Abstract
Sustained smooth-muscle contraction or its experimental counterpart, Ca2+ sensitization, by Gq/13-coupled receptor agonists is mediated via RhoA-dependent inhibition of MLC (myosin light chain) phosphatase and MLC20 (20 kDa regulatory light chain of myosin II) phosphorylation by a Ca2+-independent MLCK (MLC kinase). The present study identified the corresponding pathways initiated by Gi-coupled receptors. Somatostatin acting via Gi1-coupled sstr3 receptor, DPDPE ([D-Pen2,D-Pen5]enkephalin; where Pen is penicillamine) acting via Gi2-coupled δ-opioid receptors, and cyclopentyl adenosine acting via Gi3-coupled adenosine A1 receptors preferentially activated PI3K (phosphoinositide 3-kinase) and ILK (integrin-linked kinase), whereas ACh (acetylcholine) acting via Gi3-coupled M2 receptors preferentially activated PI3K, Cdc42 (cell division cycle 42)/Rac1, PAK1 (p21-activated kinase 1) and p38 MAPK (mitogen-activated protein kinase). Only agonists that activated ILK induced sustained CPI-17 (protein kinase C potentiated inhibitor 17 kDa protein) phosphorylation at Thr38, MLC20 phosphorylation at Ser19, and contraction, consistent with recent evidence that ILK can act as a Ca2+-independent MLCK capable of phosphorylating the MLC phosphatase inhibitor, CPI-17, at Thr38. ILK activity, and CPI-17 and MLC20 phosphorylation were inhibited by LY294002 and in muscle cells expressing ILK(R211A) or treated with siRNA (small interfering RNA) for ILK. ACh acting via M2 receptors activated ILK, and induced CPI-17 and MLC20 phosphorylation and muscle contraction, but only after inhibition of p38 MAPK; all these responses were inhibited in cells expressing ILK(R211A). Conversely, ACh activated PAK1, a step upstream of p38 MAPK, whereas the three other agonists did so only in cells transfected with ILK(R211A) or siRNA for ILK. The results demonstrate reciprocal inhibition between two pathways downstream of PI3K, with ILK inhibiting PAK1, and p38 MAPK inhibiting ILK. Sustained contraction via Gi-coupled receptors is dependent on CPI-17 and MLC20 phosphorylation by ILK.
Keywords: Gi-coupled receptor, integrin-linked kinase, myosin light chain phosphatase, p21-activated kinase, protein kinase C potentiated inhibitor 17 kDa protein (CPI-17), smooth muscle
Abbreviations: ACh, acetylcholine; Cdc42, cell division cycle 42; CPA, cyclopentyl adenosine; CPI-17, protein kinase C potentiated inhibitor 17 kDa protein; 4-DAMP, 4-diphenylacetoxy-N-methylpiperidine; Pen, penicillamine; DPDPE, [D-Pen2,D-Pen5]enkephalin; DTT, dithiothreitol; ERK, extracellular-signal-regulated kinase; ETA receptor, endothelin A receptor; ILK, integrin-linked kinase; IP3, inositol 1,4,5-trisphosphate; MAPK, mitogen-activated protein kinase; MBP, myelin basic protein; MEK, MAPK/ERK kinase; ML-9, 1-(5-chloronaphthalene-1-sulphonyl)-1H-hexahydro-1,4-diazepine hydrochloride; MLC, myosin light chain; MLC20, 20 kDa regulatory light chain of myosin II; MLCK, MLC kinase; MYPT1, myosin phosphatase targeting subunit 1; PAK1, p21-activated kinase 1; PHI-1, phosphatase holoenzyme inhibitor-1; PI, phosphoinositide; PI3K, phosphoinositide 3-kinase; PKB, protein kinase B; PKC, protein kinase C; PLC-β3, phospholipase C-β3; PP1c, protein phosphatase 1 catalytic subunit; siRNA, small interfering RNA; ZIP kinase, zipper-interacting protein kinase
INTRODUCTION
Phosphorylation of MLC20 (20 kDa regulatory light chain of myosin II) at Ser19 is a prerequisite for initiating and maintaining smooth-muscle contraction. The agonist-induced increase in cytosolic Ca2+ concentration results in Ca2+/calmodulin-dependent activation of MLCK [MLC (myosin light chain) kinase] and phosphorylation of MLC20 [1–3]. Despite a rapid decrease in cytosolic Ca2+ concentration and MLCK activity, MLC20 phosphorylation and contraction are well maintained. Studies of contraction at fixed Ca2+ concentrations (‘Ca2+ sensitization’) have identified several mechanisms that could explain agonist-induced sustained contraction and MLC20 phosphorylation [4–7]. Three essential features characterize these mechanisms: (i) G-protein activation, (ii) a regulated decrease in MLC phosphatase [PP1c (protein phosphatase 1 catalytic subunit)] activity and (iii) MLC20 phosphorylation at both Ser19 and Thr18 by a Ca2+-independent MLCK. The requirement for a Ca2+-independent MLCK was evident in studies of vascular smooth muscle where complete inhibition of MLC phosphatase in the absence of Ca2+ was accompanied by contraction that was sensitive to the non-specific kinase inhibitor, staurosporine [8]. The existence of a Ca2+-independent MLCK that was insensitive to specific inhibitors of Ca2+/calmodulin-dependent kinase was first demonstrated by Walsh and co-workers [9–11]. Two Ca2+-independent MLCKs have now been characterized: an ILK (integrin-linked kinase), associated with the myofilaments and spatially distinct from plasma membrane-associated ILK, and ZIP kinase (zipper-interacting protein kinase), which is associated with the MYPT1 (myosin phosphatase targeting subunit 1) and is also known as MYPT1-associated kinase [8–13].
Our recent studies have shown that the mechanisms involved in ‘Ca2+ sensitization’ also mediate sustained contraction and MLC20 phosphorylation via Gq/13-coupled receptor agonists [14–16]. The pathways involve sequential activation of trimeric (G13 or both Gq and G13) and monomeric (RhoA) G-proteins, leading to activation of dual RhoA-dependent pathways. The latter involve a co-ordinated phosphorylation of two regulatory proteins that inhibit MLC phosphatase activity: MYPT1 and CPI-17 [PKC (protein kinase C) potentiated inhibitor 17 kDa protein]. Phosphorylation of Thr696 in MYPT1 promotes inhibition of the catalytic subunit (PP1cδ). Phosphorylation of MYPT1 is mediated either directly by Rho kinase, or indirectly via MYPT1-associated ZIP kinase [17]. Phosphorylation of CPI-17 at Thr38 [or of its ubiquitous orthologue, PHI-1 (phosphatase holoenzyme inhibitor-1) at Thr57] by PKC greatly augments the ability of CPI-17 or PHI-1 to inhibit MLC phosphatase [18–22].
Both Ca2+-independent MLCKs (ZIP kinase and ILK) phosphorylate MLC20 at both Ser19 and Thr18 with equal affinity and induce contraction when added to permeabilized smooth-muscle strips [8–12]. Previous studies have shown that carbachol, which interacts with Gq/13-coupled muscarinic M3 receptors, stimulates ZIP kinase activity in bladder smooth muscle [8]. CPI-17 or PHI-1, thiophosphorylated by ILK in vitro, induced contraction when added to Triton X-100 skinned arterial muscle strips; alanine mutants of either CPI-17 or PHI-1 had no effect [22]. However, activation of ILK in vivo by contractile agonists and its involvement in agonist-induced sustained contraction and MLC20 phosphorylation have not been demonstrated.
Our recent studies have shown that Gi-coupled receptors activate PI3K (phosphoinositide 3-kinase) via Gβγi, but do not activate RhoA in smooth muscle [23,24]. Because ILK is a known downstream effector of PI3K, we speculated that ILK might be responsible for sustained contraction induced by Gi-coupled receptor agonists. Gi1-coupled somatostatin sstr3 receptors, Gi2-coupled δ-opioid receptors, and Gi3-coupled adenosine A1 receptors cause an initial transient contraction by activating PLC-β3 (phospholipase C-β3) via GβγI, and stimulating IP3 (inositol 1,4,5-trisphosphate)-dependent Ca2+ release [25–27]. In the present study, we show that these agonists elicit a sustained contraction by sequential activation of Gβγi, PI3K and ILK, resulting in phosphorylation of both CPI-17 and MLC20. Although Gi3-coupled muscarinic M2 receptors activated PI3K, they did not induce MLC20 phosphorylation or contraction. These receptors triggered preferentially a parallel pathway involving sequential activation of Cdc42 (cell division cycle 42)/Rac1, PAK1 (p21-activated kinase 1) and p38 MAPK (mitogen-activated protein kinase), which resulted in p38 MAPK-dependent inactivation of ILK. Blockade of p38 MAPK activity unmasked M2-mediated CPI-17 and MLC20 phosphorylation and muscle contraction.
MATERIALS AND METHODS
Intestinal smooth-muscle cell culture
Smooth-muscle cells were isolated from the circular muscle layer of rabbit intestine by sequential enzymatic digestion in 25 mM Hepes medium, filtration through 500 μM Nitex, and centrifugation at 350 g, as previously described [14–16]. Dispersed smooth-muscle cells were cultured in Dulbecco's modified Eagle's medium containing 10% (v/v) fetal bovine serum until they attained confluence and were then passaged once for use in various studies [14,16,23,24].
Vector and cDNA constructs
Dominant-negative ILK [ILK (R211A)] or protein kinase B [PKB/Akt (S473A)] was subcloned into the multiple cloning sites (EcoRI) of the eukaryotic expression vector pEXV. Recombinant plasmid DNAs (2 μg each) were transiently transfected into smooth-muscle cells in primary culture using Lipofectamine™ Plus reagent for 48 h. The cells were co-transfected with 1 μg of pGreen Lantern-1 to monitor expression. Control cells were co-transfected with 2 μg of vector (pEXV) and 1 μg of pGreen Lantern-1 DNA. Transfection efficiency (∼75–85%) was monitored by the expression of green fluorescent protein using FITC filters.
ILK-specific siRNA (small interfering RNA) was purchased from Santa Cruz Biotechnology. The siRNA transfection was performed using siRNA transfection reagent and siRNA transfection medium (Santa Cruz Biotechnology) according to the manufacturer's instructions.
ILK and PAK1 immunokinase assays
ILK and PAK1 activities were determined on immunoprecipitates from extracts of cultured muscle cells as described previously [23]. The immunoprecipitates were washed twice with a phosphorylation buffer containing 10 mM MgCl2 and 40 mM Hepes (pH 7.4) and then incubated for 5 min on ice with 5 μg of MBP (myelin basic protein). ILK activity was also determined using MLC20 (25 μM) as substrate. Kinase assays were initiated by the addition of 10 μCi of [γ-32P]ATP (3000 Ci/mmol) and 20 μM ATP, followed by incubation for 10 min at 37 °C. 32P-labelled substrate was absorbed on to phosphocellulose discs and free radioactivity was removed by repeated washing with 75 mM phosphoric acid. The extent of phosphorylation was determined from the radioactivity on phosphocellulose discs by liquid-scintillation counting. The results were expressed as c.p.m. per mg of protein in the lysates.
In-gel kinase assay for ILK
ILK activity was also determined by in-gel kinase assay using MBP as the substrate that was co-polymerized with SDS/polyacrylamide gel matrix. Briefly, ILK immunoprecipitates were resolved on an SDS/15% polyacrylamide gel containing MBP (5 μg/ml of running gel solution). The gels were washed in 50 mM Tris/HCl (pH 7.5) containing 20% (v/v) propan-2-ol for 60 min to remove SDS and then in 50 mM Tris/HCl and 1 mM DTT (dithiothreitol) for another 60 min. Gels were denatured in 6 M guanidinium chloride solution (50 mM Tris/HCl, 20 mM DTT and 2 mm EDTA) and then renatured with 50 mM Tris/HCl solution containing Tween 20. The gels were pre-incubated in kinase medium containing 20 mM Tris/HCl (pH 7.5), 0.25 mM EGTA and 1 μM calyculin for 60 min and the assay was performed in the same medium containing 300 μM [γ-32P]ATP and 5 mM MgCl2 for another 60 min at 30 °C. The gels were washed extensively with 5% (w/v) trichloroacetic acid and 1% sodium phosphate, transferred to nitrocellulose membrane and autoradiographed.
PI3K assay
PI3K was measured by TLC as described previously [23]. Freshly dispersed muscle cells were treated for 10 min with various agonists. After centrifugation for 5 min, 1 ml of lysis buffer [50 mM Tris/HCl, pH 7.5, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 2 mM sodium vanadate, 50 mM NaF and protease inhibitor cocktail (10 μg/ml)] was added to the cell suspension and incubation was maintained for 20 min at 4 °C. The cell lysates were precleared by centrifugation, and an aliquot was incubated with 5 μl of anti-PI3K antibody for 2 h at 4 °C, followed by incubation with 30 μl of Protein A/G–Sepharose for 2 h at 4 °C. The immunoprecipitates were washed with lysis buffer and Tris/HCl buffer (20 mM Tris/HCl, pH 7.5) and incubated in a medium containing 1 mg/ml phosphatidylinositol, 20 mM MgCl2, 10 μCi of [γ-32P]ATP (3000 Ci/mmol) and 20 μM ATP for 10 min at 37 °C. The organic phase containing phosphoinositol phosphates was analysed by TLC and the spots were visualized by autoradiography.
Phosphorylation of MLC20, CPI-17 and MYPT1
Phosphorylation of MLC20, CPI-17 or MYPT1 was determined by immunoblotting using phospho-specific antibodies to MLC20 (Ser19), CPI-17 (Thr38) and MYPT1 (Thr696) as described previously [14]. Freshly dispersed or cultured smooth-muscle cells were treated with various agonists for 10 min and solubilized on ice for 1 h in a medium containing 20 mM Tris/HCl (pH 8.0), 1 mM DTT, 100 mM NaCl, 0.5% SDS, 0.75% deoxycholate, 1 mM PMSF, 10 μg/ml leupeptin and 100 μg/ml aprotinin. The proteins were resolved by SDS/PAGE and electrophoretically transferred on to PVDF membranes, which were incubated for 12 h with the phospho-specific antibody and then for 1 h with a horseradish peroxidase-conjugated secondary antibody. The bands were identified by enhanced chemiluminescence.
Measurement of contraction in dispersed smooth-muscle cells
Muscle cell contraction was measured in freshly dispersed muscle cells by scanning micrometry as described previously [14,15]. A cell aliquot containing approx. 104 muscle cells/ml was added to 0.1 ml of medium containing various agonists, and the reaction was terminated with 1% acrolein. The mean length of 50 muscle cells treated with agonist was measured and compared with the mean length of untreated cells, and contraction was expressed as the decrease in mean cell length from control.
Materials
[γ-32P]ATP was obtained from NEN Life Science Products; polyclonal antibodies to MLC20, CPI-17, MYPT1, ILK and PAK1 and phospho-specific antibodies to MLC20 (Ser19), CPI-17 (Thr38) and MYPT1 (Thr696) were obtained from Santa Cruz Biotechnology. All other reagents were from Sigma.
RESULTS
Sequential activation of PI3K and ILK by Gi-coupled receptor agonists
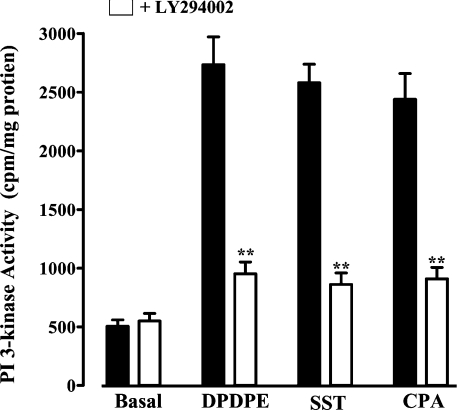
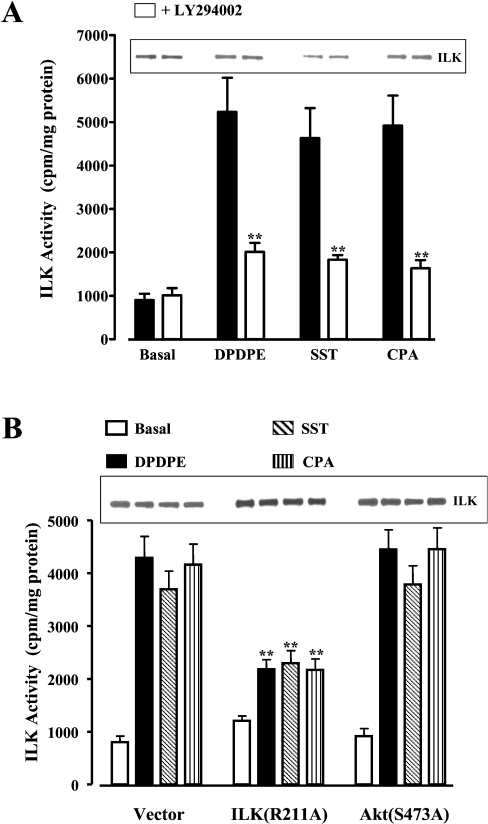
Our previous studies have shown that somatostatin sstr3, δ-opioid and adenosine A1 receptors in smooth-muscle cells are coupled respectively with Gi1, Gi2 and Gi3 [25–27]. Here we show that activation of these receptors with somatostatin, DPDPE ([D-Pen2,D-Pen5]enkephalin; where Pen is penicillamine) and CPA (cyclopentyl adenosine) respectively stimulated PI3K and ILK activities in freshly dispersed and cultured intestinal smooth-muscle cells (Figures 1 and 2A). Both activities were abolished by the selective PI3K inhibitor LY294002, implying that ILK was activated downstream of PI3K [28,29]. ILK activity induced by all three agonists was also inhibited in cells expressing ILK(R211A), but was not affected in cells expressing PKB/Akt(S473A) (Figure 2B). ILK activity was time-dependent, reaching a plateau in 5–10 min, and was sustained for at least 20 min (results not shown).
Figure 1. Activation of PI3K by Gi-coupled receptor agonists.
Cultured smooth-muscle cells were treated for 10 min with DPDPE (1 μM), somatostatin (SST; 1 μM) or CPA (1 μM) in the presence or absence of LY294002 (10 μM). PI3K activity was measured by TLC and expressed as c.p.m./mg of protein. Similar results were obtained using freshly dispersed smooth-muscle cells (results not shown). Values are means±S.E.M. for four experiments. **P<0.01, significant inhibition of agonist-stimulated PI3K activity by LY294002. Filled bars represent values in the absence of LY294002.
Figure 2. Activation of ILK by Gi-coupled receptor agonists.
(A) Cultured smooth-muscle cells were treated for 10 min with DPDPE (1 μM), somatostatin (SST; 1 μM) or CPA (1 μM) in the presence or absence of LY294002 (10 μM). Filled bars represent values in the absence of LY294002. (B) Cultured smooth-muscle cells expressing vector, dominant-negative ILK(R211A) or dominant-negative PKB/Akt(S473A) were treated for 10 min with DPDPE (1 μM), somatostatin (1 μM) or CPA (1 μM). ILK activity was measured by immunokinase assay and expressed as c.p.m./mg of protein. ILK activity was strongly inhibited by LY294002 in control cells (A) and in cells expressing ILK(R211A), but not in cells expressing PKB/Akt(S473A) (B). Inset: Western-blot analysis of anti-ILK immunoprecipitates using anti-ILK antibody. Values are means±S.E.M. for four experiments. **P<0.01, significant inhibition of agonist-stimulated ILK activity by LY294002 or in cells expressing ILK(R211A).
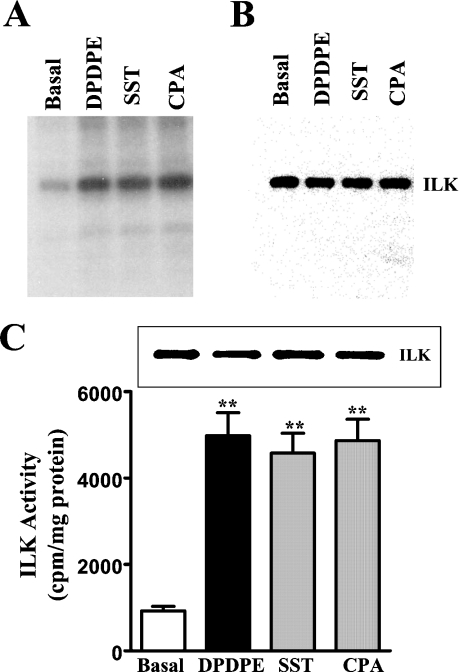
ILK activity was confirmed by in-gel kinase assay using MBP co-polymerized with SDS/polyacrylamide gel matrix (Figure 3A) and by immunokinase assay using MLC20 as substrate (Figure 3C). The results demonstrate that all three Gi-coupled receptor agonists stimulated ILK activity.
Figure 3. Activation of ILK by Gi-coupled receptor agonists.
(A) In-gel kinase assay. Cultured smooth-muscle cells were treated for 10 min with DPDPE (1 μM), somatostatin (SST; 1 μM) or CPA (1 μM), and ILK activity in the immunoprecipitates was determined by in-gel kinase assay using MBP co-polymerized with an SDS/polyacrylamide gel. The autoradiograms demonstrate specific phosphorylation of MBP by ILK and stimulation of ILK activity by Gi-coupled receptor agonists. (B) Western blot. The membrane was immunoblotted with anti-ILK antibody. (C) ILK activity using MLC20 as substrate. Cultured smooth-muscle cells were treated for 10 min with DPDPE (1 μM), somatostatin (1 μM) or CPA (1 μM), and ILK activity in the immunoprecipitates was determined by immunokinase assay. Inset: Western-blot analysis of ILK immunoprecipitates using anti-ILK antibody. Values are means±S.E.M. for four experiments. **P<0.01, significant stimulation of ILK activity by Gi-coupled receptor agonists.
Agonist-dependent, ILK-mediated CPI-17 and MLC20 phosphorylation and muscle cell contraction
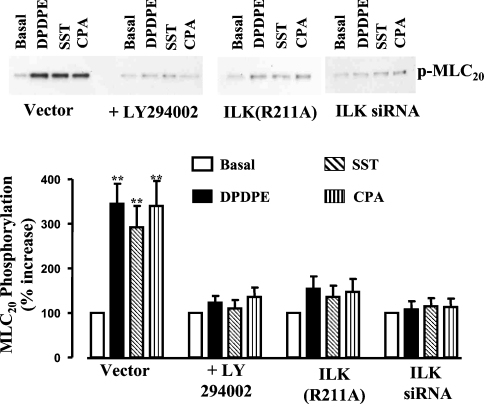
Treatment of cultured smooth-muscle cells for 10 min with DPPDE, somatostatin-14 or CPA stimulated CPI-17 phosphorylation at Thr38; the phosphorylation was abolished by LY294002 (Figure 4). Phosphorylation of CPI-17 was also inhibited in smooth-muscle cells transfected with ILK(R211A) or siRNA for ILK (Figure 4). Treatment of cultured smooth-muscle cells for 10 min with each agonist stimulated MLC20 phosphorylation at Ser19; this sustained MLC20 phosphorylation was abolished by LY294002 (Figure 5). Sustained MLC20 phosphorylation was also inhibited in smooth-muscle cells transfected with ILK(R211A) or siRNA for ILK (Figure 5). The results implied that PI3K-dependent activation of ILK mediated both CPI-17 phosphorylation and sustained MLC20 phosphorylation. Recent studies have shown that ILK can directly phosphorylate MLC20 at Ser19 and CPI-17 at Thr38 in vitro [10,11,22]. Consequently, the increase in sustained MLC20 phosphorylation observed in vivo in the present study reflected both direct phosphorylation of MLC20 by ILK and inhibition of MLC phosphatase by phosphorylated CPI-17.
Figure 4. CPI-17 phosphorylation induced by Gi-coupled receptor agonists is mediated by ILK.
Cultured smooth-muscle cells transfected with vector alone, ILK(R211A), or siRNA for ILK were treated for 10 min with DPDPE (1 μM), somatostatin (SST; 1 μM) or CPA (1 μM). Smooth-muscle cells expressing vector alone were treated with each agonist for 10 min in the presence or absence of LY294002 (10 μM). Cell lysates were analysed for CPI-17 phosphorylation using phospho(Thr38)-specific anti-CPI-17 antibody. Values are means±S.E.M. for three experiments. **P<0.01, significant increase in CPI-17 phosphorylation induced by Gi-coupled receptor agonists.
Figure 5. Sustained MLC20 phosphorylation induced by Gi-coupled receptor agonists is mediated by ILK.
Cultured smooth-muscle cells transfected with vector alone, ILK(R211A), or siRNA for ILK were treated for 10 min with DPDPE (1 μM), somatostatin (1 μM) or CPA (1 μM). Smooth-muscle cells expressing vector alone were treated with each agonist for 10 min in the presence or absence of LY294002 (10 μM). Cell lysates were analysed for MLC20 phosphorylation using phospho(Ser19)-specific anti-MLC20 antibody. Values are means±S.E.M. for three experiments. **P<0.01, significant increase in MLC20 phosphorylation induced by Gi-coupled receptor agonists.
Sustained muscle cell contraction measured 10 min after addition of each agonist was abolished by pretreatment of the cells for 60 min with pertussis toxin (results not shown) or for 10 min with LY294002 (control contraction: 23±2 to 26±3 μm decrease in cell length; agonist+LY294002: 5±3 to 2±1 μm decrease in cell length), implying that sustained contraction was mediated by Gi-dependent activation of PI3K. Sustained contraction was not affected by U73122, Y27632, bisindolylmaleimide, PD98059, SB203580, or genistein, implying that it was not dependent on PI (phosphoinositide) hydrolysis, or on activation of Rho kinase, PKC, MEK [MAPK/ERK (extracellular-signal-regulated kinase) kinase], p38 MAPK, or tyrosine kinase(s). Similarly, sustained MLC20 phosphorylation induced by DPDPE, somatostatin or CPA was abolished by LY294002 (Figure 5).
The initial transient contraction measured 30 s after addition of each agonist was abolished by pretreatment with pertussis toxin (results not shown), but in contrast with sustained contraction, it was abolished by U73122 and ML-9 [1-(5-chloronaphthalene-1-sulphonyl)-1H-hexahydro-1,4-diazepine hydrochloride] (control contraction: 31±3 to 34±2 μm decrease in cell length; agonist+U73122: 3±4 to 2±4 μm decrease in cell length; agonist+ML-9: 4±5 to 1±2 μm decrease in cell length), implying that it was dependent on PI hydrolysis and Ca2+/calmodulin activation of MLCK. The results corroborate previous studies in which all three agonists were shown to stimulate PI hydrolysis and Ca2+ mobilization via Gβγi-dependent activation of PLC-β3 [25–27]. Initial contraction was not affected by LY294002, Y27632, bisindolylmaleimide, PD98059, SB203580, or genistein.
Preferential activation of a distinct pathway by Gi3-coupled muscarinic M2 receptors
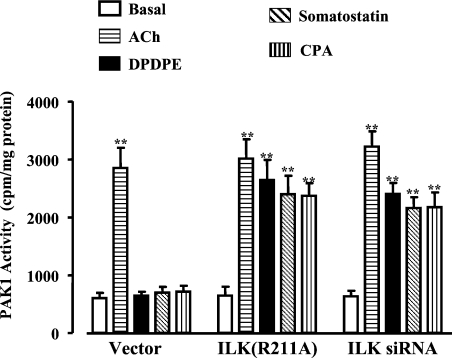
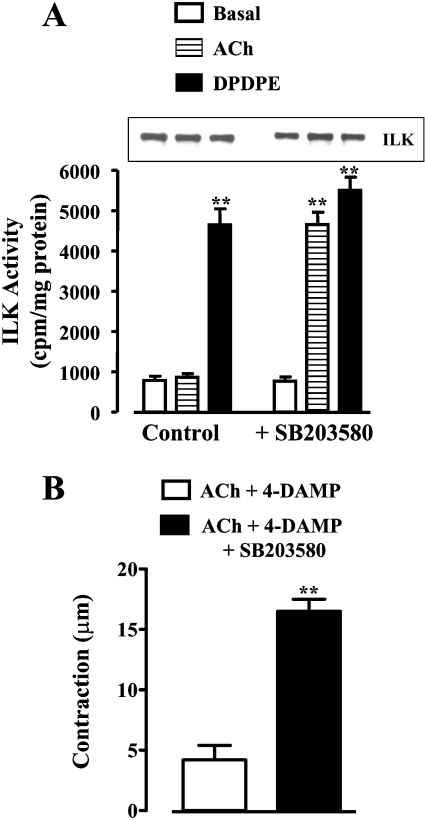
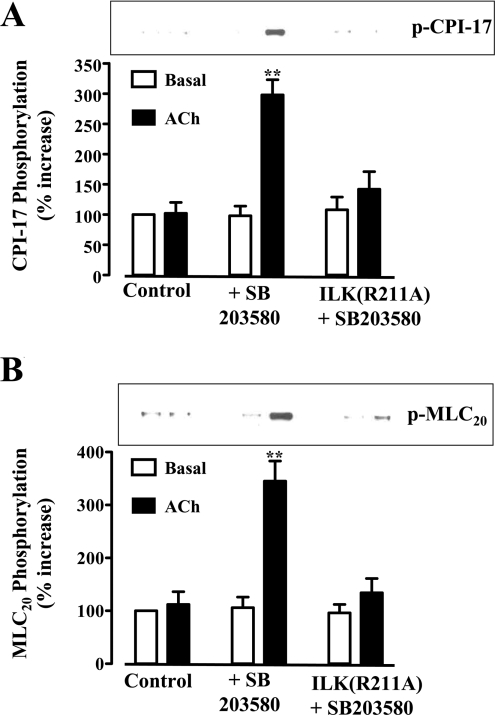
As previously shown [14], and confirmed in the present study, activation of M2 receptors did not elicit muscle cell contraction or MLC20 phosphorylation. The receptors initiated parallel, Gβγi-mediated signalling cascades, involving either activation of PLC-β3 or sequential activation of PI3K, Cdc42/Rac1, PAK1 and p38 MAPK [23]. Treatment of cultured smooth-muscle cells with ACh (acetylcholine; 0.1 μM) in the presence of the M3 receptor antagonist, 4-DAMP (4-diphenylacetoxy-N-methylpiperidine), so as to activate selectively M2 receptors, stimulated PAK1 but not ILK activity, in contrast with DPDPE, somatostatin-14 and CPA, which stimulated ILK activity but not PAK1 activity (Figure 6). The pattern suggested that M2 receptors preferentially activate a PI3K/PAK1 pathway, whereas δ-opioid, somatostatin sstr3 and adenosine A1 receptors preferentially activate a PI3K/ILK pathway. Expression of ILK(R211A) or siRNA for ILK led to stimulation of PAK1 by δ-opioid, somatostatin sstr3 and adenosine A1 receptors, implying that the inability of these receptors to activate a PI3K/PAK1 pathway resulted from suppression of PAK1 activation by ILK (Figure 6). We next examined whether the PAK1/p38 MAPK pathway preferentially activated by M2 receptors exerted a reciprocal suppression of ILK activity. Blockade of p38 MAPK activity with SB203580 led to activation of ILK via M2 receptors (Figure 7A), and resulted in CPI-17 phosphorylation at Thr38 (Figure 8A) and MLC20 phosphorylation at Ser19 (Figure 8B) and caused smooth-muscle contraction (Figure 7B). Phosphorylation of CPI-17 and MLC20 in the presence of SB203580 was blocked in cells expressing ILK(R211A) (Figure 8). Thus PAK1 acting via its downstream effector, p38 MAPK, suppressed ILK activity and blocked the ability of M2 receptors to induce sustained MLC20 phosphorylation and muscle contraction. Previously, we had shown that phosphorylation of MLCK by PAK1 inactivated MLCK and blocked the ability of M2 receptors to elicit an initial Ca2+-dependent MLC20 phosphorylation and muscle contraction [14].
Figure 6. Suppression of PAK1 activity by ILK.
Cultured smooth-muscle cells expressing vector alone or ILK(R211A) and cells transfected with siRNA for ILK were treated for 10 min with ACh (0.1 μM) plus 4-DAMP (0.1 μM), DPDPE (1 μM), somatostatin (1 μM) or CPA (1 μM). PAK1 activity was measured by immunokinase assay and expressed as c.p.m./mg of protein. Overexpression of ILK(R211A) or transfection with siRNA for ILK restored the ability of DPDPE, somatostatin and CPA to stimulate PAK1 activity. Values are means±S.E.M. for three to four experiments. **P<0.01, significant stimulation of PAK1 activity by Gi-coupled receptor agonists.
Figure 7. ILK activity and contraction by M2 receptor agonists is inhibited by p38 MAPK.
(A) Activation of ILK by M2 receptor agonists is unmasked upon inhibition of p38 MAPK. Cultured smooth-muscle cells were treated for 10 min with ACh (0.1 μM) plus 4-DAMP (0.1 μM) or with DPDPE (1 μM) in the presence or absence of the p38 MAPK inhibitor SB203580 (1 μM). ILK activity was measured by immunokinase assay and expressed as c.p.m./mg of protein. Inset: Western-blot analysis of ILK immunoprecipitates using anti-ILK antibody. Values are means±S.E.M. for three experiments. **P<0.01, significant stimulation of ILK activity by Gi-coupled receptor agonists. (B) Smooth-muscle contraction by M2 receptors unmasked by inhibition of p38 MAPK. Freshly dispersed smooth-muscle cells were treated for 10 min with ACh (0.1 μM) plus 4-DAMP (0.1 μM) in the presence or absence of SB203580 (1 μM). Contraction was measured by scanning micrometry and expressed as decrease in cell length from control (115±2 μm). Values are means±S.E.M. for four experiments. **P<0.01, significant decrease in muscle cell length.
Figure 8. CPI-17 and MLC20 phosphorylation induced by M2 receptor agonists unmasked by inhibition of p38 MAPK is abolished in cells expressing ILK(R211A).
ACh (0.1 μM) plus 4-DAMP (0.1 μM) was added for 10 min to cultured smooth-muscle cells expressing vector or ILK(R211A) in the presence or absence of SB203580. Cell lysates were analysed for (A) CPI-17 phosphorylation using phospho(Thr38)-specific CPI-17 antibody and (B) MLC20 phosphorylation using phospho(Ser19)-specific MLC20 antibody. Inhibition of p38 MAPK with SB203580 restored the ability of M2 receptors to induce phosphorylation of CPI-17 and MLC20; phosphorylation of both proteins was blocked by overexpression of ILK(R211A). Values are means±S.E.M. for three experiments. **P<0.01, significant increase in CPI-17 and MLC20 phosphorylation induced by M2 receptor agonists in the presence of SB203580.
DISCUSSION
Agonist-induced contraction at fixed Ca2+ concentration (i.e. Ca2+ sensitization) involves a Rho-dependent regulated decrease in MLC phosphatase activity, and MLC20 phosphorylation by a Ca2+-independent MLCK. The same mechanisms are responsible for the sustained response to these agonists. Sustained MLC20 phosphorylation and muscle contraction induced by Gq/G13-coupled receptor agonists involve sequential activation of RhoGEF (Rho guanine nucleotide-exchange factor) and RhoA, leading to Rho kinase-mediated phosphorylation of MYPT1 and/or PKC-mediated phosphorylation of CPI-17, and resulting in inhibition of MLC phosphatase activity [14,16]. MLC20 phosphorylation appears to be induced by a Ca2+-independent kinase, probably ZIP kinase, recently shown to be activated by the muscarinic agonist, carbachol [8]. The requirement for inhibition of MLC phosphatase via the Rho kinase/MYPT1 and/or PKC/CPI-17 pathways is agonist-specific. A co-ordinated interplay of the two pathways is involved in the sustained response to muscarinic agonists, motilin and sphingosine 1-phosphate, whereas only MYPT1 is involved in the sustained response to endothelin [30–32]. The latter activates RhoA via ETA receptors (endothelin A receptors) only; concurrent activation of ETB receptors causes dephosphorylation of CPI-17 via sequential activation of p38 MAPK and phosphatase 2A.
The present study characterized the signalling pathways that mediate sustained muscle contraction via Gi-coupled receptors that do not activate RhoA, Rho kinase or PKC [24]. Instead, they initiate various pathways dependent on activation of PI3K by Gβγi [23,25–27]. Three Gi-coupled receptor agonists used in the present study (DPDPE, somatostatin and CPA) preferentially activated PI3K and ILK, whereas ACh acting via M2 receptors preferentially activated Cdc42/Rac1, PAK1 and p38 MAPK as shown previously [23]; p38 MAPK activity was dependent on PAK1 and inhibited by anti-PAK1 antibody [23]. Only the agonists that activated ILK induced sustained MLC20 phosphorylation and contraction, consistent with ILK acting as a Ca2+-independent MLCK that is also capable of inhibiting MLC phosphatase via direct phosphorylation of CPI-17 at Thr38 [9–11,22]. The results of the present study provide the first in vivo evidence that ILK acts as a Ca2+-independent MLCK in response to activation of Gi-coupled receptors.
The evidence supporting the role of ILK in sustained contraction may be summarized as follows. Agonist-stimulated PI3K and ILK activities and sustained MLC20 phosphorylation and contraction were inhibited by the PI3K inhibitor LY294002. Furthermore, MLC20 and CPI-17 phosphorylation was inhibited in cultured smooth-muscle cells expressing ILK(R211A) or treated with siRNA for ILK. Although in vitro studies suggested that ILK could phosphorylate MYPT1 at various sites including the critical inhibitory site (Thr695 in chicken gizzard MYPT1), we were unable to detect phosphorylation of MYPT1 at Thr696 in rabbit smooth muscle in vivo [33,34]. It is possible that only CPI-17 and not MYPT1 is phosphorylated in vivo. Sustained contraction was not affected by MEK, p38 MAPK, Rho kinase, PKC and tyrosine kinase inhibitors, providing further evidence that Rho-dependent pathways were not involved in sustained contraction mediated by Gi-coupled receptor agonists. Studies on circular smooth muscle of the cat oesophageal sphincter suggested sequential involvement of ERK1/2 and ILK in PKC-mediated contraction [35]. In the present study, however, neither a MEK inhibitor, PD98059, nor the PKC inhibitor, bisindolylmaleimide, had any effect on sustained contraction.
Initial contraction whether mediated by Gq- or Gi-coupled receptors involves phosphorylation of MLC20 by a Ca2+/calmodulin-dependent MLCK. The initial contraction can be fully dissociated from sustained contraction, and is selectively suppressed by expression of Gαq or Gαi minigene, by inhibition of PLC-β activity with U73122, which effectively eliminates IP3-dependent Ca2+ release, by calmodulin inhibitors, and by selective inhibitors of Ca2+/calmodulin-dependent MLCK [14,15,30–32]. As previously shown and confirmed in the present study, initial contraction induced by DPDPE, somatostatin and CPA was inhibited by pertussis toxin, U73122 and ML-9.
Our previous studies have shown that selective activation of muscarinic M2 receptors involves sequential activation of Gβγi3, PI3K, Cdc42/Rac1, PAK1 and p38 MAPK; the latter was dependent on activation of PAK1 and was inhibited by anti-PAK1 antibody [23]. In the present study, M2 receptors, but not δ-opioid, somatostatin sstr3 or adenosine A1 receptors, activated PAK1; conversely, M2 receptors did not activate ILK or induce CPI-17 and MLC20 phosphorylation or muscle contraction. The preferential activation of PAK1 and p38 MAPK by M2 receptors suppressed the PI3K-mediated activation of ILK, since blockade of p38 MAPK activity by SB203580 restored ILK activity as well as CPI-17 and MLC20 phosphorylation and muscle contraction. Furthermore, the preferential activation of ILK by δ-opioid, somatostatin sstr3 and adenosine A1 receptors suppressed PAK1 stimulation, since expression of ILK(R211A) and treatment with siRNA for ILK restored PAK1 activity. Thus a reciprocal inhibition between these PI3K-dependent pathways determines the ability of a given Gi-coupled receptor to initiate sustained MLC20 phosphorylation and contraction. What determines the preferential activation of either pathway downstream of PI3K has not been examined. It is worth noting that M2 receptor-activated PAK1 also inhibits Ca2+-dependent MLCK, thereby also suppressing initial contraction [14,36]. The results provide an explanation for why muscarinic M2 receptors are not capable of initiating or sustaining smooth-muscle contraction.
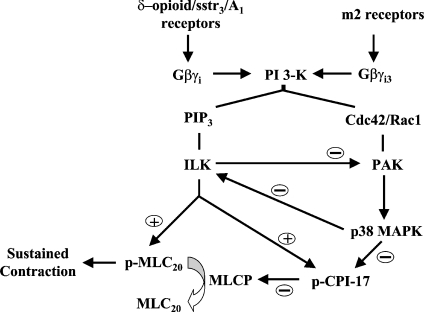
In summary, we have identified a novel pathway for sustained smooth-muscle contraction induced by Gi-coupled receptor agonists. The pathway involves sequential activation of Gβγi, PI3K and ILK, resulting in direct phosphorylation of MLC20 and CPI-17-mediated inhibition of MLC phosphatase. In contrast, Gi-coupled M2 receptors engage preferentially a PI3K/PAK1/p38 MAPK pathway that suppresses ILK activity (Scheme 1). Receptors that preferentially engage the PI3K/ILK pathway induce reciprocal suppression of the PAK1/p38 MAPK pathway.
Scheme 1. Distinct PI3K pathways for inhibition of MLC phosphatase (MLCP) and stimulation of MLC20 phosphorylation by Gi-coupled receptors.
δ-Opioid, sstr3 and adenosine A1 receptors are coupled with sequential activation of Gβγi, PI3K and ILK, resulting in direct phosphorylation of MLC20, CPI-17-mediated inhibition of MLC phosphatase, and suppression of PAK1 activity. M2 receptors are coupled with sequential activation of Gβγi, PI3K, Cdc42/Rac1, PAK1 and p38 MAPK, resulting in suppression of ILK activity. Thus there is reciprocal inhibition between two pathways downstream of PI3K, with ILK inhibiting PAK1, and p38 MAPK inhibiting ILK. Only agonists that activate ILK induce sustained MLC20 phosphorylation and contraction. PIP3, phosphatidylinositol 3,4,5-trisphosphate.
Acknowledgments
This work was supported by grant DK15564 from the National Institute of Diabetes and Digestive and Kidney Diseases (Bethesda, MD, U.S.A.).
References
- 1.Somlyo A. P., Somlyo A. V. Ca2+ sensitivity of smooth muscle and non-muscle myosin II: modulated by G proteins, kinases and phosphatases. Physiol. Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 2.Kamm K. E., Stull J. T. Dedicated myosin light chain kinase with diverse cellular functions. J. Biol. Chem. 2001;276:4527–4530. doi: 10.1074/jbc.R000028200. [DOI] [PubMed] [Google Scholar]
- 3.Tansey M. G., Word R. A., Hidaka H., Singer H. A., Schworer C. M., Kamm K. E., Stull J. T. Phosphorylation of myosin light chain kinase by the multifunctional calmodulin-dependent protein kinase II in smooth muscle cells. J. Biol. Chem. 1992;267:12511–12516. [PubMed] [Google Scholar]
- 4.Kubota Y., Nomura M., Kamm K. E., Mumby M. C., Stull J. T. GTPγS-dependent regulation of smooth muscle contractile elements. Am. J. Physiol. 1992;262:C405–C410. doi: 10.1152/ajpcell.1992.262.2.C405. [DOI] [PubMed] [Google Scholar]
- 5.Kitazawa T., Kobayashi S., Horiuti K., Somlyo A. V., Somlyo A. P. Receptor-coupled, permeabilized smooth muscle. Role of the phosphoinositide cascade, G proteins, and modulation of the contractile response to Ca2+ J. Biol. Chem. 1989;264:5339–5342. [PubMed] [Google Scholar]
- 6.Fukata Y., Amano M., Kaibuchi K. Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol. Sci. 2001;22:32–39. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- 7.Hartshorne D. J., Eto M., Erodi F. Myosin light chain phosphatase: subunit composition, interaction and regulation. J. Muscle Res. Cell Motil. 1998;19:325–341. doi: 10.1023/a:1005385302064. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald J. A., Borman M. A., Muranyi A., Somlyo A. V., Hartshorne D. J., Haystead T. A. J. Identification of the endogenous smooth muscle myosin phosphatase-associated kinase. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2419–2424. doi: 10.1073/pnas.041331498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber L. P., Van Lierop J. E., Walsh M. P. Ca2+-independent phosphorylation of myosin in rat caudal artery and chicken gizzard myofilaments. J. Physiol. (Cambridge, U.K.) 1999;516:805–824. doi: 10.1111/j.1469-7793.1999.0805u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng J. T., Van Lierop J. E., Sutherland C., Walsh M. P. Ca2+-independent smooth muscle contraction: a novel function of integrin-linked kinase. J. Biol. Chem. 2001;276:16365–16373. doi: 10.1074/jbc.M011634200. [DOI] [PubMed] [Google Scholar]
- 11.Wilson D. P., Sutherland C., Borman M. A., Deng J. T., MacDonald J. A., Walsh M. P. Integrin-linked kinase is responsible for Ca2+-independent myosin dephosphorylation and contraction of vascular smooth muscle. Biochem. J. 2005;392:641–648. doi: 10.1042/BJ20051173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niiro N., Ikebe M. Zipper-interacting protein kinase induces Ca2+-free smooth muscle contraction via myosin light chain phosphorylation. J. Biol. Chem. 2001;276:29567–29574. doi: 10.1074/jbc.M102753200. [DOI] [PubMed] [Google Scholar]
- 13.Endo A., Surks H. K., Mochizuki S., Mochizuki N., Mendelsohn M. E. Identification and characterization of zipper-interacting protein kinase as the unique vascular smooth muscle myosin phosphatase-associated kinase. J. Biol. Chem. 2004;279:42055–42061. doi: 10.1074/jbc.M403676200. [DOI] [PubMed] [Google Scholar]
- 14.Murthy K. S., Zhou H., Grider J. R., Brautigan D. L., Eto M., Makhlouf G. M. Differential signalling by muscarinic receptors in smooth muscle: m2-mediated inactivation of myosin light chain kinase via Gi3, Cdc42/Rac1 and p21-activated kinase 1 pathway, and m3-mediated MLC20 (20 kDa regulatory light chain of myosin II) phosphorylation via Rho-associated kinase/myosin phosphatase targeting subunit 1 and protein kinase C/CPI-17 pathway. Biochem. J. 2003;374:145–155. doi: 10.1042/BJ20021274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murthy K. S., Grider J. R., Kuemmerle J. F., Makhlouf G. M. Sustained muscle contraction induced by agonists, growth factors, and Ca2+ mediated by distinct PKC isozymes. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G201–G210. doi: 10.1152/ajpgi.2000.279.1.G201. [DOI] [PubMed] [Google Scholar]
- 16.Murthy K. S., Zhou H., Grider J. R., Makhlouf G. M. Sequential activation of heterotrimeric and monomeric G proteins mediates PLD activity in smooth muscle. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G381–G388. doi: 10.1152/ajpgi.2001.280.3.G381. [DOI] [PubMed] [Google Scholar]
- 17.Borman M. A., MacDonald J. A., Muranyi A., Hartshorne D. J., Haystead T. A. J. Smooth muscle myosin phosphatase-associated kinase induces Ca2+-sensitization via myosin phosphatase inhibition. J. Biol. Chem. 2002;277:23441–23446. doi: 10.1074/jbc.M201597200. [DOI] [PubMed] [Google Scholar]
- 18.Kitazawa T., Eto M., Woodsome T. P., Brautigan D. L. Agonists trigger G protein-mediated activation of the CPI-17 inhibitor phosphoprotein of myosin light chain phosphatase to enhance vascular smooth muscle contractility. J. Biol. Chem. 2000;275:9897–9900. doi: 10.1074/jbc.275.14.9897. [DOI] [PubMed] [Google Scholar]
- 19.Woodsome T. P., Eto M., Everett A., Brautigan D. L., Kitazawa T. Expression of CPI-17 and myosin phosphatase correlates with Ca2+ sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J. Physiol. (Cambridge, U.K.) 2001;535:553–564. doi: 10.1111/j.1469-7793.2001.t01-1-00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eto M., Kitazawa T., Yazawa M., Mukai H., Ono Y., Brautigan D. L. Histamine-induced vasoconstriction involves phosphorylation of a specific inhibitor protein for myosin phosphatase by protein kinase C alpha and delta isoforms. J. Biol. Chem. 2001;276:29072–29078. doi: 10.1074/jbc.M103206200. [DOI] [PubMed] [Google Scholar]
- 21.Eto M., Karginov A., Brautigan D. L. A novel phosphoprotein inhibitor of protein type-1 phosphatase holoenzymes. Biochemistry. 1999;38:16952–16957. doi: 10.1021/bi992030o. [DOI] [PubMed] [Google Scholar]
- 22.Deng J. T., Sutherland C., Brautigan D. L., Eto M., Walsh M. P. Phosphorylation of the myosin phosphatase inhibitors, CPI-17 and PHI, by integrin-linked kinase. Biochem. J. 2002;367:517–524. doi: 10.1042/BJ20020522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou H., Das S., Murthy K. S. Erk1/2- and p38 MAP kinase-dependent phosphorylation and activation of cPLA2 by m3 and m2 receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284:G472–G480. doi: 10.1152/ajpgi.00345.2002. [DOI] [PubMed] [Google Scholar]
- 24.Murthy K. S., Zhou H., Huang J., Pentyala S. Activation of PLC-δ1 by Gi/o-coupled receptor agonists. Am. J. Physiol. Cell Physiol. 2004;287:C1209–C1218. doi: 10.1152/ajpcell.00257.2004. [DOI] [PubMed] [Google Scholar]
- 25.Murthy K. S., Makhlouf G. M. Opioid μ, δ, κ receptor-induced activation of PLC-β3 and inhibition of adenylyl cyclase is mediated by Gi2 and Go in smooth muscle. Mol. Pharmacol. 1996;50:870–877. [PubMed] [Google Scholar]
- 26.Murthy K. S., Coy D. H., Makhlouf G. M. Somatostatin-receptor mediated signaling in smooth muscle: activation of PLC-β3 by Gβ and inhibition of adenylyl cyclase by Gαi1 and Gαo. J. Biol. Chem. 1996;271:23458–23463. doi: 10.1074/jbc.271.38.23458. [DOI] [PubMed] [Google Scholar]
- 27.Murthy K. S., Makhlouf G. M. Adenosine A1 receptor-mediated activation of PLC-β3 in intestinal muscle: dual requirement for α and βγ subunits of Gi. Mol. Pharmacol. 1995;47:1172–1179. [PubMed] [Google Scholar]
- 28.Delcommenne M., Tan C., Gray V., Rue L., Woodgett J., Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc. Natl. Acad. Sci. U.S.A. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu C., Dedhar S. Integrin-linked kinase (ILK) and its interactors: a new paradigm for the coupling of extracellular matrix to actin cytoskeleton and signaling complex. J. Cell Biol. 2001;155:505–510. doi: 10.1083/jcb.200108077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang J., Zhou H., Mahavadi S., Sriwai W., Lyall V., Murthy K. S. Signaling pathways mediating gastrointestinal smooth muscle contraction and MLC20 phosphorylation by motilin receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;288:G23–G31. doi: 10.1152/ajpgi.00305.2004. [DOI] [PubMed] [Google Scholar]
- 31.Zhou H., Murthy K. S. Distinctive G protein-dependent signaling in smooth muscle by sphingosine 1-phosphate receptors, S1P1 and S1P2. Am. J. Physiol. Cell Physiol. 2004;286:C1130–C1138. doi: 10.1152/ajpcell.00429.2003. [DOI] [PubMed] [Google Scholar]
- 32.Hersh E., Huang J., Grider J. R., Murthy K. S. Gq/G13 signaling by ET-1 in smooth muscle: MYPT1 phosphorylation via ETA and CPI-17 dephosphorylation via ETB. Am. J. Physiol. Cell Physiol. 2004;287:C1209–C1218. doi: 10.1152/ajpcell.00198.2004. [DOI] [PubMed] [Google Scholar]
- 33.Muranyi A., Macdonald J. A., Deng J. T., Wilson D. P., Haystead T. A. J., Walsh M. P., Erdodi F., Kiss E., Wu Y., Hartshorne D. J. Phosphorylation of the myosin phosphatase target subunit by integrin-linked kinase. Biochem. J. 2002;366:211–216. doi: 10.1042/BJ20020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiss E., Muryani A., Csortos C., Gergely P., Ito M., Hartshorne D. J., Erdodi F. Integrin-linked kinase phosphorylates myosin phosphatase target subunit at the inhibitory site in platelet cytoskeleton. Biochem. J. 2002;365:79–87. doi: 10.1042/BJ20011295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim N., Cao W., Song I. S., Kim C. Y., Harnett K. M., Cheng L., Walsh M. P., Biancani P. Distinct kinases are involved in contraction of cat esophageal and lower esophageal smooth muscles. Am. J. Physiol. Cell Physiol. 2004;287:C384–C394. doi: 10.1152/ajpcell.00390.2003. [DOI] [PubMed] [Google Scholar]
- 36.Wirth A., Schroeter M., Kock-Hauser C., Manser E., Chalovich J. M., de Lanerolle P., Pfitzer G. Inhibition of contraction and myosin light chain phosphorylation in guinea-pig smooth muscle by p21-activated kinase 1. Biochem. J. 2003;549:489–500. doi: 10.1113/jphysiol.2002.033167. [DOI] [PMC free article] [PubMed] [Google Scholar]