Abstract
pABA (p-aminobenzoate) is a precursor of folates and, besides esterification to glucose, has no other known metabolic fate in plants. It is synthesized in two steps from chorismate and glutamine, the first step being their conversion into glutamate and ADC (4-aminodeoxychorismate). In Escherichia coli, two proteins forming a heterodimeric complex are required for this reaction, but, in plants and lower eukaryotes, a single protein is involved. The Arabidopsis enzyme was expressed in E. coli and was purified to homogeneity. The monomeric enzyme (95 kDa) catalyses two reactions: release of NH3 from glutamine (glutaminase activity) and substitution of NH3 for the hydroxy group at position 4 of chorismate (ADC synthase activity). The kinetic parameters of the plant enzyme are broadly similar to those of the bacterial complex, with Km values for glutamine and chorismate of 600 and 1.5 μM respectively. As with the bacterial enzyme, externally added NH3 was a very poor substrate for the plant enzyme, suggesting that NH3 released from glutamine is preferentially channelled to chorismate. The glutaminase activity could operate alone, but the presence of chorismate increased the efficiency of the reaction 10-fold, showing the interdependency of the two domains. The plant enzyme was inhibited by dihydrofolate and its analogue methotrexate, a feature never reported for the prokaryotic system. These molecules were inhibitors of the glutaminase reaction, competitive with respect to glutamine (Ki values of 10 and 1 μM for dihydrofolate and methotrexate respectively). These findings support the view that the monomeric ADC synthase is a potential target for antifolate drugs.
Keywords: p-aminobenzoate, aminodeoxychorismate synthase, C1 metabolism, folate, methotrexate, plastid
Abbreviations: pABA, p-aminobenzoate; ADC, 4-amino-4-deoxychorismate; AtADCS, Arabidopsis thaliana ADC synthase; H2PteGlun, dihydrofolate with n glutamate residues; H4PteGlun, tetrahydrofolate with n glutamate residues; MTX, methotrexate; Ni-NTA, Ni2+-nitrilotriacetate
INTRODUCTION
Folates are a family of cofactors that are essential for all cellular one-carbon transfer reactions: they are required for the synthesis of purines, thymidylate, methionine and the interconversion of serine and glycine [1]. Deciphering folate metabolism in plants and understanding the biochemical properties of the corresponding enzymes is important for at least two major reasons. First, folate deficiency in humans is a worldwide health problem and, because plant foods are a major source of folate, it is important to understand how folate is synthesized in plants, and how the folate content in plants could be improved [1]. Secondly, several enzymes that are involved in folate biosynthesis are not present in animals and are therefore potential targets for new herbicides. Folate cofactors are made of three distinct parts: a pterin ring, a pABA (p-aminobenzoate) moiety and a glutamate residue, to which is usually attached a γ-linked polyglutamyl tail of up to approx. six residues [2]. In plants, folate synthesis presents a complex spatial organization, in which three subcellular compartments participate: the pterin part of the molecule is synthesized from GTP in the cytosol, pABA is synthesized from chorismate in plastids and the combination of pterin, pABA and glutamate is made within mitochondria [2–4]. Recently, two attempts to engineer the pterin branch of folate synthesis by overexpressing GTP cyclohydrolase I, the first enzyme of the pterin pathway, resulted in a massive accumulation of pterins, but in a modest 2–4-fold increase of the folate concentration because the amount of pABA was limiting [5,6].
pABA is synthesized from chorismate, a compound also needed to synthesize important aromatic products, including phenylalanine, tyrosine and tryptophan, and their derivatives [7–9]. The first step in the synthesis of pABA is the amination of chorismate to form ADC (4-amino-4-deoxychorismate). In this reaction (reaction 1), glutamine is the source of NH3 [10,11]. In the second step (reaction 2), ADC is aromatized with loss of pyruvate to form pABA [12,13].
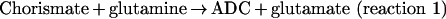 |
(reaction 1) |
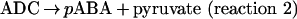 |
(reaction 2) |
In bacteria such as Escherichia coli, this biosynthesis requires three separate proteins: PabA, the amidotransferase, PabB, the ADC synthase, and PabC, the ADC lyase. Thus the synthesis of ADC (reaction 1) in bacteria requires the co-operation of two proteins, PabA and PabB, which form a heterodimeric complex [14]. This complex catalyses a reaction with an ordered Bi Bi mechanism in which chorismate binds first [10]. Whereas PabA alone has no glutaminase activity [14], PabB alone can convert chorismate into ADC in the presence of NH3 [10,12]. Until now, there has been no evidence of feedback inhibition of ADC synthesis by pABA, pABA analogues or end products of folate metabolism [10]. The crystal structure of PabB [15] indicates strong similarities with the TrpE subunit of anthranilate synthase, another chorismate-utilizing enzyme that is allosterically regulated by tryptophan [16]. Although PabB activity is not regulated by tryptophan, a tryptophan molecule was found deeply embedded in the structure, strengthening the idea that PabB and TrpE are evolutionarily related [15]. Recently, PabB was identified as an interesting target for antimicrobial action: indeed, (6S)-6-fluoroshikimate is a potent antimicrobial agent owing to its conversion into 2-fluorochorismate, a compound that strongly inhibits PabB [17].
In plants and lower eukaryotes, such as fungi and Plasmodium falciparum, fewer data are available, but the situation appears different. Indeed, genomic data show that these organisms have a bipartite protein with domains similar to PabA and PabB and respectively positioned at the N- and C-terminal part of the enzyme [18–21]. In addition, the plant protein is targeted to plastids [18]. The ADC lyase activity in plants is supported by a second enzyme, similar to PabC, also targeted to plastids [22]. Thus plastids are the unique site of pABA synthesis in plants.
In the present study, we overexpressed AtADCS (Arabidopsis thaliana ADC synthase) and we purified the enzyme to homogeneity. The main kinetic parameters of the recombinant protein were determined and were compared with those reported for the bacterial enzyme. Surprisingly, we observed that the plant enzyme was inhibited by H2PteGlun (dihydrofolate with n glutamate residues) and MTX (methotrexate), a feature that has never been reported for other ADC synthases. Thus the monomeric ADC synthase appeared as a potential target for antifolate drugs.
EXPERIMENTAL
Chemicals
Folic acid (pteroylmono-L-glutamic acid) and MTX were obtained from Sigma. Pteroylpenta-γ-L-glutamic acid was obtained from Schircks Laboratories. H2PteGlu1 and H2PteGlu5 were synthesized by reduction of pteroylmono- and penta-γ-L-glutamic acid and were purified as described by Scrimgeour [23]. Stock solutions of H2PteGlun were quantified by their typical absorption spectra [24], flushed with argon, and stored at −80 °C in the presence of 100 mM 2-mercaptoethanol. In experiments requiring H2PteGlun, all of the solutions were maintained under a stream of argon to minimize oxidation. Using this experimental procedure, there was no detectable change in the absorption spectrum of H2PteGlun during the course of the experiment.
Expression of the recombinant AtADCS in E. coli
Arabidopsis cDNA encoding AtADCS starting at Val85, without the predicted chloroplast targeting sequence, was amplified by PCR from the pET-28a plasmid described previously [18], using the following pair of primers: 5′-GGGCTAGCGTGAGGACTTTGTTGATTGAT-3′ (forward) and 5′-CCCTCGAGCTATTGTCTCCTCTGATCAC-3′ (reverse). The PCR product was ligated into the expression vector pET28b (Novagen) between the NheI and XhoI restriction sites. Using this cloning strategy, two His6-tag sequences carried by the vector were added in-frame to the 5′ and 3′ ends of the construct. Transformation of E. coli BL21-CodonPlus (DE3)-RIL cells (Stratagene) was performed according to the supplier's protocol. The cells were grown in M9 minimal medium, containing 1 mM MgSO4, 0.1 mM CaCl2, 0.2% (w/v) glucose and 50 μg/ml kanamycin, at 16 °C. Protein production was induced by adding 0.5 mM IPTG (isopropyl β-D-thiogalactoside) at an A600 of 0.1, and cells were harvested at stationary phase by centrifugation at 4000 g for 30 min at 4 °C.
Purification of the recombinant AtADCS
Cells harvested from 1 litre of culture were resuspended in 2 ml 0.1 M Tris/HCl (pH 8.0), 0.3 M NaCl, 5 mM MgCl2, 5 mM 2-mercaptoethanol, 1 mM L-glutamine, 10% (v/v) glycerol and Complete™ protease inhibitor cocktail (Roche Applied Science) at the concentration recommended by the manufacturer. Cells were disrupted by sonication and centrifuged at 15000 g for 30 min at 4 °C, and the supernatant was applied to an Ni-NTA (Ni2+-nitrilotriacetate)-affinity column (Amersham) equilibrated with buffer A [0.1 M Tris/HCl (pH 8.0), 1 mM L-glutamine, 0.3 M NaCl and 10% (v/v) glycerol]. The column was washed with the same buffer containing 5 mM imidazole, then the enzyme was eluted with 15 mM imidazole in buffer A. Fractions containing the highest activity were combined and concentrated by centrifugation (50 kDa cut-off; Microsep, Pall Filtron) to a final concentration of 2–3 mg of protein/ml. Proteins were quantified following the method of Bradford [25] using BSA as standard.
Samples collected from the Ni-NTA purification step were desalted on PD-10 columns (Amersham Biosciences) equilibrated with buffer B (buffer A without L-glutamine) and loaded on a MTX–agarose (Sigma) column equilibrated with the same buffer. After washing with 2 column vol. of buffer B, the enzyme was eluted with 2 column vol. of the same buffer containing 10 mM L-glutamine. Fractions containing the purified AtADCS were dialysed against buffer A (the presence of 1 mM glutamine increases the stability of the enzyme), concentrated and stored at −80 °C.
The quality of the purification was determined after SDS/PAGE (11% gels) analysis and staining with Coomassie Brilliant Blue R-250. Samples were analysed under non-denaturing conditions using Blue native PAGE (11% gels) analysis [26]. Size-exclusion chromatography was performed using a FPLC system (Äkta purifier; Amersham Biosciences) and a TSK-Gel Super SW3000 column (Tosoh Biosciences) equilibrated with buffer A without glycerol. Proteins were eluted with the same buffer, at a flow rate of 0.3 ml/min. The column was calibrated using a gel-filtration calibration kit from Amersham Biosciences.
Determination of ADC synthase activity
Standard assays (final volume 100 μl) contained 100 mM Tris/HCl (pH 8.0), 5 mM MgCl2, 0.01–5 mM L-glutamine, 0–50 μM chorismate as free acid or barium salt (Sigma) and 1.5–2 μg (150–200 nM) of the recombinant enzyme. In experiments where pABA and pyruvate were determined, a desalted extract from a pabA−pabB− E. coli strain (BN1163; pabA1, pabB::Kan, rpsL704, ilvG-, rfb-50, rph-1) containing PabC activity was added to the mixture [18]. In NH3-dependent assays, the above reaction medium was replaced by 40 mM triethanolamine (pH 8.0), 5 mM MgCl2, 50 μM chorismate and various concentrations of (NH4)2SO4. Assays were run for 20–30 min at 37 °C.
Measurements of pABA were as described previously [18], using a reverse-phase HPLC system. The reaction was stopped with 20 μl of 75% (v/v) ethanoic (acetic) acid. After incubation for 1 h on ice, the samples were centrifuged at 15000 g for 30 min at 4 °C. Samples were injected on the C18 reverse-phase column, and the peak corresponding to pABA was detected by its fluorescence (290 nm excitation/340 nm emission) and was quantified relative to standards.
Pyruvate and glutamate concentrations were estimated using lactate and glutamate dehydrogenase-coupled assays respectively as described in [27,28]. In both cases, the samples were first incubated for 10–15 min at 37 °C, and the reaction was stopped by boiling for 1 min. After incubation on ice for 20 min, the amount of L-glutamate or pyruvate produced during the reaction was estimated from the change in absorbance at 340 nm due to NADH [27,28].
Analyses of the kinetic data were made using EasyPlot software (Spiral Software).
RESULTS AND DISCUSSION
Purification of AtADCS
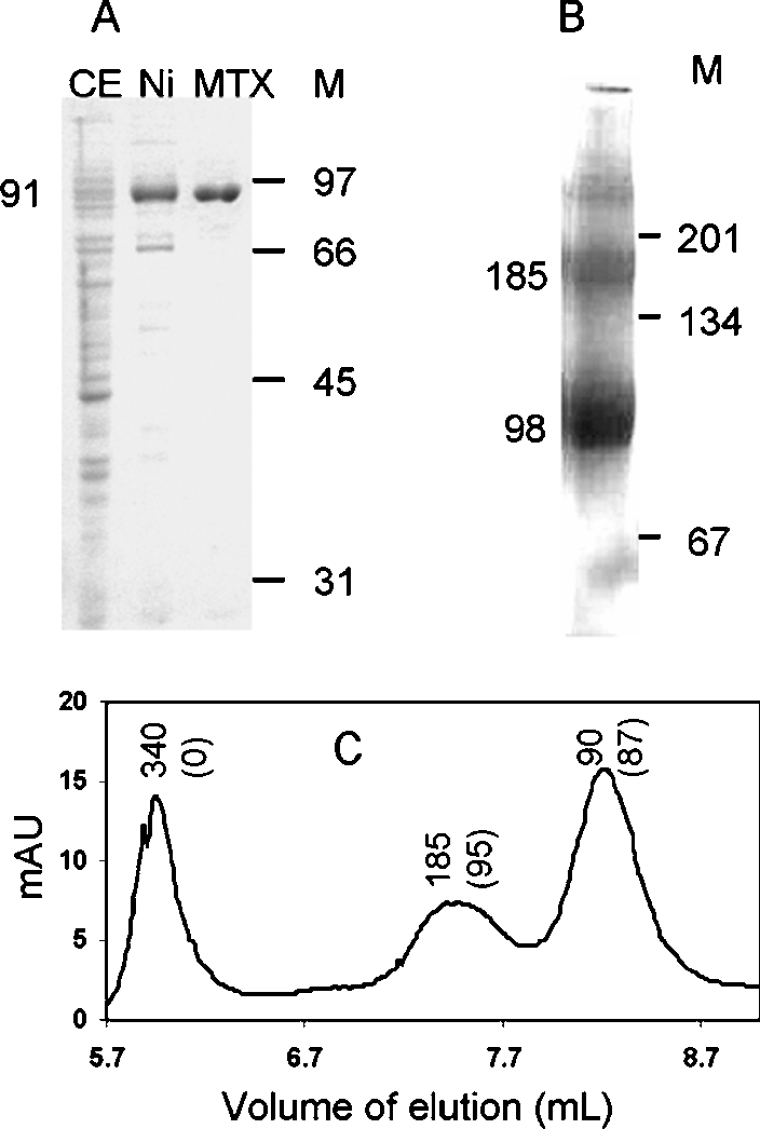
In a previous study, we have shown that the recombinant plant enzyme expressed in E. coli cells was able to rescue E. coli and yeast mutants deficient in the synthesis of pABA [18]. However, the recombinant AtADCS was weakly expressed and could not be purified. To purify the enzyme, we made a new construct adding two His6-tags at the C- and N-terminal parts of the protein. In separate control experiments, we used soluble extracts from induced cells and verified that the tagged and untagged recombinant proteins had similar Km values for glutamine and chorismate, suggesting that these additional sequences did not affect the catalytic properties of the enzyme. As shown in Figure 1(A), the recombinant AtADC was purified from E. coli in two steps, with a final yield of approx. 0.3 mg/l of culture. First, the His6-tagged recombinant enzyme was retained on an Ni-NTA column to remove most of the bacterial proteins. Then the enzyme was bound to an MTX-affinity column to eliminate residual contaminants. This purification step exploits the specific interaction between MTX and AtADCS (see below). The apparent molecular mass estimated from SDS/PAGE was approx. 91 kDa, which is close to the 94 kDa predicted from the deduced amino acid sequence. Native gel electrophoresis (Figure 1B) indicated a major band with a molecular mass of approx. 98 kDa, confirming the previous suggestion that, under given conditions, the predominant form of plant ADC synthase is a monomer [18]. However, we consistently observed on native gels and by gel filtration (Figure 1C) minor forms at approx. 180 kDa and even higher molecular masses, suggesting that the recombinant protein aggregates to form dimers or more complex structures. Separation of the different forms by gel-filtration and measurement of activity indicated that dimers and monomers had similar specific activities, but aggregates with higher molecular masses were inactive (Figure 1C).
Figure 1. PAGE analysis and gel-filtration of recombinant AtADCS.
(A) SDS/PAGE analysis of the recombinant enzyme during the course of its purification. CE, crude extract; Ni, proteins eluted from the Ni-NTA-affinity column; MTX, proteins eluted from the MTX-affinity column; M, molecular-mass markers (sizes indicated in kDa). (B) Blue native PAGE analysis of the recombinant enzyme eluted from the MTX-affinity column. (C) Size-exclusion chromatography of the purified recombinant protein eluted from the MTX column. Numbers above the peaks indicate the estimated molecular mass (kDa) corresponding to the peak fractions, and numbers into brackets are the specific activities (nmol·min−1·mg−1 of protein) present in these fractions. mAU, milli-absorbance units.
AtADCS activity
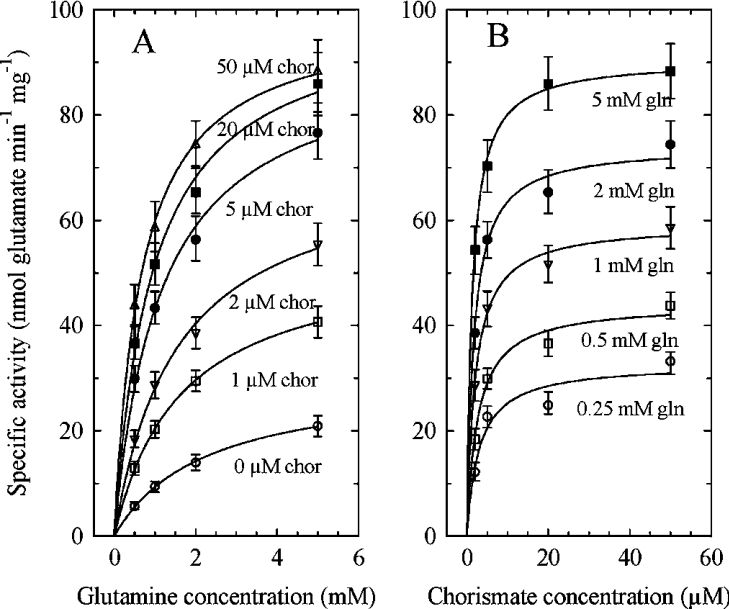
Preliminary experiments verified that the activity of the recombinant enzyme is linear with time (for at least 20 min) and proportional to the amount of protein. The synthesis of ADC requires an amidotransferase activity, producing glutamate from glutamine, and an amination activity, producing ADC from chorismate and NH3. When ADC synthase is coupled with an excess of ADC lyase, ADC produced by the former enzyme is converted into pyruvate and pABA by the latter. Thus ADC synthase activity can be estimated by detecting the formation of glutamate or, when coupled with ADC lyase, by detecting the pyruvate or pABA that accumulates in the medium. The amounts of pABA, pyruvate and glutamate produced (82±7, 75±5 and 95±5 nmol/mg of protein respectively; means±S.E.M.; n=4) measured in the presence of saturating levels of glutamine (5 mM) and chorismate (50 μM), either in association with an excess of ADC lyase activity (pABA and pyruvate) or alone (glutamate), were rather similar, as expected. The slightly higher glutamate production suggests, however, that some glutaminase activity could operate independently of the ADC synthase activity. Indeed, as shown in Figure 2(A) and Table 1, some glutamate production was detected even in the absence of chorismate. Similar results were obtained with the E. coli PabA–PabB complex [14]. Increasing the chorismate concentration resulted in a higher Vmax and a lower apparent Km for glutamine, leading at saturating chorismate concentration to a 12-fold increase of the catalytic efficiency (Table 1) and suggesting that this substrate modified the catalytic properties of the glutaminase domain. Also, the apparent Km for chorismate decreased with increasing glutamine concentration (Figure 2B and Table 1). As shown in Table 1, minimal apparent Km values for glutamine and chorismate were 600 and 1.3 μM respectively, values similar to those reported for the E. coli PabA–PabB complex [14,29]. The family of curves presented in Figure 2 is typical of a Bi Bi sequential mechanism. However, the kinetics of inhibition by products is required to determine the type of mechanism, ordered or random. Unfortunately, glutamate had no inhibitory effect at concentrations up to 10 mM and ADC is not commercially available. It was proposed that the E. coli heterodimeric complex catalyses an ordered Bi Bi mechanism in which chorismate binds first, but more experiments are required with the plant enzyme to substantiate this conclusion.
Figure 2. Effect of varying the substrate concentrations on recombinant AtADCS activity.
(A) Effects of varying glutamine at different chorismate (chor) concentrations. (B) Effects of varying chorismate at different glutamine (gln) concentrations. Each point is the mean±S.E.M. for two to four determinations. The curves were fitted according to Michaelis–Menten equation using non-linear regression and EasyPlot software.
Table 1. Steady-state kinetic parameters for AtADCS.
The rates of the reaction are expressed as nmol of glutamate produced, except for NH3 where the rates are expressed as nmol of pABA produced. Results are means±S.E.M. for triplicate determinations.
| Substrate | Vmax (nmol·min−1·mg−1 of protein) | Km (μM) | kcat (s−1) | kcat/Km (M−1·s−1) |
|---|---|---|---|---|
| Glutamine (no chorismate) | 30±10 | 2200±500 | 0.045 | 20 |
| Glutamine (+50 μM chorismate) | 90±10 | 600±100 | 0.15 | 250 |
| Chorismate (+0.25 mM glutamine) | 32±10 | 2.8±0.3 | 0.045 | 0.16×105 |
| Chorismate (+5 mM glutamine) | 90±10 | 1.3±0.2 | 0.15 | 1×105 |
| NH3 (no glutamine; +50 μM chorismate) | 65±7 | (600±100)×103 | 0.11 | 0.18 |
In the absence of chorismate, NH3 released from glutamine must accumulate. In such a situation, we observed that NH3 was a weak inhibitor of the glutaminase reaction, with a Ki value of approx. 20 mM. In the presence of chorismate and in the absence of glutamine, the E. coli PabB or PabA–PabB complex can use NH3 added to the external medium for chorismate amination [10]. This is also true for the plant enzyme, with a Km value that was nearly twice as high as that reported for the bacterial system (Table 1). Thus, in the presence of a saturating concentration of chorismate, the Km for NH3 of the plant enzyme was 1000-fold higher than the Km for glutamine. This strongly suggests that NH3 released from glutamine during the amidotransferase reaction does not equilibrate with the bulk medium but rather is channelled between the binding sites of glutamine and chorismate. Structural analysis of E. coli PabB supports this hypothesis [15].
Inhibition of AtADCS
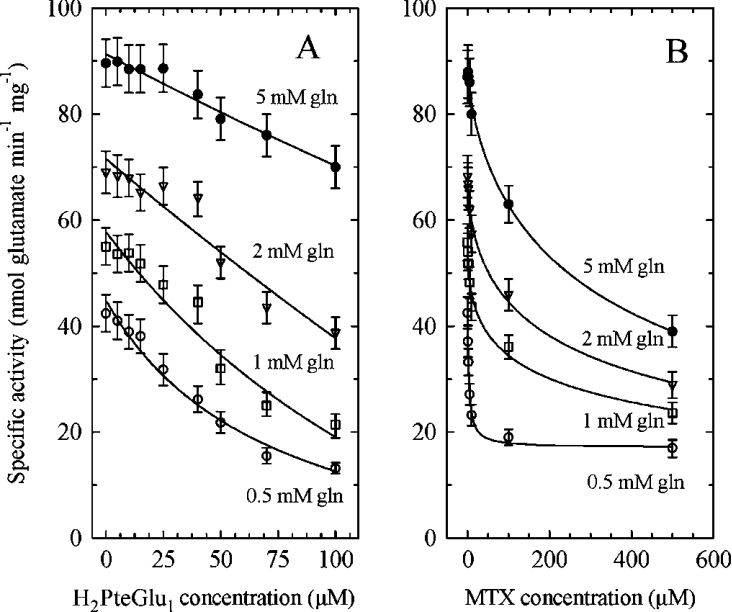
Chorismate stands at an important metabolic crossroads, leading also in plants to the synthesis of tryptophan (via anthranilate) and phenylalanine, tyrosine, tocopherols and quinones (via prephenate). All of these pathways are situated in plastids, so that the various chorismate-utilizing enzymes compete for the same substrate pool and it is likely that they are regulated. This is the case for the anthranilate synthase that catalyses the first committed step in tryptophan synthesis and that is feedback-inhibited by the end product of the pathway [30]. This is also true for the chorismate mutase that initiates the pathway leading to phenylalanine and tyrosine and that is inhibited by both amino acids [31]. However, no physiological inhibitors have been found for the E. coli ADC synthase. We tested several compounds for their inhibitory effects on the purified plant enzyme (Table 2). Neither aromatic amino acids (up to 2 mM) nor pABA and H4PteGlu1 (tetrahydrofolate) derivatives (up to 500 μM) had any effect. However, folic acid was a weak inhibitor of the enzyme and dihydro-compounds such as dihydropterin, dihydropteroate and H2PteGlun were quite effective (Table 2). H2PteGlu5 showed the strongest effect (Ki≈8 μM) and varying the number of glutamates did not markedly change the Ki value (Ki≈10 μM for the monoglutamate form). MTX, a structural analogue of H2PteGlu1, is also a potent inhibitor of AtADCS (Ki≈1 μM) (Table 2), explaining why AtADCS was retained by an MTX-affinity column. MTX had similar, but more pronounced, effects than H2PteGlu1 when varied against glutamine (Figure 3). Interestingly, even in the presence of saturating concentrations of MTX, the enzyme retained some activity, reaching a minimal plateau value (Figure 3B). This type of kinetics suggests a partial inhibition mechanism [32] where inhibitors (MTX or H2PteGlu1) and substrate (glutamine) bind at different sites, forming a ternary enzyme–substrate–inhibitor (ESI) complex that can yield product. The reciprocal plots shown in Figure 4 suggest that this partial inhibition is competitive with respect to glutamine [32]. When inhibitors were varied against chorismate, much higher concentrations were required to obtain significant inhibition, and the apparent Km values for chorismate were not significantly changed (results not shown). Taken as a whole, these data suggest a rather complex inhibition mechanism where H2PteGlun and MTX affect preferentially the glutaminase domain. This was not observed with the E. coli enzyme (results not shown), suggesting structural differences between the two systems. Alignment of the plant glutaminase domain with PabA from E. coli indicates 33% identity between the two proteins [18]. The most obvious differences are the presence in the plant enzyme of an insertion of approx. 45 residues, basic in character, near the end of the domain, followed by a rather long interdomain linker of approx. 95 residues. These differences in primary structure are, however, not conclusive, and crystallographic data are required to understand where inhibitors are binding and how they could affect the catalytic activity. Unfortunately, the crystal structures of AtADCS and PabA are not yet available.
Table 2. Effect of various metabolites on AtADCS activity.
The Ki values were calculated from the equations developed by Segel [32] for a partial competitive inhibition. Results are means±S.E.M. for triplicate determinations.
| Metabolite | Ki (μM) |
|---|---|
| Aromatic amino acids (phenylalanine, tyrosine, tryptophan) | No significant effect |
| pABA | No significant effect |
| Tetrahydrofolate derivatives (H4PteGlu1, 5-CH3-H4PteGlu1, 5-CHO-H4PteGlu1) | No significant effect |
| Folic acid (monoglutamate) | 3000±500 |
| Dihydropterin | 30±10 |
| Dihydropteroate | 15±5 |
| H2PteGlu1 | 10±3 |
| H2PteGlu5 | 8±3 |
| MTX | 1±0.3 |
Figure 3. Inhibition of AtADCS activity by H2PteGlu1 or MTX at various glutamine concentrations.
Effects of H2PteGlu1 (A) and MTX (B) on recombinant ADC synthase activity at different glutamine (gln) concentrations in the presence of 50 μM chorismate. Results are means±S.E.M. for at least three different determinations. Data were fitted using a decreasing hyperbolic function and non-linear regression with EasyPlot software.
Figure 4. Reciprocal plots for AtADCS activity at various glutamine concentrations.
The curves are replots of values shown in Figure 3. (A) Competitive inhibition of H2PteGlu1 against glutamine. The concentrations of H2PteGlu1 were: ○, 0 μM; □, 10 μM; ▽, 25 μM; ●, 50 μM; △, 100 μM. (B) Competitive inhibition of MTX against glutamine. The concentrations of MTX were: ○, 0 μM; □, 1 μM; ▽, 2 μM; ●, 5 μM; ■, 10 μM; △, 100 μM. Data were fitted using linear regression and EasyPlot software. For all of the curves, the least-square fitting values were ≥0.98. The Vmax values determined from these data ranged from 96 to 106 nmol·min−1·mg−1 when varying H2PteGlu1 concentration and from 90 to 111 nmol·min−1·mg−1 when varying MTX concentration.
That ADC synthase in plants is apparently not inhibited by the end product of the pathway (tetrahydrofolates) but by H2PteGlun raises the question of the physiological significance of this effect. H2PteGlu1 is produced in mitochondria during the course of H4PteGlu1 synthesis, whereas H2PteGlun is produced in mitochondria, plastids and, presumably, cytosol during thymidylate synthesis (in Arabidopsis, there are three isoforms of dihydrofolate reductase/thymidylate synthase: one is present in mitochondria, one in plastids and the location of the third one is not known; for a review, see [2]). Furthermore, H2PteGlun could be produced throughout the cell by spontaneous oxidation of H4PteGlun [33]. H2PteGlun is thereafter reduced back to H4PteGlun by dihydrofolate reductase. Thus there exists a cycle between H2PteGlun and H4PteGlun that is closely connected to DNA (thymidylate) synthesis. Although we can only speculate at this stage, it seems possible that H2PteGlun serves as a sensor of the folate pool, at least the pool connected to thymidylate synthesis. Nothing is known in plants about the concentration of H2PteGlun in subcellular compartments; nor is it clear whether H2PteGlun molecules are transported from one compartment to another.
Finally, our results indicate that the plant ADC synthase is a potential target for antifolate drugs such as MTX. MTX is a well-known and potent inhibitor of dihydrofolate reductase and, to our knowledge, no other enzyme was reported to be sensitive to this chemical, at least in plants. It would be interesting to know whether other monomeric ADC synthases, such as those present in the unicellular protozoan parasites that belong to the apicomplexan phylum are also potential targets for such inhibitors. Indeed, these organisms, responsible for serious human diseases, are related to the plant kingdom [34].
Acknowledgments
We thank Bernadette Gambonnet for technical assistance, Professor Roland Douce and Dr Gilles Curien for critical reading of the manuscript, and Dr Renaud Dumas and Dr Michel Matringe for helpful discussions. This work and the salary of one of us (T. S.) were supported by a grant from the European Community: European network on vitamins and cofactors synthesis, number HPRN-CT-2002-00244.
References
- 1.Scott J., Rébeillé F., Fletcher J. Folic acid and folates: the feasibility for nutritional enhancement in plant foods. J. Sci. Food Agric. 2000;80:795–824. [Google Scholar]
- 2.Ravanel S., Douce R., Rébeillé F. The uniqueness of tetrahydrofolate synthesis and one-carbon metabolism in plants. In: Day D. A., Millar H., Whelan J., editors. Plant Mitochondria from Genome to Function. Amsterdam: Kluwer Academic; 2004. pp. 277–292. [Google Scholar]
- 3.Douce R., Bourguignon J., Neuburger M., Rébeillé F. The glycine decarboxylase system: a fascinating complex. Trends Plant Sci. 2001;6:167–176. doi: 10.1016/s1360-1385(01)01892-1. [DOI] [PubMed] [Google Scholar]
- 4.Basset G. J. C., Quinlivan E. P., Gregory J. F., Hanson A. D. Folate synthesis and metabolism in plants and prospects for biofortification. Crop Sci. 2005;45:449–453. [Google Scholar]
- 5.Diaz de la Garza R., Quinlivan E. P., Klaus S. M., Basset G. J., Gregory J. F., 3rd, Hanson A. D. Folate biofortification in tomatoes by engineering the pteridine branch of folate synthesis. Proc. Natl. Acad. Sci. U.S.A. 2004;101:13720–13725. doi: 10.1073/pnas.0404208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hossain T., Rosenberg I., Selhub J., Kishore G., Beachy R., Schubert K. Enhancement of folates in plants through metabolic engineering. Proc. Natl. Acad. Sci. U.S.A. 2004;101:5158–5163. doi: 10.1073/pnas.0401342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siehl D. The biosynthesis of tryptophan, tyrosine and phenylalanine from chorismate. In: Singh B. K., editor. Plant Amino Acids. New York: Marcel Dekker Inc.; 1999. pp. 171–204. [Google Scholar]
- 8.He Z., Stigers Lavoie K. D., Bartlett P. A., Toney M. D. Conservation of mechanism in three chorismate-utilizing enzymes. J. Am. Chem. Soc. 2004;126:2378–2385. doi: 10.1021/ja0389927. [DOI] [PubMed] [Google Scholar]
- 9.Nichols B. P., Seibold A. M., Doktor S. Z. para-aminobenzoate synthesis from chorismate occurs in two steps. J. Biol. Chem. 1989;264:8597–8601. [PubMed] [Google Scholar]
- 10.Viswanathan V. K., Green J. M., Nichols B. P. Kinetic characterization of 4-amino 4-deoxychorismate synthase from Escherichia coli. J. Bacteriol. 1995;177:5918–5923. doi: 10.1128/jb.177.20.5918-5923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roux B., Walsh C. T. p-Aminobenzoate synthesis in Escherichia coli: mutational analysis of three conserved amino acid residues of the amidotransferase PabA. Biochemistry. 1993;32:3763–3768. doi: 10.1021/bi00065a031. [DOI] [PubMed] [Google Scholar]
- 12.Ye Q. Z., Liu J., Walsh C. T. p-Aminobenzoate synthesis in Escherichia coli: purification and characterization of PabB as aminodeoxychorismate synthase and enzyme X as aminodeoxychorismate lyase. Proc. Natl. Acad. Sci. U.S.A. 1990;87:9391–9395. doi: 10.1073/pnas.87.23.9391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green J. M., Nichols B. P. p-Aminobenzoate biosynthesis in Escherichia coli: purification of aminodeoxychorismate lyase and cloning of pabC. J. Biol. Chem. 1991;266:12971–12975. [PubMed] [Google Scholar]
- 14.Roux B., Walsh C. T. p-aminobenzoate synthesis in Escherichia coli: kinetic and mechanistic characterization of the amidotransferase PabA. Biochemistry. 1992;31:6904–6910. doi: 10.1021/bi00145a006. [DOI] [PubMed] [Google Scholar]
- 15.Parsons J. F., Jensen P. Y., Pachikara A. S., Howard A. J., Eisenstein E., Ladner J. E. Structure of Escherichia coli aminodeoxychorismate synthase: architectural conservation and diversity in chorismate-utilizing enzymes. Biochemistry. 2002;41:2198–2208. doi: 10.1021/bi015791b. [DOI] [PubMed] [Google Scholar]
- 16.Morollo A. A., Eck M. J. Structure of the cooperative allosteric anthranilate synthase from Salmonella typhimurium. Nat. Struct. Biol. 2001;8:243–247. doi: 10.1038/84988. [DOI] [PubMed] [Google Scholar]
- 17.Bulloch E. M., Jones M. A., Parker E. J., Osborne A. P., Stephens E., Davies G. M., Coggins J. R., Abell C. Identification of 4-amino-4-deoxychorismate synthase as the molecular target for the antimicrobial action of (6s)-6-fluoroshikimate. J. Am. Chem. Soc. 2004;126:9912–9913. doi: 10.1021/ja048312f. [DOI] [PubMed] [Google Scholar]
- 18.Basset G. J., Quinlivan E. P., Ravanel S., Rébeillé F., Nichols B. P., Shinozaki K., Seki M., Adams-Phillips L. C., Giovannoni J. J., Gregory J. F., 3rd, Hanson A. D. Folate synthesis in plants: the p-aminobenzoate branch is initiated by a bifunctional PabA-PabB protein that is targeted to plastids. Proc. Natl. Acad. Sci. U.S.A. 2004;101:1496–1501. doi: 10.1073/pnas.0308331100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Criado L. M., Martin J. F., Gil J. A. The pab gene of Streptomyces griseus, encoding p-aminobenzoic acid synthase, is located between genes possibly involved in candicidin biosynthesis. Gene. 1993;126:135–139. doi: 10.1016/0378-1119(93)90602-y. [DOI] [PubMed] [Google Scholar]
- 20.Edman J. C., Goldstein A. L., Erbe J. G. Para-aminobenzoate synthase gene of Saccharomyces cerevisiae encodes a bifunctional enzyme. Yeast. 1993;9:669–675. doi: 10.1002/yea.320090613. [DOI] [PubMed] [Google Scholar]
- 21.Triglia T., Cowman A. F. Plasmodium falciparum: a homologue of p-aminobenzoic acid synthetase. Exp. Parasitol. 1999;92:154–158. doi: 10.1006/expr.1999.4400. [DOI] [PubMed] [Google Scholar]
- 22.Basset G. J. C., Ravanel S., Quinlivan E. P., White R., Giovannoni J. J., Rébeillé F., Nichols B. P., Shinozaki K., Seki M., Gregory J. F., 3rd, Hanson A. D. Folate synthesis in plants: the last step of the p-aminobenzoate branch is catalyzed by a plastidial aminodeoxychorismate lyase. Plant J. 2004;40:453–461. doi: 10.1111/j.1365-313X.2004.02231.x. [DOI] [PubMed] [Google Scholar]
- 23.Scrimgeour K. G. Methods for reduction, stabilization, and analyses of folates. Methods Enzymol. 1980;66:517–523. doi: 10.1016/0076-6879(80)66496-9. [DOI] [PubMed] [Google Scholar]
- 24.Temple C., Jr, Montgomery J. A. Chemical and physical properties of folic acid and reduced derivatives. In: Blakley R. L., Benkovic S. J., editors. Folates and Pterins, vol. 1. New York: Wiley (Interscience); 1984. pp. 61–120. [Google Scholar]
- 25.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 26.Schagger H., von Jagow G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal. Biochem. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- 27.Bernt E., Bergmeyer H. U. L-glutamate: UV-assay with glutamate dehydrogenase and NAD. In: Bergmeyer H. U., editor. Methods of Enzymatic Analysis. New York: Academic Press; 1974. pp. 1704–1708. [Google Scholar]
- 28.Czok R., Lamprecht W. Pyruvate. Fluorimetric assay. In: Bergmeyer H., editor. Methods of Enzymatic Analysis. New York: Academic Press; 1974. pp. 1446–1451. [Google Scholar]
- 29.Kozlowski M. C., Tom N. J., Seto C. T., Sefler A. M., Bartlett P. A. Chorismate-utilizing enzymes isochorismate synthase, anthranilate synthase, and p-aminobenzoate synthase: mechanistic insight through inhibitor design. J. Am. Chem. Soc. 1995;117:2128–2140. [Google Scholar]
- 30.Romero R. M., Roberts M. F., Phillipson J. D. Anthranilate synthase in microorganisms and plants. Phytochemistry. 1995;39:263–276. doi: 10.1016/0031-9422(95)00010-5. [DOI] [PubMed] [Google Scholar]
- 31.Eberhard J., Ehrler T. T., Epple P., Felix G., Raesecke H. R., Amrhein N., Schmid J. Cytosolic and plastidic chorismate mutase isozymes from Arabidopsis thaliana: molecular characterization and enzymatic properties. Plant J. 1996;10:815–821. doi: 10.1046/j.1365-313x.1996.10050815.x. [DOI] [PubMed] [Google Scholar]
- 32.Segel I. H. New York: John Wiley and Sons; 1975. Enzyme Kinetics. [Google Scholar]
- 33.Rébeillé F., Douce R. Folate synthesis and compartmentation in higher plants. In: Kruger N. J., Hill S. A., Ratcliffe R. G., editors. Regulation of Primary Metabolic Pathways in Plants. Amsterdam: Kluwer Academic Publishers; 1999. [Google Scholar]
- 34.Archibald J. M., Keeling P. J. Recycled plastids: a ‘green movement’ in eukaryotic evolution. Trends Genet. 2002;18:577–584. doi: 10.1016/s0168-9525(02)02777-4. [DOI] [PubMed] [Google Scholar]