liver, including sinusoidal dilatation and atrophy of hepatocytes, but did not increase the risk of steatosis. Prolonged preoperative chemotherapy increased the risks of postoperative complications after major liver resection by increasing the risk of transient postoperative liver insufficiency. However, even after prolonged preoperative chemotherapy, major liver resection could be performed without mortality. If preoperative chemotherapy can be relatively short, these moderate and reversible inconveniences should be put in balance with the hope placed in neoadjuvant chemotherapy for the treatment of resectable liver metastases. A large European multicenter trial testing the effectiveness of perioperative chemotherapy on survival in this setting is now closed for inclusions and results are awaited for year 2005.
Footnotes
Reprints: Bernard Nordlinger, MD, Service de Chirurgie Digestive et Oncologique, Hôpital Ambroise Paré, 9 av Ch. De Gaulle, 92100 Boulogne Billancourt, France. E-mail: bernard.nordlinger@apr.ap-hop-paris.fr.
REFERENCES
- 1.Steele G Jr, Ravikumar TS. Resection of hepatic metastases from colorectal cancer: biologic perspective. Ann Surg. 1989;210:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheele J. Hepatectomy for liver metastases. Br J Surg. 1993;80:274–276. [DOI] [PubMed] [Google Scholar]
- 3.Fortner JG, Silva JS, Golbey RB, et al. Multivariate analysis of a personal series of 247 consecutive patients with liver metastases from colorectal cancer: I. Treatment by hepatic resection. Ann Surg. 1984;199:306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adson MA, van Heerden JA, Adson MH, et al. Resection of hepatic metastases from colorectal cancer. Arch Surg. 1984;119:647–651. [DOI] [PubMed] [Google Scholar]
- 5.Nordlinger B, Jaeck D, Guiguet M, et al. Surgical resection of hepatic metastases: multicentric retrospective study by the French Association of Surgery. In: Nordlinger B, Jaeck D, eds. Treatment of Hepatic Metastases of Colorectal Cancer. Paris: Springer-Verlag, 1992:129–146. [Google Scholar]
- 6.Scheele J, Stang R, Altendorf-HofmannA, et al. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. [DOI] [PubMed] [Google Scholar]
- 7.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minagawa M, Makuuchi M, Torzilli G, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer. Ann Surg. 2000;231:487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheithauer W, Rosen H, Kornek GV, et al. Randomized comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ. 1993;306:752–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. [DOI] [PubMed] [Google Scholar]
- 11.Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1047. [DOI] [PubMed] [Google Scholar]
- 12.Fowler WC, Eisenberg BL, Hoffman JP. Hepatic resection following systemic chemotherapy for metastatic colorectal carcinoma. J Surg Oncol. 1992;51:122–125. [DOI] [PubMed] [Google Scholar]
- 13.Elias D, Lasser P, Rougier P, et al. Frequency, technical aspects, results, and indications of major hepatectomy after prolonged intra-arterial hepatic chemotherapy for initially unresectable hepatic tumors. J Am Coll Surg. 1995;180:213–219. [PubMed] [Google Scholar]
- 14.Bismuth H, Adam R, Levi F, et al. Resection of nonresectable liver metastases from colorectal cancer after neoadjuvant chemotherapy. Ann Surg. 1996;224:509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meric F, Patt YZ, Curley SA, et al. Surgery after downstaging of unresectable hepatic tumors with intra-arterial chemotherapy. Ann Surg Oncol. 2000;7:490–495. [DOI] [PubMed] [Google Scholar]
- 16.Shankar A, Leonard P, Renaut AJ, et al. Neo-adjuvant therapy improves resectability rates for colorectal liver metastases. Ann R Coll Surg Engl. 2001;83:85–88. [PMC free article] [PubMed] [Google Scholar]
- 17.King PD, Perry MC. Hepatotoxicity of chemotherapy. Oncologist. 2001;6:162–176. [DOI] [PubMed] [Google Scholar]
- 18.Parc Y, Dugue L, Farges O, et al. Preoperative systemic 5-fluorouracil does not increase the risk of liver resection. Hepatogastroenterology. 2000;47:1703–1705. [PubMed] [Google Scholar]
- 19.Pocard M, Vincent-Salomon A, Girodet J, et al. Effects of preoperative chemotherapy on liver function tests after hepatectomy. Hepatogastroenterology. 2001;48:1406–1408. [PubMed] [Google Scholar]
- 20.Rivoire M, De Cian F, Meeus P, et al. Combination of neoadjuvant chemotherapy with cryotherapy and surgical resection for the treatment of unresectable liver metastases from colorectal carcinoma. Cancer. 2002;95:2283–2292. [DOI] [PubMed] [Google Scholar]
- 21.de Gramont A, Bosset JF, Milan C, et al. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol. 1997;15:808–815. [DOI] [PubMed] [Google Scholar]
- 22.Delva E, Camus Y, Nordlinger B, et al. Vascular occlusions for liver resections: operative management and tolerance to hepatic ischemia. Ann Surg. 1989;209:211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farges O, Belghiti J, Kianmanesh R, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bedossa P, Poynard T, French Metavir Cooperative Study Group. An algorithm for grading activity in chronic hepatitis C. Hepatology. 1996;24:289–293. [DOI] [PubMed] [Google Scholar]
- 25.Nagasue N, Kobayashi M, Iwaki A, et al. Effect of 5-fluorouracil on liver regeneration and metabolism after partial hepatectomy in the rat. Cancer. 1978;4:435–443. [DOI] [PubMed] [Google Scholar]
- 26.Hahn TL, Jacobson L, Einhorn LH, et al. Hepatic resection of metastatic testicular carcinoma: a further update. Ann Surg Oncol. 1999;6:640–644. [DOI] [PubMed] [Google Scholar]
- 27.Rivoire M, Elias D, De Cian F, et al. Multimodality treatment of patients with liver metastases from germ cell tumors: the role of surgery. Cancer. 2001;92:578–587. [DOI] [PubMed] [Google Scholar]
- 28.Belghiti J, Noun R, Zante E, et al. Portal triad clamping or hepatic vascular exclusion for major liver resection: a controlled study. Ann Surg. 1996;224:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Man K, Fan ST, Ng IO, et al. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg. 1997;226:704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delva E, Barberousse JP, Nordlinger B, et al. Hemodynamic and biochemical monitoring during major liver resection with the use of hepatic vascular exclusion. Surgery. 1984;95:309–318. [PubMed] [Google Scholar]
- 31.Suc B, Panis Y, Belghiti J, et al. ‘Natural history’ of hepatectomy. Br J Surg. 1992;79:39–42. [DOI] [PubMed] [Google Scholar]


