Abstract
Objectives:
To examine a population-based cohort for the association between clinicopathologic predictors of survival and immunohistochemical markers (IHC), and to assess changes in gene expression that are associated with lymphovascular invasion (LVI).
Summary Background Data:
LVI has been associated with poor survival and aggressive tumor behavior. The molecular changes responsible for the behavior of gastric cancer have yet to be determined. Characterization of IHC markers and gene expression profiles may identify molecular alterations governing tumor behavior.
Methods:
Clinicopathologic and survival data of 114 patients were reviewed. Archival specimens were used to construct a multitumor tissue array that was subjected to IHC of selected protein targets. Correlation of IHC with tumor thickness (T status), LVI and prognosis was studied. Microarray analysis of fresh gastric cancer tissue was conducted to examine the gene expression profile with respect to LVI.
Results:
In a multivariate analysis, nodal status (N), metastasis (M), and LVI were independent predictors of survival. LVI was associated with a 5-year survival of 13.9% versus 55.9% in patients in whom it was absent. LVI correlated with advancing T status (P = 0.001) and N status (P < 0.001). IHC staining of cyclooxygenase-2 (COX-2) correlated with T status, tumor grade, lymph node positivity, and IHC staining of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9). Microarray analyses suggested differential expression of oligophrenin-1 (OPHN1) and ribophorin-II (RPNII) with respect to LVI.
Conclusion:
LVI was an independent predictor of survival in gastric cancer. Expression of COX-2 may facilitate tumor invasion through MMP-2 and MMP-9 activation. OPHN1 and RPN II appeared to be differentially expressed in gastric cancers exhibiting LVI. The reported function of OPHN1 and RPN II makes these gene products promising candidates for future studies involving LVI in gastric cancer.
A multivariate analysis was conducted to examine clinicopathologic factors and protein immunoreactivity in a gastric cancer tissue array preparation using a population-based cohort. Microarray gene-expression profiles, with respect to lymphovascular invasion, are provided to further characterize molecular alterations and provide insight into the biologic behavior of gastric cancer.
Surgical resection for gastric cancer continues to be the primary modality, with complete locoregional control being the only chance for cure.1,2 However, even after potentially curative surgery, up to 80% of patients will develop tumor recurrence.3 This is compounded by the observation that 65% of gastric cancers in the United States present at an advanced stage, with nearly 85% of tumors accompanied by lymph node metastasis at diagnosis.4 The incidence of nodal involvement has given rise to controversy with regard to what is considered an appropriate lymphadenectomy. It is unlikely, however, that the issue of lymphadenectomy will be settled in the absence of more specific markers of biologic behavior, which may be used to improve prognostication and provide targets for improved management strategies.
Lymphovascular invasion (LVI) predicts poor outcome in several malignancies, including gastric cancer.3,5–8 In a recent review,9 LVI emerged as a prognostically promising factor, which independently predicted survival and was associated with advanced T stage, prompting some authors to suggest that LVI should be included in risk stratification and selection of patients for entry into clinical trials.3,8 In a follow-up study, our results indicated that LVI was predictive of survival in node-negative patients selected from a population-based cohort,10 and were in agreement with previous studies examining node-negative gastric cancer, further supporting LVI as a potential marker of biologic behavior.11–13 To better understand the role of LVI in gastric cancer, complete delineation of the pathways preceding lymphatic permeation is necessary.
Cyclooxygenase-1 (COX-1) is a constitutively active enzyme, involved in maintaining normal tissue homeostasis, including cytoprotection of the gastric mucosa.14 Constitutive expression of COX-1 in gastric tissue provides a useful control in protein localization studies. Cyclooxygenase-2 (COX-2) is a rate-limiting enzyme in the conversion of arachidonic acid to prostaglandins.15 COX-2 is an inducible gene product whose expression is enhanced by stimuli such as inflammation, cytokines, tumor promoters, and growth factors.15–17 Studies have shown that increased levels of COX-2 favor malignant growth in many tumor types, including gastric cancer, by giving tumor cells a survival advantage through inhibition of apoptosis and immune surveillance and promotion of angiogenesis.14,18 COX-2 levels correlate with lymphatic permeation, tumor thickness, and lymph node metastasis.14,16,17,19 Recent studies have shown that the signaling protein, integrin-linked kinase (ILK), once stimulated, is capable of inducing expression of invasion-related genes such as COX-2, which is thought to stimulate activation of the matrix metalloproteinases-2 and -9 (MMP-2 and MMP-9), thereby facilitating tumor invasion through degradation of the basement membrane and allowing access to the lymphatic and vascular spaces.20–23
We conducted a study in a population-based cohort with gastric cancer in which independent predictors of survival and the presence of COX-1, COX-2, MMP-2, and MMP-9 immunoreactivity were considered for their abilities to predict biologic behavior with respect to T status and LVI. We also conducted gene expression analysis in a small number of gastric tumor samples using oligonucleotide microarray analysis to identify potential genetic determinants of gastric cancer behavior.
MATERIALS AND METHODS
Retrospective Population-Based Cohort
From January 1994 through December 1997, 328 patients with a histologic diagnosis of gastric cancer were entered into the Northern Alberta Cancer Registry. The Registry serves a population of 1.56 million and is updated monthly with respect to survival status and histopathology. A gastric cancer database for these 328 patients was created containing Registry data and supplemented with tumor- and patient-related prognostic factors from patient charts. All patients aged >18 years of age with primary gastric adenocarcinoma undergoing surgery with curative intent were included. Exclusion criteria included operations for recurrent disease and gastric cancer confined to the gastric cardia, the gastroesophageal junction (GEJ), or the esophagus. The exclusion of proximal gastric cancer was based upon evidence from studies24,25 that suggest that tumors within 2 cm of the GEJ are a separate clinical entity from gastric cancers arising elsewhere in the stomach. To ensure that the analyzed cases were as homogeneous as possible, tumors that were judged by the aforementioned criteria to be proximal gastric cancer were excluded. Of the 328 patients, 192 (58.5%) underwent resection with curative intent. Clinicopathologic data and pathologic specimens were available for 114 patients, forming the retrospective study population.
Prospective Population-Based Cohort
Between July 2002 and November 2003, 56 consecutive patients diagnosed with gastric cancer with these criteria (age >18 years, primary gastric adenocarcinoma, no prior treatment of gastric cancer, and able to provide informed consent) were enrolled into the study. All patients provided consent for the collection of fresh gastric tumor tissue and adjacent normal mucosa for tumor banking in the PolyomX multitumor tissue bank. Thirty-six patients initially diagnosed with gastric cancer were subsequently excluded for the following reasons: 11 (19.6%) cases, despite adequate tumor harvesting, yielded insufficient total RNA for microarray analysis, 4 (7.1%) were subsequently diagnosed as lymphoma, 4 (7.1%) as gastrointestinal stromal tumor, 12 (21.4%) were unresectable at time of surgery, and 5 (8.9%) refused consent. Clinicopathologic and survival data from the Northern Alberta Cancer Registry and patient records of the remaining 20 patients were prospectively entered into a gastric cancer database.
Gastric tumors were classified according to the American Joint Committee on Cancer TNM staging criteria (5th edition).26 LVI was defined as the presence of tumor emboli within either the lymphatic or vascular channels. Ethics approval was provided by the Health Ethics Research Board at the University of Alberta, Capital Health Region and the Research Ethics Board at the Alberta Cancer Board.
Tissue Microarray and Immunohistochemistry
Original hematoxylin and eosin slides from 114 selected patients in the retrospective cohort were reviewed, and representative tumor regions were marked by an anatomic pathologist. Corresponding paraffin-embedded gastric cancer tissue blocks were obtained. Tissue cylinders with a diameter of 1.0 mm were punched from the marked areas of each donor block with a tissue arrayer (Beecher Instruments) and placed into a recipient paraffin block. Four-micron sections of the resulting multitumor tissue array blocks were transferred to glass slides. The sections were deparaffinized in xylene, rehydrated in graded concentrations of ethyl alcohol (100% 3 times, 80%, 70%, 50%, then water), and then washed in running tap water. The slides were incubated in TRIS (pH 10.0) retrieval solution (DAKO catalog no. S3307) under pressure and heated at 100°C for 10 minutes. The slides were then sequentially cooled, washed in running water for 10 minutes, and incubated in 3% H2O2 and methanol to deplete endogenous peroxidase activity. Finally, the slides were washed in running water for 10 minutes and then placed in phosphate-buffered saline. The multitumor tissue arrays were immunostained with antimouse antibodies (Novocastra Laboratories Ltd, Newcastle, UK) by the avidin-biotin peroxidase complex method. Monoclonal antibodies, purchased from Santa Cruz Biotechnology Inc. (Santa Cruz) and were directed against MMP-2 (monoclonal IgG1, 2C1), MMP-9, (monoclonal IgG1, 2C3), COX-1 (monoclonal IgG2b), and COX-2 (monoclonal IgG). Tissue arrays were scored independently by 2 pathologists blinded to the clinical outcome of the patients, using a semi-quantitative scoring system adapted from Monig et al.27 For each antibody preparation studied, the location of immunoreactivity (cytoplasmic, nuclear, or combined) was noted, and staining intensities were graded according to the proportion of positively staining tumor cells relative to a reference sample included on each multitumor tissue array slide as follows: score 0, ≤30% positive tumor cells; score 1, 30% to 70% positive tumor cells; and score 2, ≥70% positive tumor cells (Figs. 1, 2).

FIGURE 1. A, Immunohistochemical staining of COX-2 in a gastric cancer multitumor tissue array (magnification ×10). A, COX-2 immunoreactivity is evident (2+) in the cytoplasm of the tumor cells. B, Immunohistochemical staining of COX-2 in a gastric cancer multitumor tissue array (magnification ×10). B, COX-2 negative immunoreactivity.
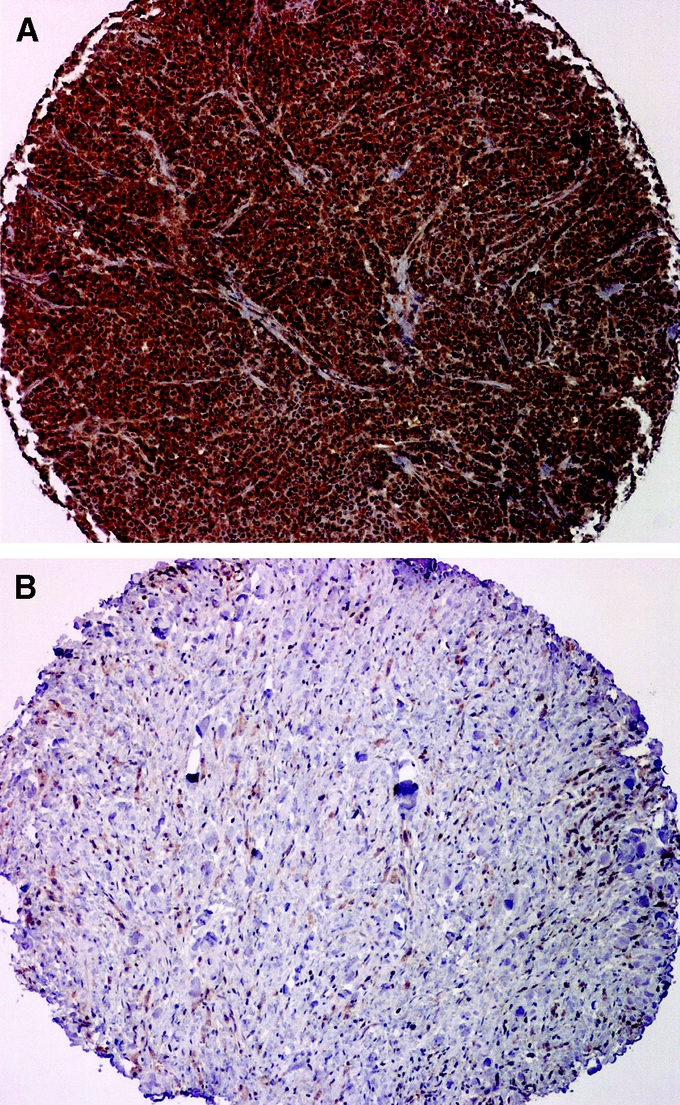
FIGURE 2. A: Immunohistochemical staining of MMP-9 in a gastric cancer multitumor tissue array (magnification ×10). A, MMP-9 immunoreactivity is evident (2+) in the cytoplasm of the tumor cell. B, Immunohistochemical staining of MMP-9 in a gastric cancer multitumor tissue array (magnification ×10). B, MMP-9 negative immunostaining.
Microarray Hybridization
Microarray slides of the Operon (Alameda, CA) human 70-mer oligonucleotide set (version 1.1), representing 13,971 genes, were printed by the Gene Array Facility of Genome British Columbia. The oligos were printed in duplicate on each ArrayIt SuperAmide slide (Telechem, Sunnyvale, CA) using a Microgrid TAS 2 (Biorobotics, Woburn, MA) array printer.
Total RNA was isolated from 20 prospective gastric cancer samples using Trizol, followed by purification on an RNeasy column (Qiagen, Mississauga, Ontario, Canada) according to the manufacturer's recommendations. A reference sample was generated from one part normal gastric mucosa added to an equal amount of total RNA prepared from 17 pooled gastric cancers. Microarray slides were probed in triplicate with labeled cDNA prepared from 30 μg each of tumor and reference total RNA. Superscipt II was used to prepare cDNA, which was then labeled with fluorescent dyes (Cy3 and Cy5) using an indirect amino-allyl technique.28 After hybridization, the microarray slides were scanned with an Axon 4000B, using GenePix 3.0 software (Molecular Devices Corporation, Sunnyvale, CA).
Analysis of microarray data was performed using the Nearest Shrunken Centroid method.29,30 Tumors were classified into LVI-negative and LVI-positive groups, and a binary classifier was built using the specified classes with one clinical parameter at a time. Leave-one-out cross-validation was performed.
Statistical Analysis
Gastric cancer database analyses were undertaken with SPSS statistical software, version 11.0 (Chicago, IL). Patient and tumor factors were entered as categorical variables. Continuous variables were assessed for linearity and, where appropriate, transformed into categorical variables. Survival curves were calculated using the Kaplan-Meier method, then compared using the log-rank test. The association of patient factors, tumor factors, and protein immunoreactivities with disease-specific survival was assessed through the Cox proportional hazard model, applying a purposeful selection method.31 The significance of the covariates was tested using the Wald test. No variables included in the final model violated the proportional hazard assumption. The association between T status and LVI with protein immunoreactivities was tested using a χ2 test. A P value <0.05 was considered statistically significant.
RESULTS
Clinicopathologic Factors
The clinicopathologic characteristics of the retrospective cohort (n = 114) are shown in Table 1. The median follow-up was 19.2 months (range, 1–120 months). At the time of analysis, 26 (22.8%) patients were alive and 88 (77.2%) were dead. There were 6 (5.3%) in-hospital mortalities not related to gastric cancer. The 5-year disease-specific survival was 29.8% (95% confidence interval [CI] 21.6–38.4).
TABLE 1. Baseline Characteristics of the Retrospective and Prospective Gastric Cancer Cohorts

TABLE 1. (Continued)

The majority of patients (68.4%) presented with locally advanced gastric cancer, of which 70 (61.4%) had T3 and 8 (7.0%) had T4 tumors (Table 1). The small number of T4 tumors was related to the exclusion of unresectable tumors, where archival tissue blocks were not available for immunohistochemical studies. Histologic grade was reported as well differentiated in 7 (6.1%), moderate in 35 (30.7%), poor in 68 (59.6%), and missing in 4 (3.5%) cases. Total gastrectomies were performed in 35 (36.7%) cases, and subtotal gastrectomies were performed in 79 (69.3%) cases. There was no significant difference in disease-specific survival between the total versus the subtotal gastrectomy groups (P = 0.269).
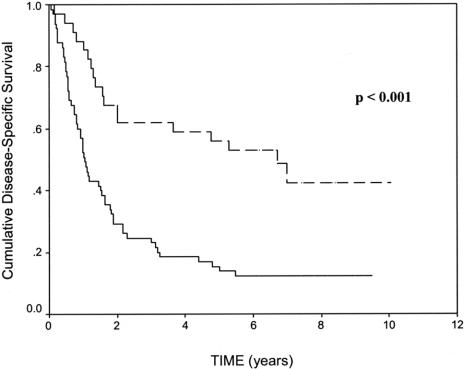
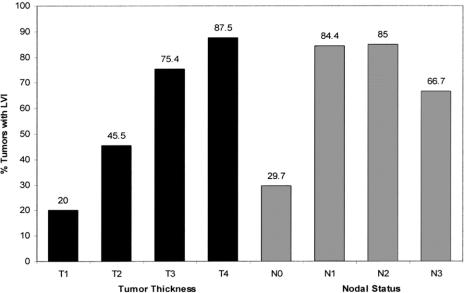
LVI was identified in 68 (59.6%), absent in 37 (32.5%), and not reported in 9 (7.9%) cases. There was a significant difference in the disease-specific 5-year survivals between LVI-positive and LVI-negative tumors (13.9% ± 8.4% versus 55.9% ± 16.7% respectively, P < 0.001, Fig. 3). LVI was significantly associated with both N status (P < 0.001) and T status (P = 0.001). The relationship between increasing T status and increasing N status with LVI is shown in Figure 4. The mean number of lymph nodes resected was 9.8 (range, 2–30) during the retrospective study period (1994–1997), compared with 13.6 (range, 3–29) during the prospective study period (2002–2003). This difference was statistically significant (P < 0.01).
FIGURE 3. The relationship between LVI and disease-specific survival in a population of patients with resected gastric cancer. The 5-year survivals of LVI-positive patients (n = 68, solid line) and LVI-negative patients (n = 37, dashed line) were 13.9% ± 8.4% versus 55.9% ± 16.7% respectively.
FIGURE 4. Relationship of LVI with tumor thickness and lymph node status in a cohort of 114 patients with gastric cancer. The number above each bar represents the percentage of patients with LVI within each stage category. The chart demonstrates a significant association between advancing T status (P = 0.001) and advancing N status (P < 0.001) with LVI.
Gastric Cancer Tissue Array Analyses
COX-2 immunoreactivity was localized predominantly in the cytoplasm of gastric cancer cells (Fig. 1B) and was not detectable within the tumor stroma. In contrast, COX-1 immunoreactivity was more variable, demonstrating cytoplasmic and/or nuclear localization. The presence of COX-1 and COX-2 immunoreactivity was demonstrated in 64.1% and 82.5% of gastric cancer specimens, respectively. COX-1 immunoreactivity correlated significantly with tumor grade (P = 0.003), whereas 32 (80%) of 40 poorly differentiated tumors failed to exhibit COX-1 immunoreactivity. COX-2 immunoreactivity significantly correlated with T status (P = 0.02) and tumor grade (P = 0.01). Although COX-2 immunoreactivity was not significant with respect to N status, there was a significant association when nodal involvement was stratified into positive versus negative (P = 0.02, Table 2) tumor involvement. COX-2 immunoreactivity was significantly associated with both MMP-2 (P < 0.001) and MMP-9 (P < 0.001) immunoreactivity.
TABLE 2. Association Between Lymphovascular Invasion, Tumor Thickness, and Immunoreactivities Among 114 Gastric Cancer Cases Studied With a Multitumor Tissue Array

Of 114 cases studied, MMP-2 immunoreactivity was absent in 29 (25.4%), weak in 56 (49.1%), and strong in 29 (25.4%) patients. MMP-2 immunoreactivity was preferentially localized to the cytoplasm. MMP-2 immunoreactivity was not associated with any clinicopathologic factors examined (Table 2).
MMP-9 immunoreactivity was localized primarily to the cytoplasm of gastric cancer cells (Fig. 2B) with little to no stromal staining. Of 114 cases studied, 32 (28.1%) had no immunoreactivity, 56 (49.1%) had weak staining, and 26 (22.8%) had strong staining (Fig. 2A). MMP-9 exhibited borderline significance with both T status (P = 0.07) and lymph node positivity (P = 0.08).
There was a significant association in the pattern of immunoreactivity between MMP-2 and MMP-9 (P < 0.001), where there was concordance in the immunoreactivity in 64.5% of tumors with weak staining, 63% with moderate staining, and 48.3% among tumors staining strongly with MMP-2 and MMP-9.
Univariate and Multivariate Survival Analysis
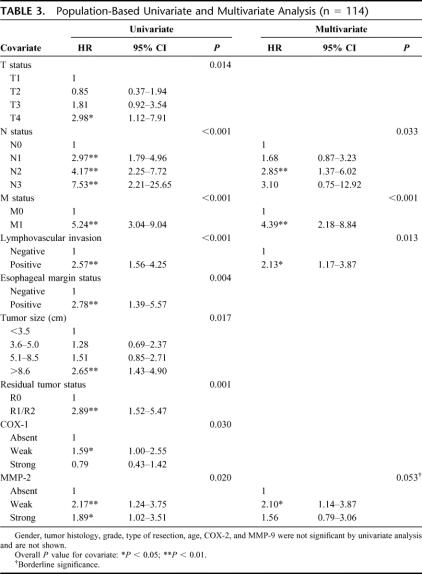
Table 3 shows the results of the univariate and multivariate Cox proportional hazard model. In the univariate analysis T, N, M, LVI, esophageal margins, tumor size, residual tumor status (R status), and COX-1 and MMP-2 immunoreactivity were significantly associated with survival. In contrast, gender, age, histologic subtype, tumor grade, type of surgical resection, and immunoreactivity of COX-2 and MMP-9 were not significant prognostic factors.
TABLE 3. Population-Based Univariate and Multivariate Analysis (n = 114)
In multivariate analyses, M status was the most significant independent prognostic factor (P < 0.001), followed by LVI (P = 0.013, Fig. 3) and N status (P = 0.033). MMP-2 was a borderline significant (P = 0.053) predictor of survival.
Gene-Expression Analysis
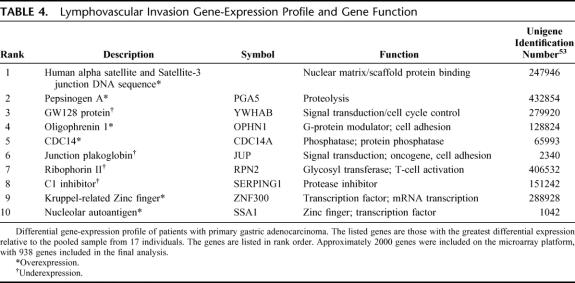
The clinicopathologic characteristics of the microarray cohort (n = 20) are shown in Table 1. The gene-expression analysis was undertaken to determine if patterns in the gene expression profile relate to the LVI status of the samples studied. LVI was present in 15 (75.0%) and absent in 5 (25.0%) tumors analyzed. Unfortunately, it was not possible to construct a classifier that could reliably predict LVI; this may have been due to the small sample size. Nevertheless, the gene-expression profile and reported gene functions of the genes whose class means differed most between the 2 classes are shown in Table 4. The 6 genes whose class means had the greatest expression in LVI-positive relative to that in LVI-negative cases, included genes associated with proteolysis, G-protein modulation, and cell-adhesion/migration. Conversely, 4 genes whose class means had the least expression in LVI-positive relative to that in LVI-negative cases are shown in Table 4. Of these, the most biologically relevant was ribophorin-II, which is associated with a glycosyl transferase involved in T-cell activation.32
TABLE 4. Lymphovascular Invasion Gene-Expression Profile and Gene Function
DISCUSSION
This study examined the clinicopathologic predictors of survival in a population-based cohort and correlated these factors on tissue arrays for marker protein immunoreactivity. In addition, oligonucleotide microarray analyses were conducted to look for gene expression patterns that could be correlated with clinical parameters such as LVI.
The incidence of LVI in gastric cancer varies from 5.4% to 86%, with the lowest incidence reported in patients with node-negative tumors.12,33 In our analysis, 59.6% of patients resected for cure were found to have evidence of LVI. LVI has previously been reported to be an independent risk factor for long-term survival34 and for the risk of lymph node metastasis.35 It has been suggested that LVI may be a clinically useful marker of biologic aggressiveness.13 This observation was subsequently supported in a study by Hyung et al,12 which showed LVI to be an adverse prognostic indicator, independent of clinicopathologic factors in node-negative gastric cancer. This study concluded that LVI may provide useful information for prognosis and clinical management in the subset of patients with node-negative gastric cancer.12 More recently, Kooby et al11 showed that vascular invasion in node-negative patients was an independent predictor of poor outcome and identified more aggressive lesions independent of tumor size and depth of invasion. This finding was consistent with our earlier results, in which a subgroup analysis demonstrated that LVI was independently associated with poor outcome in node-negative gastric cancer.10
Our earlier studies differed from the present one in that we included all patients with gastric cancer resected for cure, regardless of nodal status. By multivariate analysis, our results indicated that N status (P = 0.033), M status (P < 0.001), and LVI (P = 0.013) were independently associated with survival in patients with resected gastric cancer. This observation is supported by Talamonti et al3 who recently showed that, along with other clinicopathologic factors, LVI is independently associated with disease-free survival. The latter study reported 5-year overall survival rates of 26.2% in LVI positive compared with 49.9% in LVI-negative tumors. In the present study, we demonstrated a strong correlation between LVI and T status (P = 0.001, Fig. 4) and N status (P < 0.001). Furthermore, we showed that patients with LVI had significantly worse 5-year survival compared with LVI-negative tumors (13.9% ± 8.4% versus 55.9% ± 16.7%, respectively, P < 0.001, Fig. 3).
Examining protein immunoreactivity provides an opportunity to correlate observed clinicopathologic characteristics with specific gene products governing tumor behavior. The present study demonstrated a significant association between COX-2 immunoreactivity and gastric cancer with respect to depth of tumor invasion, tumor grade, and the presence of lymph node involvement. Tumor grade14,18 and depth of tumor invasion14,17,18,36 have been previously shown to correlate with COX-2 expression in gastric cancer. It has been proposed that the activity of COX-2 is facilitated through enhanced activity of the matrix metalloproteinases.16 In the present study, we showed a significant association between COX-2 and MMP-2 (P < 0.001) or MMP-9 immunoreactivity (P < 0.001), which was consistent with the results of gene transfection studies that showed that COX-2 expression increased the metastatic potential of colon cancer through activation of the MMP-2.37 It has recently been demonstrated that COX-2 and the MMPs are up-regulated following mitogenic stimulation of phosphoinositide 3-kinase (PI3K).20,38 Activated PI3K is thought to act through a pleckstrin homology domain (PH) of an integrin-linked kinase (ILK) with subsequent activation of protein kinase B (PKB/Akt).23 Once activated, PKB/Akt up-regulates the transcriptional factor NF-kB.23,39 Importantly, MMP-9 has been shown to be expressed in a NF-kB-dependent manner.23,39 The observed association between COX-2 and MMP-9 may therefore suggest a role of COX-2 in the activation of MMP-9 as previously suggested.23,39
We did not show an association between COX-1, COX-2, MMP-2, or MMP-9 immunoreactivity and LVI. MMP-9, however, demonstrated borderline significance with both lymph node positivity and depth of tumor invasion, as previously shown.21,40 Overall, this pattern of expression was consistent with the concept that both COX-2 and MMP-9 may function at an early stage in gastric cancer, thereby giving invasive cells a survival advantage through early access to the lymphatic and vascular spaces, facilitated through degradation of the basement membrane.21,40,41 Furthermore, there was a significant concordance between MMP-2 and MMP-9 immunoreactivities, perhaps suggesting codependence in the process involved with tumor penetration and LVI.
Oligophrenin-1 is a GTPase-activating protein that stimulates GTP hydrolysis of signaling intermediates such as Rac1.42,43 Once activated, these intermediates regulate functions such as cell-cell and cell-matrix adhesion, membrane trafficking, and transcriptional regulation.43 Recently, Oligophrenin-1 was demonstrated in the enteric plexus, where it was hypothesized to be involved with gastrointestinal disease and recovery after injury.42 Pinheiro et al,44 using cDNA microarray analysis, demonstrated its overexpression of in colorectal tumors. In the present study, we identified the gene encoding Oligophrenin-1 in gastric cancer tissue to be differentially expressed in tumors exhibiting LVI. Although its exact role in gastric cancer is unknown, previous studies have shown the ability of Oligophrenin-1 to activate Rac1.45 Rac1, following stimulation by PI3K, activates the Akt/PKB intracellular pathway mediating cellular migration and invasion through MMP-9 modulation.39 Interestingly, Oligophrenin-1 contains a pleckstrin homology domain (PH), and some PH domains bind PI3K products with high affinity.46 The differential expression of the Oligophrenin-1 gene in colorectal cancer,44 gastric cancer,47 and in the present study provide potential insight into the behavior of gastric cancer. The association between COX-2 and MMP-9 immunoreactivities observed in the present study, in view of the proposed pathway of MMP-9 activation, makes OPHN1 an interesting candidate for future studies with respect to LVI.
The late events of T-cell activation are thought to be associated with N-linked glycosylation, mediated by an oligosaccharyltransferase (OT),32 a protein complex consisting of ribophorin I, ribophorin II, and a 50-kDa protein.32 OT activity has been show to increase 10-fold during cytotoxic T-cell activation, and tumor-infiltrating lymphocytes produced up to 20-fold more glycoprotein than resting lymphocytes when stimulated by OT.32 Since the majority of tumors express major histocompatibility complex type I, they are susceptible to destruction by activated T cells,48 which therefore play a key role in immune surveillance and antitumor activity in many human malignancies, including gastric cancer.48–51 In the present study, when examining the class means of LVI-positive relative to LVI-negative gastric tumors, we identified ribophorin-II, a protein that has not been previously documented in gastric cancer. Maehara et al50 suggested that when gastric cancer cells advance into the lymphatic space, an immunosuppressive activity is exerted and local defense mechanisms are suppressed. The precise role of ribophorin-II in gastric cancer is not known. The reported role of immune surveillance in mediating antitumor activity makes the documentation of ribophorin-II expression in gastric cancers exhibiting LVI of considerable interest for future studies.
CONCLUSION
This study demonstrated the significance of LVI as an independent predictor of survival in a population-based cohort of patients with gastric cancer. We also document a significant association between COX-2 abundance and T status, tumor grade, lymph node positivity, and MMP-2 and MMP-9 abundance. Finally, we have presented preliminary findings from gene expression profiles of gastric cancer patients, which revealed the expression of oligophrenin-I, a gene product potentially involved in mediating cellular migration and invasion of cancer cells through basement membrane. In addition, we demonstrated the expression of ribophorin-II, a protein complex involved with T-cell activation, immune surveillance, and potential antitumor activity. The identification of these gene products provides potential insight into the biology of gastric cancer. More detailed microarray and tissue array profiles, using larger sample sizes, are needed to clarify the role of the molecular alterations identified in this study in gastric cancer.
ACKNOWLEDGMENTS
The authors thank Laith Dabbagh for his assistance in tissue array construction and immunohistochemistry; Dr. John Mackey for suggestions; Lillian Cook, Jennifer Dufour, and Diana Carandang for help with microarray experiments and tumor banking; and Kathryn Calder for help with clinical research protocols.
Footnotes
Dr. Cass is Canada Research Chair in Oncology. Supported by the University of Alberta Hospital Foundation, the Alberta Cancer Board and the Clinical Investigator Program of the Royal College of Physicians and Surgeons of Canada.
Reprints: Carol Cass, PhD, Department of Oncology, Cross Cancer Institute, 11560 University Avenue, Edmonton, Alberta, Canada T6G 1Z2. E-mail: carol.cass@cancerboard.ab.ca.
REFERENCES
- 1.Volpe CM, Driscoll DL, Douglass HO Jr. Outcome of patients with proximal gastric cancer depends on extent of resection and number of resected lymph nodes. Ann Surg Oncol. 2000;7:139–144. [DOI] [PubMed] [Google Scholar]
- 2.Msika S, Benhamiche A, Jouve J-L, et al. Prognostic factors after curative resection of gastric cancer: a population-based study. Eur J Cancer. 2000;36:390–396. [DOI] [PubMed] [Google Scholar]
- 3.Talamonti MS, Kim SP, Yao KA, et al. Surgical outcomes of patients with gastric carcinoma: the importance of primary tumor location and microvessel invasion. Surgery. 2003;134:720–727; discussion 727–729. [DOI] [PubMed]
- 4.Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the ‘different disease’ hypothesis. Cancer. 2000;88:921–932. [PubMed] [Google Scholar]
- 5.Chambers WM, Khan U, Gagliano A, et al. Tumour morphology as a predictor of outcome after local excision of rectal cancer. Br J Surg. 2004;91:457–459. [DOI] [PubMed] [Google Scholar]
- 6.Mirza AN, Mirza NQ, Vlastos G, et al. Prognostic factors in node-negative breast cancer: a review of studies with sample size more than 200 and follow-up more than 5 years. Ann Surg. 2002;235:10–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radespiel-Troger M, Hohenberger W, Reingruber B. Improved prediction of recurrence after curative resection of colon carcinoma using tree-based risk stratification. Cancer. 2004;100:958–967. [DOI] [PubMed] [Google Scholar]
- 8.Maehara Y, Kabashima A, Koga T, et al. Vascular invasion and potential for tumor angiogenesis and metastasis in gastric carcinoma. Surgery. 2000;128:408–416. [DOI] [PubMed] [Google Scholar]
- 9.Dicken BJ, Bigam DL, Cass C, et al. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg. 2005;241:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dicken BJ, Saunders LD, Jhangri GS, et al. Gastric cancer: establishing predictors of biologic behavior with use of population-based data. Ann Surg Oncol. 2004;11:629–635. [DOI] [PubMed] [Google Scholar]
- 11.Kooby DA, Suriawinata A, Klimstra DS, et al. Biologic predictors of survival in node-negative gastric cancer. Ann Surg. 2003;237:828–835; discussion 835–837. [DOI] [PMC free article] [PubMed]
- 12.Hyung WJ, Lee JH, Choi SH, et al. Prognostic impact of lymphatic and/or blood vessel invasion in patients with node-negative advanced gastric cancer. Ann Surg Oncol. 2002;9:562–567. [DOI] [PubMed] [Google Scholar]
- 13.Yokota T, Kunii Y, Teshima S, et al. Significant prognostic factors in patients with node-negative gastric cancer. Int Surg. 1999;84:331–336. [PubMed] [Google Scholar]
- 14.Han SL, Tang HJ, Hua YW, et al. Expression of COX-2 in stomach cancers and its relation to their biological features. Dig Surg. 2003;20:107–114. [DOI] [PubMed] [Google Scholar]
- 15.Joo YE, Rew JS, Seo YH, et al. Cyclooxygenase-2 overexpression correlates with vascular endothelial growth factor expression and tumor angiogenesis in gastric cancer. J Clin Gastroenterol. 2003;37:28–33. [DOI] [PubMed] [Google Scholar]
- 16.Chen CN, Sung CT, Lin MT, et al. Clinicopathologic association of cyclooxygenase 1 and cyclooxygenase 2 expression in gastric adenocarcinoma. Ann Surg. 2001;233:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohno R, Yoshinaga K, Fujita T, et al. Depth of invasion parallels increased cyclooxygenase-2 levels in patients with gastric carcinoma. Cancer. 2001;91:1876–1881. [PubMed] [Google Scholar]
- 18.Joo YE, Oh WT, Rew JS, et al. Cyclooxygenase-2 expression is associated with well-differentiated and intestinal-type pathways in gastric carcinogenesis. Digestion. 2002;66:222–229. [DOI] [PubMed] [Google Scholar]
- 19.Murata H, Kawano S, Tsuji S, et al. Cyclooxygenase-2 overexpression enhances lymphatic invasion and metastasis in human gastric carcinoma. Am J Gastroenterol. 1999;94:451–455. [DOI] [PubMed] [Google Scholar]
- 20.Persad S, Dedhar S. The role of integrin-linked kinase (ILK) in cancer progression. Cancer Metastasis Rev. 2003;22:375–384. [DOI] [PubMed] [Google Scholar]
- 21.Kabashima A, Maehara Y, Kakeji Y, et al. Clinicopathological features and overexpression of matrix metalloproteinases in intramucosal gastric carcinoma with lymph node metastasis. Clin Cancer Res. 2000;6:3581–3584. [PubMed] [Google Scholar]
- 22.Allgayer H, Babic R, Beyer BC, et al. Prognostic relevance of MMP-2 (72-kD collagenase IV) in gastric cancer. Oncology. 1998;55:152–160. [DOI] [PubMed] [Google Scholar]
- 23.Troussard AA, Costello P, Yoganathan TN, et al. The integrin linked kinase (ILK) induces an invasive phenotype via AP-1 transcription factor-dependent upregulation of matrix metalloproteinase 9 (MMP-9). Oncogene. 2000;19:5444–5452. [DOI] [PubMed] [Google Scholar]
- 24.Ichikura T, Ogawa T, Kawabata T, et al. Is adenocarcinoma of the gastric cardia a distinct entity independent of subcardial carcinoma? World J Surg. 2003;27:334–338. [DOI] [PubMed] [Google Scholar]
- 25.Stein HJ, Feith M, Siewert JR. Cancer of the esophagogastric junction. Surg Oncol. 2000;9:35–41. [DOI] [PubMed] [Google Scholar]
- 26.AJCC. AJCC Cancer Staging Manual. Philadelphia: Lippincott-Raven, 1997. [Google Scholar]
- 27.Monig SP, Baldus SE, Hennecken JK, et al. Expression of MMP-2 is associated with progression and lymph node metastasis of gastric carcinoma. Histopathology. 2001;39:597–602. [DOI] [PubMed] [Google Scholar]
- 28.Botwell D, Sambrook J. DNA Microarrays: A Cloning Manual. Cold Spring Harbor, NY: Cold Springs Harbor Laboratory, 2003. [Google Scholar]
- 29.Tibshirani R, Hastie T, Narasimhan B, et al. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci USA. 2002;99:6567–6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Listgarten J, Graham K, Damaraju S, et al. Clinically validated benchmarking of normalization techniques for two-color oligonucleotide spotted microarray slides. Appl Bioinformatics. 2003;2:219–228. [PubMed] [Google Scholar]
- 31.Hosmer D, Lemeshow S. Applied Survival Analysis. New York: Wiley, 1999. [Google Scholar]
- 32.Kumar V, Heinemann FS, Ozols J. Interleukin-2 induces N-glycosylation in T-cells: characterization of human lymphocyte oligosaccharyltransferase. Biochem Biophys Res Commun. 1998;247:524–529. [DOI] [PubMed] [Google Scholar]
- 33.Noguchi Y. Blood vessel invasion in gastric carcinoma. Surgery. 1990;107:140–148. [PubMed] [Google Scholar]
- 34.Gabbert HE, Meier S, Gerharz CD, et al. Incidence and prognostic significance of vascular invasion in 529 gastric-cancer patients. Int J Cancer. 1991;49:203–207. [DOI] [PubMed] [Google Scholar]
- 35.Maehara Y, Orita H, Okuyama T, et al. Predictors of lymph node metastasis in early gastric cancer. Br J Surg. 1992;79:245–247. [DOI] [PubMed] [Google Scholar]
- 36.Uefuji K, Ichikura T, Mochizuki H. Expression of cyclooxygenase-2 in human gastric adenomas and adenocarcinomas. J Surg Oncol. 2001;76:26–30. [DOI] [PubMed] [Google Scholar]
- 37.Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piccolo E, Vignati S, Maffucci T, et al. Inositol pentakisphosphate promotes apoptosis through the PI 3-K/Akt pathway. Oncogene. 2004;23:1754–1765. [DOI] [PubMed] [Google Scholar]
- 39.Kim D, Kim S, Koh H, et al. Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J. 2001;15:1953–1962. [DOI] [PubMed] [Google Scholar]
- 40.Zhang S, Li L, Lin JY, et al. Imbalance between expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in invasiveness and metastasis of human gastric carcinoma. World J Gastroenterol. 2003;9:899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torii A, Kodera Y, Ito M, et al. Matrix metalloproteinase 9 in mucosally invasive gastric cancer. Gastric Cancer. 1998;1:142–145. [DOI] [PubMed] [Google Scholar]
- 42.Xiao J, Neylon CB, Hunne B, et al. Oligophrenin-1, a Rho GTPase-activating protein (RhoGAP) involved in X-linked mental retardation, is expressed in the enteric nervous system. Anat Rec. 2003;273A:671–676. [DOI] [PubMed] [Google Scholar]
- 43.Fauchereau F, Herbrand U, Chafey P, et al. The RhoGAP activity of OPHN1, a new F-actin-binding protein, is negatively controlled by its amino-terminal domain. Mol Cell Neurosci. 2003;23:574–586. [DOI] [PubMed] [Google Scholar]
- 44.Pinheiro NA, Caballero OL, Soares F, et al. Significant overexpression of oligophrenin-1 in colorectal tumors detected by cDNA microarray analysis. Cancer Lett. 2001;172:67–73. [DOI] [PubMed] [Google Scholar]
- 45.Billuart P, Bienvenu T, Ronce N, et al. Oligophrenin-1 encodes a rhoGAP protein involved in X-linked mental retardation. Nature. 1998;392:923–926. [DOI] [PubMed] [Google Scholar]
- 46.Mitsuuchi Y, Johnson SW, Sonoda G, et al. Identification of a chromosome 3p14. 3–21. 1 gene, APPL, encoding an adaptor molecule that interacts with the oncoprotein-serine/threonine kinase AKT2. Oncogene. 1999;18:4891–4898. [DOI] [PubMed] [Google Scholar]
- 47.Oien KA, Vass JK, Downie I, et al. Profiling, comparison and validation of gene expression in gastric carcinoma and normal stomach. Oncogene. 2003;22:4287–4300. [DOI] [PubMed] [Google Scholar]
- 48.Nakakubo Y, Miyamoto M, Cho Y, et al. Clinical significance of immune cell infiltration within gallbladder cancer. Br J Cancer. 2003;89:1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okuyama T, Maehara Y, Kakeji Y, et al. Interrelation between tumor-associated cell surface glycoprotein and host immune response in gastric carcinoma patients. Cancer. 1998;82:1468–1475. [DOI] [PubMed] [Google Scholar]
- 50.Maehara Y, Tomisaki S, Oda S, et al. Lymph node metastasis and relation to tumor growth potential and local immune response in advanced gastric cancer. Int J Cancer. 1997;74:224–228. [DOI] [PubMed] [Google Scholar]
- 51.Menon AG, Janssen-van Rhijn CM, Morreau H, et al. Immune system and prognosis in colorectal cancer: a detailed immunohistochemical analysis. Lab Invest. 2004;84:493–501. [DOI] [PubMed] [Google Scholar]
- 52.Fenogilo-Preiser C, Carneiro F, Correa P, et al. Gastric carcinoma. In: Hamilton S, Aaltonin L, eds. Pathology and Genetics: Tumors of the Digestive System, vol. 1. Lyon, France: IARC Press, 2000:37–52. [Google Scholar]