Abstract
Activation of the superoxide-producing phagocyte NADPH oxidase, crucial for host defence, requires an SH3 (Src homology 3)-domain-mediated interaction of the regulatory protein p47phox with p22phox, a subunit of the oxidase catalytic core flavocytochrome b558. Although previous analysis of a crystal structure has demonstrated that the tandem SH3 domains of p47phox sandwich a short PRR (proline-rich region) of p22phox (amino acids 151–160), containing a polyproline II helix, it has remained unknown whether this model is indeed functional in activation of the oxidase. In the present paper we show that the co-operativity between the two SH3 domains of p47phox, as expected from the model, is required for oxidase activation. Deletion of the linker between the p47phox SH3 domains results not only in a defective binding to p22phox but also in a loss of the activity to support superoxide production. The present analysis using alanine-scanning mutagenesis identifies Pro152, Pro156 and Arg158 in the p22phox PRR as residues indispensable for the interaction with p47phox. Pro152 and Pro156 are recognized by the N-terminal SH3 domain, whereas Arg158 contacts with the C-terminal SH3 domain. Amino acid substitution for any of the three residues in the p22phox PRR abrogates the superoxide-producing activity of the oxidase reconstituted in intact cells. The bis-SH3-mediated interaction of p47phox with p22phox thus functions to activate the phagocyte oxidase. Furthermore, we provide evidence that a region C-terminal to the PRR of p22phox (amino acids 161–164), adopting an α-helical conformation, participates in full activation of the phagocyte oxidase by fortifying the association with the p47phox SH3 domains.
Keywords: bis-SH3 domain, flavocytochrome b558, NADPH oxidase, proline-rich region, p22phox, p47phox
Abbreviations: AIR, auto-inhibitory region; CBB, Coomassie Brilliant Blue; CGD, chronic granulomatous disease; CHO, Chinese-hamster ovary; GST, glutathione S-transferase; MBP, maltose-binding protein; p47-F, full-length p47phox; p47-ΔC, amino acid residues 1–286 of p47phox; p47-(SH3)2, tandem SH3 (Src homology 3) domains of p47phox; PPII, polyproline II; PRR, proline-rich region; ROS, reactive oxygen species; SH3(C), C-terminal SH3 domain; SH3(N), N-terminal SH3 domain; SOD, superoxide dismutase
INTRODUCTION
Professional phagocytes such as neutrophils and macrophages play a crucial role in the first line of innate immune defence against invading microbes. One of the mechanisms for microbe elimination is via production of ROS (reactive oxygen species). The phagocyte NADPH oxidase, which is dormant in resting cells, becomes activated during phagocytosis to produce superoxide, a precursor of powerful microbicidal ROS [1–5]. The significance of the oxidase in host defence is exemplified by recurrent and life-threatening infections that occur in patients with CGD (chronic granulomatous disease) because of the lack of the superoxide-producing system in phagocytes. Because inappropriate or excessive production of ROS, on the other hand, results in inflammatory disorders, the activity of the phagocyte oxidase should be strictly regulated. It is thus important to understand in detail the molecular mechanism of oxidase regulation.
The catalytic core of the oxidase is flavocytochrome b558, comprising the two membrane-integrated proteins gp91phox and p22phox; the subunit association is essential for stabilization of both proteins [1–5]. Whereas gp91phox harbours a complete electron-transferring apparatus from NADPH to molecular oxygen for superoxide production, p22phox is not directly involved in the electron transfer, but participates in oxidase regulation by serving as an anchoring site for regulatory proteins. Activation of the phagocyte oxidase requires the specific regulatory proteins p47phox and p67phox, and the small GTPase Rac; these exist in the cytoplasm of resting phagocytes and translocate upon cell stimulation to the membrane to interact with flavocytochrome b558. The interaction allows gp91phox to transport electrons, leading to superoxide production. In this process, p47phox plays a central role: p47phox by itself moves to the membrane to interact directly with cytochrome b558, whereas p67phox is recruited via its association with p47phox [6]. In contrast, Rac is independently targeted to the membrane [7,8] and thus participates in the oxidase assembly [9–13].
p47phox harbours two SH3 (Src homology 3) domains, which are arranged in tandem [p47-(SH3)2; see Figure 1A] and are capable of interacting with a PRR (proline-rich region) in the C-terminus of p22phox [14–17]. This interaction is crucial for both membrane recruitment of p47phox and oxidase activation. For example, substitution of Gln for Pro156 (P156Q) in the p22phox PRR, a mutation found in a patient with CGD [18], abrogates the interaction with p47phox and the translocation of p47phox [14–17]. p47-(SH3)2 are normally masked by an intramolecular association with the AIR (auto-inhibitory region) that exists between the SH3(C) (C-terminal SH3 domain) of p47phox [p47-SH3(C)] and the PRR (see Figure 1A) [19,20]; via the PRR, p47phox associates with p67phox [21]. Upon cell stimulation, p47phox undergoes phosphorylation on multiple serines in the AIR [22–27], an event that induces a conformational change of this protein to render the SH3 domains in a state accessible to the target p22phox [19,26]. The SH3-mediated interaction between p47phox and p22phox is strictly regulated so that it functions as a switch of phagocyte oxidase activation.
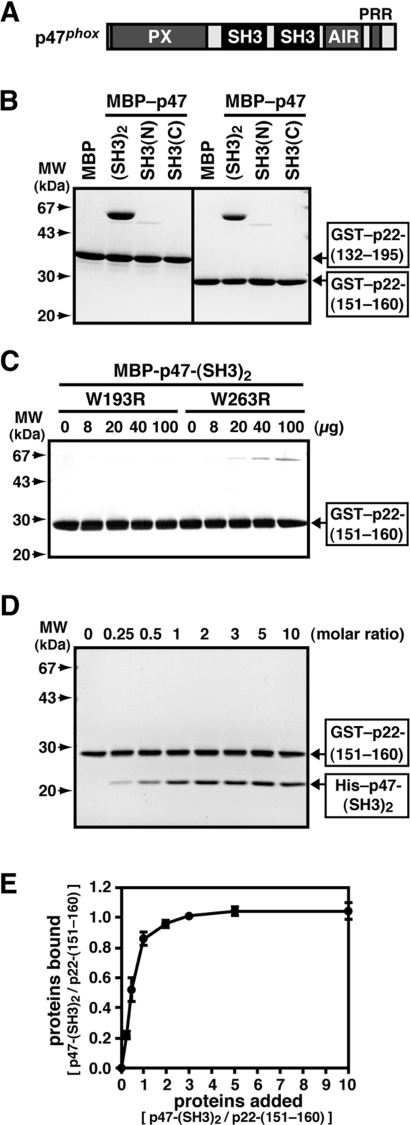
Figure 1. Interaction of the SH3 domains of p47phox with the proline-rich core of p22phox.
The interaction of the SH3 domains of p47phox with the p22phox PRR was estimated by an in vitro pull-down assay using purified proteins. (A) A representation of the domain arrangement of p47phox. PX, phox homology domain. (B) MBP alone, MBP–p47-(SH3)2, MBP–p47-SH3(N), or MBP–p47-SH3(C) (8 μg) was incubated with 10 μg of GST–p22-(132–195) or GST–p22-(151–160) and separated by pull-down assay with gluthathione–Sepharose beads. The precipitated proteins were subjected to SDS/10% PAGE, followed by staining with CBB. Molecular masses (MW) of marker proteins are indicated. (C) MBP–p47-(SH3)2 carrying the W193R or W263R substitution (8 μg) was incubated with GST–p22-(151–160) (10 μg) and separated by pull-down assay with gluthathione–Sepharose beads. The precipitated proteins were subjected to SDS/10% PAGE, followed by staining with CBB. Molecular masses (MW) of marker proteins are indicated. (D) GST–p22-(151–160) (100 nM) was incubated with His–p47-(SH3)2 at the indicated molar ratio. The proteins were precipitated with glutathione–Sepharose beads and eluted from the beads with gluthathione. The eluates were subjected to SDS/10% PAGE and stained with CBB. Molecular masses (MW) of marker proteins are indicated. (E) Intensities of the bands on the gel in (D) were quantified by the image analyser. The experiments were repeated three times with similar results.
With hundreds of SH3 domains in the human genome, binding selectivity is a key issue in understanding the molecular basis of SH3-domain interactions [28–32]. Typical SH3 domains interact directly with a PRR containing a conserved PxxP motif (x is any amino acid) in a PPII (polyproline type II) helix conformation. Because most SH3–PxxP interactions are of relatively low affinity, ligand peptides show high cross-reactivity with several SH3 domains [29–32]. To obtain high affinity and selectivity, SH3 domains are considered to usually make additional contacts with regions outside the proline-rich core, albeit there exist only a few known examples in which an SH3 domain simultaneously interacts with PxxP and extra-PxxP regions of a natural ligand [21,33–35].
It is known that the SH3(N) (N-terminal SH3 domain) of p47phox [p47-SH3(N)] alone can bind directly to the ten-amino-acid proline-rich core of p22phox (amino acids 151–160; PPSNPPPRPP), while p47-SH3(C) by itself fails to interact with p22phox [17,36]. Consistent with the former observation, the amino acid substitution of Arg for Trp193 (W193R) in p47-SH3(N), the invariant residue among SH3 domains, results in a complete loss of the activity to support oxidase activation [17,36,37]. On the other hand, a mutant p47phox carrying the corresponding W263R substitution in SH3(C) has a significant, but extremely weak, activity [36,37], suggesting that p47-SH3(C) also plays a role. Recent analysis of a crystal structure of p47-(SH3)2 complexed with a proline-rich peptide of p22phox [38] has revealed that the two SH3 domains bind to the PRR in a PPII helix conformation at the same time, thereby conferring high affinity and specificity, although the SH3(N) forms an intertwined dimer in the crystal. The proposed target recognition via the bis-SH3 domain appears to be a novel mode of improvement of affinity and selectivity in SH3-mediated interactions. However, no functional analysis has been performed and thus it has remained unknown whether the bis-SH3-mediated recognition indeed functions in activation of the phagocyte oxidase, especially in vivo.
In the present paper we demonstrate that the co-operativity between the two SH3 domains of p47phox, which is expected from the bis-SH3-mediated recognition model, participates in both interaction with p22phox and activation of the phagocyte oxidase. The present functional analysis identifies Pro152 and Arg158 in the p22phox PRR, besides Pro156, as residues crucial for this mode of recognition. Importantly, each of the three residues plays an indispensable role in the oxidase activation in vivo. Thus the bis-SH3-mediated interaction functions to activate the phagocyte oxidase. In addition to the PRR, we show that its C-terminally flanking region in p22phox (amino acids 161–164), adopting an α-helix in the p47phox–p22phox complex in solution [39], is also involved in full activation of the phagocyte oxidase by fortifying the interaction with the p47phox SH3 domains.
EXPERIMENTAL
Plasmid construction
The DNA fragments encoding the full-length of p47phox (p47-F; amino acid residues 1–390), p47-ΔC (amino acid residues 1–286 of p47phox), p47-(SH3)2; (amino acid residues 151–286), SH3(N) (amino acid residues 151–219) and SH3(C) (amino acid residues 223–286) were amplified from a cloned cDNA encoding human p47phox by PCR using specific primers [17,19]. Similarly, the DNA fragments that encode the full-length of p22phox (amino acid residues 1–195), p22-(132–160), p22-(132–165), p22-(132–195), p22-(150–195) and p22-(151–160) were constructed from a cloned cDNA encoding human p22phox by PCR using specific primers [17,19]. Mutations leading to the indicated amino acid substitutions were introduced by PCR-mediated site-directed mutagenesis. The cDNAs encoding p47-ΔC lacking amino acid residues 219–222 or 217–224, or those encoding p47-(SH3)2 lacking amino acid residues 220–221, 219–222, 218–223, 217–224 or 216–225, or containing two additional serine residues at the positions between 220 and 221, were also prepared with PCR-mediated site-directed mutagenesis. All of the constructs were sequenced for confirmation of their identities.
In vitro pull-down binding assays using purified proteins
For expression in Escherichia coli, cDNA fragments were ligated to the following vectors: pGEX-2T (Amersham Biosciences) for GST (gluthathione S-transferase)-fusion protein; pMALc2 (New England BioLab) for MBP (maltose-binding protein)-fusion protein; and pProEX-HTb (Invitrogen) for His6-tagged protein. GST-, MBP- or His6-tagged proteins were expressed in E. coli strain DH5 and purified by glutathione–Sepharose-4B (Amersham Biosciences), amylose resin (New England BioLab) or His-bind resin (Novagen) respectively, according to the manufacturers’ instructions. For in vitro pull-down binding assays, a pair of a GST-fusion proteins and an MBP-fusion protein, or a GST- and a His6-tagged protein were mixed in PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4 and 1.4 mM KH2PO4, pH 7.4) containing 1% Triton X-100 and incubated for 30 min at 4 °C. For the pull-down assay of a GST-fusion protein, a slurry of glutathione–Sepharose-4B beads was added to the mixture and then incubated for 30 min at 4 °C. After washing three times with PBS, GST-fusion and its interacting proteins were eluted from glutathione–Sepharose-4B beads by 20 mM Tris/HCl (pH 8.0) containing 10 mM glutathione. The eluates were subjected to SDS/10% PAGE, followed by protein staining with CBB (Coomassie Brilliant Blue). To estimate the binding ratio of p22-(151–160) to p47-(SH3)2, amounts of the stained proteins on the gel were quantified by the image analyser LAS1000 (Fuji).
Cell-free activation of the phagocyte NADPH oxidase
The membrane fraction of human neutrophils was prepared as previously described [9,17,19,26]. The membranes (0.43 μg/ml) were mixed with: 90 nM GST-fused full-length p67phox (GST–p67-F); 90 nM His6-tagged Rac2 carrying the Q61L substitution, namely His–Rac2 (Q61L) [9]; and the indicated concentration of GST–p47-ΔC or its mutant protein lacking amino acids 219–222 or 217–224. The mixture was incubated with 100 μM SDS for 2.5 min at 25 °C in 100 mM potassium phosphate (pH 7.0), containing 75 μM cytochrome c, 1.0 μM FAD, 1.0 mM EGTA, 1.0 mM MgCl2, 100 μM GTPγS and 1.0 mM NaN3, and the reaction was initiated by the addition of 1.0 mM NADPH. The NADPH-dependent superoxide production was measured by determining the rate of SOD (superoxide dismutase)-inhibitable ferricytochrome c reduction at 550–540 nm using a Hitachi 557 dual-wavelength spectrophotometer [9,17,19,26]. The superoxide-producing activity was represented as mol of superoxide produced/s per mol of cytochrome b558 haem; the haem content was calculated from the reduced absorption value minus the oxidized absorption value at 558 nm [40].
Whole-cell activation of the phagocyte NADPH oxidase in CHO (Chinese-hamster ovary) cells
Experiments for activation of the phagocyte NADPH oxidase in a whole-cell system were performed as described previously [41] with minor modifications. The cDNAs encoding the wild-type and mutant proteins of p22phox, the wild-type p47phox, and the wild-type p67phox were ligated to the expression vector pEF-BOS [42], whereas the cDNA of the wild-type gp91phox was ligated to pcDNA3.0 (Invitrogen). The CHO cells were transfected with pEF-BOS–p47phox, pEF-BOS–p67phox, pcDNA3.0–gp91phox and pEF-BOS encoding various forms of p22phox. After culture for 30 h at 37 °C, adherent cells were harvested by incubating with trypsin/EDTA for 1 min at 37 °C, and washed with Hepes-buffered saline (120 mM NaCl, 5 mM KCl, 5 mM glucose, 1 mM MgCl2, 0.5 mM CaCl2 and 17 mM Hepes, pH 7.4).
For detection of p22phox, CHO cells (1×105 cells) were lysed by sonication, and the sonicates were analysed by SDS/12% PAGE. Proteins were transferred to a polyvinylidene difluoride membrane (Millipore) and probed with anti-p22phox antibodies (Santa Cruz Biotech). The blots were developed using ECL-plus (Amersham Biosciences) to visualize the antibodies. Expression of gp91phox was estimated by using 7D5 (from Professor Michio Nakamura, Nagasaki University), a monoclonal antibody that recognizes gp91phox functionally complexed with p22phox [43,44], as described below. Transfected cells were fixed for 15 min at 25 °C in 3.7% (w/v) formaldehyde. The fixed cells were washed four times with PBS and blocked with PBS containing 3% (w/v) BSA for 60 min. The sample was subsequently incubated with 7D5 for 1 h at 25 °C, and probed with Alexa Flour®-488-labelled goat anti-(mouse IgG) antibodies (Molecular Probes) as secondary antibodies. Images were visualized with an LSM5 PASCAL confocal laser scanning microscope (Carl Zeiss).
Superoxide production by the cells was determined by SOD-inhibitable chemiluminescence with an enhancer-containing luminol-based detection system (DIOGENES; National Diagnostics) as previously described [9,27,41]. After the addition of the enhanced luminol-based substrate, the cells were stimulated with 200 ng/ml PMA. The chemiluminescence was assayed using a luminometer (Auto Lumat LB953; EG&G Berthold).
RESULTS
Role of the p47-(SH3)2 in binding to the proline-rich core of p22phox
Structural analysis has demonstrated that, in the p47-(SH3)2–p22-PRR complex, the two SH3 domains of p47phox sandwich the PRR of p22phox, which probably confers high affinity and specificity [38]. According to this model, the two SH3 domains should function co-operatively in binding to p22phox and probably in activation of the phagocyte oxidase. We first examined the co-operativity between the p47phox SH3 domains in the interaction with p22phox using a pull-down binding assay with purified proteins. As shown in Figure 1(B), MBP–p47-(SH3)2 bound to the entire C-terminal cytoplasmic region of p22phox (amino acids 132–195) expressed as a GST-fusion protein; it also interacted with the ten-amino-acid proline-rich core of p22phox (amino acids 151–160; PPSNPPPRPP) but to a lesser extent. In contrast, MBP–p47-(SH3)2 was not pulled down by GST alone (results not shown). MBP–p47-SH3(N) bound to both GST–p22-(132–195) and GST–p22-(151–160) much more weakly than MBP–p47-(SH3)2 bound (Figure 1B), whereas p47-SH3(C) was incapable of interacting with p22phox (Figure 1B). In addition, the W193R substitution in SH3(N), the invariant residue that is known to make a contact with a proline of SH3-target proteins, led to a completely impaired interaction with the proline-rich core of p22phox, whereas the corresponding substitution in SH3(C) (W263R) led to a severe but incomplete loss of the binding to p22phox (Figure 1C). Thus, under the present experimental conditions, p47-SH3(N) primarily binds to the ten-amino-acid proline-rich core of p22phox, and p47-SH3(C) supports the interaction.
To confirm that the two SH3 domains of p47phox bind to the single proline-rich core of p22phox at the same time, we accessed the stoichiometry of the interaction by a pull-down assay using purified His6-tagged p47-(SH3)2 and GST-fused p22phox (Figure 1D). As shown in Figure 1(E), increasing the concentration of His–p47-(SH3)2 in a solution containing 100 nM GST–p22-(151–160) yielded a profile exhibiting a saturating plateau, consistent with the formation of a complex of 1:1 stoichiometry. The present experimental conditions thus allow the two SH3 domains of p47phox to bind simultaneously to the ten-amino-acid proline-rich core of p22phox. From the binding profile (Figure 1E), the KD value was calculated at 0.2 μM, which is in good agreement with the values obtained by other methods such as isothermal titration calorimetry and a binding assay using a fluorescein-labelled p22phox peptide [38]. We also tried to estimate the KD value for the p47-SH3(N)–p22phox interaction using the pull-down assay. Although the increase in the concentration of His–p47-SH3(N) resulted in an elevated binding to GST–p22-(151–160), the binding was not saturated even under the conditions where 50 times higher concentrations of His–p47-SH3(N) were used (results not shown); its KD value was calculated at more than 5 μM, which agrees with results obtained by other methods [38].
Effect of truncation of the linker region between the p47-(SH3)2 in the interaction with p22phox
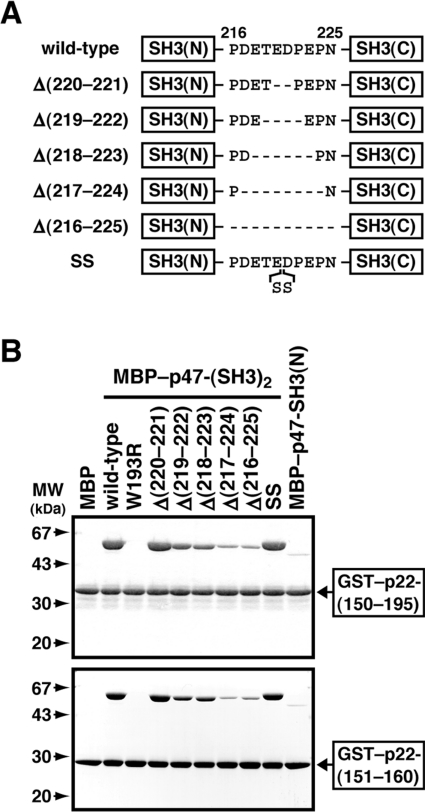
To study further the co-operativity between the p47-(SH3)2 in the interaction with p22phox, we prepared mutant p47phox proteins with various lengths of the linker region between the domains (amino acids 216–225; PDETEDPEPN) as MBP-fusions (Figure 2A) and investigated their activity to bind to p22phox. As shown in Figure 2(B), a mutant p47phox containing two additional serine residues at the position between 220 and 221 was as active as the wild-type. Similarly, a mutant protein lacking the two amino acids Glu220 and Asp221, Δ(220–221), fully bound to p22phox (Figure 2B). Further deletion in the linker region led to an impaired interaction with p22phox: mutant p47phox proteins without four and six amino acids, Δ(219–222) and Δ(218–223) respectively, associated with p22phox, but to a slightly lesser extent than did the wild-type p47-(SH3)2. On the other hand, a mutant protein lacking eight or ten amino acids, Δ(217–224) and Δ(216–225) respectively, only weakly bound to p22phox, to an extent similar to that of p47-SH3(N) (Figure 2B). This shortening of the linker region prevents the tandem SH3 domains from simultaneously recognizing p22phox.
Figure 2. Role of the linker region between the SH3 domains of p47phox in the binding to p22phox.
(A) Representation of the mutant p47phox proteins used in the present study. They have various lengths of the linker region between the SH3(N) and SH3(C). (B) MBP alone, the wild-type p47-(SH3)2 fused to MBP, or mutant MBP–p47-(SH3)2 (8 μg) with the indicated length of the linker was incubated with 10 μg of GST–p22-(150–195) or GST–p22-(151–160) and separated by pull-down assay with gluthathione–Sepharose beads. The precipitated proteins were subjected to SDS/10% PAGE followed by staining with CBB. Molecular masses (MW) of marker proteins are indicated. The experiments were repeated three times with similar results.
Effect of truncation of the linker region between the p47-(SH3)2 in activation of the phagocyte NADPH oxidase
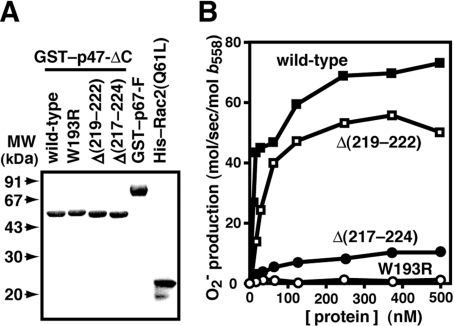
To clarify the role of the co-operativity between the p47-(SH3)2 in phagocyte oxidase activation, we next tested the activity of linker-deleted mutant p47phox proteins to support superoxide production in a cell-free system, which was reconstituted with human neutrophil membranes rich in cytochrome b558 and the recombinant proteins p67phox and Rac2 (Figure 3A). As shown in Figure 3(B), a mutant p47phox lacking four amino acids, Δ(219–222), was capable of supporting superoxide production but to a lesser extent than the wild-type p47phox. Furthermore, deletion of eight amino acids in the linker, Δ(217–224), resulted in a severe loss of superoxide production (Figure 3B). The effect of the deletions on oxidase activation (Figure 3B) is well correlated with that on the ability to bind to p22phox (Figure 2B). Shortening of the linker region between the tandem SH3 domains prevents them from sandwiching p22phox, which leads to defective activation of the phagocyte oxidase. Thus oxidase activation requires the bis-SH3-mediated recognition, in which the p47phox SH3 domains co-operatively bind to the p22phox PRR.
Figure 3. Role of the linker region between the two SH3 domains of p47phox in cell-free activation of the phagocyte NADPH oxidase.
(A) SDS/10% PAGE analysis of purified GST-fusion proteins that were used in a cell-free system for activation of the phagocyte NADPH oxidase: wild-type p47-ΔC (amino acid residues 1–286); W193R, p47-ΔC carrying the W193R substitution; Δ(219–222), p47-ΔC lacking amino acid residues 219–222; Δ(217–224), p47-ΔC lacking amino acid residues 217–224; GST–p67-F, the full-length p67phox fused to GST; and His–Rac2(Q61L), His6-tagged Rac2 carrying the Q61L substitution. The proteins were subjected to SDS/10% PAGE and stained with CBB. Molecular masses (MW) of marker proteins are indicated. (B) Various p47phox proteins at the indicated concentrations were mixed with human neutrophil membranes (0.43 μg/ml), His–Rac2(Q61L) (90 nM), and GST–p67-F (90 nM) in the presence of FAD (1.0 μM) and GTPγS (100 μM), followed by incubation with 100 μM SDS for 2.5 min at 25 °C. The superoxide production was initiated by the addition of NADPH (1.0 mM) to the reaction mixture. The NADPH-dependent superoxide-generating activity was represented as mol of superoxide produced/s per mol of cytochrome b558 haem, as described in the Experimental section.
Role of each individual amino acid residue of the p22phox PRR in the interaction with the p47phox SH3 domains
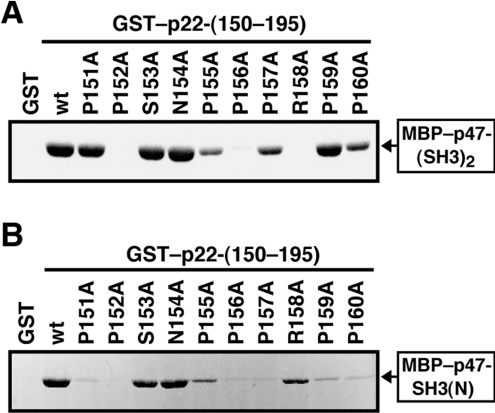
Whereas structural analysis of p47-(SH3)2–p22-PRR complexes revealed a number of contacts between p47-(SH3)2 and residues of the proline-rich core of p22phox (amino acids 151–160; PPSNPPPRPP) [38], functional analysis has been expected to provide information on relative contribution of each individual residue in the p22phox PRR to the interaction with p47phox. To obtain such information, we prepared a series of mutant proteins carrying the substitution of alanine for one of the residues and tested their activity to bind to p47-(SH3)2. As shown in Figure 4(A), a mutant p22phox with the alanine substitution for Pro152, Pro156 or Arg158 was incapable of interacting with p47-(SH3)2, indicative of crucial roles of the three residues. Whereas the KD value for the wild-type p22phox binding to p47-(SH3)2 was estimated at 0.2 μM from the present data as described above (Figure 1E), similar experiments revealed that KD values for mutant proteins carrying the P152A, P156Q or R158A substitution were more than 5 μM (results not shown). In addition, the substitution for Pro155, Pro157 or Pro160 resulted in a partial loss of the binding activity. On the other hand, the replacement of Ser151, Ser153, Asn154 or Pro159 did not affect the interaction (Figure 4A), indicating that the side chains of these residues do not play a major role.
Figure 4. Roles of residues of the p22phox proline-rich core in the interaction with p47phox.
(A) MBP–p47-(SH3)2 (10 μg) was incubated with 8 μg of GST–p22-(150–195) wild-type (wt) or carrying the indicated amino acid substitution. The proteins were precipitated with glutathione–Sepharose beads and eluted from the beads with gluthathione. The eluates were subjected to SDS/10% PAGE and stained with CBB. The experiments were repeated three times with similar results. (B) MBP–p47-SH3(N) (80 μg) was incubated with 20 μg of GST–p22-(150–195) wild type (wt) or carrying the indicated amino acid substitution. The proteins were precipitated with glutathione–Sepharose beads and eluted from the beads with gluthathione. The eluates were subjected to SDS/10% PAGE and stained with CBB. The experiments were repeated three times with similar results.
To know which SH3 domain of p47phox recognizes the individual residues in the p22phox PRR, we tested the effect of the substitutions in p22phox on the interaction with p47-SH3(N). The binding of p22phox to p47-SH3(N) was completely abolished by the substitution for Pro152, and strongly reduced by that for Pro151, Pro156, Pro157, Pro159 or Pro160 (Figure 4B). On the other hand, the replacement of Ser153, Asn154 or Arg158 exhibited a small effect on the interaction with p47-SH3(N) (Figure 4B).
These findings suggest that, among the three residues crucial in the interaction with p47phox, Pro152/Pro156 and Arg158 are probably recognized in a distinct manner: Pro152 and Pro156 appear to be recognized mainly by p47-SH3(N), because the substitution for either proline residue led to a drastic loss of the interaction not only with p47-(SH3)2 but also with p47-SH3(N); Arg158 probably makes a direct contact with p47-SH3(C), since the R158A substitution resulted in a completely impaired interaction with p47-(SH3)2 but not with p47-SH3(N) (for details, see the Discussion). Among the residues that play a significant but minor role in the interaction with p47phox (Figure 4B), Pro157 and Pro160 probably associate with p47-SH3(N), whereas Pro155 appears to bind mainly to p47-SH3(C) (Figure 4B).
Role of Pro152, Pro156 and Arg158 in activation of the phagocyte NADPH oxidase
It is well established that gp91phox tightly associates with p22phox in phagocytic membranes, the association being essential for the stabilization of both proteins: one protein can not exist in the absence of the other [1–5]. To investigate the role of Pro152, Pro156 and Arg158 in p22phox activation of the phagocyte NADPH oxidase in vivo, we used the CHO cells to functionally reconstitute the phagocyte oxidase system by expressing the membranous and cytosolic oxidase factors [41]. Although the mRNA of p22phox exists in a wide variety of cells, CHO cells scarcely express the message [41]. The phagocyte oxidase reconstitution in CHO cells, therefore, is totally dependent on ectopic expression of p22phox [41].
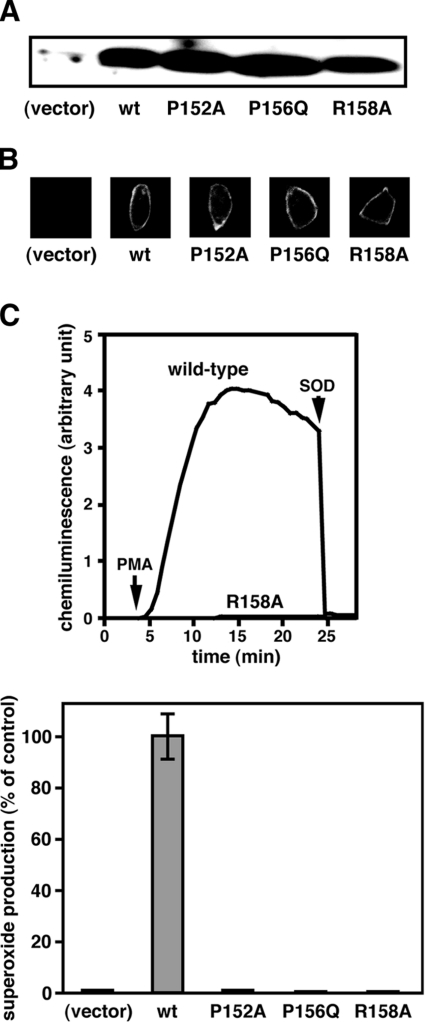
We transfected CHO cells with cDNA encoding the wild-type p22phox or a mutant protein with the P152A, P156Q or R158A substitution, and found that these proteins were expressed at essentially the same level (Figure 5A). The mutant proteins, as well as the wild-type one, seem to associate properly with gp91phox: at the plasma membrane of the cells transfected with the wild-type or mutant p22phox cDNA, gp91phox was detected by 7D5, a monoclonal antibody that recognizes gp91phox functionally complexed with p22phox [43,44]. In contrast, the gp91phox protein was not detected without expression of p22phox (Figure 5B). Thus these mutant p22phox proteins probably retain the conformational integrity. When the wild-type p22phox was co-expressed with gp91phox, p47phox and p67phox in CHO cells, superoxide was produced by the cells in response to PMA (Figure 5C). On the other hand, the superoxide production was not observed in the cells expressing a p22phox carrying the P156Q substitution (Figure 5D), a mutation that occurs in a patient with CGD [18]. Similarly, the P152A or R158A substitution resulted in a complete loss of the superoxide-producing activity (Figures 5C and 5D). These findings clearly show that Pro152 and Arg158 in the proline-rich core of p22phox participate in binding to p47phox in intact cells, thereby playing a crucial role in activation of the phagocyte NADPH oxidase.
Figure 5. Role of Pro152 and Arg158 of p22phox in whole-cell activation of the phagocyte NADPH oxidase.
(A) CHO cells were transfected simultaneously with pcDNA3.0–gp91phox, pEF-BOS–p47phox, pEF-BOS–p67phox, and pEF-BOS encoding the wild-type p22phox (wt) or a mutant p22phox carrying the P152A, P156Q or R158A substitution. Lysates of the cells transfected with the indicated form of p22phox were subjected to SDS/12% PAGE, followed by immunoblot with anti-p22phox antibodies. See the Experimental section for details. These experiments were repeated more than three times with similar results. (B) The cells transfected with cDNA for the wild-type p22phox (wt) or a mutant p22phox carrying the P152A, P156Q or R158A substitution were fixed and stained with the anti-gp91phox monoclonal antibody 7D5. The experiments were repeated more than three times with similar results. (C) The cells expressing the wild-type p22phox or a mutant p22phox carrying the R158A substitution (1×105 cells) were incubated for 5 min at 37 °C and then stimulated with PMA (200 ng/ml). Upper panel: chemiluminescence change was continuously monitored with DIOGENES, and SOD (50 μg/ml) was added where indicated (see the Experimental section for details). The experiments were repeated more than three times with similar results. Lower panel: the amount of superoxide produced by the cells expressing the indicated form of p22phox was expressed as the percent activity relative to control cells transfected with pcDNA3.0–gp91phox, pEF-BOS–p47phox, pEF-BOS–p67phox and pEF-BOS encoding the wild-type p22phox. Values are means±S.D. for three independent transfections.
Role of the α-helical region C-terminal to the PRR in the interaction with p47-(SH3)2
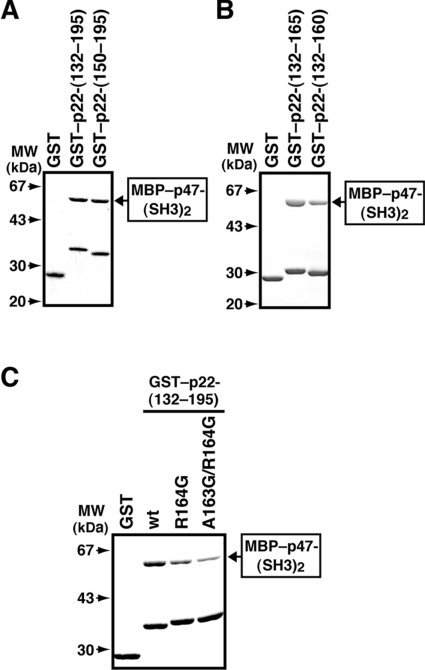
We next investigated the role of regions outside of the PRR of p22phox in the interaction with p47phox. The region N-terminal to the PRR does not seem to be involved in the interaction, because GST–p22-(150–195) bound to p47-(SH3)2 to the same extent as GST–p22-(132–195) (Figure 6A). On the other hand, the addition of the C-terminal five amino acids (161–165) enhanced the interaction with p47-(SH3)2 (Figure 6B), a finding that is consistent with a previous observation that the p22phox peptide consisting of amino acids 151–165 binds to p47phox more strongly than the peptide of 149–163 [45].
Figure 6. Role of a region C-terminal to the p22phox PRR in the interaction with the p47phox SH3 domains.
(A) MBP–p47-(SH3)2 (10 μg) was incubated with 8 μg of GST alone, GST–p22-(132–195) or GST–p22-(150–195). The proteins were precipitated with glutathione–Sepharose beads and eluted from the beads with gluthathione. The eluates were subjected to SDS/10% PAGE and stained with CBB. The experiments were repeated three times with similar results. (B) MBP–p47-(SH3)2 (10 μg) was incubated with 8 μg of GST alone, GST–p22-(132–160) or GST–p22-(132–165). The proteins were precipitated with glutathione–Sepharose beads and eluted from the beads with glutathione. The eluates were subjected to SDS/10% PAGE and stained with CBB. The experiments were repeated three times with similar results. (C) MBP–p47-(SH3)2 (10 μg) was incubated with 1.5 μg of GST alone or GST-fused protein of the p22phox C-terminal region (132–195): the wild-type p22phox (wt) or a mutant p22phox carrying the R164G or A163G/R164G substitution. The proteins were precipitated with glutathione–Sepharose beads and eluted from the beads with gluthathione. The eluates were subjected to SDS/10% PAGE and stained with CBB. The experiments were repeated three times with similar results. Molecular masses (MW) of marker proteins are indicated.
The region of amino acids 161–164 of p22phox (AEAR) adopts an α-helical structure in a solution structure of p47-(SH3)2 complexed with a p22phox peptide, as revealed by our NMR analysis [39]. To investigate the role of the α-helix in the interaction with p47-(SH3)2, we replaced Arg164 with Gly, a residue known to destabilize α-helical structures. As shown in Figure 6(C), the R164G substitution led to a decreased binding to p47-(SH3)2. The binding was further impaired by the additional replacement of Ala163 with Gly (A163G/R164G) (Figure 6C). Thus α-helical conformation of the region C-terminal to the p22phox PRR (amino acids 161–164) is likely to be involved in full interaction with p47-(SH3)2.
Role of the α-helical region C-terminal to the p22phox PRR in oxidase activation
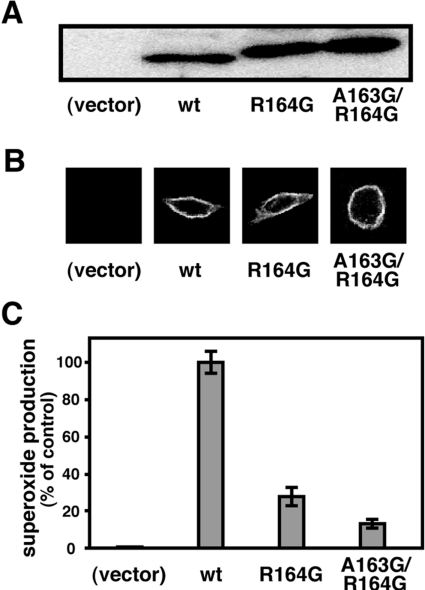
To understand the role of the p22phox α-helix of amino acids 161–164 in activation of the phagocyte NADPH oxidase, we transfected CHO cells with cDNA that encodes the wild-type p22phox or a mutant protein carrying the R164G or A163G/R164G substitution. These mutant p22phox proteins were expressed to the same extent as the wild-type one at the protein level (Figure 7A) and formed a proper complex with gp91phox at the plasma membrane (Figure 7B). As shown in Figure 7(C), CHO cells expressing a mutant p22phox with the R164G substitution produced a small amount of superoxide, compared with that produced by cells expressing the wild-type p22phox. In addition, the double substitution A163G/R164G led to a further decrease in superoxide production (Figure 7C). These findings indicate that the α-helix C-terminal to the core PRR of p22phox participates in full activation of the phagocyte NADPH oxidase.
Figure 7. Role of an α-helix C-terminal to the p22phox PRR in whole-cell activation of the phagocyte NADPH oxidase.
(A) CHO cells were transfected simultaneously with pcDNA3.0–gp91phox, pEF-BOS–p47phox, pEF-BOS–p67phox, and pEF-BOS encoding the wild-type p22phox (wt) or a mutant p22phox carrying the R164G or A163G/R164G substitution. Lysates of the cells transfected with the indicated form of p22phox were subjected to SDS/12% PAGE, followed by immunoblot with anti-p22phox antibodies (see the Experimental section for details). The experiments were repeated more than three times with similar results. (B) Cells transfected with cDNA for the wild-type p22phox (wt) or a mutant p22phox carrying the R164G or A163G/R164G substitution were fixed and stained with the anti-gp91phox monoclonal antibody 7D5. The experiments were repeated more than three times with similar results. (C) Cells transfected with cDNA for the wild-type p22phox (wt) or a mutant p22phox carrying the R164G or A163G/R164G substitution were incubated for 5 min at 37 °C and then stimulated with PMA (200 ng/ml). Chemiluminescence change was continuously monitored with DIOGENES. The amount of superoxide by the cells expressing the indicated form of p22phox was expressed as the percentage activity relative to control cells transfected with pcDNA3.0–gp91phox, pEF-BOS–p47phox, pEF-BOS–p67phox and pEF-BOS encoding the wild-type p22phox. Values are means±S.D. for three independent transfections.
DISCUSSION
In the present study, we showed that activation of the phagocyte NADPH oxidase required co-operative binding of the two SH3 domains of p47phox to the ten-amino-acid PRR of p22phox (amino acids 151–160). The target recognition by the bis-SH3 domain was proposed originally on the basis of a crystal structure of p47-(SH3)2 in complex with a p22phox-derived peptide: the tandem SH3 domains share an interface, which gives rise to a shallow groove that constitutes the peptide-binding surface and is lined with residues of the conserved SH3 domain ligand-binding surfaces [38]. Although p47-(SH3)2 exists as an intertwined dimer in the crystal [38], this target-recognition model is supported by the present functional analysis using various mutant proteins of p47phox and p22phox (Figures 1–5; and see below). Furthermore, we also demonstrated that α-helical conformation of the region C-terminal to the p22phox PRR (amino acids 161–164) is involved in full activation of the phagocyte oxidase (Figures 6 and 7).
The conclusion that the co-operation between the two SH3 domains in binding to p22phox plays a crucial role in phagocyte oxidase activation is drawn from several lines of evidence obtained in the present study. First, deletion of the flexible linker connecting the SH3 domains of p47phox led to a drastic decrease in both the interaction with p22phox and oxidase activation (Figures 2 and 3). The shortening of distance between the SH3 domains probably prevents their ligand-binding surfaces from being arranged such that they can make contacts with the p22phox PRR at the same time. Secondly, the co-operative role of p47-SH3(C) is also supported by the finding that the interaction of p47-(SH3)2 with the p22phox PRR is profoundly suppressed by W263R substitution in the SH3(C) (Figure 1), a mutation which is known to result in a significant loss of the ability to support activation of the NADPH oxidase [36,37]. The significance of p47-SH3(C) is consistent with the finding that a p47phox peptide encompassing Trp263 (amino acids 253–267) moderately inhibits cell-free activation of the oxidase [46]. Finally, oxidase activation is abolished when alanine is substituted not only for the p47-SH3(N)-interacting residue Pro152 or Pro156 in the p22phox PRR, but also for Arg158, a residue that makes a close contact with p47-SH3(C) but not with p47-SH3(N) (Figure 4; and see below).
A number of direct contacts between residues of the p22phox PRR and those of the p47phox SH3 domains have been revealed by analysis of the crystal structure of p47-(SH3)2–p22phox-PRR complexes [38]. However, it remained unknown to what extent each individual residue in the p22phox PRR contributed to the interaction with p47phox and oxidase activation, except that replacement of Pro156 by glutamine, a mutation found in a patient with CGD [18], resulted in a complete loss of the interaction [14,15]. The present analysis using alanine-scanning mutagenesis showed the contribution of each individual residue in the p22phox PRR. In addition to Pro156, Pro152 and Arg158 are essential for binding to p47-(SH3)2, whereas Pro155, Pro157 and Pro160 play a modest role (Figure 4). In contrast, the side chains of Ser153 and Asn154 do not appear to be involved, since mutations of these residues to alanine have no effect (Figure 4). These findings agreed with those provided by the structural analysis of the p47phox–p22phox complexes [38], in which both Ser153 and Asn154 point away from the binding surfaces of the p47phox SH3 domains, making no stabilizing contacts. Although Pro151 and Pro159 probably bind to p47-SH3(N) as indicated by the structural analysis [38] and the present binding assay (Figure 4B), these residues do not seem to make a major contribution to the interaction with p47-(SH3)2 (Figure 4A). Among the three essential residues, Pro152 and Pro156 appear to make a direct contact with p47-SH3(N) whereas Arg158 associates with p47-SH3(C) (Figure 8): the alanine substitution for Pro152 or Pro156 abrogates the binding to p47-SH3(N) (Figure 4B) as well as that to p47-(SH3)2 (Figure 4A); on the other hand, the substitution for Arg158 affects the interaction with p47-SH3(N) to a much lesser extent (Figure 4B). Consistent with these findings, Pro152 and Pro156 of p22phox in the complex with p47phox engage in van der Waals interactions with Trp193 of p47-SH3(N), whereas Arg158 makes salt bridges with Asp243 and Glu244 on p47-SH3(C) [38]. The present study further demonstrates that the substitutions for Pro152 and for Arg158, as well as those for Pro156, completely abrogate in vivo activation of the phagocyte NADPH oxidase without affecting the protein integrity (Figure 5). Taken together with the above-mentioned co-operation between the two SH3 domains of p47phox, we conclude that the bis-SH3-mediated target recognition is crucial for the oxidase activation both in vivo and in vitro.
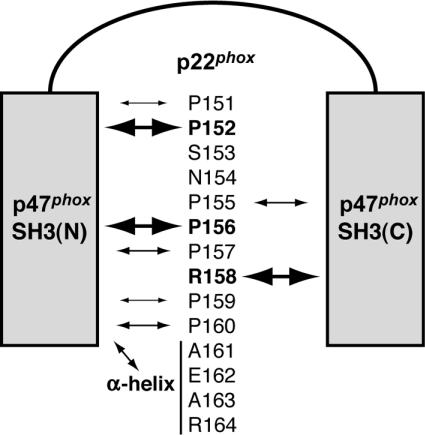
Figure 8. A model for interaction of the p47phox bis-SH3 domain with p22phox, which is essential for activation of the phagocyte NADPH oxidase.
The p47phox bis-SH3 domain, comprising SH3(N) and SH3(C), binds to the PRR (amino acids 151–160) and its C-terminal α-helical region (amino acids 161–164) of p22phox. Among residues in p22phox, Pro152, Pro156 and Arg158 are essential for the interaction with p47phox (as indicated by the bold text and largest arrows) and activation of the phagocyte NADPH oxidase: Pro152 and Pro156 make a direct contact with p47-SH3(N), whereas Arg158 associates with p47-SH3(C). Although Pro155, Pro157 and Pro160 play a modest role (indicated by medium-sized arrows), Ser153 and Asn154 are not involved in the interaction with the p47phox SH3 domains. The α-helix C-terminal to the p22phox PRR binds to p47-SH3(N). This association also participates in oxidase activation.
In the present paper we also provide evidence that the C-terminal extension of the p22phox PRR plays an important role in activation of the phagocyte NADPH oxidase. The extended region of amino acids 161–164 adopts an α-helical conformation in the complex of p47-(SH3)2 with the p22phox-derived peptide of amino acids 149–168 in solution, as shown in our NMR analysis [39]. On the other hand, the structure of this region is not determined in the crystal structure of p47-(SH3)2 that is complexed with the p22phox peptide of amino acids 149–166 [38]. Although the reason for this is presently unclear, it may be due to the difference in conformation between the solution and the crystal or the difference in length between the p22phox-derived peptides used. The α-helix C-terminal to the PRR in p22phox appears to participate in binding to p47-(SH3)2 via hydrophobic interactions with p47-SH3(N) [39]. The interactions are considered to be important because comparison of the p47phox-binding activity between p22-(132–160) and p22-(132–165) reveals that the addition of the α-helical region to the PRR increases the affinity for p47-(SH3)2 (Figure 6), which is in agreement with a previous observation that the p22phox peptide consisting of amino acids 151–165 binds to p47phox more strongly than the peptide of 149–163 [45]. Replacement of Ala163 and Arg164 with glycine, a residue that is expected to destabilize α-helical structures, results in an incomplete but significant loss of the ability to bind to p47phox (Figure 6) and to support superoxide production (Figure 7). These mutations, however, do not seem to affect the protein integrity of p22phox: these mutant proteins expressed ectopically exist in cells to the same extent as the wild-type one (Figure 7A), and are targeted to the plasma membrane to form a properly arranged complex with gp91phox (Figure 7B). Thus the α-helical structure of the region adjacent to the PRR of p22phox is required for full activation of the phagocyte NADPH oxidase.
In general, the interaction of an SH3 domain with its proline-rich target in a PPII helix conformation tends to be fairly weak, with typical KDs in the μM range [29–32], whereas affinity and specificity can be enhanced by additional contacts outside the proline-rich core forming the PPII helix, as reported in some SH3-mediated interactions [21,33–35]. By contrast, in the interaction between p47phox and p22phox, the high affinity and high specificity are largely conferred by co-operative binding of the two SH3 domains of p47phox to the single PRR of p22phox, which plays a crucial role in activation of the phagocyte NADPH oxidase, as shown in the present study (Figure 8). Similar co-operativity between the p47phox SH3 domains also participates in keeping them in an inactive state via an intramolecular interaction with AIR, which lies C-terminal to the tandem SH3 domains [19,38,47,48]. Besides the bis-SH3-mediated recognition with the proline-rich core, the interaction of p47phox with p22phox is further facilitated by a contact of SH3(N) with an α-helix, immediately adjacent to the PPII region; this additional contact contributes to full activation of the phagocyte oxidase (Figure 8). It is presently obscure how this highly specific, strong SH3-mediated interaction leads to triggering of electron transfer by gp91phox, a complete electron-transporting apparatus containing NADPH-, FAD-, and haem-binding sites [1–5]. Once the active oxidase complex is formed, gp91phox transfers electrons from NADPH to molecular oxygen, thereby generating superoxide [12,49–52]. The strong binding to p22phox may allow p47phox to stably interact with gp91phox, the partner of p22phox in flavocytochrome b558, which might facilitate electron flow. Alternatively, because p47phox associates with p67phox via a tail-to-tail interaction [21], p47phox in activated cells may serve solely as an adaptor molecule that forms a bridge between p67phox and p22phox, thereby stabilizing the attachment of p67phox to gp91phox, an event that is considered to induce electron transport [10,12,52]. Elucidation of the precise architecture of the membrane-assembled, active oxidase complex consisting of gp91phox, p22phox, p47phox, p67phox and Rac awaits further studies.
Acknowledgments
Supported in part by Grants-in-Aid for Scientific Research and National Project on Protein Structural and Functional Analyses from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by CREST (Core Research for Evolutional Science and Technology) of JST (Japan Science and Technology Agency) and BIRD (Institute for Bioinformatics Research and Development) of JST. We are grateful to Yohko Kage (Kyushu University and JST), Miki Nakashima (Kyushu University), Natsuko Yoshiura (Kyushu University) and Namiko Kubo (Kyushu University) for technical assistance, and to Minako Nishino (Kyushu University and JST) for secretarial assistance. We thank Professor Michio Nakamura, Nagasaki University, for the provision of the monoclonal antibody 7D5.
References
- 1.Nauseef W. M. Assembly of the phagocyte NADPH oxidase. Histochem. Cell. Biol. 2004;122:277–291. doi: 10.1007/s00418-004-0679-8. [DOI] [PubMed] [Google Scholar]
- 2.Quinn M. T., Gauss K. A. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J. Leukoc. Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- 3.Cross A. R., Segal A. W. The NADPH oxidase of professional phagocytes-prototype of the NOX electron transport chain systems. Biochim. Biophys. Acta. 2004;1657:1–22. doi: 10.1016/j.bbabio.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambeth J. D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 5.Sumimoto H., Miyano K., Takeya R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem. Biophys. Res. Commun. 2005;338:677–686. doi: 10.1016/j.bbrc.2005.08.210. [DOI] [PubMed] [Google Scholar]
- 6.Heyworth P. G., Curnutte J. T., Nauseef W. M., Volpp B. D., Pearson D. W., Rosen H., Clark R. A. Neutrophil nicotinamide adenine dinucleotide phosphate oxidase assembly: translocation of p47-phox and p67-phox requires interaction between p47-phox and cytochrome b558. J. Clin. Invest. 1991;87:352–356. doi: 10.1172/JCI114993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heyworth P. G., Bohl B. P., Bokoch G. M., Curnutte J. T. Rac translocates independently of the neutrophil NADPH oxidase components p47phox and p67phox: evidence for its interaction with flavocytochrome b558. J. Biol. Chem. 1994;269:30749–30752. [PubMed] [Google Scholar]
- 8.Kuribayashi F., Nunoi H., Wakamatsu K., Tsunawaki S., Sato K., Ito T., Sumimoto H. The adaptor protein p40phox as a positive regulator of the superoxide-producing phagocyte oxidase. EMBO J. 2002;21:6312–6320. doi: 10.1093/emboj/cdf642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koga H., Terasawa H., Nunoi H., Takeshige K., Inagaki F., Sumimoto H. Tetratricopeptide repeat (TPR) motifs of p67phox participate in interaction with the small GTPase Rac and activation of the phagocyte NADPH oxidase. J. Biol. Chem. 1999;274:25051–25060. doi: 10.1074/jbc.274.35.25051. [DOI] [PubMed] [Google Scholar]
- 10.Nisimoto Y., Motalebi S., Han C. H., Lambeth J. D. The p67phox activation domain regulates electron flow from NADPH to flavin in flavocytochrome b558. J. Biol. Chem. 1999;274:22999–23005. doi: 10.1074/jbc.274.33.22999. [DOI] [PubMed] [Google Scholar]
- 11.Lapouge K., Smith S. J., Walker P. A., Gamblin S. J., Smerdon S. J., Rittinger K. Structure of the TPR domain of p67phox in complex with Rac GTP. Mol. Cell. 2000;6:899–907. doi: 10.1016/s1097-2765(05)00091-2. [DOI] [PubMed] [Google Scholar]
- 12.Diebold B. A., Bokoch G. M. Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat. Immunol. 2001;2:211–215. doi: 10.1038/85259. [DOI] [PubMed] [Google Scholar]
- 13.Sarfstein R., Gorzalczany Y., Mizrahi A., Berdichevsky Y., Molshanski-Mor S., Weinbaum C., Hirshberg M., Dagher M. C., Pick E. Dual role of Rac in the assembly of NADPH oxidase, tethering to the membrane and activation of p67phox: a study based on mutagenesis of p67phox–Rac1 chimeras. J. Biol. Chem. 2004;279:16007–16016. doi: 10.1074/jbc.M312394200. [DOI] [PubMed] [Google Scholar]
- 14.Sumimoto H., Kage Y., Nunoi H., Sasaki H., Nose T., Fukumaki Y., Ohno M., Minakami S., Takeshige K. Role of Src homology 3 domains in assembly and activation of the phagocyte NADPH oxidase. Proc. Natl. Acad. Sci. U.S.A. 1994;91:5345–5349. doi: 10.1073/pnas.91.12.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leto T. L., Adams A. G., de Mendez I. Assembly of the phagocyte NADPH oxidase: binding of Src homology 3 domains to proline-rich targets. Proc. Natl. Acad. Sci. U.S.A. 1994;91:10650–10654. doi: 10.1073/pnas.91.22.10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leusen J. H., Bolscher B. G., Hilarius P. M., Weening R. S., Kaulfersch W., Seger R. A., Roos D., Verhoeven A. J. 156Pro→Gln substitution in the light chain of cytochrome b558 of the human NADPH oxidase (p22-phox) leads to defective translocation of the cytosolic proteins p47-phox and p67-phox. J. Exp. Med. 1994;180:2329–2334. doi: 10.1084/jem.180.6.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sumimoto H., Hata K., Mizuki K., Ito T., Kage Y., Sakaki Y., Fukumaki Y., Nakamura M., Takeshige K. Assembly and activation of the phagocyte NADPH oxidase: specific interaction of the N-terminal Src homology 3 domain of p47phox with p22phox is required for activation of the NADPH oxidase. J. Biol. Chem. 1996;271:22152–22158. doi: 10.1074/jbc.271.36.22152. [DOI] [PubMed] [Google Scholar]
- 18.Dinauer M. C., Pierce E. A., Erickson R. W., Muhlebach T. J., Messner H., Orkin S. H., Seger R. A., Curnutte J. T. Point mutation in the cytoplasmic domain of the neutrophil p22-phox cytochrome b subunit is associated with a nonfunctional NADPH oxidase and chronic granulomatous disease. Proc. Natl. Acad. Sci. U.S.A. 1991;88:11231–11235. doi: 10.1073/pnas.88.24.11231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ago T., Nunoi H., Ito T., Sumimoto H. Mechanism for phosphorylation-induced activation of the phagocyte NADPH oxidase protein p47phox: triple replacement of serines 303, 304, and 328 with aspartates disrupts the SH3 domain-mediated intramolecular interaction in p47phox, thereby activating the oxidase. J. Biol. Chem. 1999;274:33644–33653. doi: 10.1074/jbc.274.47.33644. [DOI] [PubMed] [Google Scholar]
- 20.Huang J., Kleinberg M. E. Activation of the phagocyte NADPH oxidase protein p47phox: phosphorylation controls SH3 domain-dependent binding to p22phox. J. Biol. Chem. 1999;274:19731–19737. doi: 10.1074/jbc.274.28.19731. [DOI] [PubMed] [Google Scholar]
- 21.Kami K., Takeya R., Sumimoto H., Kohda D. Diverse recognition of non-PxxP peptide ligands by the SH3 domains from p67phox, Grb2 and Pex13p. EMBO J. 2002;21:4268–4276. doi: 10.1093/emboj/cdf428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rotrosen D., Leto T. L. Phosphorylation of neutrophil 47-kDa cytosolic oxidase factor: translocation to membrane is associated with distinct phosphorylation events. J. Biol. Chem. 1990;265:19910–19915. [PubMed] [Google Scholar]
- 23.El Benna J., Faust L. P., Babior B. M. The phosphorylation of the respiratory burst oxidase component p47phox during neutrophil activation: phosphorylation of sites recognized by protein kinase C and by proline-directed kinases. J. Biol. Chem. 1994;269:23431–23436. [PubMed] [Google Scholar]
- 24.Inanami O., Johnson J. L., McAdara J. K., Benna J. E., Faust L. R., Newburger P. E., Babior B. M. Activation of the leukocyte NADPH oxidase by phorbol ester requires the phosphorylation of p47PHOX on serine 303 or 304. J. Biol. Chem. 1998;273:9539–9543. doi: 10.1074/jbc.273.16.9539. [DOI] [PubMed] [Google Scholar]
- 25.Johnson J. L., Park J. W., Benna J. E., Faust L. P., Inanami O., Babior B. M. Activation of p47PHOX, a cytosolic subunit of the leukocyte NADPH oxidase: phosphorylation of Ser-359 or Ser-370 precedes phosphorylation at other sites and is required for activity. J. Biol. Chem. 1998;273:35147–35152. doi: 10.1074/jbc.273.52.35147. [DOI] [PubMed] [Google Scholar]
- 26.Shiose A., Sumimoto H. Arachidonic acid and phosphorylation synergistically induce a conformational change of p47phox to activate the phagocyte NADPH oxidase. J. Biol. Chem. 2000;275:13793–13801. doi: 10.1074/jbc.275.18.13793. [DOI] [PubMed] [Google Scholar]
- 27.Ago T., Kuribayashi F., Hiroaki H., Takeya R., Ito T., Kohda D., Sumimoto H. Phosphorylation of p47phox directs phox homology domain from SH3 domain toward phosphoinositides, leading to phagocyte NADPH oxidase activation. Proc. Natl. Acad. Sci. U.S.A. 2003;100:4474–4479. doi: 10.1073/pnas.0735712100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pawson T., Nash P. Assembly of cell regulatory systems through protein interaction domains. Science. 2003;300:445–452. doi: 10.1126/science.1083653. [DOI] [PubMed] [Google Scholar]
- 29.Zarrinpar A., Bhattacharyya R. P., Lim W. A. The structure and function of proline recognition domains. Sci. STKE. 2003;2003:RE8. doi: 10.1126/stke.2003.179.re8. [DOI] [PubMed] [Google Scholar]
- 30.Musacchio A. How SH3 domains recognize proline. Adv. Protein Chem. 2002;61:211–268. doi: 10.1016/s0065-3233(02)61006-x. [DOI] [PubMed] [Google Scholar]
- 31.Mayer B. J. SH3 domains: complexity in moderation. J. Cell Sci. 2001;114:1253–1263. doi: 10.1242/jcs.114.7.1253. [DOI] [PubMed] [Google Scholar]
- 32.Kay B. K., Williamson M. P., Sudol M. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 2000;14:231–241. [PubMed] [Google Scholar]
- 33.Lee C. H., Saksela K., Mirza U. A., Chait B. T., Kuriyan J. Crystal structure of the conserved core of HIV-1 Nef complexed with a Src family SH3 domain. Cell. 1996;85:931–942. doi: 10.1016/s0092-8674(00)81276-3. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Z. S., Manser E., Lim L. Interaction between PAK and nck: a template for Nck targets and role of PAK autophosphorylation. Mol. Cell. Biol. 2000;20:3906–3917. doi: 10.1128/mcb.20.11.3906-3917.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghose R., Shekhtman A., Goger M. J., Ji H., Cowburn D. A novel, specific interaction involving the Csk SH3 domain and its natural ligand. Nat. Struct. Biol. 2001;8:998–1004. doi: 10.1038/nsb1101-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Mendez I., Homayounpour N., Leto T. L. Specificity of p47phox SH3 domain interactions in NADPH oxidase assembly and activation. Mol. Cell. Biol. 1997;17:2177–2185. doi: 10.1128/mcb.17.4.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hata K., Ito T., Takeshige K., Sumimoto H. Anionic amphiphile-independent activation of the phagocyte NADPH oxidase in a cell-free system by p47phox and p67phox, both in C terminally truncated forms: implication for regulatory Src homology 3 domain-mediated interactions. J. Biol. Chem. 1998;273:4232–4236. doi: 10.1074/jbc.273.7.4232. [DOI] [PubMed] [Google Scholar]
- 38.Groemping Y., Lapouge K., Smerdon S. J., Rittinger K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell. 2003;113:343–355. doi: 10.1016/s0092-8674(03)00314-3. [DOI] [PubMed] [Google Scholar]
- 39.Ogura K., Nobuhisa I., Yuzawa S., Takeya R., Torikai S., Saikawa K., Sumimoto H., Inagaki F. NMR solution structure of the tandem SH3 domains of p47phox complexed with a p22phox-derived proline-rich peptide. J. Biol. Chem. 2006;281:3660–3668. doi: 10.1074/jbc.M505193200. [DOI] [PubMed] [Google Scholar]
- 40.Sumimoto H., Sakamoto N., Nozaki M., Sakaki Y., Takeshige K., Minakami S. Cytochrome b558, a component of the phagocyte NADPH oxidase, is a flavoprotein. Biochem. Biophys. Res. Commun. 1992;186:1368–1375. doi: 10.1016/s0006-291x(05)81557-8. [DOI] [PubMed] [Google Scholar]
- 41.Takeya R., Ueno N., Kami K., Taura M., Kohjima M., Izaki T., Nunoi H., Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J. Biol. Chem. 2003;278:25234–25246. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- 42.Mizushima S., Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamauchi A., Yu L., Potgens A. J., Kuribayashi F., Nunoi H., Kanegasaki S., Roos D., Malech H. L., Dinauer M. C., Nakamura M. Location of the epitope for 7D5, a monoclonal antibody raised against human flavocytochrome b558, to the extracellular peptide portion of primate gp91phox. Microbiol. Immunol. 2001;45:249–257. doi: 10.1111/j.1348-0421.2001.tb02614.x. [DOI] [PubMed] [Google Scholar]
- 44.Burritt J. B., DeLeo F. R., McDonald C. L., Prigge J. R., Dinauer M. C., Nakamura M., Nauseef W. M., Jesaitis A. J. Phage display epitope mapping of human neutrophil flavocytochrome b558: identification of two juxtaposed extracellular domains. J. Biol. Chem. 2001;276:2053–2061. doi: 10.1074/jbc.M006236200. [DOI] [PubMed] [Google Scholar]
- 45.Dahan I., Issaeva I., Gorzalczany Y., Sigal N., Hirshberg M., Pick E. Mapping of functional domains in the p22phox subunit of flavocytochrome b559 participating in the assembly of the NADPH oxidase complex by “peptide walking”. J. Biol. Chem. 2002;277:8421–8432. doi: 10.1074/jbc.M109778200. [DOI] [PubMed] [Google Scholar]
- 46.Morozov I., Lotan O., Joseph G., Gorzalczany Y., Pick E. Mapping of functional domains in p47phox involved in the activation of NADPH oxidase by “peptide walking”. J. Biol. Chem. 1998;273:15435–15444. doi: 10.1074/jbc.273.25.15435. [DOI] [PubMed] [Google Scholar]
- 47.Yuzawa S., Suzuki N. N., Fujioka Y., Ogura K., Sumimoto H., Inagaki F. A molecular mechanism for autoinhibition of the tandem SH3 domains of p47phox, the regulatory subunit of the phagocyte NADPH oxidase. Genes Cells. 2004;9:443–456. doi: 10.1111/j.1356-9597.2004.00733.x. [DOI] [PubMed] [Google Scholar]
- 48.Yuzawa S., Ogura K., Horiuchi M., Suzuki N. N., Fujioka Y., Kataoka M., Sumimoto H., Inagaki F. Solution structure of the tandem Src homology 3 domains of p47phox in an autoinhibited form. J. Biol. Chem. 2004;279:29752–29760. doi: 10.1074/jbc.M401457200. [DOI] [PubMed] [Google Scholar]
- 49.Koshkin V., Lotan O., Pick E. Electron transfer in the superoxide-generating NADPH oxidase complex reconstituted in vitro. Biochim. Biophys. Acta. 1997;1319:139–146. doi: 10.1016/s0005-2728(96)00154-5. [DOI] [PubMed] [Google Scholar]
- 50.Cross A. R., Erickson R. W., Curnutte J. T. Simultaneous presence of p47phox and flavocytochrome b−245 are required for the activation of NADPH oxidase by anionic amphiphiles: evidence for an intermediate state of oxidase activation. J. Biol. Chem. 1999;274:15519–15525. doi: 10.1074/jbc.274.22.15519. [DOI] [PubMed] [Google Scholar]
- 51.Hashida S., Yuzawa S., Suzuki N. N., Fujioka Y., Takikawa T., Sumimoto H., Inagaki F., Fujii H. Binding of FAD to cytochrome b558 is facilitated during activation of the phagocyte NADPH oxidase, leading to superoxide production. J. Biol. Chem. 2004;279:26378–26386. doi: 10.1074/jbc.M309724200. [DOI] [PubMed] [Google Scholar]
- 52.Dang P. M., Cross A. R., Quinn M. T., Babior B. M. Assembly of the neutrophil respiratory burst oxidase: a direct interaction between p67phox and cytochrome b558 II. Proc. Natl. Acad. Sci. U.S.A. 2002;99:4262–4265. doi: 10.1073/pnas.072345299. [DOI] [PMC free article] [PubMed] [Google Scholar]