Abstract
Objective:
To study the effect of bariatric surgery on the entero-hypothalamic endocrine axis of humans and rodents.
Background:
Bariatric surgery is the most effective obesity treatment as it achieves substantial and sustained weight loss. Glycemic control and enhanced satiation improve before substantial weight loss occurs. Gut peptides, acting both peripherally and centrally, contribute to glycemic control and regulate food intake.
Methods:
We examined meal-stimulated responses of insulin, ghrelin, peptide YY (PYY), glucagon-like-peptide-1 (GLP-1), and pancreatic polypeptide (PP) in humans and rodents following different bariatric surgical techniques.
Results:
Compared with lean and obese controls, patients following Roux-en-Y gastric bypass (RYGB) had increased postprandial plasma PYY and GLP-1 favoring enhanced satiety. Furthermore, RYGB patients had early and exaggerated insulin responses, potentially mediating improved glycemic control. None of these effects were observed in patients losing equivalent weight through gastric banding. Leptin, ghrelin, and PP were similar in both the surgical groups. Using a rodent model of jejuno-intestinal bypass (JIB), we showed elevated PYY and GLP-1 in JIB rats compared with sham-operated rats. Moreover, exogenous PYY reduced food intake and blockade of endogenous PYY increased food intake. Thus, higher plasma PYY following JIB may contribute to reduced food intake and contribute to weight loss.
Conclusions:
Following RYGB and JIB, a pleiotropic endocrine response may contribute to the improved glycemic control, appetite reduction, and long-term changes in body weight.
Bariatric surgery changes the entero-hypothalamic endocrine axis of humans and rodents. Following Roux-en-Y gastric bypass in humans and jejuno-intestinal bypass in rodents, a pleiotropic endocrine response may partly explain the improved glycemic control, appetite reduction, and long-term changes in body weight.
The rising prevalence of obesity in developed societies is causing a major health threat in terms of morbidity and mortality.1 The complications of obesity, especially type 2 diabetes mellitus (T2DM), are placing growing demand on healthcare resources.1,2 Existing medical therapeutic strategies to achieve and maintain clinically significant weight loss remain limited.3 Surgical procedures for the treatment of obesity are, however, highly effective in achieving substantial and sustained weight loss,4,5 but they are technically demanding and costly and carry small but significant rates of morbidity and mortality.6
Dramatic improvements in glycemic control have been observed in subjects with T2DM following bariatric surgery, and specifically the Roux-en-Y gastric bypass (RYGB) procedure.7–11 In the early postoperative period following RYGB, many patients with T2DM discontinue all antidiabetic medication, and may achieve normal fasting plasma glucose concentrations even before substantial weight loss has occurred.10,11 It has been postulated that the improvements in glycemic control, reduction in appetite, and subsequent weight loss following bypass surgery may be due to changes in circulating gut hormones.10,12–14
A number of peptides released from the gastrointestinal tract have recently been shown to regulate appetite and food intake, effecting both orexigenic and anorexic outcomes through actions on the hypothalamic arcuate nucleus.15,16 Ghrelin, a hormone produced from the stomach in the preprandial state, increases expression of the orexigenic hypothalamic neuropeptide Y (NPY) and stimulates food intake in rodents and humans.17,18 In contrast, peptide YY (PYY), released postprandially from the distal gastrointestinal tract, acts within the arcuate nucleus to inhibit the release of NPY19. Intravenous PYY3–36 infusions into humans and intraperitoneal injections into rodents induce satiety and reduce food intake.19,20 Glucagon-like peptide 1 (GLP-1) acts mainly as an incretin, promoting postprandial insulin release21 and improving pancreatic β-cell function,22 and has also been reported to inhibit food intake in humans.23 Pancreatic polypeptide (PP) has recently been shown to inhibit appetite and food intake and promotes energy expenditure.24,25
We sought to determine the changes in the entero-hypothalamic endocrine axis in human subjects following bariatric surgery, examining the meal-stimulated release of PYY, GLP-1, and PP. We used a rodent model of jejuno-intestinal bypass (JIB) surgery to further examine gut hormone changes and to investigate the potential role of PYY in mediating the food intake and weight reducing effects of surgery.
MATERIALS AND METHODS
Human Studies
All human studies were performed according to the principles of the Declaration of Helsinki. The Local Research and Ethics committee at King's College London approved the bariatric surgery study (02/174). Written informed consent was obtained from all subjects. Subjects had undergone either RYGB or an adjustable gastric banding (GB) 6 to 36 months prior to the study. In this cross-sectional study, patients were routinely offered a choice of surgical procedures. There was a small, nonsignificant difference in the preoperative weight of the RYGB and GB groups (Table 1). The subjects included in the study had very similar outcomes in terms of weight loss compared with other patients who had the same obesity surgery in our clinic but who were not recruited (data not shown). The RYGB procedures were performed laparoscopically using an omega loop technique as described by Olbers et al.26 The Roux limb was 112 cm with a 30-mL stomach pouch and a cut omega loop. GB was performed laparoscopically using the Lap-Band system (BioEnterics). Bands were positioned in the perigastric area to create a proximal gastric pouch of approximately 30 mL. Obese control subjects were recruited following attendance at a surgical obesity clinic. These obese control subjects had been accepted for surgery and were on the waiting list for admission; however, they were not engaged in any active weight loss program. Lean controls were recruited through local advertising. All control subjects were at a stable body mass for 3 months prior to this study. The obese control group was matched for body mass index (BMI) to the preoperative BMI of surgical patients. Exclusion criteria included chronic medical or psychiatric illness, pregnancy, substance abuse, more than 2 alcoholic drinks per day, and aerobic exercise for more than 30 minutes 3 times per week.
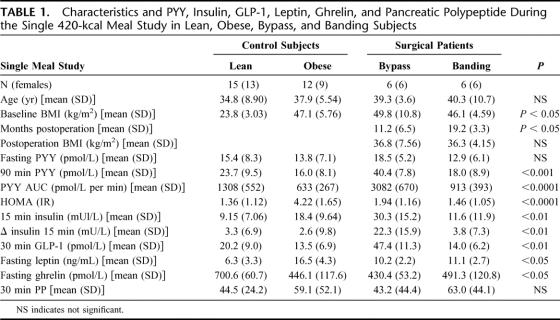
TABLE 1. Characteristics and PYY, Insulin, GLP-1, Leptin, Ghrelin, and Pancreatic Polypeptide During the Single 420-kcal Meal Study in Lean, Obese, Bypass, and Banding Subjects
For the meal response studies, subjects were admitted to the research center after a 12-hour fast, and venous blood was collected before the meal, 15 minutes after, and at 30 minute intervals up to 3 hours following a mixed 420-kcal meal. Plasma levels of the gut hormones PYY, GLP-1, PP, and ghrelin were compared at each time point.
Animal Studies
Animal work was performed under a license issued by the Home Office UK (PL 70-5569). Male Wistar rats of 387 ± 11.7 g were randomized to an established protocol for JIB and sham bypass (sham) surgery.13 In the JIB group, the jejunum was divided and anastomosed to the terminal ileum end-to-side creating a blind limb. Sham animals underwent division of the jejunum but with immediate reanastomosis maintaining normal anatomic continuity. The duration of both procedures was similar, and each operation lasted between 25 and 30 minutes. Postoperatively, gentamicin 8 mg/kg and carprofen 0.01 mL were administered intraperitoneally (ip) as prophylaxis for postoperative infection.
Animals had ad libitum access to normal rat chow (RM1 diet, Special Diet Services Ltd, UK) and water unless otherwise indicated. In each experiment, on the day of termination, animals were fasted for 12 hours from the beginning of the light cycle and were killed at the end of the light cycle. Blood was obtained, immediately centrifuged at 3000 rpm for 10 minutes at 4°C, and stored at −20°C until analysis.
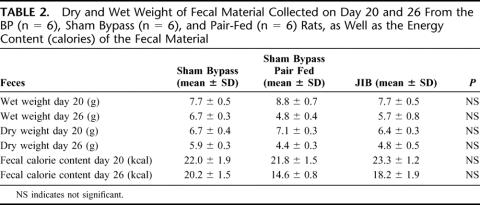
In accordance to previously published protocol,13 rectal temperature was measured preoperatively, 10 and 26 days after the operation in conscious restrained rats. Measurements of C-reactive protein and white blood cell counts were made to assess inflammation. To evaluate malabsorption, fecal material was collected on day 20 and 26 from the JIB, sham bypass and pair fed groups. The feces were dried in an oven and weighed; calorie content was measured using a ballistic bomb calorimeter.27
Ten days after the operation, 6 JIB rats received ip injections 1 hour before the dark phase of purified PYY rabbit antibody (0.2 mL). In these experiments, control sham rats received 0.2 mL of purified nonimmune rabbit serum. Food intake was measured at 1, 2, 4, 8, 12, and 24 hours following injection.
Where indicated, rodent PYY3–36 (5 μg/100 g; Bachem UK) was administered ip as a 0.2 mL solution with 0.9% saline, 1 hour before the beginning of the dark phase. JIB animals received 0.2 mL of 0.9% saline ip. In the chronic exposure experiment, 5 μg/100 g PYY3–36 was given ip as a 0.2-mL solution with 0.9% saline to the sham rats 1 hour before the beginning of the dark phase for 10 days and then twice daily for a further 8 days, 1 hour before the onset of the dark or light phase. The JIB rats received 0.2 mL of 0.9% saline ip. Food intake was measured daily for 28 days.
Hormone Assays
All samples were assayed in duplicate. PYY-like immunoreactivity was measured with a specific and sensitive radioimmunoassay, which measures, both the full length (PYY1–36) and the fragment (PYY3–36).20,28 GLP-1 and PP were measured in duplicate by established in-house radioimmunoassays.21,29 Plasma immunoreactive ghrelin was measured in duplicate with the commercially available Phoenix Pharmaceutical assay kit and plasma leptin with a Linco Research assay kit. Insulin (Abbott, UK) and glucose (Olympus, UK) were measured using commercial immunoassays.
Statistical Analysis
Hormone levels are expressed as means ± SD. Values for the area under the curve were calculated with the use of the trapezoidal rule. End points were compared with the use of 2-tailed, paired Student t tests or ANOVA, and exact binomial methods were used where indicated (Graphpad Prism, USA).
RESULTS
Human Studies
The characteristics of the lean (n = 15), obese (n = 12), RYGB (n = 6), or GB (n = 6) patients are shown in Table 1. Control obese subjects were matched to the preoperative BMI of patients in the surgical groups. Patients with either operation had an equivalent postoperative BMI, although the duration following the procedure was greater for the GB patients. Fasting leptin levels were equivalent in the RYGB and GB subjects (P = 0.68), and although lower than obese control subjects, leptin levels were significantly higher than lean subjects (P < 0.05) (Table 1).
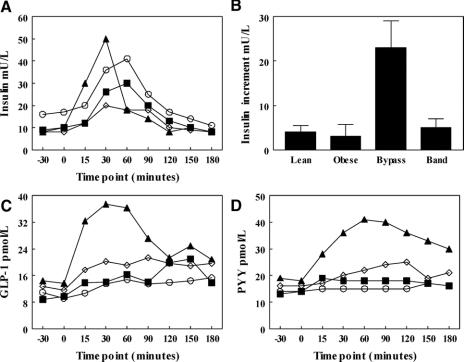
Two patients in the RYGB and 2 in the GB group had diabetes before the surgery, but no patient satisfied the WHO criteria for diabetes at the time of the study. Obese subjects demonstrated elevated fasting insulin and basal insulin resistance (assessed by HOMA),30 whereas postsurgical subjects were found to be as insulin sensitive as lean subjects, despite a significantly higher BMI postoperatively. Subjects did not experience hypoglycemia or symptoms of the dumping syndrome following a test meal, and all postprandial glucose values were above 4.4 mmol/L. Significant differences were observed between groups in the dynamic insulin response to a mixed meal. In the RYGB group, an early rise in insulin secretion was observed (Fig. 1A) with a significantly higher increase in plasma insulin at 15 minutes compared with the GB, lean and obese subjects (P < 0.001; Table 1; Fig. 1B).
FIGURE 1. A, The insulin response, C, GLP-1 responses and D, PYY response to test a meal in RYGB (▴), GB (▪), lean (⋄), and obese (O) subjects. B, The insulin increment between baseline and 15 minutes in the 4 groups.
The pronounced early insulin peak in RYGB patients prompted further investigation of the release of the incretin, GLP-1. In response to a 420-kcal meal, lean control subjects achieved a 30-minute GLP-1 level of 20.2 ± 9.0 pmol/L (66% above baseline, P = 0.02) (Table 1; Fig. 1C). However, RYGB patients had an exaggerated GLP-1 response of 47.4 ± 11.3 pmol/L (230% above baseline, P < 0.001), significantly higher than other groups (P < 0.001, Fig. 1C). In contrast, obese control subjects exhibited an attenuated response (13.5 ± 6.9 pmol/L, 22% above baseline, P = 0.1). GB subjects achieved GLP-1 levels of 14.0 ± 6.2 pmol/L (50% above baseline) equivalent to obese controls.
Similar to GLP-1, following the test meal, plasma PYY concentrations were exaggerated in the RYGB patients. Lean control subjects achieved a 90-minute PYY level of 23.7 ± 9.5 pmol/L (49% above baseline, P < 0.01; Table 1; Fig. 1D). RYGB patients had an exaggerated PYY response of 40.4 ± 7.8 pmol/L (162% above baseline, P < 0.001) and significantly higher than lean subjects, (P = 0.002; Fig. 1D). In contrast, PYY did not rise postprandially in obese control subjects (16.0 ± 8.1 pmol/L, 14% above baseline, P = 0.19), while GB subjects achieved PYY levels of 18.0 ± 8.9 pmol/L (22% above baseline, P = 0.001, no different from lean subjects, P = 0.37). PP response was similar in all 4 groups (Table 1).
Fasting ghrelin levels in the lean group were 700.6 ± 15.5 pmol/L (Table 1), whereas obese control subjects had a lower fasting ghrelin of 446.1 ± 41.9 pmol/L (P = 0.002). We found no differences in the fasting ghrelin levels among the obese and surgical treated groups (Table 1).
Animal Studies
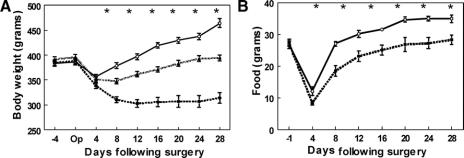
To further evaluate the potential importance of the observed hormonal responses in human subjects, an established rat model of bariatric surgery was used in which rats either received a JIB or a sham bypass.13 Although anatomically distinct, like the RYGB, the JIB procedure bypasses a significant segment of small bowel. The JIB differs from the RYGB in that there is no reduction in stomach size, which gives a restrictive component to the RYGB. In the immediate 4 days postoperatively, both JIB and sham rats had comparable weight loss. At day 8, however, the sham animals returned to baseline weight, which continued to increase on ad libitum feeding (Fig. 2A). In contrast, JIB animals lost 20% of their preoperative weight at day 8 and then achieved a plateau around 314 ± 11 g. This was significantly lower than the final weight of 464 ± 9 g in the sham rats (P < 0.001). Interestingly, the food consumption followed similar patterns but was consistently lower in the JIB group (Fig. 2B). Another group of sham animals (n = 6) were pair-fed to the JIB group for 28 days (Fig. 2A). The pair-fed sham rats weighed significantly more than the JIB animals; therefore, infection or malabsorption was evaluated among the bypass animals.
FIGURE 2. A, The weight changes for the JIB (--■--) (n = 12), sham bypass (⦵) (n = 12), and sham bypass pair fed (⋯▲⋯) (n = 10) rats over 28 days. B, The average daily food intake of the JIB (--■--) and sham bypass (⦵) rats over 28 days. *P < 0.05.
Malabsorption was investigated by fecal weight and fecal calorie content using bomb calorimetry. There was no evidence for an increase in either fecal mass or fecal calorie content in the JIB animals, thus excluding malabsorption as a major cause for the weight loss (Table 2). Survival after surgery was similar in both groups, and there was no evidence of an inflammatory response reflecting anastomotic leakage since body temperature, C-reactive protein, and neutrophil count were similar in both groups (data not shown). An inference can be drawn that energy expenditure may be influenced by surgery, although we were unable to measure this directly in this study.
TABLE 2. Dry and Wet Weight of Fecal Material Collected on Day 20 and 26 From the BP (n = 6), Sham Bypass (n = 6), and Pair-Fed (n = 6) Rats, as Well as the Energy Content (calories) of the Fecal Material
The potential role of gut hormones on mediating the observed weight loss following JIB surgery was determined. Figure 3 demonstrates that the JIB rats had significantly higher fasting plasma levels of both PYY and GLP-1. To further evaluate the potential causative effect of the increase in PYY following JIB surgery, two experimental paradigms were used. First, a PYY neutralizing antibody was injected into JIB animals in an attempt to abrogate the effect of surgery. Second, PYY levels were raised through exogenous PYY administered in the sham animals. In a preliminary study, following injection of neutralizing antibody, JIB rats ate significantly more than the previous day (30.0 ± 1.0 g versus 25.0 ± 1.9 g, P = 0.04), whereas there was no difference in food consumed for 24 hour in sham rats after injection of control serum (30.3 ± 0.8 g versus 29.4 ± 1.6 g, P = 0.54). Elevating circulating PYY in another group of sham rats was achieved by daily administration of PYY from day 10 to 20 and then twice daily for days 21 to 28. Mean food intake after the 10th day postoperatively among saline treated JIB rats were 32.2 ± 1.0 g/d, which was similar to the PYY treated sham rats that ate 31.8 ± 1.3 g/day (P = 0.76).
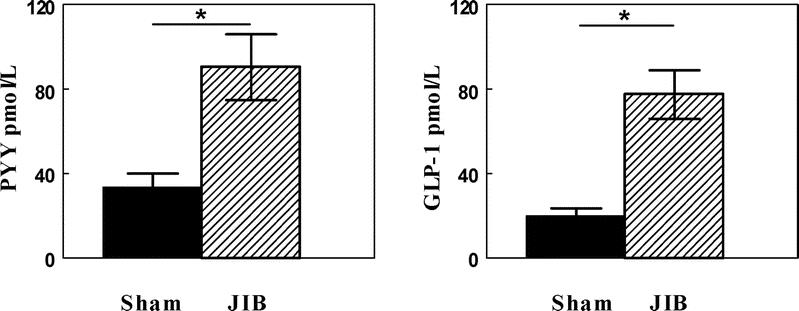
FIGURE 3. Fasting plasma PYY and GLP-1 levels in the sham bypass (▪) (n = 6) and JIB (□) rats (n = 6). *P < 0.05.
DISCUSSION
The RYGB leads to a reduced obese state with the anticipated changes in fuel homeostasis, but without the compensatory increased appetite that usually leads to weight regain following most forms of intentional weight loss. We have demonstrated a pleiotropic endocrine response to bariatric surgery, which might account for the appetite reduction that leads to long-term changes in body weight. We have shown that following RYGB, patients have increased postprandial PYY and GLP-1 favoring enhanced satiety to a meal. Furthermore, RYGB patients had early and exaggerated insulin responses, potentially mediating the improved glycemic control in patients with T2DM. None of these effects were observed in patients losing similar weight through GB, suggesting that the hormonal changes are not secondary to weight loss alone. We further demonstrated in a rodent model of intestinal bypass that elevated PYY may have a causative role in mediating reduced food intake.
Weight loss following JIB surgery is due to decreased appetite and calorie intake,31 and clinical malabsorption is not usually a feature of RYGB.32 PYY, a gut hormone with highest tissue concentrations in distal segments of the gastrointestinal tract, is released into the circulation proportional to food intake.33 Chronically elevated fasting levels of PYY have been described in several gastrointestinal diseases associated with loss of appetite.34,35 Established actions of PYY include reduced gastric emptying, delayed gastrointestinal transit,36 reduced food intake,19,20 and may be a major factor influencing postprandial satiety.15,37 All of these actions could be considered an appropriate response to acute small intestinal disease or shortened small bowel as they would decrease the nutrient load and increase absorption time. Following RYGB, postprandial PYY concentrations were elevated to levels previously shown to have appetite-reducing effects.19,20 We propose that the higher postprandial PYY response after gastric bypass surgery due to the altered intestinal anatomy may contribute to the patients’ increased satiety and weight loss. RYGB is thought to be significantly more effective than restrictive procedures such as vertical banded gastroplasty for weight loss in morbid obesity, especially for patients addicted to sweets.38 The banding patients did not have an exaggerated PYY response. There are no direct comparisons of satiety between RYGB and GB, although reports indicate that patients do experience sensations reported as enhanced satiety after GB39,40 and RYGB.41,42
Our studies in humans fall short of establishing changes in PYY as having a mechanistic role in the appetite changes and weight loss after RYGB. To investigate a potential causative role for PYY, we used an established rodent model of JIB.13 At the time of the experiments, JIB was the only established model of bariatric surgery in rodents. Both JIB and RYGB lead to weight loss and reduced food intake,32,41,42 which were previously suggested to be due to a hormonally mediated mechanism.13 Thus, despite the species and surgical procedure being different, the rodent model of intestinal bypass was used to examine the mechanisms for weight loss in RYGB patients. Both the humans and the rodents show reduced food intake and weight loss, while plasma PYY and GLP-1 were elevated. In a preliminary rodent study, a specific neutralizing PYY antibody resulted in an increased food intake in the bypass rats, while the nonimmune serum in the sham bypass rats caused a nonsignificant reduction. Exogenous administration of PYY to the sham bypass rats led to a reduction in food intake to a similar level seen in the bypass group. These results are consistent with PYY having a role in enhancing satiety following intestinal bypass surgery. Note, however, that findings in the JIB rodent model cannot be directly extrapolated to the human RYGB, which has an additional restrictive component over and above the potential effect of an exaggerated PYY response. The previous data reported in humans after JIB showing elevated fasting and postprandial PYY concentrations43 are, however, consistent with the findings in both our rodent model of JIB and human model of RYGB. The lower body weight in the bypass group was not due to decreased food intake alone, as the sham bypass pair fed group weighed more than the bypass group. The difference in weight despite the same food intake raises the possibility of enhanced energy expenditure following intestinal bypass surgery. These observations will require further investigation.
In both rodent and human studies, high GLP-1 was observed in parallel with PYY. GLP-1 is produced from the same entero-endocrine cells (L cells) as PYY and acts as an incretin, releasing insulin21 and improving pancreatic β-cell function in rodents.22 Reduction in food intake has also been reported.44 Previously, increased GLP-1 concentrations have been shown following intestinal bypass.9,43 Consistent with previous RYGB studies, our study found no difference in fasting GLP-1 levels11 but demonstrates an exaggerated GLP-1 response following a 420-kcal meal in gastric bypass subjects. The increased GLP-1 response may contribute as a satiety signal and incretin. Glycemic control in T2DM patients improves following RYGB surgery7,10,11 and often before significant weight loss occurs.10 A recent study in a nonobese diabetic rodent model following duodenal-jejunal exclusion demonstrated improvement in glycemic control that appeared independent of weight loss.14 Following weight loss, lower body weight per se most likely also contributes to improved insulin sensitivity.12
The normal postprandial glucose concentrations in all the individuals studied here are consistent with the absence of symptoms of the dumping syndrome. Although the BMI of both the bypass and banding patients was in the obese range, the fasting glucose homeostasis (HOMA) was similar to that observed in the lean control subjects. However, the RYGB group had significantly higher insulin levels 15 minutes after the meal, which might partly explain the improvement in glycemic control observed in other studies.7,10,11 The early and increased release of insulin in the bypass patients may be mediated by direct action of nutrients in the jejunum and/or a hormonal signal such as GLP-1.
Ghrelin, produced in the stomach, was initially associated with appetite reduction following gastric bypass.12 The reduced appetite following gastric bypass was explained by the failure of ghrelin to show the expected rise observed in diet-induced weight loss.12,45 However, a series of publications have since shown inconsistent ghrelin changes after RYGB and GB. These studies have recently been reviewed, and the heterogeneity of the findings makes it unlikely that ghrelin has a major role in the weight loss after RYGB.46 In our study, compared with lean control subjects, both bypass and banding patients had lower fasting ghrelin levels, although these were similar to those of the obese control subjects. In agreement with the majority of studies, we did not find an anticipated compensatory rise in ghrelin among surgical subjects.
Our data show that patients treated for obesity with gastric bypass therefore have alterations of several peripheral signals that potentially contribute to their reduced appetite and enhanced glucose homeostasis. An exaggerated postprandial PYY and GLP-1 might combine to enhance satiety, leading to a long-term reduction in calorie intake, while increased GLP-1 and insulin might contribute to immediate improvements in glycemic control.
Our studies demonstrate that obese subjects treated with GB had equivalent weight loss and improvements in insulin resistance as measured by HOMA to bypass patients, but did not have the postprandial insulin or gut hormone responses indicating that the endocrine changes are a consequence of the particular type of surgery rather than weight loss. Although it has been suggested that bypass operations are more effective,47 restrictive procedures undoubtedly cause significant weight loss39 and affect appetite. It remains to be established whether neural mechanisms possibly through vagal afferents are involved in mediating increased satiety following GB.
The obese subjects in our study had a blunted postprandial PYY and GLP-1 response, which may reflect a functional deficiency state. Following bypass surgery, it is likely that multiple mechanisms act in concert to achieve sustainable weight loss. Our rodent model indicates that, although PYY may contribute, other additional hormonal and/or neural factors may be involved. Replicating the endocrine milieu that arises as a consequence of gastric bypass surgery through pharmacologic means holds promise for future medical intervention in morbid obesity.
Footnotes
Reprints: Stephen R. Bloom, FRCP, DSc, Department of Metabolic Medicine, Hammersmith Hospital, Du Cane Road, Imperial College London, U.K. E-mail: s.bloom@imperial.ac.uk.
REFERENCES
- 1.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. [DOI] [PubMed] [Google Scholar]
- 2.Kortt MA, Langley PC, Cox ER. A review of cost-of-illness studies on obesity. Clin Ther. 1998;20:772–779. [DOI] [PubMed] [Google Scholar]
- 3.Bray GA, Tartaglia LA. Medicinal strategies in the treatment of obesity. Nature. 2000;404:672–677. [DOI] [PubMed] [Google Scholar]
- 4.Vage V, Solhaug JH, Berstad A, et al. Jejunoileal bypass in the treatment of morbid obesity: a 25-year follow-up study of 36 patients. Obes Surg. 2002;12:312–318. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell JE, Lancaster KL, Burgard MA, et al. Long-term follow-up of patients’ status after gastric bypass. Obes Surg. 2001;11:464–468. [DOI] [PubMed] [Google Scholar]
- 6.Craig BM, Tseng DS. Cost-effectiveness of gastric bypass for severe obesity. Am J Med. 2002;113:491–498. [DOI] [PubMed] [Google Scholar]
- 7.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it?An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pories WJ, MacDonald KG Jr, Morgan EJ, et al. Surgical treatment of obesity and its effect on diabetes: 10-y follow-up. Am J Clin Nutr. 1992;55(suppl):582–585. [DOI] [PubMed] [Google Scholar]
- 9.Mason EE. Ilial transposition and enteroglucagon/GLP-1 in obesity (and diabetic?) surgery. Obes Surg. 1999;9:223–228. [DOI] [PubMed] [Google Scholar]
- 10.Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clements RH, Gonzalez QH, Long CI, et al. Hormonal changes after Roux-en Y gastric bypass for morbid obesity and the control of type-II diabetes mellitus. Am Surg. 2004;70:1–4. [PubMed] [Google Scholar]
- 12.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. [DOI] [PubMed] [Google Scholar]
- 13.Atkinson RL, Brent EL. Appetite suppressant activity in plasma of rats after intestinal bypass surgery. Am J Physiol. 1982;243:R60–R64. [DOI] [PubMed] [Google Scholar]
- 14.Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz MW, Morton GJ. Obesity: keeping hunger at bay. Nature. 2002;418:595–597. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz MW, Woods SC, Porte D Jr, et al. Central nervous system control of food intake. Nature. 2000;404:661–671. [DOI] [PubMed] [Google Scholar]
- 17.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. [DOI] [PubMed] [Google Scholar]
- 18.Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. [DOI] [PubMed] [Google Scholar]
- 19.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–654. [DOI] [PubMed] [Google Scholar]
- 20.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–948. [DOI] [PubMed] [Google Scholar]
- 21.Kreymann B, Williams G, Ghatei MA, et al. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300–1304. [DOI] [PubMed] [Google Scholar]
- 22.Farilla L, Bulotta A, Hirshberg B, et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144:5149–5158. [DOI] [PubMed] [Google Scholar]
- 23.Gutzwiller JP, Goke B, Drewe J, et al. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut. 1999;44:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asakawa A, Inui A, Yuzuriha H, et al. Characterization of the effects of pancreatic polypeptide in the regulation of energy balance. Gastroenterology. 2003;124:1325–1336. [DOI] [PubMed] [Google Scholar]
- 25.Batterham RL, Le Roux CW, Cohen MA, et al. Pancreatic polypeptide reduces appetite and food intake in humans. J Clin Endocrinol Metab. 2003;88:3989–3992. [DOI] [PubMed] [Google Scholar]
- 26.Olbers T, Lonroth H, Fagevik-Olsen M, et al. Laparoscopic gastric bypass: development of technique, respiratory function, and long-term outcome. Obes Surg. 2003;13:364–370. [DOI] [PubMed] [Google Scholar]
- 27.Jackson RJ, Davis WB, Macdonald I. The energy values of carbohydrates: should bomb calorimeter data be modified? Proc Nutr Soc. 1977;36:90A. [PubMed] [Google Scholar]
- 28.Savage AP, Adrian TE, Carolan G, et al. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut. 1987;28:166–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adrian TE, Bloom SR, Bryant MG, et al. Distribution and release of human pancreatic polypeptide. Gut. 1976;17:940–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 31.Naslund E, Melin I, Gryback P, et al. Reduced food intake after jejunoileal bypass: a possible association with prolonged gastric emptying and altered gut hormone patterns. Am J Clin Nutr. 1997;66:26–32. [DOI] [PubMed] [Google Scholar]
- 32.Kenler HA, Brolin RE, Cody RP. Changes in eating behavior after horizontal gastroplasty and Roux-en-Y gastric bypass. Am J Clin Nutr. 1990;52:87–92. [DOI] [PubMed] [Google Scholar]
- 33.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, et al. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89:1070–1077. [DOI] [PubMed] [Google Scholar]
- 34.Adrian TE, Savage AP, Fuessl HS, et al. Release of peptide YY (PYY) after resection of small bowel, colon, or pancreas in man. Surgery. 1987;101:715–719. [PubMed] [Google Scholar]
- 35.Adrian TE, Savage AP, Bacarese-Hamilton AJ, et al. Peptide YY abnormalities in gastrointestinal diseases. Gastroenterology. 1986;90:379–384. [DOI] [PubMed] [Google Scholar]
- 36.Imamura M. Effects of surgical manipulation of the intestine on peptide YY and its physiology. Peptides. 2002;23:403–407. [DOI] [PubMed] [Google Scholar]
- 37.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. [DOI] [PubMed] [Google Scholar]
- 38.Sugerman HJ, Londrey GL, Kellum JM, et al. Weight loss with vertical banded gastroplasty and Roux-Y gastric bypass for morbid obesity with selective versus random assignment. Am J Surg. 1989;157:93–102. [DOI] [PubMed] [Google Scholar]
- 39.Ren CJ. Controversies in bariatric surgery: evidence-based discussions on laparoscopic adjustable gastric banding. J Gastrointest Surg. 2004;8:396–397. [DOI] [PubMed] [Google Scholar]
- 40.Dixon AF, Dixon JB, O'Brien PE. Laparoscopic adjustable gastric banding induces prolonged satiety: a randomized blind crossover study. J Clin Endocrinol Metab. 2005;90:813–819. [DOI] [PubMed] [Google Scholar]
- 41.Rand CS, Macgregor AM, Hankins GC. Eating behavior after gastric bypass surgery for obesity. South Med J. 1987;80:961–964. [DOI] [PubMed] [Google Scholar]
- 42.Halmi KA, Mason E, Falk JR, et al. Appetitive behavior after gastric bypass for obesity. Int J Obes. 1981;5:457–464. [PubMed] [Google Scholar]
- 43.Naslund E, Gryback P, Hellstrom PM, et al. Gastrointestinal hormones and gastric emptying 20 years after jejunoileal bypass for massive obesity. Int J Obes Relat Metab Disord. 1997;21:387–392. [DOI] [PubMed] [Google Scholar]
- 44.Gutzwiller JP, Goke B, Drewe J, et al. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut. 1999;44:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Faraj M, Havel PJ, Phelis S, et al. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88:1594–1602. [DOI] [PubMed] [Google Scholar]
- 46.Aylwin SJB. Gastrointestinal surgery and gut hormones. Curr Opin Endocrinol Diabetes. 2005;12:89–98. [Google Scholar]
- 47.Biertho L, Steffen R, Ricklin T, et al. Laparoscopic gastric bypass versus laparoscopic adjustable gastric banding: a comparative study of 1,200 cases. J Am Coll Surg. 2003;197:536–544. [DOI] [PubMed] [Google Scholar]