Abstract
TRPC3 (canonical transient receptor potential protein 3) has been suggested to be a component of cation channel complexes that are targeted to cholesterol-rich lipid membrane microdomains. In the present study, we investigated the potential role of membrane cholesterol as a regulator of cellular TRPC3 conductances. Functional experiments demonstrated that cholesterol loading activates a non-selective cation conductance and a Ca2+ entry pathway in TRPC3-overexpressing cells but not in wild-type HEK-293 (human embryonic kidney 293) cells. The cholesterol-induced membrane conductance exhibited a current-to-voltage relationship similar to that observed upon PLC (phospholipase C)-dependent activation of TRPC3 channels. Nonetheless, the cholesterol-activated conductance lacked negative modulation by extracellular Ca2+, a typical feature of agonist-activated TRPC3 currents. Involvement of TRPC3 in the cholesterol-dependent membrane conductance was further corroborated by a novel dominant-negative strategy for selective blockade of TRPC3 channel activity. Expression of a TRPC3 mutant, which contained a haemagglutinin epitope tag in the second extracellular loop, conferred antibody sensitivity to both the classical PLC-activated as well as the cholesterol-activated conductance in TRPC3-expressing cells. Moreover, cholesterol loading as well as PLC stimulation was found to increase surface expression of TRPC3. Promotion of TRPC3 membrane expression by cholesterol was persistent over 30 min, while PLC-mediated enhancement of plasma membrane expression of TRPC3 was transient in nature. We suggest the cholesterol content of the plasma membrane as a determinant of cellular TRPC3 activity and provide evidence for cholesterol dependence of TRPC3 surface expression.
Keywords: canonical transient receptor potential protein 3 (TRPC3), Ca2+ signalling, dominant-negative knockdown, lipid microdomain, membrane cholesterol, non-selective cation channel
Abbreviations: BFA, brefeldin A; DMEM, Dulbecco's modified Eagle's medium; DPBS, Dulbecco's PBS; FCS, fetal calf serum; fura 2/AM, fura 2 acetoxymethyl ester; HA, haemagglutinin; HEK-293 cells, human embryonic kidney 293 cells; HEK-TSA cells, T-cell surface antigen 201-expressing HEK-293 cells; MβCD, methyl-β-cyclodextrin; PLC, phospholipase C; TRP, transient receptor potential; TRPC, canonical TRP protein
INTRODUCTION
Members of the canonical TRP (transient receptor potential) family [TRPC (canonical TRP protein) subfamily] have repeatedly been shown to form cation channels that lack voltage sensitivity and are typically activated in response to stimulation of PLC (phospholipase C)-coupled receptors [1,2]. Most TRPC isoforms were reported to constitute pores that display significant permeability for Ca2+ ions and are therefore considered as pivotal elements of Ca2+ signalling in excitable [3,4] as well as non-excitable tissue [5]. Recent investigations suggest that, in addition to its Ca2+ permeability, non-selective TRPC3 channels might govern cellular Ca2+ homoeostasis via Na+ entry and by utilizing a locally coupled Na+/Ca2+ exchange process [6].
TRPC3 as well as its close relative TRPC6 have been proposed as ‘lipid-regulated’ ion channels, which are activated by diacylglycerol [7]. Consistent with this, these TRPC channels are considered as part of a signalling complex that includes PLC and the inositol 1,4,5-trisphosphate receptor [8,9] and are targeted to the specific lipid environment of lipid rafts and caveolae. Targeting to cholesterol-rich membrane microdomains has been reported for TRPC1, TRPC3 and TRPC4 [10–12]. Moreover, reduction of membrane cholesterol content by cyclodextrins, an intervention that is typically associated with disruption of lipid raft structures, was found to impair TRPC signalling processes [9,13]. Thus TRPC channels appear sensitive to alterations in membrane structures that are dependent on membrane cholesterol content. Cholesterol and other microdomain-resident lipids may interact with proteins on a level beyond simple platform formation. Indeed, membrane cholesterol was found to determine functional properties of a variety of ion channels including nucleotide-gated channels [14], P/Q-type calcium channels [15], epithelial sodium channels [16] and potassium channels [17–20]. Consequently, membrane lipid composition was found to affect cellular functions such as contractility [21,22] and cellular volume regulation [23], and pathophysiological consequences of hypercholesterolaemia have been linked to lipid modulation of channels [24]. Membrane cholesterol content is likely to undergo changes during tissue maturation and is associated with cellular functions, as cultured cells that approach confluence were found to display a substantial increase in membrane cholesterol concomitant with altered protein tyrosine phosphorylation [25].
It is expected that ion channel proteins that are targeted to specialized microdomains of the plasma membrane display particular sensitivity to membrane lipids and are subject to lipid-dependent control of gating. Members of the TRPC family are prototypical examples of such raft-resident and lipid-sensitive ion channels. Moreover, TRPC conductances were found to be regulated by exocytotic insertion of channels into the plasma membrane [26,27]. Since caveolae-mediated cellular targeting and traffic of proteins are considered to be dependent on membrane lipid composition [28], it is tempting to speculate about the dependency of TRPC targeting on membrane cholesterol. Thus TRPC channels may sense perturbations in membrane cholesterol due to altered membrane structure and/or may serve as direct sensors for cholesterol and/or other structural membrane lipids. Here, we present for the first time evidence for activation of a TRPC channel in response to increases in membrane cholesterol content. We propose that cellular regulation of TRPC3 channel surface expression is governed by a cholesterol-dependent mechanism.
EXPERIMENTAL
Cell culture and transfections
HEK-293 (human embryonic kidney 293) cells stably expressing C-terminally HA (haemagglutinin)-tagged TRPC3, designated as T3-9 cells, were established and maintained as previously described [29]. An extracellularly HA-tagged TRPC3 (exoHA–TRPC3) construct was made by inserting a sequence encoding YPYDVPD before the codons for Tyr524-Ala525 of human TRPC3, thus creating a complete HA epitope YPYDVPDYA in the putative second extracellular loop of the channel subunit. For some experiments, T3-9 cells or HEK-TSA cells (T-cell surface antigen 201-expressing HEK-293 cells) were transiently co-transfected with exoHA–TRPC3 and yellow fluorescent protein as an expression marker using Transfast™ Transfection reagent (Promega, Madison, WI, U.S.A.) according to the manufacturer's instructions. The cells were cultured in DMEM (Dulbecco's modified Eagle's medium; Sigma–Aldrich, Vienna, Austria) supplemented with 10% (v/v) FCS (fetal calf serum; PAA Laboratories, Linz, Austria) and 0.25 g/l Geneticin (G418 sulphate; PAA Laboratories). Some experiments were performed with wild-type HEK-293 cells, which were cultured in the absence of Geneticin.
Saturation of MβCD (methyl-β-cyclodextrin) with cholesterol
Complexes of cholesterol and MβCD (Sigma–Aldrich) were prepared by a modification of the method described by Klein et al. [30]. Briefly, 25 ml of an aqueous solution of MβCD (45 mM) and 750 μl of isopropyl alcohol containing 45 mg of cholesterol was heated to 80 °C. The cholesterol solution was added drop by drop into MβCD solution while stirring. After dispersal of cholesterol, the solution was allowed to cool to 37 °C under constant stirring and the volume was re-adjusted to 25 ml by adding distilled water. Aliquots of 5 ml of the solution were placed in 50 ml centrifuge tubes, frozen with liquid nitrogen and freeze-dried for 24–30 h. The freeze-dried MβCD–cholesterol complexes were stored at −20 °C. Each aliquot was dissolved in 22.5 ml of buffer [PBS containing (in mM): 137 NaCl, 2.7 KCl, 8 Na2HPO4·2H2O and 1.5 KH2PO4] to obtain an MβCD concentration of 10 mM prior to experiments.
Neutral lipid analysis
T3-9 cells were grown to confluence for 48 h and incubated in FCS-free DMEM in the presence or absence of cholesterol-saturated MβCD (10 mM, 45 min). Cells were washed twice with ice-cold PBS, scraped off in PBS and centrifuged at 4 °C. Subcellular fractionation was performed by differential centrifugation by the method of Lockwich et al. [9]. Briefly, cells were suspended in 0.3 M NaOH, homogenized by sonication, and a plasma membrane-rich fraction was obtained by removing larger cell fragments, nuclei and mitochondria by centrifugation at 10000 g for 10 min at 4 °C followed by a second centrifugation at 130000 g for 1 h at 4 °C using a Sorvall AH-650 rotor. Lipids were extracted in hexane/propan-2-ol (3:2, v/v) at 4 °C (30 min, twice, using 4 ml of solvent), dried under argon and extracted a second time in methanol/hexane (1:5, v/v) [31]. The hexane phase (containing the neutral lipid fraction) was removed, dried, redissolved in mobile phase [hexane, 2% (v/v) ethanol and 0.005% acetic acid] and subjected to straight phase HPLC analysis (Lichrosphere 5 μm column; flow rate, 1 ml/min) with UV detection at 210 nm on a Waters Alliance 2690 separation module. Quantification was performed by peak area comparison with external standards.
Biotinylation of cell surface membrane proteins and immunoblotting
T3-9 cells were grown to confluence for 48 h and either pre-incubated with cholesterol-saturated MβCD (10 mM, 45 min) in FCS-free DMEM or stimulated with carbachol (200 μM, 1 min) in Ca2+-free buffer (137 mM NaCl, 5.4 mM KCl, 10 mM Hepes, 10 mM glucose and 1 mM MgCl2). Cells were washed three times with ice-cold PBS containing 1 mM MgCl2 and 0.8 mM CaCl2 [DPBS (Dulbecco's PBS), pH 8] and incubated on ice for 30 min with 1 mg/ml EZ-Link Sulpho-NHS-SS-biotin [sulphosuccinimidyl 2-(biotinamido)-ethyl-1,3-dithiopropionate; Pierce] in DPBS. The biotinylation reaction was terminated by washing the cells three times with ice-cold DPBS containing 100 mM glycine. Cells were then washed twice with ice-cold PBS, scraped off from the dishes in PBS and centrifuged at 4 °C. The pellets were resuspended in 0.5 ml of ice-cold lysis buffer [50 mM Tris, pH 7.4, 150 mM NaCl, 1% Triton X-100, 60 mM n-octyl β-D-glucopyranoside, 1 mM PMSF and protease inhibitor mixture (Complete™; Roche Applied Science)] and incubated for 30 min on ice. Cell lysates were sonicated and centrifuged at 10000 g for 10 min at 4 °C. Clear lysates (500 μg of protein) were rotated overnight at 4 °C with 50 μl of streptavidin–agarose beads (Pierce). The beads were recovered by centrifugation for 1 min at 10000 g and supernatants were collected as intracellular protein fractions. The pellets, representing surface-expressed proteins, were washed three times with ice-cold lysis buffer, eluted from the streptavidin–agarose beads in 50 μl of 2× Laemmli buffer [31a], incubated at 60 °C for 30 min and then analysed by SDS/PAGE and transferred to nitrocellulose sheets. Nitrocellulose membranes were incubated with TBST buffer (25 mM Tris, 137 mM NaCl, 2.7 mM KCl, and 0.1% Tween 20, pH 7.4) containing 5% (w/v) Bio-Rad blocking reagent overnight at 4 °C and incubated with primary rat monoclonal antibody against HA (1:1.000; Roche Applied Science, U.S.A.) for 1 h at room temperature (22 °C). After washing with TBST buffer, horseradish peroxidase-conjugated anti-rat IgG was used as secondary antibody. Membranes were detected via Chemi Glow™ West detection system and developed using a Herolab RH-5.2 Darkroom Hood, equipped with an EASY 1.3 HC camera.
Measurement of intracellular Ca2+
Cells were loaded with fura 2/AM (fura 2 acetoxymethyl ester; Molecular Probes, Eugene, OR, U.S.A.) for 30 min at 37 °C and 5% CO2. Thereafter, cells were washed and constantly perfused during experiments with buffer containing either 137 mM NaCl, 5.4 mM KCl, 10 mM Hepes, 10 mM glucose and 1 mM MgCl2 (nominally Ca2+-free) or 137 mM NaCl, 5.4 mM KCl, 10 mM Hepes, 10 mM glucose, 1 mM MgCl and 1 or 2 mM CaCl2 (+Ca2+). For some experiments, calcium in the solution was replaced with barium (2 mM Ba2+). The free Ca2+ concentration of the nominally Ca2+-free solutions was determined using a Ca2+-sensitive electrode and was approx. 20 μM (15–30 μM). Excitation light was supplied via a Polychrome II polychromator (TILL Photonics, Gräfelfing, Germany) and emission was detected by a Sensicam CCD (charged-coupled device) camera (PCO Computer Optics, Kehlheim, Germany). Ca2+-sensitive fura 2/AM fluorescence was measured ratiometrically at 340 and 380 nm excitation wavelengths and emission was collected at 510 nm. Digital image recordings were analysed using Axon Imaging Workbench (Axon Instruments, Foster City, CA, U.S.A.). Experiments were performed at room temperature.
Measurement of membrane currents
Patch pipettes were pulled from borosilicate glass (Clark Electromedical Instruments, Reading, U.K.), had a resistance of 3–5 MΩ and were filled with a solution containing 120 mM caesium methanesulphonate, 20 mM CsCl, 10 mM Hepes, 1 mM MgCl2 and 1 mM EGTA (pH adjusted to 7.4). Bath solutions were similar to solutions described in the ‘Measurement of intracellular Ca2+’ subsection. For some experiments, cells were incubated with an anti-HA antibody (for 1 h; 1:200 in bath solution) and constantly perfused (1:200 in bath solution) during the experiment. Currents were recorded using a List EPC7 (electronic patch clamp 7) amplifier (List-Medical-Electronic, Darmstadt, Germany). Signals were low-pass filtered at 1 kHz and digitized with 5 kHz. Voltage-clamp protocols (voltage ramps from −100 to +80 mV/0.6 V per s, 0.2 Hz, holding potential −70 mV) were controlled by pClamp software (Axon Instruments). Experiments were performed at room temperature.
Statistical analysis
Results were expressed as mean values ±S.E.M. Differences were considered statistically significant at P<0.05 using Student's t test for unpaired values.
RESULTS
Cholesterol activates a cation conductance in TRPC3-expressing cells
Measurement of membrane currents in HEK-293 cells stably transfected to express TRPC3 channels (T3-9) revealed that acute administration of cholesterol-saturated MβCD (10 mM), an established tool for elevation of cellular cholesterol [32], induced a whole-cell conductance in these TRPC3-overexpressing cells (Figure 1A, left panel).
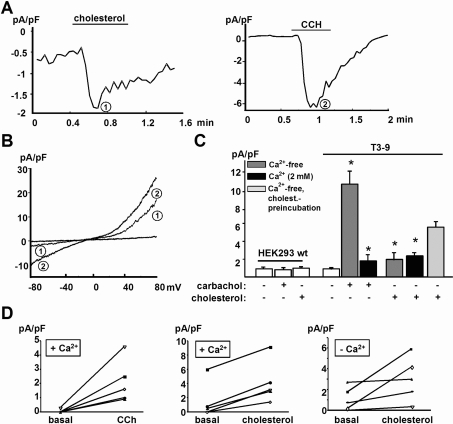
Figure 1. Acute administration of MβCD-saturated cholesterol induces a cation current in TRPC3-overexpressing HEK-293 cells.
(A) Representative time courses of membrane currents recorded at −70 mV showing acute stimulation of TRPC3-overexpressing HEK-293 cells (T3-9) by either cholesterol-saturated MβCD (left panel) or 200 μM carbachol (CCH; right panel) as indicated. (B) Representative current responses recorded using ramp-like voltage protocols in T3-9 cells after challenge with cholesterol (1) and carbachol (2). Experiments were performed at room temperature. (C) Mean values for peak current density recorded in T3-9 cells are shown for cells exposed to cholesterol or carbachol in the absence or presence of extracellular calcium. Values represent the means±S.E.M. (n=5–17). Asterisks denote significant difference versus non-stimulated T3-9 cells. (D) Pairs of T3-9 whole-cell current values measured at −70 mV before and after administration of cholesterol or carbachol in the presence and absence of extracellular calcium.
The current-to-voltage relationship of this membrane conductance was similar to that displayed by the typical TRPC3 currents activated by carbachol in these cells (Figure 1B). Current densities measured at −70 mV during application of cholesterol amounted to approx. 20% of the carbachol responses (Figures 1B and 1C). Figure 1(B) shows representative current responses to voltage ramps in T3-9 cells challenged with cholesterol (1) and carbachol (2), both displaying slightly outward rectifying properties and reversal potentials near zero as typically observed for TRPC3 currents under these conditions. Both carbachol- and cholesterol-induced currents were virtually absent in wild-type HEK-293 cells, indicating a pivotal contribution of TRPC3 in these conductances. As evident from the time courses of current activation (Figure 1A), acute administration of both compounds gave rise to transient channel activation. Nonetheless, cholesterol-induced currents tended to decline more slowly and reversibility of activation (deactivation) after cholesterol washout was slow and incomplete, while carbachol-induced responses displayed fast inactivation. Interestingly, the cholesterol-induced conductance activation was barely sensitive to changes of extracellular Ca2+ concentration (from 20 μM to 2 mM), while carbachol effects were significantly suppressed at elevated extracellular Ca2+ [33] as shown in Figure 1(C). The magnitude of the TRPC3-mediated inward currents at physiological Ca2+ concentrations was similar for both the lipid- and the PLC-induced activation as shown in Figure 1(D). Thus divergent Ca2+ sensitivity was observed for cholesterol- and carbachol-induced activation of TRPC3.
Prolonged incubation of T3-9 cells (45 min) with cholesterol-saturated MβCD induced substantially larger whole-cell currents than acute administration (40% of carbachol stimulation; Figure 1C). To verify the concept that MβCD–cholesterol-induced conductance activation was due to cholesterol loading of the cells [32], we performed control experiments with methyl-cyclo-α-dextrin, a related compound that lacks affinity for cholesterol, which provided evidence against cholesterol-independent effects of the cyclodextrin. Cyclo-α-dextrin (10 mM, 45 min) did not activate membrane conductances in T3-9 cells (n=6, results not shown). Moreover, MβCD-mediated loading of cell membranes with cholesterol was monitored by HPLC analysis. Consistent with previous studies [34], a clear increase in total cellular cholesterol content (1.5-fold) and a prominent elevation of cholesterol in the plasma membrane fraction (3.2-fold; n=2) were measured in cells exposed for 45 min to cholesterol–MβCD (10 mM).
Repeated challenge of cells with carbachol resulted in declining current responses, indicating that this transient activation of TRPC3 is subject to desensitization.
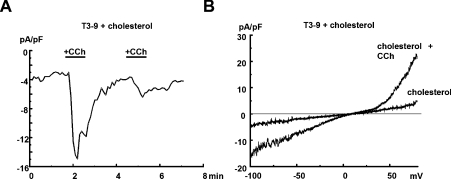
Cholesterol-loaded cells responded to carbachol with further transient increases in conductance as shown in Figure 2. The magnitude of carbachol-induced current increases was slightly but not significantly smaller in cholesterol-loaded cells as compared with controls, and currents deactivated in both situations rapidly below 10% of peak current densities within 1 min after washout. Thus carbachol responses remained largely unchanged in cholesterol-loaded cells, indicating that cholesterol loading does not significantly affect PLC-mediated conductance activation. Maximum current densities measured in the presence of cholesterol plus carbachol (−70 mV, 200 μM; 11.3±3.4 pA/pF; n=6; results not shown) were not significantly different from those obtained with 200 μM carbachol alone (10.2±1.6 pA/pF; n=6), indicating that the two activating stimuli are likely to converge at the same conductance.
Figure 2. Combined stimulation of TRPC3-expressing HEK-293 cells with cholesterol and carbachol.
(A) Time course of whole-cell current recorded at −70 mV from a TRPC3-overexpressing HEK-293 cell (T3-9) during repetitive stimulation with 200 μM carbachol (CCh), as indicated, after incubation with cholesterol-saturated MβCD for 45 min. (B) Representative current responses recorded using ramp-like voltage protocols in T3-9 cells pre-incubated with cholesterol and challenged with carbachol as indicated.
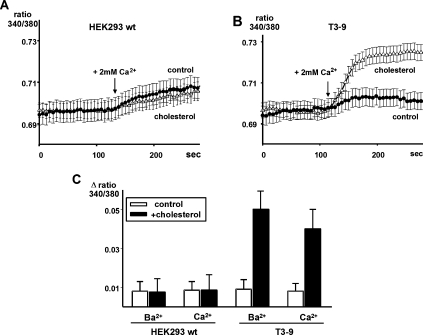
Besides generating a measurable membrane conductance, cholesterol loading of T3-9 cells promoted entry of Ca2+ and Ba2+, a phenomenon that was not detectable in wild-type HEK-293 cells. Elevation of extracellular Ca2+ from 20 μM to 2 mM induced minute elevation of intracellular Ca2+ in wild-type HEK-293 cells, and the observed changes in intracellular Ca2+ were indistin-guishable from controls (Figure 3A). In contrast, promotion of Ca2+ entry was clearly evident in cholesterol-loaded cells expressing TRPC3 (T3-9) (Figure 3B). Moreover, enhanced Ba2+ entry was observed in cholesterol-loaded T3-9 cells but not in cholesterol-loaded wild-type HEK-293 cells (Figure 3C). Ca2+ imaging during acute administration of cholesterol-loaded MβCD showed that stimulation of T3-9 cells by cholesterol did not lead to a detectable mobilization of Ca2+ from internal stores within the observation timeframe (results not shown). Although we cannot exclude a slow depletion of intracellular Ca2+ by cholesterol, these experiments indicate that cholesterol-dependent channel activation does not involve elevation of cellular inositol 1,4,5-trisphosphate levels and rapid depletion of intracellular Ca2+ stores.
Figure 3. Membrane loading with cholesterol promotes basal Ca2+ entry into T3-9 cells.
Representative traces of Ca2+-sensitive fura 2/AM fluorescence ratios (F340/380) recorded in HEK-293 wild-type (wt) cells (A) and TRPC3-overexpressing HEK-293 cells (T3-9) (B) incubated in serumfree medium in the absence (control; ●) or presence (△) of cholesterol-saturated MβCD (cholesterol; 10 mM, 45 min). During the experiments, cells were initially kept in Ca2+-free solution, and Ca2+-containing solution was added to initiate calcium influx at the time point indicated. (C) Average of maximal Ca2+ or Ba2+ net influx signal derived from fura 2/AM imaging in wild-type and T3-9 cells under control conditions (open bars) and loaded with cholesterol-saturated MβCD (filled bars). Values represent means±S.E.M. (n=40–70).
Expression of exoHA-tagged TRPC3 confers anti-HA sensitivity to both carbachol- and cholesterol-induced membrane conductances
The above-described membrane effects of cholesterol in TRPC3-overexpressing cells strongly suggest that TRPC3 generates cation channels that are regulated by cholesterol- as well as by PLC-dependent signalling pathways.
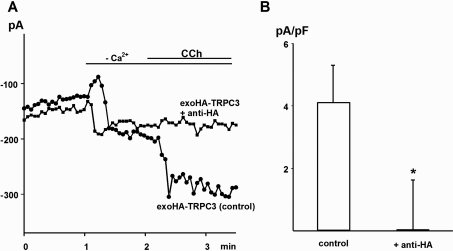
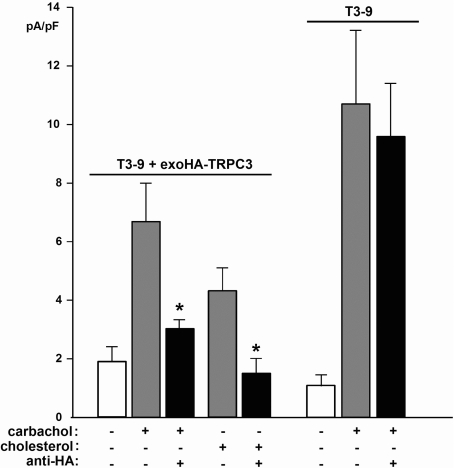
To verify further the involvement of TRPC3 in the observed conductance, we employed a novel technique for conditional knockdown of TRPC3 conductances. A TRPC3 mutant that contained an HA epitope in its second extracellular loop was tested for its sensitivity to blockade by extracellularly applied anti-HA antibody. HEK-TSA cells were transiently transfected with the exoHA–TRPC3 construct and displayed a typical non-selective cation conductance upon stimulation with 200 μM carbachol (Figure 4). This conductance was eliminated in cells pre-incubated with anti-HA antibody (1:200, 30 min; Figures 4A and 4B). We hypothesized that integration of this construct into TRPC3 multimers may transfer the anti-HA sensitivity of the mutant protein to a large fraction of TRPC3-containing channel complexes, even in cells expressing native or wild-type TRPC3 channels. As illustrated in Figure 5, expression of exoHA–TRPC3 in T3-9 cells, which overexpress TRPC3, indeed resulted in a profound sensitivity to anti-HA of both the carbachol- and the cholesterol-induced membrane conductance. Importantly, incubation with anti-HA antibody did not prevent carbachol responses in sham-transfected T3-9 cells. It is of note that the transfection itself resulted in increased basal leak conductance of the cells, which was not affected by anti-HA incubation. Thus expression of exo-epitope-tagged TRPC species appears to be a suitable approach to confer antibody sensitivity to channels formed by the respective species, and this method of ‘conditional dominant-negative knockdown’ appears valuable to explore the role of TRPC in specific membrane conductances. The present results suggest TRPC3 as a subunit of cation channels that are regulated by membrane cholesterol.
Figure 4. ExoHA-tagged TRPC3 cation channels are sensitive to anti-HA antibody.
(A) Representative time course of carbachol (CCh; 200 μM)-induced current recorded at −70 mV in an HEK-TSA cell transfected to express exoHA–TRPC3. Experiments were performed with cells pre-incubated for 30 min in the presence or absence of anti-HA antibody (1:200). (B) Comparison of carbachol-induced current densities measured at −70 mV in exoHA–TRPC3-expressing cells after pre-incubation in the absence and presence of anti-HA antibody. Values represent the means±S.E.M. (n=6–8). The asterisk denotes significant difference versus control.
Figure 5. Cholesterol-induced TRPC3-mediated currents in exoHA-tagged TRPC3-expressing cells are sensitive to anti-HA antibody.
Shown are mean carbachol- and cholesterol-induced current densities recorded in T3-9 cells transiently transfected with exoHA-tagged TRPC3. Responses in T3-9 wild-type cells are shown for comparison. The cells were kept in Ca2+-free solution when stimulated with carbachol, and in Ca2+-containing solution when challenged by acute administration of cholesterol-saturated MβCD. Carbachol- and cholesterol-mediated conductances in T3-9 cells transiently transfected with exoHA-tagged TRPC3 were significantly inhibited by pre-incubation with anti-HA antibody (1:200, 30 min), while carbachol-induced currents in T3-9 cells were not affected by anti-HA antibody. Values represent the means±S.E.M. (n=7–10). Asterisks denote significant differences versus carbachol- and cholesterol-induced conductance in the absence of antibody.
Cholesterol and carbachol promote surface expression of TRPC3
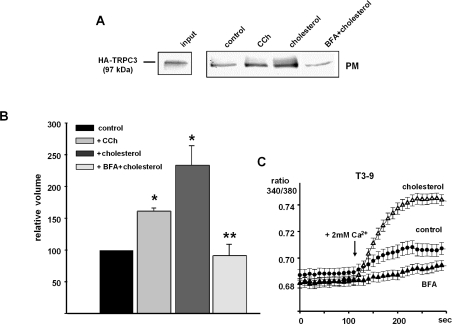
Cholesterol-induced activation of TRPC3 displayed a striking insensitivity to changes in extracellular Ca2+ and a rather slow and persistent component of activation, which is in clear contrast with channel activation via PLC stimulation. This may be explained by the involvement of distinct mechanisms of TRPC3 conductance activation. As recent studies suggested regulated insertion of TRPC channels into the plasma membrane as a key mechanism of TRPC activation [27], we set out to test for possible effects of cholesterol loading on plasma membrane expression of TRPC3 by cell surface biotinylation experiments (Figures 6A and 6B). Surface presentation of TRPC3 was significantly enhanced in T3-9 cells exposed to cholesterol-saturated MβCD. The cholesterol-induced promotion of plasma membrane presentation of TRPC3 was comparable with that observed after stimulation of the cells with carbachol in low extracellular Ca2+. Interestingly, carbachol-induced translocation of TRPC3 into the biotinylated fraction of cellular proteins was reduced by approx. 30% in the presence of extracellular Ca2+ (results not shown), while cholesterol-induced translocation was fairly insensitive to extracellular Ca2+. Cholesterol-induced promotion of surface expression of TRPC3 was persistent, i.e. clearly detectable after 45 min of incubation, while carbachol elicited only a transient increase in TRPC3 immunoreactivity in the biotinylated protein fraction, typically detectable within 2–5 min after agonist stimulation. As enhanced membrane presentation of TRPC3 in response to PLC activation was recently demonstrated to be effectively suppressed by BFA (brefeldin A) [27], we used this tool to investigate the contribution of TRPC3 membrane recruitment to cholesterol-induced Ca2+ entry. BFA inhibited TRPC3 membrane presentation in cholesterol-loaded cells (Figure 6A) and markedly suppressed cholesterol-induced Ca2+ signals as shown in Figure 6(C). These results suggest that cholesterol-induced activation of TRPC3 conductances and TRPC3-mediated Ca2+ entry involves, at least in part, the recruitment of TRPC3 to the plasma membrane.
Figure 6. Effects of carbachol and cholesterol on plasma membrane presentation of TRPC3 and analysis of the effects of BFA on membrane presentation and function of TRPC3.
(A) T3-9 cells were either stimulated with carbachol (CCh; 200 μM, 1 min) in Ca2+-free buffer or incubated with FCS-free DMEM containing cholesterol–MβCD (10 mM, 45 min), or with FCS-free DMEM containing cholesterol–MβCD subsequent to a pre-incubation with BFA (10 μg/ml, 60 min). HA–TRPC3 abundance in total lysates (150 μg; input) and biotinylated fractions (PM, plasma membrane fraction) was measured by immunoblotting with anti-HA antibody. Results are representative of three individual experiments. (B) Statistical analysis of the TRPC3 immunoreactivity detected in biotinylated fractions of controls, in cells after stimulation with carbachol or cholesterol and in BFA-treated cells stimulated with cholesterol (BFA+cholesterol). * Indicate values that are significantly different from control; ** indicates significant difference versus cholesterol stimulation. (C) Inhibition of cholesterol-induced Ca2+ signalling by BFA. Representative traces of Ca2+-sensitive fura 2/AM fluorescence ratios (F340/380) recorded in TRPC3-overexpressing HEK-293 cells pre-incubated in FCS-free medium in the absence (control; ●) or presence (△) of cholesterol–MβCD (10 mM, 45 min) subsequent to pre-incubation with BFA (10 μg/ml, 60 min) (BFA, filled triangles).
DISCUSSION
In the present study, we present evidence for a novel function of TRPC3 in terms of lipid sensitivity. We show that a cellular TRPC3 conductance is promoted at elevated membrane cholesterol content. In addition, we report on a novel strategy for the analysis of the physiological function of TRP proteins. We demonstrate suppression of TRPC3 channels by expression of a conditional dominant-negative species, which renders TRPC3 channels sensitive to blockade by specific antibodies.
Identification of a cholesterol-sensitive TRPC3 conductance
It is well established that the cholesterol content and/or the cholesterol/phospholipid ratio of biological membranes represents a key determinant of membrane functions [35], and alterations in cholesterol homoeostasis have been implicated in the pathogenesis of a variety of diseases, including cardiovascular dysfunctions [36] as well as neurodegeneration [37] and Alzheimer's disease [38]. Importantly, cholesterol is not uniformly distributed in biological membranes but is strictly organized, including asymmetry regarding leaflet distribution and accumulation within specific microdomains [39–42]. Membrane cholesterol is considered essential for the generation of specific types of membrane architecture and apparently enables accumulation as well as segregation of signalling proteins within the plasma membrane [15,16,21–24]. The TRPC channel proteins TRPC1, TRPC3 and TRPC4 have been identified as residents of a specific subset of membrane microdomains, which contain the cholesterol-binding scaffold protein caveolin-1, termed caveolae [9,11,12]. From these reports, it appears likely that membrane cholesterol might affect TRPC channels, as suppression of caveolae-associated signalling processes by reduction of membrane cholesterol has been reported for TRPC1-mediated Ca2+ signalling [9,13,43]. The present study demonstrates that an increase in membrane cholesterol content results in activation of a TRPC3 cation conductance. An experimental increase in cellular cholesterol content was accomplished by exposure of cells to an MβCD–cholesterol complex, an agent that enables modulation of cellular cholesterol levels [34]. Besides classical liposome-mediated control of cellular cholesterol, cyclodextrin-mediated cholesterol loading has been established as a suitable approach to elevate the cholesterol content of cell membranes [32]. For both strategies, cholesterol-independent effects of the vehicle, such as effects due to modification of membrane phospholipid composition, need to be considered and evaluated. Thus we performed control experiments with cyclo-α-dextrin, a structural analogue of MβCD, which specifically lacks affinity for cholesterol. The results of these control experiments provided evidence against dextrin-mediated effects on TRPC3 other than cholesterol shuttling. Analysis of neutral lipid content in subcellular fractions of cholesterol-loaded HEK-293 cells confirmed the efficiency of MβCD-mediated cholesterol loading, which produced an approx. 3-fold increase in plasma membrane cholesterol as compared with control cells. It is of note that comparable increases in cellular cholesterol content have been observed in pathophysiologically relevant animal models [44,45], indicating that the experimentally introduced changes in cellular cholesterol are likely to be of pathophysiological relevance.
We suggest that increased cellular cholesterol levels represent an activating stimulus for a non-selective cation channel that involves the TRPC3 protein. In Ca2+ imaging experiments, cholesterol loading clearly promoted bivalent cation entry, indicating that the cholesterol-activated conductance is able to control cellular Ca2+ homoeostasis. This may be of significance in pathophysiological situations that are associated with disturbed cholesterol homoeostasis and tissue accumulation of cholesterol such as diabetes and neurodegenerative diseases [44,46,47].
The identification of TRPC3 channels as the cholesterol-sensing element underlying the observed membrane conductance is based on three lines of evidence: (i) cholesterol-induced activation of cation channels was observed exclusively in TRPC3-overexpressing cells but not in wild-type HEK-293 cells, which do not display detectable TRPC3 protein expression (M. Poteser and K. Groschner, unpublished work); (ii) the current to voltage characteristic measured for the cholesterol-activated membrane conductance in TRPC3-overexpressing cells was reminiscent of that typically measured for the PLC-dependent TRPC3 conductance expressed in these cells [33,48]; (iii) a conditional dominant-negative construct of TRPC3 inhibited the cholesterol-activated conductance. Thus we suggest that TRPC3 is able to generate a cholesterol-sensitive cation conductance.
A novel strategy for conditional knockdown of cellular TRPC channel activity
One line of evidence supporting our concept of a cholesterol-sensitive TRPC3 complex in which TRPC3 is part of the ion-conducting structure was provided by experiments using a TRPC3 mutant that contains an HA epitope in the second extracellular loop (exoHA–TRPC3) for conditional knockdown of TRPC3 channels. Incubation of T3-9 cells, transfected to express exoHA–TRPC3, with anti-HA antibody completely suppressed the PLC-activated conductance. Our results are consistent with the assumption that exoHA–TRPC3 is incorporated into TRPC3 channel complexes and transfers a specific anti-HA sensitivity to these channels, thereby acting as a ‘conditional’ (antibody-dependent) dominant-negative species. We suggest this approach as a useful test for physiological functions of native TRPC channel complexes.
Mechanism of cholesterol-dependent activation of cellular TRPC3 conductances
Cholesterol-induced TRPC3 activation displayed insensitivity to changes in extracellular Ca2+ along with an unusually prominent persistent component. As these features are different from those of PLC-dependent activation of the channels, we considered a mode of conductance activation different from that initiated by PLC stimulation. Indeed, carbachol-induced current activation was clearly detectable in cholesterol-loaded cells. Thus the receptor agonist apparently generates an activating stimulus distinct from that of cholesterol. It is of note that carbachol-induced current activation displayed a pronounced transient component, while cholesterol elicited only small transient responses upon acute administration. These observations prompted us to speculate about the involvement of multiple activating mechanisms, which may be differently affected by cholesterol and carbachol. PLC-dependent activation of TRPC conductances may involve direct gating of plasma membrane channels by either protein–protein interactions or binding of second messengers as well as enhanced surface presentation of the channels, which has recently been suggested as a key process in activation [27]. Here, we identified increased surface expression of TRPC3 molecules as a prominent event associated with cholesterol-induced TRPC3 activation. Interestingly, we observed that the cholesterol-induced TRPC3 translocation and insertion into the plasma membrane were fairly stable over time and insensitive to the Ca2+ concentration in the extracellular solution, while PLC-mediated translocation was transient and substantially suppressed by extracellular Ca2+. BFA was found to suppress both cholesterol-induced increases in TRPC3 membrane expression as well as Ca2+ entry; it is therefore tempting to speculate that the control of membrane recruitment of TRPC3 as the main mechanism of the observed cholesterol effects. Nonetheless, our results do not exclude the existence of additional effects of cholesterol on TRPC3 channel gating, which may play a prominent role in PLC-dependent TRPC3 activation. It is of note that direct effects of cholesterol on ion channel gating have recently been described for other channel species such as voltage-gated K+ channels [49,50], and sequence analysis of TRPC3 revealed that the protein hosts seven potential cholesterol-binding motifs (cholesterol recognition amino acid consensus, [51,52]), with some of these motifs located in transmembrane spanning regions. Control of surface presentation of TRPC3 was suggested to involve rapid vesicular trafficking and an exocytosis-like mechanism [27], processes that are probably dependent on membrane cholesterol [53–55]. Thus cholesterol-dependent facilitation of vesicular transport and/or membrane fusion events may be considered as key processes underlying lipid-mediated channel activation. It remains to be clarified if cholesterol activates TRPC3 conductances exclusively via effects on regulated membrane insertion/retrieval of TRPC3 or by promotion of both TRPC3 membrane presentation and modulation of channel gating.
The present demonstration of cholesterol regulation of TRPC3-mediated cation transport suggests a novel sensor function of TRPC proteins, which enables the integration of receptor-dependent stimuli and information on cellular lipid homoeostasis. In view of the potential role of TRPC3 heteromers as native cation channels in various cellular systems, we suggest that the cholesterol dependence of TRPC3 conductances is an important link between lipid metabolism and cellular ion homoeostasis.
Acknowledgments
This project was funded by FWF [Fonds zur Förderung der Wissenschaftlichen Forschung (Austrian Science Fund)] projects 14950 and 18280 (to K. G.) and FWF project P18169 (to C. R.).
References
- 1.Vennekens R., Voets T., Bindels R. J., Droogmans G., Nilius B. Current understanding of mammalian TRP homologues. Cell Calcium. 2002;31:253–264. doi: 10.1016/s0143-4160(02)00055-6. [DOI] [PubMed] [Google Scholar]
- 2.Nilius B., Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol. Rev. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 3.Sergeeva O. A., Korotkova T. M., Scherer A., Brown R. E., Haas H. L. Co-expression of non-selective cation channels of the transient receptor potential canonical family in central aminergic neurones. J. Neurochem. 2003;85:1547–1552. doi: 10.1046/j.1471-4159.2003.01806.x. [DOI] [PubMed] [Google Scholar]
- 4.Schilling W. P. TRP proteins: novel therapeutic targets for regional blood pressure control? Circ. Res. 2001;88:256–259. doi: 10.1161/01.res.88.3.256. [DOI] [PubMed] [Google Scholar]
- 5.Montell C. Physiology, phylogeny, and functions of the TRP superfamily of cation channels. Science STKE 2001. 2001:RE1. doi: 10.1126/stke.2001.90.re1. [DOI] [PubMed] [Google Scholar]
- 6.Rosker C., Graziani A., Lukas M., Eder P., Zhu M. X., Romanin C., Groschner K. Ca2+ signaling by TRPC3 involves Na+ entry and local coupling to the Na+/Ca2+ exchanger. J. Biol. Chem. 2004;279:13696–13704. doi: 10.1074/jbc.M308108200. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann T., Obukhov A. G., Schaefer M., Harteneck C., Gudermann T., Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature (London) 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 8.Trebak M., Vazquez G., Bird G. S., Putney J. W., Jr The TRPC3/6/7 subfamily of cation channels. Cell Calcium. 2003;33:451–461. doi: 10.1016/s0143-4160(03)00056-3. [DOI] [PubMed] [Google Scholar]
- 9.Lockwich T. P., Liu X., Singh B. B., Jadlowiec J., Weiland S., Ambudkar I. S. Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J. Biol. Chem. 2000;275:11934–11942. doi: 10.1074/jbc.275.16.11934. [DOI] [PubMed] [Google Scholar]
- 10.Brazer S. C., Singh B. B., Liu X., Swaim W., Ambudkar I. S. Caveolin-1 contributes to assembly of store-operated Ca2+ influx channels by regulating plasma membrane localization of TRPC1. J. Biol. Chem. 2003;278:27208–27215. doi: 10.1074/jbc.M301118200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lockwich T., Singh B. B., Liu X., Ambudkar I. S. Stabilization of cortical actin induces internalization of transient receptor potential 3 (Trp3)-associated caveolar Ca2+ signaling complex and loss of Ca2+ influx without disruption of Trp3-inositol trisphosphate receptor association. J. Biol. Chem. 2001;276:42401–42408. doi: 10.1074/jbc.M106956200. [DOI] [PubMed] [Google Scholar]
- 12.Torihashi S., Fujimoto T., Trost C., Nakayama S. Calcium oscillation linked to pacemaking of interstitial cells of Cajal: requirement of calcium influx and localization of TRP4 in caveolae. J. Biol. Chem. 2002;277:19191–19197. doi: 10.1074/jbc.M201728200. [DOI] [PubMed] [Google Scholar]
- 13.Bergdahl A., Gomez M. F., Dreja K., Xu S. Z., Adner M., Beech D. J., Broman J., Hellstrand P., Sward K. Cholesterol depletion impairs vascular reactivity to endothelin-1 by reducing store-operated Ca2+ entry dependent on TRPC1. Circ. Res. 2003;93:839–847. doi: 10.1161/01.RES.0000100367.45446.A3. [DOI] [PubMed] [Google Scholar]
- 14.Brady J. D., Rich T. C., Le X., Stafford K., Fowler C. J., Lynch L., Karpen J. W., Brown R. L., Martens J. R. Functional role of lipid raft microdomains in cyclic nucleotide-gated channel activation. Mol. Pharmacol. 2004;65:503–511. doi: 10.1124/mol.65.3.503. [DOI] [PubMed] [Google Scholar]
- 15.Taverna E., Saba E., Rowe J., Francolini M., Clementi F., Rosa P. Role of lipid microdomains in P/Q-type calcium channel (Cav2.1) clustering and function in presynaptic membranes. J. Biol. Chem. 2004;279:5127–5134. doi: 10.1074/jbc.M308798200. [DOI] [PubMed] [Google Scholar]
- 16.Shlyonsky V. G., Mies F., Sariban-Sohraby S. Epithelial sodium channel activity in detergent-resistant membrane microdomains. Am. J. Physiol. Renal Physiol. 2003;284:F182–F188. doi: 10.1152/ajprenal.00216.2002. [DOI] [PubMed] [Google Scholar]
- 17.Sampson L. J., Hayabuchi Y., Standen N. B., Dart C. Caveolae localize protein kinase A signaling to arterial ATP-sensitive potassium channels. Circ. Res. 2004;95:1012–1018. doi: 10.1161/01.RES.0000148634.47095.ab. [DOI] [PubMed] [Google Scholar]
- 18.Chemin J., Patel A. J., Duprat F., Lauritzen I., Lazdunski M., Honore E. A phospholipid sensor controls mechanogating of the K+ channel TREK-1. EMBO J. 2005;24:44–53. doi: 10.1038/sj.emboj.7600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molina M. L., Encinar J. A., Barrera F. N., Fernandez-Ballester G., Riquelme G., Gonzalez-Ros J. M. Influence of C-terminal protein domains and protein–lipid interactions on tetramerization and stability of the potassium channel KcsA. Biochemistry. 2004;43:14924–14931. doi: 10.1021/bi048889+. [DOI] [PubMed] [Google Scholar]
- 20.Lam R. S., Shaw A. R., Duszyk M. Membrane cholesterol content modulates activation of BK channels in colonic epithelia. Biochim. Biophys. Acta. 2004;1667:241–248. doi: 10.1016/j.bbamem.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Jennings L. J., Xu Q. W., Firth T. A., Nelson M. T., Mawe G. M. Cholesterol inhibits spontaneous action potentials and calcium currents in guinea pig gallbladder smooth muscle. Am. J. Physiol. 1999;277:G1017–G1026. doi: 10.1152/ajpgi.1999.277.5.G1017. [DOI] [PubMed] [Google Scholar]
- 22.Babiychuk E. B., Smith R. D., Burdyga T., Babiychuk V. S., Wray S., Draeger A. Membrane cholesterol regulates smooth muscle phasic contraction. J. Membr. Biol. 2004;198:95–101. doi: 10.1007/s00232-004-0663-1. [DOI] [PubMed] [Google Scholar]
- 23.Levitan I., Christian A. E., Tulenko T. N., Rothblat G. H. Membrane cholesterol content modulates activation of volume-regulated anion current in bovine endothelial cells. J. Gen. Physiol. 2000;115:405–416. doi: 10.1085/jgp.115.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heaps C. L., Tharp D. L., Bowles D. K. Hypercholesterolemia abolishes voltage-dependent K+ channel contribution to adenosine-mediated relaxation in porcine coronary arterioles. Am. J. Physiol. Heart Circ. Physiol. 2005;288:H568–H576. doi: 10.1152/ajpheart.00157.2004. [DOI] [PubMed] [Google Scholar]
- 25.Corvera S., DiBonaventura C., Shpetner H. S. Cell confluence-dependent remodeling of endothelial membranes mediated by cholesterol. J. Biol. Chem. 2000;275:31414–31421. doi: 10.1074/jbc.M001708200. [DOI] [PubMed] [Google Scholar]
- 26.Bezzerides V. J., Ramsey I. S., Kotecha S., Greka A., Clapham D. E. Rapid vesicular translocation and insertion of TRP channels. Nat. Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- 27.Singh B. B., Lockwich T. P., Bandyopadhyay B. C., Liu X., Bollimuntha S., Brazer S. C., Combs C., Das S., Leenders A. G., Sheng Z. H., et al. VAMP2-dependent exocytosis regulates plasma membrane insertion of TRPC3 channels and contributes to agonist-stimulated Ca2+ influx. Mol. Cell. 2004;15:635–646. doi: 10.1016/j.molcel.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Predescu S. A., Predescu D. N., Palade G. E. Endothelial transcytotic machinery involves supramolecular protein–lipid complexes. Mol. Biol. Cell. 2001;12:1019–1033. doi: 10.1091/mbc.12.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu X., Jiang M., Birnbaumer L. Receptor-activated Ca2+ influx via human Trp3 stably expressed in human embryonic kidney (HEK)293 cells. Evidence for a non-capacitative Ca2+ entry. J. Biol. Chem. 1998;273:133–142. doi: 10.1074/jbc.273.1.133. [DOI] [PubMed] [Google Scholar]
- 30.Klein U., Gimpl G., Fahrenholz F. Alteration of the myometrial plasma membrane cholesterol content with beta-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry. 1995;34:13784–13793. doi: 10.1021/bi00042a009. [DOI] [PubMed] [Google Scholar]
- 31.Sattler W., Mohr D., Stocker R. Rapid isolation of lipoproteins and assessment of their peroxidation by high-performance liquid chromatography postcolumn chemiluminescence. Methods Enzymol. 1994;233:469–489. doi: 10.1016/s0076-6879(94)33053-0. [DOI] [PubMed] [Google Scholar]
- 31a.Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 32.Christian A. E., Haynes M. P., Phillips M. C., Rothblat G. H. Use of cyclodextrins for manipulating cellular cholesterol content. J. Lipid Res. 1997;38:2264–2272. [PubMed] [Google Scholar]
- 33.Lintschinger B., Balzer-Geldsetzer M., Baskaran T., Graier W. F., Romanin C., Zhu M. X., Groschner K. Coassembly of Trp1 and Trp3 proteins generates diacylglycerol- and Ca2+-sensitive cation channels. J. Biol. Chem. 2000;275:27799–27805. doi: 10.1074/jbc.M002705200. [DOI] [PubMed] [Google Scholar]
- 34.Cruz J. C., Thomas M., Wong E., Ohgami N., Sugii S., Curphey T., Chang C. C., Chang T. Y. Synthesis and biochemical properties of a new photoactivatable cholesterol analog 7,7-azocholestanol and its linoleate ester in Chinese hamster ovary cell lines. J. Lipid Res. 2002;43:1341–1347. [PubMed] [Google Scholar]
- 35.Bastiaanse E. M., Hold K. M., Van der Laarse A. The effect of membrane cholesterol content on ion transport processes in plasma membranes. Cardiovasc. Res. 1997;33:272–283. doi: 10.1016/s0008-6363(96)00193-9. [DOI] [PubMed] [Google Scholar]
- 36.Takeda S., Mochizuki S., Saini H. K., Elimban V., Dhalla N. S. Modification of alterations in cardiac function and sarcoplasmic reticulum by vanadate in ischemic-reperfused rat hearts. J. Appl. Physiol. 2005;99:999–1005. doi: 10.1152/japplphysiol.00234.2005. [DOI] [PubMed] [Google Scholar]
- 37.Koudinov A. R., Koudinova N. V. Cholesterol homeostasis failure as a unifying cause of synaptic degeneration. J. Neurol. Sci. 2005;229–230:233–240. doi: 10.1016/j.jns.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 38.Kirsch C., Eckert G. P., Koudinov A. R., Muller W. E. Brain cholesterol, statins and Alzheimer's disease. Pharmacopsychiatry. 2003;36(Suppl. 2):S113–S119. doi: 10.1055/s-2003-43058. [DOI] [PubMed] [Google Scholar]
- 39.Brown D. A., London E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell. Dev. Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 40.Jacobson K., Dietrich C. Looking at lipid rafts? Trends Cell Biol. 1999;9:87–91. doi: 10.1016/s0962-8924(98)01495-0. [DOI] [PubMed] [Google Scholar]
- 41.Simons K., Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 42.Simons K., Ikonen E. Functional rafts in cell membranes. Nature (London) 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 43.Brownlow S. L., Harper A. G., Harper M. T., Sage S. O. A role for hTRPC1 and lipid raft domains in store-mediated calcium entry in human platelets. Cell Calcium. 2004;35:107–113. doi: 10.1016/j.ceca.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Levi O., Lutjohann D., Devir A., von Bergmann K., Hartmann T., Michaelson D. M. Regulation of hippocampal cholesterol metabolism by apoE and environmental stimulation. J. Neurochem. 2005;95:987–997. doi: 10.1111/j.1471-4159.2005.03441.x. [DOI] [PubMed] [Google Scholar]
- 45.Grimm M. O., Grimm H. S., Patzold A. J., Zinser E. G., Halonen R., Duering M., Tschape J. A., Strooper B. D., Muller U., Shen J., et al. Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nat. Cell Biol. 2005;7:1118–1123. doi: 10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- 46.Wang Z., Jiang T., Li J., Proctor G., McManaman J. L., Lucia S., Chua S., Levi M. Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes. 2005;54:2328–2335. doi: 10.2337/diabetes.54.8.2328. [DOI] [PubMed] [Google Scholar]
- 47.Hartmann T. Cholesterol, A beta and Alzheimer's disease. Trends Neurosci. 2001;24:S45–S48. doi: 10.1016/s0166-2236(00)01990-1. [DOI] [PubMed] [Google Scholar]
- 48.Hurst R. S., Zhu X., Boulay G., Birnbaumer L., Stefani E. Ionic currents underlying HTRP3 mediated agonist-dependent Ca2+ influx in stably transfected HEK293 cells. FEBS Lett. 1998;422:333–338. doi: 10.1016/s0014-5793(98)00035-0. [DOI] [PubMed] [Google Scholar]
- 49.Hajdu P., Varga Z., Pieri C., Panyi G., Gaspar R., Jr Cholesterol modifies the gating of Kv1.3 in human T lymphocytes. Pflugers Arch. 2003;445:674–682. doi: 10.1007/s00424-002-0974-y. [DOI] [PubMed] [Google Scholar]
- 50.Xia F., Gao X., Kwan E., Lam P. P., Chan L., Sy K., Sheu L., Wheeler M. B., Gaisano H. Y., Tsushima R. G. Disruption of pancreatic beta-cell lipid rafts modifies Kv2.1 channel gating and insulin exocytosis. J. Biol. Chem. 2004;279:24685–24691. doi: 10.1074/jbc.M314314200. [DOI] [PubMed] [Google Scholar]
- 51.Li H., Yao Z., Degenhardt B., Teper G., Papadopoulos V. Cholesterol binding at the cholesterol recognition/interaction amino acid consensus (CRAC) of the peripheral-type benzodiazepine receptor and inhibition of steroidogenesis by an HIV TAT-CRAC peptide. Proc. Natl. Acad. Sci. U.S.A. 2001;98:1267–1272. doi: 10.1073/pnas.031461598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jamin N., Neumann J. M., Ostuni M. A., Vu T. K., Yao Z. X., Murail S., Robert J. C., Giatzakis C., Papadopoulos V., Lacapere J. J. Characterization of the cholesterol recognition amino acid consensus sequence of the peripheral-type benzodiazepine receptor. Mol. Endocrinol. 2005;19:588–594. doi: 10.1210/me.2004-0308. [DOI] [PubMed] [Google Scholar]
- 53.Chamberlain L. H., Burgoyne R. D., Gould G. W. SNARE proteins are highly enriched in lipid rafts in PC12 cells: implications for the spatial control of exocytosis. Proc. Natl. Acad. Sci. U.S.A. 2001;98:5619–5624. doi: 10.1073/pnas.091502398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lang T., Bruns D., Wenzel D., Riedel D., Holroyd P., Thiele C., Jahn R. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J. 2001;20:2202–2213. doi: 10.1093/emboj/20.9.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lafont F., Verkade P., Galli T., Wimmer C., Louvard D., Simons K. Raft association of SNAP receptors acting in apical trafficking in Madin–Darby canine kidney cells. Proc. Natl. Acad. Sci. U.S.A. 1999;96:3734–3738. doi: 10.1073/pnas.96.7.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]