Recent breakthroughs in research have resulted in the clinical approval of a number of chemotherapeutic and biologic agents, including irinotecan, oxaliplatin, cituximab (Erbitux), and bevacizumab (Avastin) that have produced great impact upon the survival of the patient with hepatic colorectal metastases.1 These agents that target the cell cycle, paracrine growth factors, as well as angiogenic factors, not only represent effective palliative treatment of the unresectable patient, but can also downstage hepatic tumors to allow for resection of a subset of patients who were previously unresectable.2 It is now common that patients are subjected to a number of chemotherapies prior to consideration for hepatectomy. Along with the benefits of such chemotherapies has come the challenge of postoperative management of the patient with hepatic damage from use of these agents. The paper from Karoui et al3 published in this issue represents a growing body of data4,5 warning against the detrimental effects of preoperative chemotherapy on recovery after hepatectomy.
The liver damage that can result from systemic therapy is not restricted to the current generation of chemotherapies. There has been a long history of reports supporting the notion that most chemotherapeutic agents, even5-fluorouracil, can cause hepatic damage.6 The change that better salvage therapies bring about is that many patients subjected to second- and third-line therapies remain candidates for liver resection, whereas in years past, most patients failing first-line therapy were unlikely to be offered surgery. Thus, chemotherapy-associated steatohepatitis (CASH) has become more ubiquitous. It is incumbent for the clinician to recognize this syndrome. If a patient is noted to have hepatic attenuation lower than the spleen (Fig. 1), fatty infiltration can be assumed.7 The damage can progress to fibrosis and frank cirrhosis of the liver. This is accompanied by clinical findings of splenomegaly easily recognizable by imaging. There is also consumptive thrombocytopenia not related to bone marrow suppression and therefore not corrected even when chemotherapy is stopped.
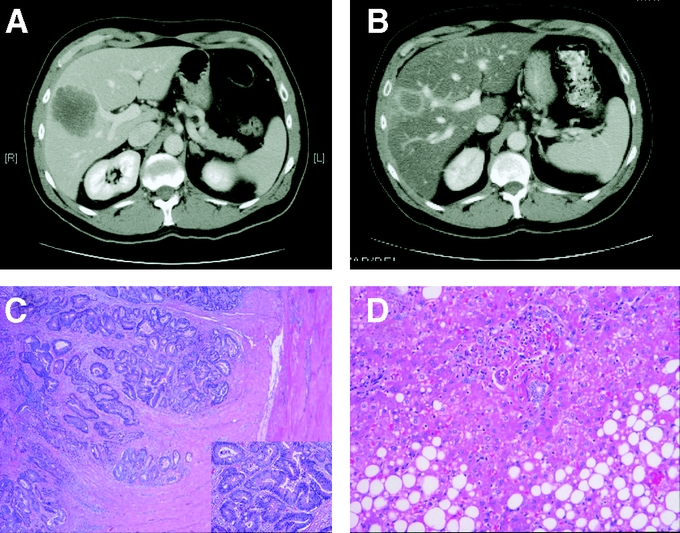
FIGURE 1. Development of CASH in a patient subjected to neoadjuvant chemotherapy. The development of steatohepatitis is clearly demonstrated by the decreased attenuation in the liver after 2 months of chemotherapy (B) as compared with before (A). The effects of chemotherapy on tumor are clear as demonstrated by the decrease in size of tumor in the scans (A, B), and in the tumor killing seen histologically (C). The effects on the noncancerous liver in terms of fat deposits are also clear (D).
Much data support the assertion that treatment of unresectable liver metastases can convert a percentage of patients with hepatic colorectal metastases to resectable.1,2 Use of 5-fluorouracil, leucovorin, and oxaliplatin can convert 25% of patients from terminal stage 4 disease to potentially curable patients.2 Even more aggressive options that include the use of cituximab and/or bevacizumab are now being tested for their ability to downstage unresectable disease. Some have used these data to justify a neoadjuvant approach or the use of preoperative chemotherapy in otherwise resectable patients. Note that in the paper by Karoui et al, 24 of the patients were subjected to preoperative chemotherapy in the setting of resectable disease.3 The theoretical benefits of neoadjuvant therapy are clear: 1) early treatment may allow potential eradication of microscopic disease even prior to resection; 2) treatment with measurable disease allows determination of effectiveness of the chosen regimen; 3) time delay to surgery allows declaration of occult disease; and 4) downsizing tumor may allow easier resection or ablation. However, costs must also be taken into account, including potential development of CASH and associated complications. Furthermore, the expenditure of monetary cash can also be considerable. Some of the combinations of current therapy can cost $20,000 to $30,000 per month. Thus, a 6-month neoadjuvant course of chemotherapy can cost as much as $180,000. For select patients, such as the patient with a high clinical risk score8 for recurrence, particularly patients with synchronous disease discovered during resection of a lymph node positive primary, such neoadjuvant therapy, can almost be justified on clinical ground. For all others, justification should be derived from future clinical trials.
Detection of CASH is not purely academic. Recognition of CASH may lead to active interventions to improve outcome. For example, one possible intervention is use of portal vein embolization (PVE). Makuuchi first proposed using preoperative, interventional occlusion of the one branch of the portal vein as a means of producing atrophy on the side of liver to be resected and hypertrophy of the contralateral remnant liver.9 It has been proposed that such portal vein embolization be used for patients whose calculated remnant liver is less than 25% of total functional liver.10 The presence of CASH would encourage consideration of such PVE. Future studies should define the relationship between size of remnant liver, the severity of CASH, and the benefit derived from PVE. One immediate benefit is that PVE may act as a “stress test” for the liver, allowing preoperative determination of the likelihood of liver regeneration prior to surgery.
Some studies have also attempted to dispel the notion that chemotherapy does not produce significant liver damage.5 If this were so, then liver enzymes would not need to be part of the ongoing evaluation of the patient subjected to chemotherapy. We need to accept that liver damage is a product of these potentially life-saving therapies. Only then can we move on to the essential studies that are necessary in this field. We need to study the pathogenesis of CASH, so that we may determine if anti-inflammatories or other therapies can be used to modulate these toxicities. We need to study the recovery of patients with CASH after resections so that we can determine how long these agents have to be withheld before surgery is safe. For now, we need to be judicious with our use of neoadjuvant chemotherapy, both to prevent CASH and also to decrease an unjustified financial burden to our healthcare system.
Footnotes
Reprints: Yuman Fong, MD, Murray F. Brennan Chair in Surgery, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10021. E-mail: fongy@mskcc.org.
REFERENCES
- 1.Leonard GD, Brenner B, Kemeny NE. Neoadjuvant chemotherapy before liver resection for patients with unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2005;23:2038–2048. [DOI] [PubMed] [Google Scholar]
- 2.Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karoui M, Penna C, Amin-Hashem M, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal metastases. Ann Surg. 2006;243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez FG, Ritter J, Goodwin JW, et al. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surgeons. 2005;200:845–853. [DOI] [PubMed] [Google Scholar]
- 5.Parikh AA, Gentner B, Wu TT, et al. Perioperative complications in patients undergoing major liver resection with or without neoadjuvant chemotherapy. J Gastrointest Surg. 2003;7:1082–1088. [DOI] [PubMed] [Google Scholar]
- 6.Peppercorn PD, Reznek RH, Wilson P, et al. Demonstration of hepatic steatosis by computerized tomography in patients receiving 5-fluorouracil-based therapy for advanced colorectal cancer. Br J Cancer. 1998;77:2008–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panicek DM, Giess CS, Schwartz LH. Qualitative assessment of liver for fatty infiltration on contrast-enhanced CT: is muscle a better standard of reference than spleen? J Comput Assist Tomogr. 1997;21:699–705. [DOI] [PubMed] [Google Scholar]
- 8.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–527. [PubMed] [Google Scholar]
- 10.Hemming AW, Reed AI, Howard RJ, et al. Preoperative portal vein embolization for extended hepatectomy. Ann Surg. 2003;237:686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]


