Abstract
Objective:
To prevent bile duct injury by using a cold 5% glucose isotonic solution cooling in the bile ducts when radiofrequency (RF) is performed in a porcine model.
Summary Background Data:
Complications that may arise during liver RF ablation include biliary stenosis and abscesses.
Methods:
The RITA 1500 generator was used for the experiments. Two lesions were performed in the left liver. The pigs were killed 1 or 3 weeks after the procedure. An ex vivo cholangiogram was obtained by direct injection into the main bile duct. Samples of RF lesions, of liver parenchyma near and at a distance from the RF lesions, underwent pathologic studies. Two groups of 20 pigs each were treated: one without perfusion of the bile ducts and the other with perfusion of cold 5% glucose isotonic solution into the bile ducts. The Pringle maneuver was used in 50% of the RF procedures. Radiologic lesions were classified as biliary stenosis, complete interruption of the bile duct, or extravasation of the radiologic contrast liquid.
Results:
Histologic lesions of the bile ducts were observed near the ablated RF lesion site and at a distance from the RF lesions when a Pringle maneuver was performed. Radiologic and histologic lesions of the bile ducts were significantly reduced (P < 0.0001) when the bile ducts were cooled.
Conclusions:
Cooling of the bile ducts with a cold 5% glucose isotonic solution significantly protects the intrahepatic bile ducts from damages caused by the heat generated by RF when performed close to the bile ducts.
Biliary stenosis and abscesses may occur after radiofrequency ablation of liver tumors. This experimental study showed that bile ducts cooling using a cold 5% glucose isotonic solution protects intrahepatic bile ducts from damages caused by the heat generated by radiofrequency.
Radiofrequency (RF) ablation is a new technique used to destroy locally malignant hepatic tumors. The RF current is a high frequency sinusoidal current (400–500 kHz) that produces ionic agitation and results in frictional heat, distributed by conduction into the tissue about the electrode.1 At a temperatures near 60°C, the intracellular proteins and collagen are denatured, lipids are dissolved, and cellular death becomes irreversible. Coagulation starts at 70°C and tissue desiccation at 100°C, thus causing coagulation necrosis of the tumor tissue and the surrounding parenchyma.2–4 The morbidity rate of this technique varies according to the studies from 3% to 14%.1,5–9 The bile ducts cannot withstand the heating effect, leading to either ductal stenosis with dilation of the upper bile duct or abscess formation.10,11 Most authors recommend a minimal distance of 1.5 to 2 cm, from a proximal intrahepatic bile duct, to perform RF ablation without any risk.1,12–14
An experimental model was designed for secondary biliary lesions in pig.15 RF ablation in proximity to the bile duct consistently induced radiologic and histologic lesions. The radiologic lesions observed were either biliary stenosis with upstream dilation or complete interruption of the bile duct, or destruction of the bile duct with extravasation of contrast. Histologic examination showed desquamation of the biliary epithelium associated with destruction of the biliary duct wall. The aim of this study was to evaluate the role of bile ducts cold 5% glucose perfusion during RF ablation on secondary post-RF biliary lesions.
MATERIALS AND METHODS
Animals
Forty-four pigs French hybrid 54 EYL (Einville aux Jards, France 54 EYL), 12 to 14 weeks old, with an average weight of 32.8 ± 2.1 kg (range, 22–44.5 kg) were used. All animals received care in accordance with French legal requirements. All procedures were performed in the laboratory for experimental surgery (UPRES EA 2403) of the Faculty of Medicine in Nancy (54500 Vandoeuvre-lès-Nancy). Upon arrival, each group of pigs were kept in sheds for 7 days prior to any experiment.
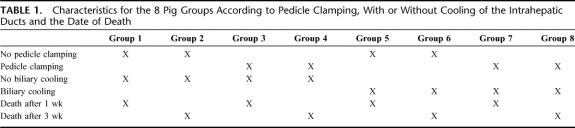
The animals were divided into 8 groups of pigs. Groups 1 to 4 were selected to the experimental model of intrahepatic lesions and groups 5 to 8 to the experimental model with cooling of the hepatic duct. The characteristics of each group are reported in Table 1.
TABLE 1. Characteristics for the 8 Pig Groups According to Pedicle Clamping, With or Without Cooling of the Intrahepatic Ducts and the Date of Death
Animal Preparation and Anesthesia
Twenty-four hours before and after RF, the food intake of the animal was restricted with free access to water. The animals were premedicated by injecting intramuscular ketamine hydrochloride (Ketalar, Parke-Davis, Courbevoie, France), midazolam (Hypnovel, Roche, Neuilly-sur-Seine, France), and atropine sulfate (Atropine Laboratory Aquettant, Aquettant, Lyon, France) at respective doses of 22 mg/kg, 1.5 mg/kg, and 0.25 mg. A 6.5-French catheter (Willy Rusch, Waiblingen, Germany) was placed on an ear vein. A dose of 2 mg/kg of 1% propofol (Diprivan, AstraZeneca, Rueil Malmaiso, France) was administered just before intubation. The pig was in a dorsal decubitus position.
Anesthesia was maintained with halothane (Halothane Belamont, Belamont, Paris, France) concentrated at 1.5%. Ventilation was performed with a respirator Monal A type (Compagnie Française de Produits Oxygène, Paris, France) with a 50% air/oxygen mix. The tidal volume was 15 mL/kg with a respiratory rate of 18 to 20; 750 mg of cefamandol (Kefandol, Lilly France, St. Cloud, France) was given intravenously prior to the cutaneous incision, with a second identical dose of cefamandol being injected during the closure of the laparotomy. One gram of paracetamol (Pro-dafalgan, Upsa, Agen, France) was administrated 20 minutes before the end of the surgical procedure by intravenous slow perfusion, for postoperative analgesia. Monitoring during anesthesia and wake-up was monitored with ECG from Hewlett Packard 78353A (Agilent Technology, Palo Alto, CA).
Surgical Procedure
A 15-cm median laparotomy was performed from the xiphoïd process. The hepatic pedicle was isolated on lace when clamping was programmed (hepatic artery and portal vein). The common bile duct was dissected when a choledochotomy was planned; 7.5 MHz linear probe was used to localize the intrahepatic bile ducts (Focus linear small parts probe, Siemens, Erlangen, Germany). The probe was connected to a Sonoline SL-2 ultrasound machine (Siemens, Erlangen, Germany). The RF electrode was placed under ultrasound imaging in contact with a bile duct. Every main bile ducts in pig liver are parallel to and in contact with the vessels of the portal system and the hepatic arterial system.16 At the end of the procedure, ultrasound imaging confirmed that the ablated zone overlaps the intrahepatic bile duct. Two lesions were created in each liver: one on the medial left lobe and another on the lateral left lobe. After placing the electrode accurately, the RF procedure was activated. The needle array was deployed to 2 cm, power was set at 50 W, and the target temperature was set at 80°C for a period of 5.5 minutes. Thirty seconds after the end of this first period, the electrode arrays were deployed to 3 cm, the power was set at 70 W, and target temperature at 105°C for a period of 5 minutes. Thirty seconds after the end of this second period, the temperatures displayed on the generator was expected to be greater than 60°C to validate the procedure.17 This protocol is recommended by the manufacturer of the RF device for a 3-cm tumor ablation in humans.
Cooling of the bile duct was performed by perfusing cold 5% glucose solution (Fresenius Kabi France, Sèvres, France). A transverse choledochotomy enabled the placement of a Seldicath 4F (Plastimed, Saint-Lieu-la-Forêt, France) catheter (4 cm long, section 1.3 mm) that was pushed into the common hepatic bile duct in the hepatic hilum. Selective catheterization, of the left or right hepatic bile ducts, was not performed. The diameter of the intrahepatic bile duct in a 40-kg pig was too small to move the catheter up the bile ducts. Second, the left and right intrahepatic bile ducts in pigs do not join in one left bile duct or one right bile duct, and every bile duct flows separately into the common bile duct. The right bile ducts flow into the common bile duct forming a 90° angle. The left bile ducts meet in a very short, left bile duct with a wider angle. We consequently chose to create 2 lesions on the left liver lobe to have a better cooling quality. The catheter was connected to a 1-L pouch of 5% glucose isotonic solution, connected via a perfusion set with stop cock (Codan, Lensahm, Germany). Through the stop cock, samples of the serum glucose, and temperature measurements could be performed. The isotonic glucose was introduced using a pressure infusor (Infusable Vital Signs, Barnham, UK) allowing fluid delivery control at exactly 90 mL/min. We used 2 pouches per RF procedure. Each procedure lasted 15 minutes, and cooling of the bile ducts was initiated 5 minutes prior to the RF procedure, thereby providing a total cooling time of 20 minutes. The isotonic glucose solution pouches were exchanged before they were completely emptied. We used 900 mL per pouch: 1800 mL/20 minutes = 90 mL/min. The catheter was fixed at the peritoneum of the hepatic pedicle with a polyamide monofilament 3.0 stitches (Ethilon Johnson&Johnson, Brussels, Belgium). The bile and the glucose solution were drained through the choledochotomy and removed from the peritoneal cavity. The choledochotomy was sutured with separate stitches with a polypropylene 1.0 suture (Prolène Johnson&Johnson, Brussels, Belgium). When the pedicle was clamped using the Pringle maneuver, it was maintained during the 2 RF procedures.
RF System
The RITA 1500: RF generator (RITA Medical Systems, Inc., Mountain View, CA) is a monopolar system rated at 460 kHz 150 W at 50 ohms. Two dispersive electrodes were placed on the anterior face of the pig back legs. We used a Starburst XL (RITA Medical Systems, Inc.) 15 gauge, 15 cm length with 7 retractable arrays. The needle is insulated on its whole length except the distal 0.5 cm of the tip, allowing us to ablate the needle track when retracting the electrode at the end of the procedure. Four of the 7 electrode arrays have thermocouples that monitor tissue temperature in real time. Impedance of the necrotic zone and the power delivered by the generator were displayed in real time on the generator screen. The preoperative settings of the generator (target temperature, impedance, power delivery, time set, control mode, thermocouple selection) were saved on the computer. The RITA generator adjusted the power delivery according to the tissue impedance (the power delivery decreased when the impedance increased) and according to the target temperatures set for the thermocouples situated at the tip of 4 of the 7 arrays that were deployed in the liver. The generator delivered the maximum set power until the target temperature was reached and then automatically adjusted the power to maintain the target temperature at the tip of the 4 thermocouples.16 The generator calculates the average temperature in the ablation zone. When one of the 4 thermocouples was placed into contact with or inside a cooled hepatic duct, the temperature did not rise over 45°C to 50°C. In such cases, the electrode was removed from the algorithm. As soon as the target temperature was reached, the countdown started.
Temperature Measurement
The temperature of the perfusion liquid was measured as it came out of the refrigerator during setup, and as it was removed from the catheter. A thermocouple thermometry sensor (Digi-Sense Cole Parmer Instrument Company, Chicago, IL) was used for this purpose.
Liver Removal
The pig was premedicated using the same procedure described for the RF application. The death was performed by intravenous injection of 500 mg of thiopental sodium (Penthotal Abbot, Rungis, France) with 4 mg pancuronium bromide (Pavulon, Laboratories Rion, Rion, France), followed by the injection of 20 mL of potassium chloride at 10% (Fresenius Kabi, Sèvres, France).
Data Analyses
Radiologic Study
A cholangiography was performed immediately after the liver removal. A cholangiographic drain 10-French and 25 cm long (Pedinelli, Porgès, Le Plessis Robinson, France) was placed into the common bile duct. The cholangiography was performed by injecting ioxalate sodium and meglumine concentrated at 320 mg of iodine per milliliter (Hexabrix, Guerbet, Aulnay-sous-Bois, France). Two milliliters of ethylene blue dye at 1% (Renaudin, Itxassou, France) was also added. The ethylene blue dye allowed viewing of the bile ducts during the histologic microscopic dissection prior to fixing. By studying the cholangiograms, we could determine whether there was a biliary lesion at the ablated zone, with or without any impact on the upper bile duct causing dilation. Biliary stenosis was defined by the ratio equal or less than 50% of the diameter of the ablated zone over the diameter of the bile duct situated close to the inferior ablated zone.
Histologic Study
Each hepatic lesion was sectioned transversally. Necrotic lesion dimensions were determined by caliper measurements. The volume of each necrotic lesion was calculated using the following formula: V = 4/3 × Π × (a/2) × (b/2) × (c/2) (where a is the longitudinal diameter, b is the short diameter, and c is the thickness). The hepatic sample was fixed with alcoholic Bouin liquid (Dubosq-Brasil, Elvetec Services, Genas, France) for 24 hours. The studies focused on the ablated lesions, at the periphery of these lesions, up and down the bile ducts in contact with the lesions, and at a distance from the lesions on the left liver and on the right liver. The samples were fixed in paraffin and then cut up in series of 6 μm. They were colored with saffron hematoxylin and eosin before microscopic analysis.
Statistic Tests
Data are given as mean values ± SEM. The pigs that died before the hepatic removal were excluded except for the mortality criteria. The effective comparison was made by the χ2 test and the average comparison using the Mann-Whitney U test. The significance level for all tests was fixed at P = 0.05. Statistical analysis was done using BMDP software (BMDP Statistical Software, Inc., Los Angeles, CA).
RESULTS
Postoperative Complications
Four pigs died (9%) before the end of follow-up. The first died 15 days after the experiment, after a fight with other pigs. Hepatic removal was not performed because of the decomposition of the cadaver. The second one died 4 days after the experiment, probably due to prolonged hepatic clamping considering its weight. During the autopsy, no peritonitis was detected, but the liver showed signs of ischemia. The third pig was found dead 6 days after the experiment. It had tracheal injury during intubation resulting in subcutaneous emphysema. Upon awakening and during the first few days after the experiment, the injury did not cause any problems and the autopsy did not show any signs of peritonitis or ischemia. The fourth pig died during the experiment from preoperative cardiac disorders (tachycardia, fibrillation, and heart failure) secondary to a malignant hyperthermia of the halothane. The histologic study of the excised liver showed a shocked liver with diffused distress to the hepatic cells. These 4 pigs were excluded from the radiologic and histologic analysis.
The morbidity rate was 12.5%: 4 subcutaneous abscesses and 1 biliary peritonitis. The biliary peritonitis, secondary to a leak of the choledochotomy suture, was detected when the liver was removed 1 week after the experiment.
The Cooling Impact on the Volume of Necrosis
The average necrosis volume after RF ablation (Fig. 1) after 1 week and after 3 weeks were 16.4 ± 4.8 cm3 and 7.7 ± 2.8 cm3 (P = 0.001), respectively. The average volume of necrosis was 6.2 ± 1.4 cm3 without clamping and 17.9 ± 5.1 cm3 with clamping (P = 0.0005). The necrotic volume was 14.1 ± 5.1 cm3 without cooling and 10 ± 2.7 cm3 with cooling of the bile ducts (P = 0.9, not significant). The average temperature at the beginning of the perfusion was 7.1°C ± 0.8°C with extremes from 2°C to 13°C. The average temperature at the end of the perfusion was 10.1°C ± 0.8°C (SD = 2.6°C) with extremes from 5.5°C to 16.5°C.
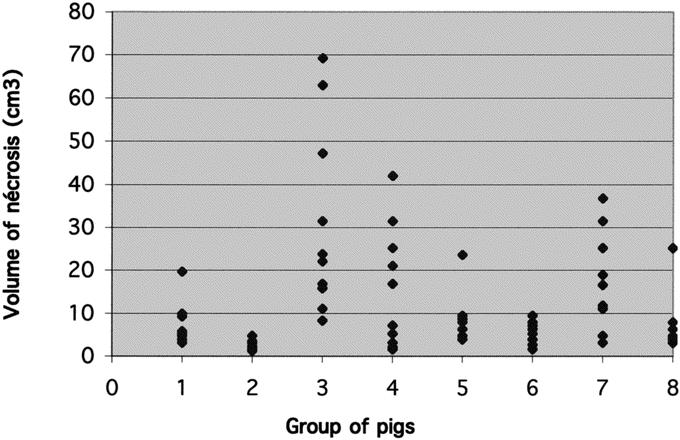
FIGURE 1. Distribution of the necrosed volumes (cm3) per pig group. In groups 1, 3, 5, and 7, the liver was removed 1 week after the RF procedure. In groups 2, 4, 6, and 8, 3 weeks after the RF ablation. Pedicle clamping: groups 3, 4, 7, and 8. Biliary cooling: groups 5–8.
The Cooling Impact on the Radiologic Study
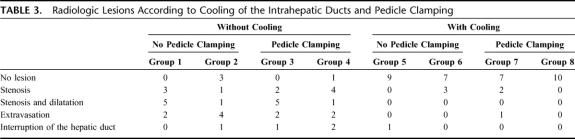
We observed that cooling of the bile ducts strongly influenced the radiologic lesions (Table 2). We observed 36 radiologic lesions (90%) among 40 liver ablations without bile ducts cooling and 7 lesions (18%) with bile ducts cooling. Furthermore, no radiologic lesion was observed at distance from the ablated zone when the bile ducts were cooled. Radiologic lesions according to cooling of the intrahepatic ducts and pedicle clamping are reported in Table 3. The clamping of the hepatic pedicle and the date of removal of the liver did not influence radiologic biliary lesions.
TABLE 2. Radiologic Lesions According to Cooling of the Intrahepatic Ducts
TABLE 3. Radiologic Lesions According to Cooling of the Intrahepatic Ducts and Pedicle Clamping
Cooling Impact on Histologic Studies
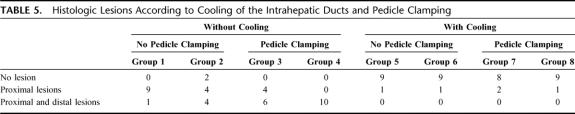
We observed that cooling of the bile ducts strongly influenced the histologic lesions (Table 4). We observed 38 histologic lesions (95%) among 40 ablations without cooling of the bile ducts and 5 histologic lesions with cooling of the bile ducts (13%). We observed no histologic lesions at distance from the ablated zone when the cooling of the bile ducts was performed during the ablation independent of clamping of the pedicle.
TABLE 4. Histologic Lesions According to Cooling of Intrahepatic Ducts
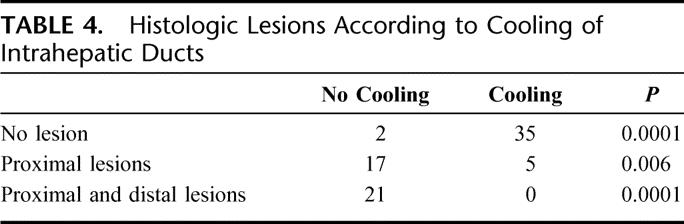
The histologic study of the necrotic zones with cooling of the bile ducts showed a certain lack of homogeneity of the necrosis. The necrotic zones in contact with the cooled bile duct were incomplete. Cells structure persisted, but they were not functional since the intracellular cytoplasmic structure had coagulated (Fig. 2A). The necrosis at the center of the lesion was total (Fig. 2B). This lack of homogeneity was only observed when the cooling of the bile duct without clamping of the pedicle was performed. When clamping and cooling were performed, the necrosis was total and accurate, with persistence of some phantoms of cell nucleus.
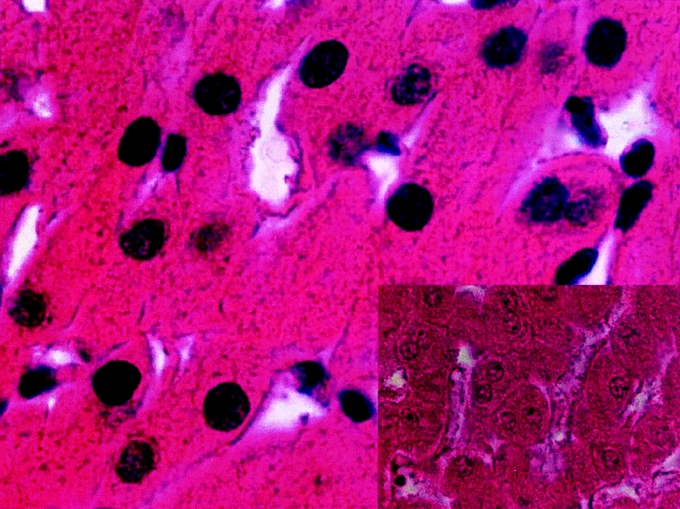
FIGURE 2. A, Histologic appearance of incomplete necrosis of hepatic parenchyma. Radiofrequency was performed without hepatic pedicle clamping. The bile ducts (BD) were cooled with cold 5% glucose isotonic serum. Hepatic removal was performed 1 week after RF (hematoxylin and eosin stain). The cell structures indicated that the intracellular cytoplasmic structures had coagulated (original magnification ×40). B, Histologic appearance in the center of the same area of hepatic necrosis: the necrosis was total (original magnification ×40) (inset).
When the bile duct was cooled (with or without clamping), the histologic study of the bile duct in contact with the necrotic area showed that the integrity of the bile duct had been preserved in 35 of 40 cases (Fig. 3). In 5 cases, we observed biliary duct lesions in contact with the necrotic area. These lesions were desquamations of the epithelium or glossy aspect of the epithelium. The structure of the bile ducts crossing the necrotic zone was preserved (Fig. 4).
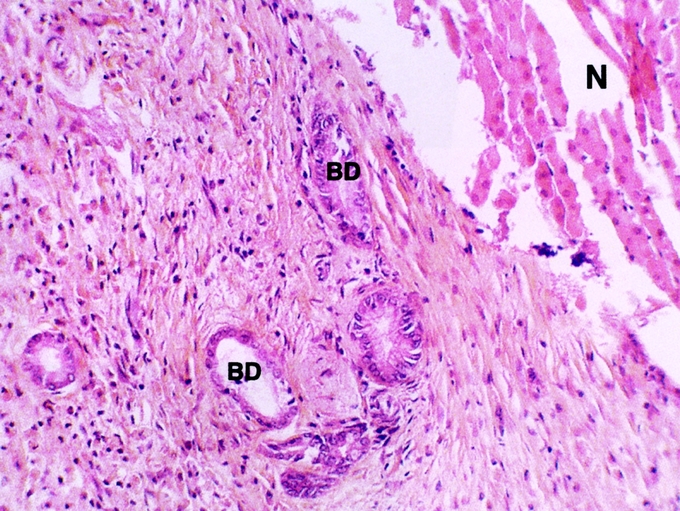
FIGURE 3. Histologic appearance of bile duct (BD) after hepatic radiofrequency (RF) necrosis with hepatic pedicle clamping. The bile ducts were cooled with cold 5% glucose isotonic serum. Hepatic removal was performed 3 weeks after RF (hematoxylin and eosin stain). The bile ducts in contact with the necrotic area were preserved. Necrosis (N) of hepatic parenchyma (original magnification ×20).
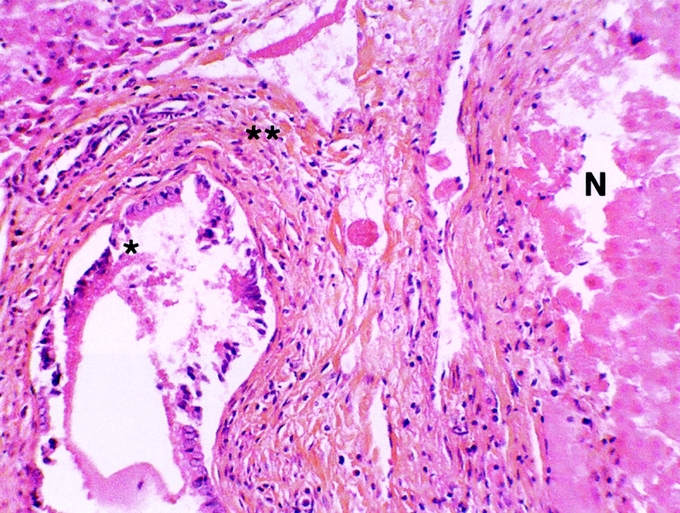
FIGURE 4. Histologic appearance of damaged bile duct (BD) in contact with the hepatic radiofrequency (RF) necrosis. RF was performed with hepatic pedicle clamping. The bile ducts were cooled with cold 5% glucose isotonic serum. Hepatic removal was performed 3 weeks after RF (hematoxylin and eosin stain). *The epithelium was dislocated. **The bile duct wall was preserved. Necrosis (N) of hepatic parenchyma (original magnification ×20).
Histologic lesions according to cooling of the intrahepatic ducts and pedicle clamping are reported in Table 5. The localization of the histologic lesions on the biliary tree was influenced by the clamping of the pedicle with a bigger proportion of distanced lesions of the biliary tree when the clamping of the pedicle without cooling was associated. The liver removal date also influenced the localization of the biliary lesions on the biliary tree. There were more distal lesions associated to proximal lesions when the liver was removed after 3 weeks.
TABLE 5. Histologic Lesions According to Cooling of the Intrahepatic Ducts and Pedicle Clamping
DISCUSSION
In 2005, the only possibly curative approach for patients with liver tumor is surgical resection.8 However, following extended or repeated hepatectomies or severe cirrhosis, some patients have poor hepatic reserve. To avoid the risk of hepatic failure that can follow hepatic resection in such patients, RF ablation could be an alternative treatment.14 RF is on the way to be considered as a first-line local palliative therapy for unresectable liver tumors owing to be a safety procedure with acceptable complication rates.4,8,14 Mulier et al6 described the complications of RF ablation of liver tumors from 82 series found in the literature, published from 1990 to December 2001. The bile ducts represented 1% of the complications in 3670 patients. This rate was probably underestimated since peripheral biliary lesions are often clinically asymptomatic. Furthermore, biliary lesions have delayed presentation up to 4 months after RF.5,18
We observed that cooling the intrahepatic bile ducts with a cold 5% glucose isotonic solution prevented radiologic and histologic lesions during an RF ablation in contact with the intrahepatic bile ducts. Indeed, the radiologic and histologic lesions observed without biliary cooling were not observed anymore when the bile ducts were chilled. The group of pigs that had their intrahepatic bile ducts cooled showed more radiologic biliary lesions (7 cases) than histologic lesions (5 cases). The radiologic lesions associated with no histologic lesions were hepatic ducts that crossed the necrotic zone. Indeed, the bile ducts surrounding the ablated zone seemed protected from the heat, and we observed that only the bile ducts included in the necrosis were damaged. Since liver tumors do not invade the biliary structures but tend to press them back,19 it may be argued that these radiologic lesions that we observed in the pig19,20 would not exist in humans. Intrahepatic bile ducts are more suspected to surround human liver tumors and consequently be more protected from the heat. Interestingly, 3 of these 5 histologic lesions occurred on clamped livers. Elias et al21 emphasized that hepatic flow (no pedicle clamping) could prevent the heat diffusion to the bile duct, but with a potentially higher risk of tumor recurrence.22,23
Moreover, the pig intrahepatic bile ducts run along and in contact with the hepatic vessels. The vascular flow is also considered to protect the bile ducts from the thermal effects of RF by dissipating the heat generated in the ablated area (“cooling effect” or “heat-sink effect”).17,19,20,24,25 To neutralize the “cooling effect” and to evaluate more specifically the role of bile ducts cooling, we also clamped the hepatic pedicle during the present study. The use of pedicle clamping had a great effect on the lesion size. We also observed a relation between pedicle clamping and presence of histologic lesions at longer distance from the RF zone.15 The tissue reaction created by the ablation continues through 7 days after the procedure;26 thus, the time to remove the liver was fixed at 1 week and 3 weeks after the ablation. This allowed to follow up the evolution of the lesions and particularly the necrosis of the lesions that shrinks and modifies over time.27
We are not aware of other experimental studies in the literature evaluating bile duct protection during RF ablation. However, Seifert et al28 protected bile ducts with hot serum perfusion in a pig ex vivo liver model using cryotherapy in vicinity of the bile ducts. This model consisted in the perfusion of the bile duct with a saline-based isotonic serum heated at 37°C, via a catheter introduced through a choledochotomy.28 Cryotherapy (instead of RF), type, and temperature of cooling liquid and bile ducts cooling liquid flow rates were different in this study and make comparison unreliable.
Several authors have proposed different solutions when an ablation was considered near the bile ducts to prevent secondary lesions. Bilchick et al29 recommended placing a stent before the ablation of central lesions dangerous for the bile ducts. Elias et al21 reported the cooling of the bile ducts in 3 cases. In follow-up over 3 months, the 3 patients did not show any evidence of biliary complications or local tumor recurrences.
Studies evaluating local recurrences after RF ablation of primary tumors (hepatocellular carcinoma) or secondary tumors (mainly from colorectal tumors) showed a correlation with the size of the tumor (up to 35% for tumors with a diameter over 2–3 cm).30–32 Several solutions have been developed to lower the local recurrence rate. These solutions included the use of sodium chloride, either by perfusing the tumor with an isotonic physiologic serum during the RF procedure33 or by injecting, prior to the procedure, hypertonic physiologic serum into the tumor.34 Sodium chloride, whatever its concentration,34 modifies the electric conductivity inside the hepatic tissue. For this reason, physiologic serum (sodium chloride 0.9 g/100 mL) and ringer lactate (containing sodium chloride 0.6 g/100 mL) were not used in this study to cool the bile ducts. We demonstrated that cooling of the bile ducts with a cold 5% glucose isotonic solution effectively prevented biliary lesions during an ablation. However, the key point is still to set up the optimal bile duct cooling temperature that could prevent biliary lesions but should also guarantee the intended therapeutic effect (tumor necrosis volume and low recurrence rate) at the same time. Another issue to evaluate is to know whether the less complete necrotic zones observed in contact with the cooled hepatic ducts are viable by studying the enzymatic activity of the mitochondria24 or the respiratory metabolism by using a NADH-diaphoresis tinting (nicotinamide adenine dinucleotide-diaphorase).35
CONCLUSION
Cooling of the intrahepatic bile ducts with a cold 5% glucose isotonic solution during an RF ablation of liver tissue in contact with intrahepatic bile ducts protects them from secondary effects of hyperthermia. This protection is effective for the bile ducts situated in contact with the ablated zones and on bile ducts at distance. The total volume of necrosis achieved is not significantly different with or without biliary cooling.
ACKNOWLEDGMENTS
The authors thank Dominique Marius Leprince and Liane Goergen for manuscript assistance, and Yasmine Elsari, Bruno Di-Cientio, and the Radiology and Pathology's staff for technical assistance.
Footnotes
Reprints: Frederic Marchal, MD, Department of Surgery, Centre Alexis Vautrin, Av. de Bourgogne, 54511 Vandoeuvre-lès-Nancy, France. E-mail: f.marchal@nancy.fnclcc.fr.
REFERENCES
- 1.De Baere T, Elias D, Dromain C, et al. Radiofrequency ablation of 100 hepatic metastases with a mean follow-up of more than 1 year. AJR Am J Roentgenol. 2000;175:1619–1625. [DOI] [PubMed] [Google Scholar]
- 2.Curley SA. Radiofrequency ablation of malignant liver tumors. Oncologist. 2001;6:14–23. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg SN, Gazelle GS, Dawson SL, et al. Tissue ablation with radiofrequency: effect of probe size, gauge, duration and temperature on lesion volume. Acad Radiol. 1995;2:399–404. [DOI] [PubMed] [Google Scholar]
- 4.Parikh AA, Curley SA, Fornage BD, et al. Radiofrequency ablation of hepatic metastases. Semin Oncol. 2002;29:168–182. [DOI] [PubMed] [Google Scholar]
- 5.Machi J, Uchida S, Sumida K, et al. Ultrasound-guided radiofrequency thermal ablation of liver tumors: percutaneous, laparoscopic and open surgical approaches. J Gastrointest Surg. 2001;5:477–489. [DOI] [PubMed] [Google Scholar]
- 6.Mulier S, Mulier P, Ni Y, et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206–1222. [DOI] [PubMed] [Google Scholar]
- 7.Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood TF, Rose M, Chung M, et al. Radiofrequency ablation of 231 unserectable hepatic tumors: indications, limitations and complications. Ann Surg Oncol. 2000;7:593–600. [DOI] [PubMed] [Google Scholar]
- 9.Stippel DL, Töx U, Gossmann A, et al. Successful treatment of radiofrequency-induced biliary lesions by interventional endoscopic retrograde cholangiography (ERC). Surg Endosc. 2003;17:1965–1970. [DOI] [PubMed] [Google Scholar]
- 10.Bilchik AJ, Rose DM, Allegra DP, et al. Radiofrequency ablation: a minimally invasive technique with multiple applications. Cancer J Sci Am. 1999;5:356–361. [PubMed] [Google Scholar]
- 11.Nordlinger B, Rougier P. Nonsurgical methods for liver metastases including cryotherapy, radiofrequency ablation, and infusional treatment: what's new in 2001? Curr Opin Oncol. 2002;14:420–423. [DOI] [PubMed] [Google Scholar]
- 12.Dodd GD, Soulen MC, Kane RA, et al. Minimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough. RadioGraphics. 2000;20:9–27. [DOI] [PubMed] [Google Scholar]
- 13.Pearson AS, Izzo F, Fleming D, et al. Intraoperative radiofrequency ablation or cryoablation for hepatic malignancies. Am J Surg. 1999;178:592–599. [DOI] [PubMed] [Google Scholar]
- 14.Elias D, De Baere T, Mutillo I, et al. Intraoperative use of radiofrequency treatment allows an increase in the rate of curative liver resection. J Surg Oncol. 1998;67:190–191. [DOI] [PubMed] [Google Scholar]
- 15.Marchal F, Elias D, Rauch P, et al. Biliary lesions during radiofrequency ablation in liver: study on the pig. Eur Surg Res. 2004;36:88–94. [DOI] [PubMed] [Google Scholar]
- 16.Baronne R. Glandes annexes de l'intestin: foie. In: Baronne R, ed. Anatomie comparé des mammifères domestiques. Paris: Vigot, 1984:507–561. [Google Scholar]
- 17.De Baere T, Denys A, Wood BJ, et al. Radiofrequency liver ablation: experimental comparative study of water-cooled versus expandable systems. AJR Am J Roentgenol. 2001;176:187–192. [DOI] [PubMed] [Google Scholar]
- 18.Livraghi T, Solbiati L, Meloni MF, et al. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–451. [DOI] [PubMed] [Google Scholar]
- 19.Hansen PD, Rogers S, Corless CL, et al. Radiofrequency ablation lesions in a pig liver model. J Surg Res. 1999;87:114–121. [DOI] [PubMed] [Google Scholar]
- 20.Patterson EJ, Scudamore CH, Nagy AG, et al. Radiofrequency ablation of porcine liver in vivo-effects of blood flow and treatment time on lesion size. Ann Surg. 1998;227:559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elias D, El Otmany A, Goharin A, et al. Intraductal cooling of the main bile ducts during intraoperative radiofrequency ablation. J Surg Oncol. 2001;76:297–300. [DOI] [PubMed] [Google Scholar]
- 22.Heisterkamp J, Van Hillgersberg R, Ijzermans JNM. Critical temperature and heating time for coagulation damage: implications for interstitial laser coagulation (ILC) of tumors. Lasers Surg Med. 1999;25:257–262. [DOI] [PubMed] [Google Scholar]
- 23.Prudhomme M, Rouy S, Tang J, et al. Biliary structures lead to tumour recurrences after laser-induced interstitial thermotherapy. Lasers Surg Med. 1999;24:269–275. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg SN, Hahn PF, Tanabe KK, et al. Percutaneous radiofrequency tissu ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol. 1998;9:101–111. [DOI] [PubMed] [Google Scholar]
- 25.Chinn SB, Lee FT, Kennedy GD, et al. Effect of vascular occlusion on radiofrequency ablation of the liver: results in a porcine model. AJR Am J Roentgenol. 2001;176:789–795. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg SN, Gazelle GS, Campton CC, et al. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer. 2000;88:2452–2463. [PubMed] [Google Scholar]
- 27.McGahan JP, Brock JM, Tesluk H, et al. Hepatic ablation with use of radio-frequency electrocautery in the animal model. J Vasc Interv Radiol. 1992;3:291–297. [DOI] [PubMed] [Google Scholar]
- 28.Seifert JK, Dutkowski P, Junginger T, et al. Bile duct warmer in hepatic cryosurgery: a pig liver model. Cryobiology. 1997;35:299–302. [DOI] [PubMed] [Google Scholar]
- 29.Bilchik AJ, Wood TF, Allegra DP. Radiofrequency ablation of unresectable hepatic malignancies: lessons learned. Oncologist. 2001;6:24–33. [DOI] [PubMed] [Google Scholar]
- 30.Solbiati L, Goldberg SN, Ierace T, et al. Hepatic metastases: percutaneous radio-frequency ablation with cooled-tip electrodes. Radiology. 1997;205:367–373. [DOI] [PubMed] [Google Scholar]
- 31.Livraghi T, Goldberg SN, Lazzaroni S, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214:761–768. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg SN, Gazelle GS, Solbiati L, et al. Ablation of liver tumors using percutaneous RF therapy. AJR Am J Roentgenol. 1998;170:1023–1028. [DOI] [PubMed] [Google Scholar]
- 33.Haensler JM, Strobel D, Wein A, et al. Percutaneous ultrasound guided radio frequency tissue ablation (RFTA) with a new applicator type-treatment of hepatocellular (HCC) and liver metastases. Ultrasound Med Biol. 2000;26:2761A. [Google Scholar]
- 34.Goldberg SN, Ahmed M, Gazelle GS, et al. Radio-frequency thermal ablation with NaCl solution injection: effect of electrical conductivity on tissue heating and coagulation-phantom and porcine liver study. Radiology. 2001;219:157–165. [DOI] [PubMed] [Google Scholar]
- 35.Neumann RA, Knobler RM, Pieczkowski F, et al. Enzyme histochemical analysis of cell viability after argon laser-induced coagulation necrosis of the skin. J Am Acad Dermatol. 1991;25:991–998. [DOI] [PubMed] [Google Scholar]