Abstract
Objective:
The objective of this study was to test the hypothesis that hepatomegaly in burned children can be attenuated or reversed by blocking lipolysis and reducing free fatty acids delivered to the liver.
Summary Background Data:
Accelerated lipolysis in severely burned children has been shown to play an important role in the accumulation of hepatic TGs. Severely burned children who survive 10 days or more after injury commonly have enlarged livers often twice or more normal size for their sex, age, and weight.
Methods:
Ninety-eight children, 2 to 18 years of age, with burns covering more than 40% of their body surface and who received either propranolol (β-adrenergic blockade) or placebo were studied. Liver weights were measured by ultrasonic scanning. Body composition changes were identified by dual-image x-ray absorptiometry and validated by whole-body potassium-40 scintillation counting. Discarded abdominal cutaneous adipose tissue was collected before and after propranolol or placebo for microarray analysis.
Results:
In 80% of severely burned children studied not receiving propranolol, liver sizes increased by 100% or more while 86% of burned children receiving propranolol showed a decrease or no change in liver size over the same period of time after injury. Gene expression patterns of adipose tissue after propranolol treatment showed that all of the identified genes related to lipid metabolism were down-regulated.
Conclusions:
Data reported here support the hypothesis that β-adrenergic blockade can reduce delivery of fatty acids to the liver and hepatic congestion commonly found in severely burned children by inhibiting lipolysis and reducing hepatic blood flow.
Hepatomegaly is a consistent postmortem finding in severely burned children. Hepatocyte enlargement, found at autopsy, represented 85% to 90% of the hepatomegaly observed with total fat contributing up to 20% of the increase in hepatocyte size. Triglycerides, as a percent of total fat, increased with the severity of hepatomegaly.
Catecholamine-mediated hypermetabolism,1–3 lipolysis4, and fatty liver5–7 are striking characteristics of the stress response to a major thermal injury. The deposition of fat in the liver is a common pathologic finding, as is liver size, which may vary 2- to 3-fold.6,8–11 Hepatic fat accumulation (fatty liver) may be due to a high rate of peripheral lipolysis coupled with a lack of increased fat oxidation.12,13 The extent to which triglycerides (TGs) are produced and the capacity of the liver to excrete them into the blood via very low density lipoproteins (VLDLs) is important in determining net fat accumulation in the liver. It has previously been shown that plasma free fatty acid (FFA) is the primary precursor for hepatic TG synthesis.14 The increase in basal lipolysis in burn patients is caused by excessive catecholamine production, which can be diminished by beta-adrenergic blockade with propranolol.12,13,15 Acute use of β-blocking agents precipitously decreases the rate of release or appearance of plasma FFAs, indicating the important role that preexisting sympathetic activity plays in the mobilization of FFAs.4 We recently found in an acute study that β-blockade decreased TG accumulation in the liver by decreasing the supply of FFAs to the liver.16 The effect of chronic treatment with β-blockade on hepatic TG accumulation has not been assessed.
Cardiac output and heart rate have been shown to be higher in the dynamic phase of a thermal injury than most other hyperdynamic conditions.3 In the hypermetabolic phase of burn recovery, cardiac dysfunction contributes significantly to mortality.7,17 Beta-adrenergic blockade has been used with success in pathologic states produced by excessive quantities of endogenous or exogenous thyroid hormone or catecholamines by decreasing the myocardial workload.18,19 We have previously shown that, after adrenalectomy or catecholamine depletion by chronic reserpine administration, rats given a 50% total body surface area (TBSA) burn had lower metabolic rates and increased mortality.20 Wilmore et al1 have shown that the dose of β-blocking agent required for decreasing heart work in patients with major burns also decreases metabolic rate, core and skin temperatures, and may be beneficial in maintaining the responsiveness of subcellular enzymes to catecholamine control.12
The purpose of this study was to test the hypothesis that propranolol, given to children with severe thermal injuries, could be beneficial by attenuating peripheral lipolysis, portal blood flow, and hepatomegaly.
METHODS
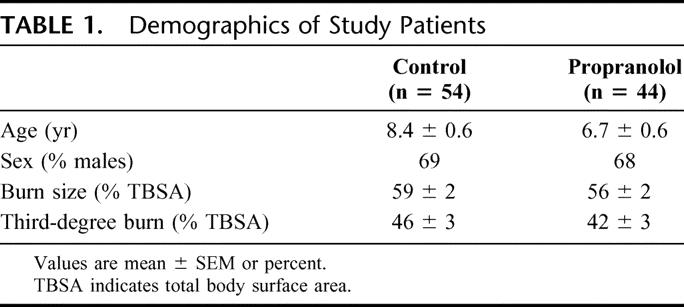
Ninety-eight children ranging in age from 2 to less than 18 years with flame or scald burns covering more than 40% of their TBSA and who were admitted to our hospital within 3 days of burn were studied. Patients with other severe injuries not related to the burn or preexisting conditions such as asthma or pneumonia were excluded.
In a randomized trial, each patient underwent burn-wound excision and grafting with skin autografts and allografts within 72 hours after admission. Sequential staged grafting procedures were performed until the burn wounds were closed. All patients were fed a commercial enteral formula (Vivonex T.E.N., Sandoz Nutritional, Minneapolis, MN) through a nasoduodenal tube. The daily caloric intake was calculated to deliver 1500 kcal per square meter of body surface area burned plus another 1500 kcal per square meter of total body surface area. Enteral nutrition was started at admission and continued until the wounds healed. Patients remained in bed for 5 days after each excision and grafting procedure, and then were allowed to walk daily. Nude weights were recorded daily until discharge using a standard sling scale. Forty-four received propranolol, and 54 received placebo as controls. Randomization followed a random-number protocol. Immediately after the second operation, the children in the drug group began to receive propranolol by nasogastric tube at a dose ranging from 0.3 to 1.0 mg/kg body weight every 4 or 6 hours. The dose was adjusted to achieve a 12% to 15% decrease in heart rate compared with the 24-hour average heart rate before drug treatment. Heart rate and blood pressure were monitored continuously throughout the study. When the mean blood pressure fell below 65 mm Hg, the dose of propranolol was withheld or decreased. The drug was then restarted and increased incrementally to meet the study goal, or the patient was dropped from the study.
Body Composition
The total-body lean mass, bone mineral content, fat mass, and trunk fat were measured by dual-image x-ray absorptiometry (model QDR-4500W, Hologic, Waltham, MA) with a pediatric software package. This system has a minimal mean error with respect to the measurement of fat-free mass in children.21 Systematic deviations were identified by calibrating each day against a spinal phantom (Hologic) in the anteroposterior, lateral, and single-beam modes.
Fat-free body mass (lean body mass + bone mass) was confirmed by whole-body potassium-40 scintillation counting in a low background noise environment and computed data analysis, which was validated for use in children.22,23 Instrument precision was within less than 1.5% and was calibrated each day using a bottle-manikin absorption phantom (Canberra Industries, Meriden, CT) with stimulated fat overlays. Feeding and intravenous fluids were discontinued during the bone mineral content studies to minimize exogenous potassium contamination.
Plasma Concentrations for Catecholamines, Glucose, Urea Nitrogen, TG, and FAA
TG and glucose blood levels were measured on a multianalyzer (Technicon RA-1000, Terrytown, NY). FFAs were determined using a copper nitrate in ethanolamine atomic absorption technique. Plasma catecholamine concentrations were determined on erythrocyte-free plasma using high-pressure liquid chromatography (Smith Kline Bio-Science Laboratories, Van Nuys, CA).
Liver Image Measures
Liver sizes, using a laser Doppler one-dimensional measure, were obtained within 48 hours of admission and weekly thereafter until the burn wound was 95% healed. Laser Doppler liver measurements were made with the patient in the supine position with a Hewlett Packard Sonas 100CF with a Sony Video Graphic Printer (Hewlett Packard 3000 Minuteman Road, Andover, MA). The ultrasound probe was placed at 90° to the surface on the midclavicular line and then located the right at the lower margin of the liver.6 The probe was then angled until the superior border of the liver was identified before scanning. The distance from the lower to upper borders of the liver was calculated and recorded. The same research nurse was used to consistently approximate the initial probe placement and deflection to the upper border of the liver. Ultrasonic scanning was repeated 3 times and the resulting values averaged. Measurements were made within 3 days of admission and weekly thereafter. The formula used for estimating liver weight from the single longitudinal scan along the right nipple line was wt = (1.15l)3/density/2 where l3/2 represents the volume of a cube cut diagonally in half to visualize the approximate shape of the normal liver in situ.6 The density of 6 enlarged livers at autopsy averaged 1.036 g/mL. Baseline values were compared with weekly determinations until the burn wound was 95% healed.
Gene Chip Analysis
Abdominal cutaneous adipose tissue, discarded during early excision of burned children, was collected prior to scheduled drug treatment and used for baseline values. Six patients received placebo and 6 received propranolol. All tissue samples were snap-frozen and stored at −80°C for microarray analysis. RNA isolation and high-density oligonucleotide array analysis methodology have been described in detail.24
Statistical Analysis
Data are presented as mean ± SE. The Wilcoxon signed-rank test was used for comparisons within groups. Comparisons between groups used the 2-tailed unpaired t test or Fisher exact test. Microarray data used paired t test to compare baseline versus placebo. Statistical significance was accepted at P less than 0.05.
This study was conducted with permission and in compliance with the requirements for institutional review and informed consent at the University of Texas Medical Branch, Galveston, TX.
RESULTS
Age, gender distribution, weight, burn size, admission time from injury, and delay time to first study were not significantly different between placebo and drug (Table 1). Initially, propranolol significantly decreased heart rate by 12% to 15% compared with the average for 3 days prior to the study (P < 0.01). An 11% lower heart rate was maintained in the drug groups compared with control until patients were 95% healed. Blood pressure, temperature, and glucose levels did not significantly differ between groups. Neither the control group nor the propranolol group required mechanical ventilation except for brief periods perioperatively, and none had clinically identified pneumonia.
TABLE 1. Demographics of Study Patients
Body Composition
All patients underwent whole-body potassium scanning to confirm changes in body composition identified by dual-image x-ray absorptiometry. Children receiving propranolol increased their trunk fat by 16% during their hospital stay compared with baseline while those receiving placebo increased trunk fat by 11% (Fig. 1). Total body fat was increased nearly 6% for propranolol therapy and 4% for placebo. Percent change in bone mineral content was −1.5% for propranolol and −3.9% for placebo. Lean body mass and fat free mass did not significantly change with treatment.
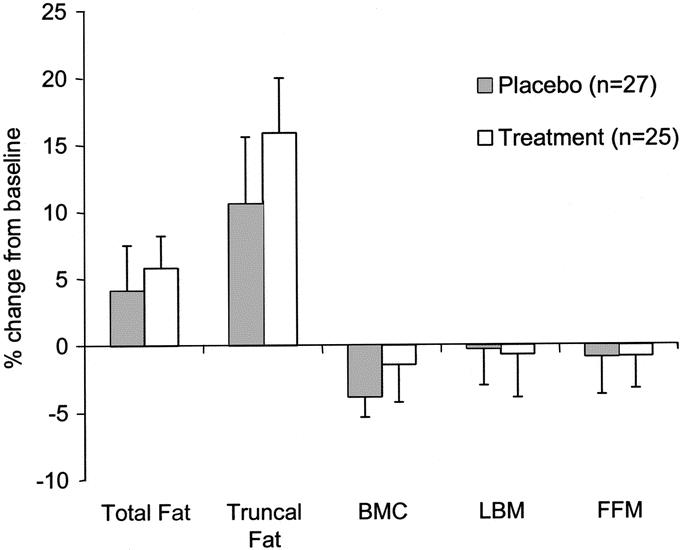
FIGURE 1. Values are mean ± SEM. Dual-image x-ray absorptiometry (DEXA) measures of changes in total fat, truncal fat, bone mineral content (BMC), and lean body mass (LBM). Comparisons of placebo (n = 27) and propranolol treatment (n = 25) show no significant difference between groups. A change in fat-free mass (FFM), measured by whole-body potassium 40 scintillation counting, verifies the LBM DEXA determination. All measurements were made as close to admission (baseline) as possible and at 95% healed.
Liver Size
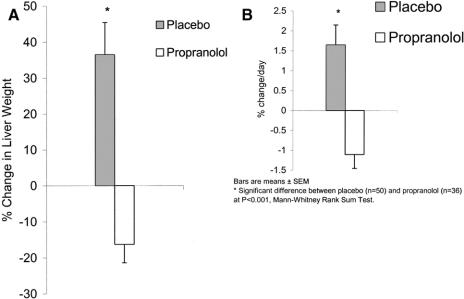
Ninety-six children with burns covering over 40% of their TBSA were tested for changes in liver size during their hospital stay. Of 49 receiving placebo, 39 showed an increase (80%) in liver size, while 11 showed no change or a decrease in size. In those receiving propranolol, 5 showed an increase in liver size, while 31 (86%) showed a decrease in liver size (P < 0.001).
The percent change in liver weights (converted from size) and percent change per day for placebo versus propranolol are depicted in Figure 2. These changes reflect the earliest liver measures after admission to the maximal increase or decrease measured before discharge.
FIGURE 2. A, Bars represent mean ± SEM. a, Percent change in liver weights. *Significant difference between placebo (n = 50) and propranolol therapy (n = 36) at P < 0.001. Confidence intervals for differences are 30.242, 75,358. Mean ± SEM days after burn until the first measurement was 12.8 ± 1.3 days (range, 2–28 days). B, Percent change per day in liver weights. *Significant difference between placebo (n = 49) and propranolol therapy (n = 36) at P < 0.0001. Confidence intervals for differences are 1.927, 4.063.
To validate the use of one-dimensional ultrasound measurements for estimating liver weights, the relationship of autopsy weight, from another study, was regressed against those calculated from ultrasound measurements (R = 0.871, P < 0.0001).6
Plasma Analysis
No significant change in blood urea nitrogen or FFAs could be shown for propranolol treatment or placebo when compared with baseline. Significant changes were, however, shown in plasma glucose concentrations with glucose decreasing compared with baseline in both those receiving placebo and propranolol (P < 0.001). Plasma TGs significantly increased by 54% compared with baseline in burned children receiving placebo (P < 0.0001), while in those receiving propranolol TGs increase by only 12% compared with baseline (P = 0.12). Epinephrine, norepinephrine, and dopamine were all elevated in children receiving propranolol compared with controls, but the differences were not statistically different.
Ancillary Study of Gene Expression Patterns
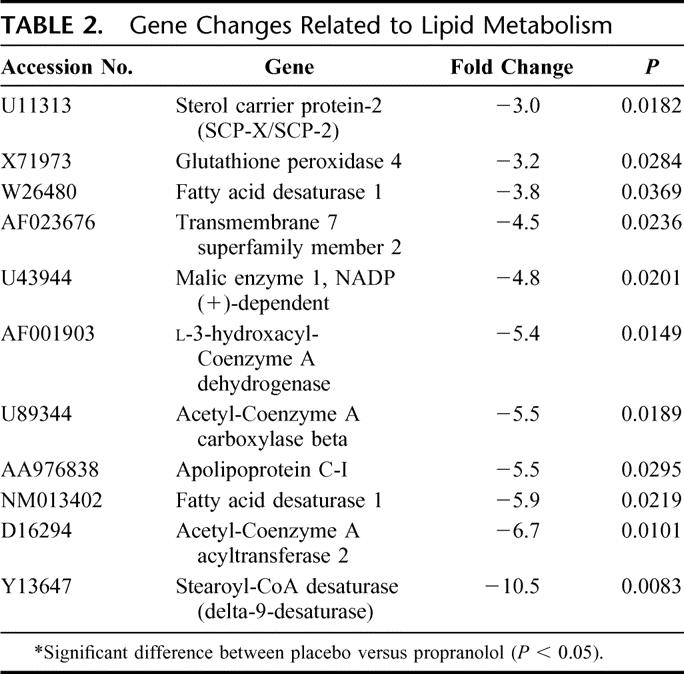
Age, gender distribution, burn size, and incidence of inhalation injury were not significantly different between groups used for RNA isolation and high-density oligonucleotide array analysis. Comparison of 12,626 genes expressed in the adipose tissue of burned children receiving placebo showed an increase in the expression of 13 genes with time compared with baseline, while 25 genes decreased. The categories of genes affected include structural proteins, genes related to metabolic processes, apoptosis, immune response, signal transduction, stress response modulators, and cytoskeleton-associated proteins. There were 147 genes that were affected by propranolol treatment as determined by DNA microarray analysis. As verification of the effect of propranolol on fat metabolism, 11 genes of interest that were related to lipid metabolism were all significantly down-regulated with propranolol therapy with no genes related to lipid metabolism up-regulated. Gene changes associated with lipid metabolism are depicted in Table 2. A decrease in mRNA was verified through a decrease in the expression of related proteins by Western Blot analysis.
TABLE 2. Gene Changes Related to Lipid Metabolism
Confirmation by RT-PCR
To confirm these findings using a different methodology, the level of expression of 3 selected genes was evaluated. Analysis showed significant differences after propranolol treatment in human adipose tissue. Messenger RNA expression for monoamine oxidase-A was increased from 0.58 ± 0.31 to 1.91 ± 0.21 (P < 0.05), while the expression of osteopontin was decreased with propranolol treatment from mRNA/β-actin ratio of 2.33 ± 0.09 to 0.65 ± 0.02 for the control group (P < 0.05). Propranolol treatment did not affect IGF-1 mRNA/β-actin ratios, 0.91 ± 0.019 and 1.1 ± 0.28 in the control group, which supports microarray data.
DISCUSSION
Increased heart rates, myocardial work, and lipolysis are responses to the high levels of catecholamines associated with thermal injury. Propranolol, given during acute hospitalization, has been proven effective in children for the control of cardiovascular derangements. Our patients underwent continuous hemodynamic and respiratory monitoring, which indicated no complications related to therapy. No significant decrease in blood pressure was observed with propranolol treatment. The mean heart rate reduction in this study was 11%, which was similar to that obtained by Baron et al.25 Validation of the lean body mass measures by DEXA was by determining fat free mass using whole-body potassium–40 scintillation counting. Further, the DEXA changes were predicted by cross leg protein-kinetic studies to measure muscle protein synthesis and breakdown.26 Neither study identified a significant effect of propranolol on lean body mass, protein synthesis, or protein breakdown in placebo controls (n = 20) versus those receiving propranolol therapy (n = 15).
Hepatomegaly occurs in severely burned patients, often resulting in 2-fold or more increases in liver size3,8 and may cause clinical problems. Metabolic failure of the liver can complicate recovery from a burn injury or trauma, particularly when steatosis develops because of an imbalance between the rate of TG formation in the liver and the rate of secretion of VLDL-TG into the blood. Fatty liver, as the result of the abnormal accumulation of TGs in the cytoplasm of hepatocytes, causes them to enlarge, and often compromises hepatic microcirculation and blood flow.27 Studies have shown that in severely burned children, plasma FFA is the primary precursor for hepatic TG synthesis.14,16 Even when patients receive prolonged high-carbohydrate feeding, de novo synthesis of fatty acids plays a minor role in the production of VLDL-TG.14 Prolonged treatment of burn patients receiving rhGH plus propranolol effectively decreased the availability of FFA to the liver by reducing the release of fatty acids into the blood. Concomitantly, plasma FFAs were converted more efficiently into VLDL-TG and secreted when propranolol was given along with rhGH.14 Principally, the net balance of fat across the liver is a function of FFA uptake from plasma, intrahepatic fatty acid consumption (oxidation), and VLDL-TG secretion. Because hepatic fatty acid uptake is closely correlated to plasma FFA availability, we have shown that propranolol acutely reduces net fat accumulation in the liver by decreasing hepatic FFA uptake while maintaining VLDL-TG secretion without affecting fat oxidation.16 To assess the extent to which fat might be retained in the liver, we reviewed the autopsy reports of 14 severely burned children. The mean burn size was 76% ± 5% TBSA. Liver weights were 75.6 ± 6.0 g/kg body weight in burn victims compared with 34.3 ± 1.1 for predicted healthy values.14 We have previously shown a decrease in blood flow to the gut using beta-blockade; and more recently, postmortem studies have demonstrated a direct relationship between liver size and fat content with normal sized livers containing 2% to 3% total fat compared with 16% to 19% total fat in livers that are 300% to 400% of predicted size at autopsy. In a blinded study, we also examined histologic slides of the livers of severely burned children. Findings indicate 7 with diffuse, 3 with centrilobular, and 2 with periportal mild to marked liver fat changes. Mild to moderate cholestasis was shown in 5 of the 14 autopsies reviewed.14
The adipose tissue used in these studies was from discarded abdominal fat, which is primarily composed of adipocytes. This cell type stores excess energy in the form of lipids and is capable of increasing in size without an underlying transformed cellular phenotype, which gives it an almost unlimited capacity for growth. Adipose tissue is composed of 50% to 60% adipocytes, and 40% to 50% of stromal-vascular fraction consisting of fibroblasts and macrophages.28 It is possible that most of the change reported here may have occurred in adipocytes while some changes occurred in the stromal-vascular fraction of adipose tissue.
The importance of fat in this study is that it is mediated through the relationship of FFAs to adipose tissue and the production of inflammatory mediators, which are the same factors determining insulin-mediated glucose uptake. Tumor necrosis factor-α is a cytokine produced by fat that directly suppresses insulin signaling through serine phosphorylation of insulin receptor substrate-1, thus decreasing insulin receptor kinase activity and inducing an insulin resistance.29
The signal transducers and activators of transcription-1 mRNA levels are increased with propranolol treatment. In fat cells, expression of STAT1, STAT5A, and STAT5B is highly induced during differentiation and correlates with lipid buildup.30 Although the functions of STATs in adipose tissue are not well known, studies have suggested that these transcription factors are important regulators of adipocyte gene expression.
The current study showed that, in severely burned children, liver weights increased by 1 to 2 g × kg−1× day−1 and treatment with propranolol prevented or attenuated this increase. Both intercellular large fat droplets, cholestasis, and congestion are common histologic findings that correlate with increases in liver weights.6 From the above discussion, it is likely that part of the effect of propranolol on the liver is its suppression of lipolysis. In addition, a decrease in portal blood flow may also play a role by working through 2 different mechanisms. The decreased delivery of FFA to the liver is in part due to inhibition of lipolysis and in part due to the decrease in portal blood flow. Second, the vascular pressure associated with the higher portal blood flow without propranolol may lead to congestion of the liver.
ACKNOWLEDGMENTS
The authors thank Julie L. Bailey for assistance in preparing and editing this manuscript; Mary Kelly, and Wes Benjamin RN, BSN, for technical help.
Footnotes
Supported by NIH R01 GM56687 and Shriners Hospitals for Children Grant Nos. 8480 and 8460, SHC Grant No. 8660, NIH Center Grant No. 1P50-GM60338-01, and NIH Grant No. R01-GM56687.
Reprints: Robert E. Barrow, PhD, Shriners Hospitals for Children, 815 Market St., Galveston, TX 77550. E-mail: rbarrow@utmb.edu.
REFERENCES
- 1.Wilmore DW, Long JM, Mason AD Jr, et al. Catecholamines: mediator of the hypermetabolic response to thermal injury. Ann Surg. 1974;180:653–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arturson G. Prostaglandins in human burn wound secretions. Burns. 1977;3:112–118. [Google Scholar]
- 3.Goodall MC, Stone C, Haynes BW Jr. Urinary output of adrenaline and non-adrenaline in severe thermal injuries. Ann Surg. 1957;145:479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolfe RR, Herndon DN, Peters EJ, et al. Regulation of lipolysis in severely burned children. Ann Surg. 1987;290:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barret JP, Jeschke MG, Herndon DN. Fatty infiltration of the liver in severely burned pediatric patients: autopsy findings and clinical implications. J Trauma. 2001;51:736–739. [DOI] [PubMed] [Google Scholar]
- 6.Barrow RE, MlCak R, Barrow LN, et al. Increased liver weights in severely burned children: comparison of ultrasound and autopsy measurements. Burns. 2004;30:565–568. [DOI] [PubMed] [Google Scholar]
- 7.Linares HA. A report of 115 consecutive autopsies in burned children, 1966–1980. Burns. 1982;8:263–270. [DOI] [PubMed] [Google Scholar]
- 8.Talaat SM, Beheri GE, Zaki MS, et al. Prevention of early histopathological changes in the liver in extensive burns. Br J Plast Surg. 1973;26:132–139. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe WA, Elkinton JR, Rhoads JE. Liver damage and dextrose tolerance in severe burns. Ann Surg. 1940;112:158–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James GW, Purnell O, Evans EI. The anemia of the thermal injury: II. Studies of liver function. J Clin Invest. 1951;30:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burke JF, Wolfe RR, Mullany CJ, et al. Glucose requirements following burn injury: parameters of optimal glucose infusion and possible hepatic and respiratory abnormalities following excessive glucose intake. Ann Surg. 1979;190:274–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfe RR, Herndon DN, Jahoor F, et al. Effect of severe burn injury on substrate cycling by glucose and fatty acids. N Engl J Med. 1987;317:403–408. [DOI] [PubMed] [Google Scholar]
- 13.Wolfe RR, Herndon DN, Peters EJ, et al. Regulation of lipolysis in severely burned children. Ann Surg. 1987;206:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aarsland A, Chinkes D, Wolfe RR, et al. Beta-blockade lowers peripheral lipolysis in burn patients receiving growth hormone. Ann Surg. 223:777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herndon DN, Nguyen TT, Wolfe RR, et al. Lipolysis in burned patients is stimulated by the beta-2-receptor for catecholamines. Arch Surg. 1994;129:1301–1305. [DOI] [PubMed] [Google Scholar]
- 16.Morio B, Irtun O, Herndon DN, et al. Propranolol decreases splanchnic triacylglycerol storage in burn patients receiving a high-carbohydrate diet. Ann Surg. 2002;236:218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herndon DN, Barrow RE, Rutan TC, et al. Effect of propranolol administration on hemodynamic and metabolic responses of burned pediatric patients. Ann Surg. 1988;208:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi VV. Effects of burns on the heart. JAMA. 1970;211:2130–2134. [PubMed] [Google Scholar]
- 19.Gelfand RA, Hutchinson-Williams KA, Bonde AA, et al. Catabolic effects of thyroid hormone excess: the contribution of adrenergic activity to hypermetabolism and protein breakdown. Metabolism. 1987;36:562–569. [DOI] [PubMed] [Google Scholar]
- 20.Herndon DN, Mason AD Jr, Wilmore DW, et al. Humoral mediators of non-temperature dependent hypermetabolism in fifty percent burned adult rats. Surg Forum. 1977;28:37–39. [PubMed] [Google Scholar]
- 21.Wells JC, Fuller NJ, Dewit O, et al. Four-component model of body composition in children: density and hydration of fat-free mass and comparison with simpler models. Am J Clin Nutr. 1999;69:904–912. [DOI] [PubMed] [Google Scholar]
- 22.Ellis KJ, Shypailo RJ. Total body potassium in the infant. J Radioanal Nucl Chem 1992;161:61–9. [Google Scholar]
- 23.Forbes GB, Lewis AM. Total sodium, potassium, and chloride in adult man. J Clin Invest. 1956;35:596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrow RE, Dasu MR, Ferrando AA, et al. Gene expression patterns in skeletal muscle of thermally injured children treated with oxandrolone. Ann Surg. 2003;237:422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baron P, Barrow RE, Herndon DN. Prolonged use of propranolol safely decreases cardiac work in burned children. J Burn Care Rehabil. 1997;18:223–227. [DOI] [PubMed] [Google Scholar]
- 26.Biolol G, Chinkes D, Zhang XJ, et al. Vars Research Award: a new model to determine in vivo the relationship between amino acid transmembrane transport and protein kinetics in muscle. JPEN J Parenter Enteral Nutr. 1992;16:305–315. [DOI] [PubMed] [Google Scholar]
- 27.Ijaz S, Yang W, Winslet MC, et al. Impairment of hepatic microcirculation in fatty liver. Microcirculation. 2003;10:447–456. [DOI] [PubMed] [Google Scholar]
- 28.Hausman GJ. The comparative anatomy of adipose tissue. In: Cryer A, Van RL, eds. New Perspectives in Adipose Tissue: Structure, Function, and Development. London: Butterworths, 1985:1–21. [Google Scholar]
- 29.Hsueh WA, Law R. The central role of fat and effect of peroxisome proliferator-activated receptor-gamma on progression of insulin resistance and cardiovascular disease. Am J Cardiol. 2003;92:3J–9J. [DOI] [PubMed] [Google Scholar]
- 30.Stephens JM, Morrison RF, Pilch PF. The expression and regulation of STATs during 3T3-L1 adipocyte differentiation. J Biol Chem. 1996;271:10441–10444. [DOI] [PubMed] [Google Scholar]