Abstract
Objective:
To assess the immediate (0–4 hours) postoperative pain level in patients after laparoscopy and laparotomy whose analgesic requirement in the Post-Anesthesia Care Unit (PACU) exceeds standard morphine therapy.
Background data:
Clinical observation has raised the suspicion that laparoscopic surgery may be associated with more intense immediate postoperative pain than expected.
Methods:
This prospective study assessed the 24-hour pain intensity and analgesia requirements in patients who underwent similar abdominal surgery via laparoscopy or laparotomy under standardized general anesthesia and whose pain in the PACU was resistant to 120 μg/kg intravenous morphine.
Results:
Of 145 sampled PACU patients, 67 were in pain (≥6 of 10 VAS) within a 30-minute postoperative period. They were then given up to 4 intravenous boluses of 15 μg/kg morphine + 250 μg/kg ketamine. The pain VAS of 36 laparotomy patients was 4.14 ± 2.14 (SD) and 1.39 ± 0.55 at 10 and 120 minutes, respectively, after 1.33 ± 0.59 doses of morphine + ketamine; the pain VAS of 31 laparoscopy patient was 6.06 ± 1.75 and 2.81 ± 1.14, respectively (P < 0.0005) following 2.0 ± 0.53 doses (P = 0.0005). Diclofenac 75 mg intramuscular usage was similar (P = 0.43) between the groups up to 9 hours after surgery but was higher in the laparotomy group by 24 hours (P = 0.01). Pain scores at 24 hours after surgery were lower for the laparoscopy patients (3.01 ± 0.87) compared with their laparotomy counterparts (4.45 ± 0.98, P < 0.001).
Conclusions:
Among patients after abdominal surgery with severe immediate (0–4 hours) postoperative pain, laparoscopic patients are a significant (46%) proportion, and their pain is more intense, requiring more analgesics than painful patients (54%) do after laparotomy. By 24 hours, the former are in less pain than the latter.
Severe pain resistant to standard morphine treatment was encountered immediately (0–4 hours) after abdominal surgery in 67 of 145 sampled PACU patients. Among these painful patients, the 4-hour mean pain intensity after laparoscopy (31 of 69 individuals) was higher by 60% compared with laparotomy (36 of 76 patients); 33% more doses of analgesics were required to satisfactorily control such pain in the former. The 24-hour parameters after laparoscopy were better than after laparotomy.
Observation in the Post-Anesthesia Care Unit (PACU) suggests sustained nociceptive input in an unspecified number of postlaparoscopy patients, sometimes more intense than after open laparotomy (Weinbroum AA, unpublished data), and requiring more analgesia. This observation apparently questioned clinical conviction and previous data1,2 of an overall better recovery and lower rate of analgesic consumption by patients who undergo laparoscopy rather than laparotomy. Since less pain is one of the advantages associated with laparoscopic surgery,3,4 we thought that this issue of immediate postoperative pain must be addressed. We specifically aimed at evaluating the magnitude of pain in laparoscopy versus laparotomy populations that is uncontrollable by a standard postoperative morphine dose.
PATIENTS AND METHODS
We prospectively studied ASA physical status I to III patients scheduled for elective cholecystectomy, small bowel resection, or nephrectomy during morning prime-shifts (between 8 AM and 3 PM) during August to October 2002. The decision of which surgical technique would be used was exclusively taken by the surgeons who were experienced in either surgical technique (>100 procedures/surgeon); the same surgical and anesthesia teams took care of all patients. Laparoscopy was performed with abdominal insufflation of CO2 at 12 mm Hg using a standard automated insufflator, and no local anesthetic was used perioperatively.
Exclusion criteria included morbid obesity (BMI > 35), disturbances of the central nervous system or psychiatric diseases, chemical substance abuse, chronic or recent (≤2 months) use of analgesics, chronic pain, cardiovascular, hepatic or renal insufficiency, pregnancy, and age < 18 years.
Departmental standard general anesthesia consisted of intravenous administration of propofol 1.5 to 2 mg/kg for induction, rocuronium infusion to facilitate tracheal intubation and obtain intraoperative muscle relaxation, fentanyl 2 to 3 μg/kg for intraoperative analgesia, and inhaled anesthesia consisting of nitrous oxide in oxygen (ratio of 2:1) enriched with isoflurane as deemed necessary by the attending anesthetist. Neuromuscular relaxation was reversed pharmacologically at the end of surgery with atropine and neostigmine.
We have recently shown that patients in the early postoperative period may require substantial amounts of IV morphine to satisfactorily control pain.5 If 120 μg/kg morphine was insufficient, IV administration of combined low-dose ketamine and morphine promptly and most efficaciously controlled such pain and minimized further rescue drug supplementation. This has now become our routine protocol of analgesia in such patients.
While recovering in the PACU, all patients initially received morphine IV (according to patient request) consisting of 2-mg increments every 5 minutes. All patients whose self-rated pain intensity ≤ 5/10 after 120 μg/kg of morphine within a 30-minute period were considered patients with controllable pain and were not included in the study.6 Conversely, patients who complained of pain (≥6/10 VAS) after the above-mentioned amount of morphine had been administered were considered to be suffering from severe pain and were the subject of the present study.
To be further treated, the study patients had to have an acceptable cognitive state (≥5 in an objective 1–10 VAS) according to the attending physician (a single measurement to enter the study protocol) and to self-rate themselves awake (≥5/10 VAS). A cutoff pain VAS score of ≥ 6/10 was chosen based on previous experience in acute pain control, where a 4 to 5/10 VAS expressed sustained but not severe pain.5,7 The analgesia regimen in these patients consisted of 15 μg/kg morphine plus 250 μg/kg ketamine available every 5 minutes, as is currently applied in our PACU to painful patients. Patients could receive up to 4 such IV boluses until either pain VAS became ≤ 5/10 or they received the maximum of 4 doses. If pain was still intense 5 minutes after their fourth dose, a rescue dose of intramuscular diclofenac 75 mg was available to the patient. Painful patients with lower than 5/10 objective cognitive state or subjective level of wakefulness were observed until their levels increased. At that point, they were eligible for further pain management as described.
The PACU physician and nurses, who were blinded to the study goals, assessed the parameters listed below during the patients' stay in the PACU. VASs were assessed using the 10-cm chiroscience pain gauge every 5 minutes for the first hour and every 15 minutes thereafter:
Subjective pain intensity that was graded on a self-rated VAS, ranging between 0 (no pain) and 10 (worst possible pain).
Patient's subjective level of wakefulness was assessed by a self-rated VAS from 1 (heavily sedated) to 10 (fully awake).
Subjective feeling of well being was recorded by a VAS of 1 (sad and gloomy) to 10 (happy and content).
If the patient was asleep, he/she was awakened to obtain his/her rating; the data of a patient who became uncooperative were excluded from the study from that time point onward.
Study vital signs included noninvasive blood pressure, a 5-lead electrocardiogram, respiratory rate, and fingertip pulse-derived O2 saturation (SpO2) on air (CardiocapTM, Datex, Helsinki, Finland). A SpO2 <92% under 40% oxygen by facemask caused the withdrawal of the patient from the study from that time point onward. Untoward effects were recorded by the nurses and treated if deemed necessary by the attending physician (eg, metoclopramide 10 mg IV for nausea or vomiting).
On-ward parameters were recorded every hour, the nurses being blinded as to the aim of the study. Intramuscular diclofenac 75 mg was available to the patients every 6 hours.
All patients were kept for observation in the PACU for 4 hours, and then transferred to the ward. Side effects were recorded by the medical staff, using a standardized checklist, which included all known side effects of the surgical procedures and the administered drugs.
The statistical analyses were performed at the Statistical Laboratory of the School of Mathematics, Tel-Aviv University, using the SPSS Release for Windows, Version 11.01 (Chicago, IL, 2001). A prestudy power table (using a 2-sample t test) where delta (mean difference in pain scale recorded in a pilot study) = 2.0 ± 0.8, alpha = 0.05, and power = 0.97 resulted in the need for a minimum of 10 patients per group. The demographic data (age, weight) and background characteristics (eg, amounts of intraoperative fentanyl and sedation and feeling of well-being VAS) were compared using the one-way ANOVA. Because part of the pain VASs did not distribute normally, these and the number of injections of morphine + ketamine per group were analyzed using the Mann-Whitney U test. Gender and group distribution of the type of procedure as well as the incidence of side effects and drugs to treat them were analyzed using the Fisher exact test. All physiologic parameters during the observation period in the PACU were analyzed using the one-way ANOVA with repeated measures; this test was always followed by the post hoc Tukey's Honest Significant Difference method. Analyses of PACU and on-ward use of diclofenac were carried out using Fisher exact test. Values are expressed as mean ± SD, median and range values, or absolute numbers, with significance defined as P ≤ 0.05.
RESULTS
Of the 145 3-month PACU sample (mean age 59 ± 15 years, weight 73 ± 13 kg), 67 (46% of the population sample, 31 laparoscopy, and 36 laparotomy) patients fulfilled the study entry criteria. The other 78 laparoscopy and laparotomy patients had their pain satisfactorily controlled by morphine alone. None of the 67 eligible patients was later withdrawn from the study.
The demographic and surgical data were similar between the 2 surgical groups (Table 1). All baseline vital signs and the physician-rated cognitive state (data not shown), patients' self-rated pain intensity scores, and levels of wakefulness and feeling before morphine + ketamine administration was started were also similar (Figs. 1A, 2). The amounts of IV analgesics that were requested during patients' PACU stay were associated with the surgical technique. Specifically, the laparoscopy individuals required 35% more injections of morphine + ketamine than those used by the laparotomy group (P = 0.001, Table 1). Furthermore, 20 of the 31 laparoscopy patients demanded 2 or 3 doses compared with only 10 among the 36 laparotomy patients (P = 0.001, Table 1). In addition, the maximal number of injections was 4/patient in the former group (in 3 patients) but only 3/patient in 2 of the latter. The number of injections within the different types of surgery showed the same distribution as for the study group itself (data not shown); diclofenac in the PACU was similarly used (Table 1). This higher analgesia consumption in the laparoscopy group was limited, however, to the period of the PACU stay. Between 5 and 9 hours postoperatively, 5 patients in each group requested diclofenac on the ward, whereas such demand during the following 15 hours was significantly (P = 0.01) higher among the laparotomy patients compared with the laparoscopy ones (Table 1).
TABLE 1. Demographic and Perioperative Data of the Study Patients
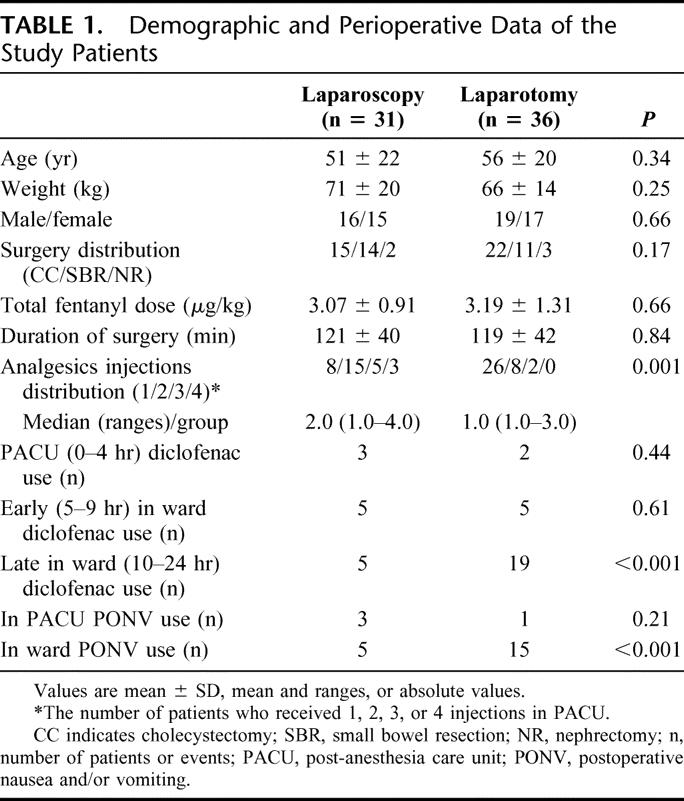
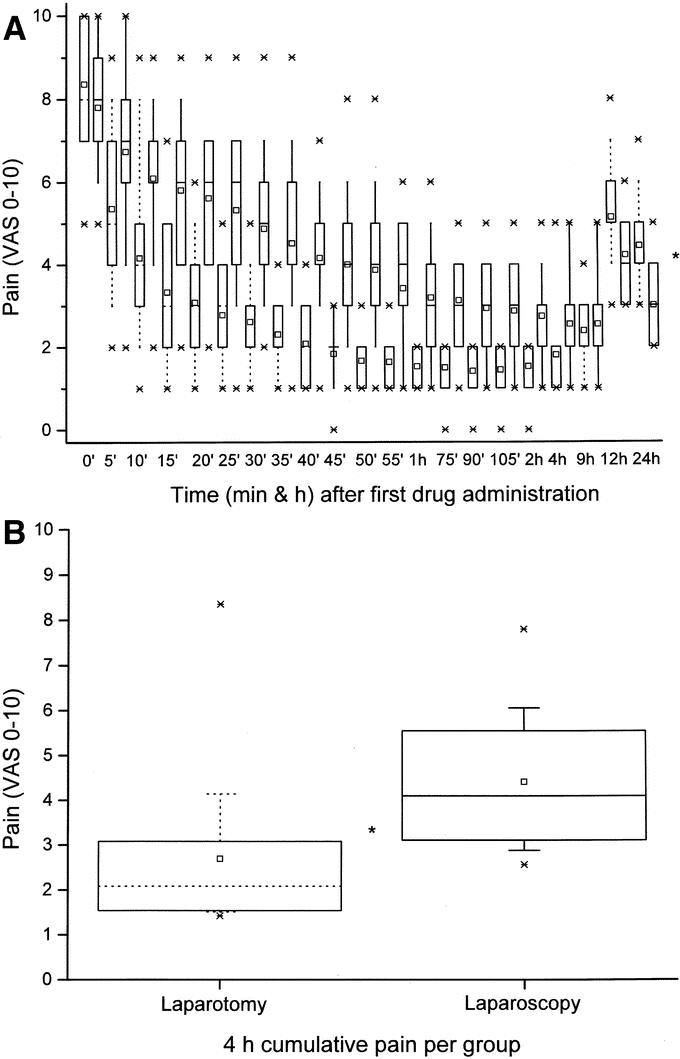
FIGURE 1. A, Self-rated (by 0–10 VAS) pain intensity (median, ranges, minimum and maximum values, and 25–75 percentiles). Dotted boxes are laparotomy-collected, and solid line boxes represent laparoscopy patient data. *P = 0.001 (by Mann-Whitney U test) except for times 0 and 9 hours. B, Self-rated (by 0–10 VAS) 4-hour cumulative pain intensity (median, ranges, minimum and maximum values, and 25–75 percentiles). *P = 0.009 (by Mann-Whitney U test).
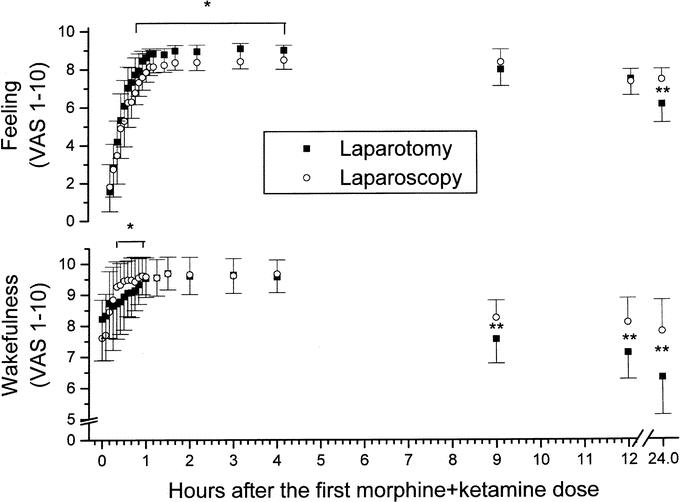
FIGURE 2. Self-rated levels (by 1–10 VAS) of wakefulness (lower half) and feeling of well-being (upper half) (mean ± SD). *P = 0.03 between the groups (by ANOVA) between 20 and 60 minutes (wakefulness), and from 30 minutes on (feeling), respectively. **P < 0.01 between the groups (by Mann-Whitney U test).
Overall, the data showed that the difference in pain intensity between the 2 groups during the PACU stay was surgery dependent (Fig. 1A, B). Initially, VASs slowly declined in both groups, then remained stable up to the PACU 4-hour stay (P = 0.003). By 12 and 24 hours after surgery, scores reversed: the laparoscopy patients were in significantly (P = 0.01, Fig. 1A) less pain than their laparotomy counterparts. The cumulative pain intensity was lower for the open-surgery compared with the laparoscopy counterparts, despite the higher amounts of analgesics administered to the latter group (P = 0.009, Fig. 1B).
The patients' subjectively rated wakefulness and well-being (Fig. 2) indicated that the laparoscopy patients were more awake starting 25 minutes after surgery but had rather lower good feeling scores than the laparotomy ones. Improvement in the 2 parameters was recorded, however, over time in both groups, probably indicating an overall sustained effect of the analgesics administered during their PACU stay. At 24 hours postoperatively, both parameters were better scored in the laparoscopy group (P < 0.01, Fig. 2)
Respiratory rates and SpO2 levels were affected by the surgical technique (Fig. 3). The mean respiratory rate was significantly (P = 0.04) lower for the laparoscopy patients for the first 30 minutes after their first dose of morphine + ketamine. After that time, respiratory rates were similar between groups and remained so for the duration of the 4-hour PACU period. SpO2 on air increased more significantly (P = 0.02) in the laparotomy group 5 minutes after starting treatment; it remained so for the next 20 minutes, reaching a plateau level thereafter. Heart rate and blood pressures were similar in all patients throughout the study period (data not shown).
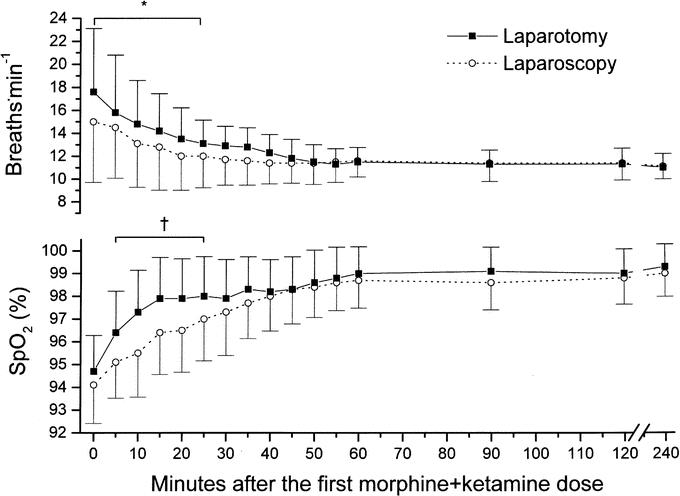
FIGURE 3. Nurse-assessed respiratory rate (upper half) and fingertip-derived arterial blood saturation on air (lower half) (mean ± SD). *P = 0.04, †P = 0.02 between groups (by ANOVA) within the first 20 minutes (respiration) and between 5 and 25 minutes (saturation), respectively.
Three of the 31 laparoscopy patients had nausea and/or vomiting during the PACU stay compared with 1 of 36 laparotomy patients (with 2 events, P = 0.44, Table 1). Conversely, between 9 and 24 hours postoperatively, nausea and/or vomiting rates among the laparotomy individuals was 3 times higher compared with the laparoscopy group (P = 0.02); metoclopramide was effective in all cases. One laparoscopy patient described a case of lightheadedness that lasted less than 2 minutes, and 1 laparotomy patient experienced a bad dream after a second injection of morphine + ketamine; both side effects resolved spontaneously. None reported hallucinations throughout the 24-hour follow-up period.
All patients were discharged from the PACU to the ward at the protocol-dictated time; all but 1 laparoscopy patient were discharged home 24–36 hours after surgery and as is customary in our institution; the latter patient was discharged 48 hours after surgery because of suspected bleeding. Laparotomy patients were discharged 3 to 7 days after the procedure.
DISCUSSION
Review of our data of 2 different techniques of abdominal surgery demonstrates that 1) 46% of the sample reported severe pain, accounting for 47.4% of postlaparotomy and 44.9% of the postlaparoscopy individuals; 2) the postlaparoscopy painful individuals required 33% more doses of pain medication and their pain was more intense than the postlaparotomy patients for the first 4 hours postoperatively; 3) from 9 hours after surgery onward, the laparotomy patients used more analgesics than their laparoscopy counterparts; 4) by 24 hours after surgery, laparoscopic patients had lower pain intensity and sedation and had better scores of well-being than their laparotomy counterparts. These results are especially interesting since past data have shown that the overall postoperative pain intensity and the amounts of analgesics used are lower after laparoscopy compared with laparotomy, these being 2 of the advantages of the laparoscopic technique,1,2 and since the proportion of abdominal surgery using laparoscopy is expected to increase in the years ahead.
Laparoscopic procedures have become very popular in recent years because of the proven quicker postoperative recovery, low rates of early and late postoperative complications, early mobilization, and discharge home.5,8 Even elderly and sicker patients (ASA III–IV) are currently more frequently scheduled for this type of procedure despite the possible temporary intraoperative cardiovascular and ventilatory disturbances.9 By 24 hours postoperatively, patients are comfortable with minimal pain medication,1 as was shown in the present study. This, however, does not contradict the early more severe postoperative pain that was encountered in 31 of 69 laparoscopy patients compared with 36 of 76 laparotomy patients, and the need for more analgesics in the former compared with the latter patients.
We and others have previously demonstrated that some postoperative patients perceive sustained pain.5,6 They are more likely to suffer consequences of severe pain such as hemodynamic and respiratory alterations and delayed return to normal activity. It is therefore important to separate patients with pain controllable by standard morphine regimens in the PACU from those presenting with severe pain, resistant to the same standard IV morphine. Our experience demonstrated that postlaparoscopy individuals might complain of severe pain; we therefore planned this study to concentrate specifically on those patients who suffer refractory pain. This allowed us, apparently for the first time, to demonstrate the incidence of severe pain immediately postlaparoscopy. Our initial suspicion was confirmed, although laparoscopy patients were discharged home earlier and expressed satisfaction from that technique.1 The steady reversal of the pain and well-being trends 24 hours after surgery (Figs. 1A, 2) is another new aspect of the data presented here that is apparently counterintuitive and therefore deserves consideration.
High pain scores in the PACU are either the result of more intense postoperative pain perception per se or of undertreatment of intraoperative pain. This would lead to more intense pain in the PACU and the generation of “central sensitization,” which further perpetuates and enhances pain, apparently in an increasing number of patients in the coming years. Since our data show that the duration of surgery was similar between the groups, as was the amount of intraoperative fentanyl, and the immediate postoperative morphine doses given were also similar, one has to conclude that the difference in the technique of surgery per se was the most plausible cause for the difference in pain intensity and, consequently, for the early postoperative higher amount of analgesic requirement in these laparoscopy patients.
More recent characterizations of pain suggest that pain is of multifactorial physical origin and has an important subjective experiential component that is associated with highly sophisticated peripheral and central neural feedback mechanisms of sensitization and inhibition.10,11 Morphine and ketamine, used here to treat refractory pain, involve diverse central mechanisms and sites of action and have been demonstrated to most effectively treat PACU pain resistant to standard morphine therapy.5,12 The thesis that painful stimuli are objectively quantifiable and predictable has been questioned13 and a subjectively rated pain score is the only valid method to measure pain14 (as was done here), even at the cost of awakening the sleeping patient to obtain self-rating.
High pain scores may also be related to patient preparation, pain preemption, and personal expectations.11,15 Since all patients were similarly prepared, as is customary in our institution, our findings cannot be explained by differences in patient preparation or preemption. Demographic differences have also been proposed to cause diversity in postoperative pain perception and (theoretically) could explain some of our results;11 these again must be ruled out in our patients because the relevant data were similar. One of the explanations for intense pain in the PACU and the generation of “central sensitization,” which further perpetuates and enhances pain, could arise from carbon dioxide (CO2) insufflation itself, which is known to cause direct hemodynamic effects often manifested by intraoperative hypertension. This occurrence could obscure the true requirement for anesthetics; however, our anesthesia protocol was the same and intraoperative fentanyl use was comparable between the 2 groups.
Finally, the severe pain we encountered in the laparoscopy patients during their 4-hour stay in the PACU may be secondary to tissue injury. One possibility is peritoneal irritation largely due to 1) CO2 pressure in the abdomen, 2) blood left in the abdomen after surgery, and 3) diaphragmatic irritation. A higher intraperitoneal CO2 pressure has been shown to generate more intense pain compared with a lower one, and laparoscopic procedures done without CO2 insufflation at all are associated with less pain.16,17 All of our laparoscopy patients had CO2 introduced at a fixed pressure. Old blood present within the abdominal cavity is a known source of irritation to the vastly innervated peritoneum;18 this variable was excluded since the same surgeons operated on all patients and there were no “rebleeding” cases among the enrolled patients. In addition, surgery closer to the diaphragm has been shown to cause more pain than that following maneuvers at lower sites in the abdomen.19 The painful patients comprised similar types of surgery in the 2 groups, thus excluding differences in their distribution within the groups (eg, more cholecystectomies in one or the other group) as a cause for overall difference in pain perception between groups. One more possible source of pain in the laparoscopy patients is pain from sustained intraoperative pressure on capillary beds in the abdominal and possibly retroperitoneal viscera, causing nociception, which is still present when patients arrive in the PACU, but resolves over the first few postoperative hours.17 There are no data here to support or negate this possibility.
CONCLUSION
Laparoscopic surgery, although relatively “painless” after 24 hours as depicted by others and in this study, may indeed be more painful in the immediate (0–4 hours) postoperative period in 45% (31 of 69) of the laparoscopic patients compared with a nearly similar sample of laparotomy individuals (36 of 76). The former thus may request more analgesia than the latter during the mentioned period only. Further physiologic studies, both in animals and in humans, are warranted to understand the precise mechanism(s) by which a laparoscopic procedure may induce intense pain immediately after surgery but not 24 hours later.
ACKNOWLEDGMENTS
The authors thank Esther Eshkol for editorial work.
Footnotes
Reprints: Avi A. Weinbroum, MD, Post-Anesthesia Care Unit, Tel Aviv Sourasky Medical Center, 6 Weizman Street, Tel Aviv 64239, Israel. E-mail: draviw@tasmc.health.gov.il.
REFERENCES
- 1.Schwenk W, Bohm B, Muller JM. Postoperative pain and fatigue after laparoscopic or conventional colorectal resections: a prospective randomized trial. Surg Endosc. 1998;12:1131–1136. [DOI] [PubMed] [Google Scholar]
- 2.Vara-Thorbeck C, Garcia-Caballero M, Salvi M, et al. Indications and advantages of laparoscopy-assisted colon resection for carcinoma in elderly patients. Surg Laparosc Endosc. 1994;4:110–118. [PubMed] [Google Scholar]
- 3.Mais V, Ajossa S, Guerriero S, et al. Laparoscopic versus abdominal myomectomy: a prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol. 1996;174:654–658. [DOI] [PubMed] [Google Scholar]
- 4.Ortega AE, Peters JH, Incarbone R, et al. A prospective randomized comparison of the metabolic and stress hormonal responses of laparoscopic and open cholecystectomy. J Am Coll Surg. 1996;183:249–256. [PubMed] [Google Scholar]
- 5.Weinbroum AA. A single small dose of postoperative ketamine provides rapid and sustained improvement in morphine analgesia in the presence of morphine-resistant pain. Anesth Analg. 2003;96:789–795. [DOI] [PubMed] [Google Scholar]
- 6.Kapfer B, Alfonsi P, Guignard B, et al. Nefopam and ketamine comparably enhance postoperative analgesia. Anesth Analg. 2005;100:169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinbroum AA, Lalayev G, Yashar T, et al. Combined pre-incisional oral dextromethorphan and epidural lidocaine for postoperative pain reduction and morphine sparing: a randomised double-blind study on day-surgery patients. Anaesthesia. 2001;56:616–622. [DOI] [PubMed] [Google Scholar]
- 8.Eypasch E, Sauerland S, Lefering R, et al. Laparoscopic versus open appendectomy: between evidence and common sense. Dig Surg. 2002;19:518–522. [DOI] [PubMed] [Google Scholar]
- 9.Andersson LE, Baath M, Thorne A, et al. Effect of carbon dioxide pneumoperitoneum on development of atelectasis during anesthesia, examined by spiral computed tomography. Anesthesiology. 2005;102:293–299. [DOI] [PubMed] [Google Scholar]
- 10.Sorkin LS. Basic pharmacology and physiology of acute pain processing. Anesthesiol Clin North Am. 1997;15:235–249. [Google Scholar]
- 11.Caumo W, Schmidt AP, Schneider CN, et al. Preoperative predictors of moderate to intense acute postoperative pain in patients undergoing abdominal surgery. Acta Anaesthesiol Scand. 2002;46:1265–1271. [DOI] [PubMed] [Google Scholar]
- 12.Portenoy RK, Bennett GJ, Katz NP, et al. Enhancing opioid analgesia with NMDA-receptor antagonists: clarifying the clinical importance. A roundtable discussion. J Pain Symptom Manage. 2000;19(suppl):57–64. [DOI] [PubMed] [Google Scholar]
- 13.Haughton VM, Fine J. Measuring the effect of novel therapies for back pain. AJNR Am J Neuroradiol. 2003;24:784–787. [PMC free article] [PubMed] [Google Scholar]
- 14.Katz J, Melzak R. Measurement of pain. Surg Clin North Am. 1999;79:231–252. [DOI] [PubMed] [Google Scholar]
- 15.Holdcroft A, Power I. Recent developments: management of pain. BMJ. 2003;326:635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swift G, Healey M, Varol N, et al. A prospective randomised double-blind placebo controlled trial to assess whether gas drains reduce shoulder pain following gynaecological laparoscopy. Aust N Z J Obstet Gynaecol. 2002;42:267–270. [DOI] [PubMed] [Google Scholar]
- 17.Sarli L, Costi R, Sansebastiano G, et al. Prospective randomized trial of low-pressure pneumoperitoneum for reduction of shoulder-tip pain following laparoscopy. Br J Surg. 2000;87:1161–1165. [DOI] [PubMed] [Google Scholar]
- 18.Kressel HY, Gatenby RA, Troupin RH. Correlative imaging conference: Hospital of the University of Pennsylvania.Abdominal pain and blood loss. AJR Am J Roentgenol. 1981;137:769–775. [DOI] [PubMed] [Google Scholar]
- 19.Laurberg S, Sorensen KE. Cervical dorsal root ganglion cells with collaterals to both shoulder skin and the diaphragm: a fluorescent double labeling study in the rat. A model for referred pain? Brain Res. 1985;331:160–163. [DOI] [PubMed] [Google Scholar]


