Abstract
Hypochlorous acid (HOCl) is produced by the neutrophil enzyme, myeloperoxidase, and reacts with amines to generate chloramines. These oxidants react readily with thiols and methionine and can affect cell-regulatory pathways. In the present study, we have investigated the ability of HOCl, glycine chloramine (Gly-Cl) and taurine chloramine (Tau-Cl) to oxidize IκBα, the inhibitor of NF-κB (nuclear factor κB), and to prevent activation of the NF-κB pathway in Jurkat cells. Glycine chloramine (Gly-Cl) and HOCl were permeable to the cells as determined by oxidation of intracellular GSH and inactivation of glyceraldehyde-3-phosphate dehydrogenase, whereas Tau-Cl showed no detectable cell permeability. Both Gly-Cl (20–200 μM) and HOCl (50 μM) caused oxidation of IκBα methionine, measured by a shift in electrophoretic mobility, when added to the cells in Hanks buffer. In contrast, a high concentration of Tau-Cl (1 mM) in Hanks buffer had no effect. However, Tau-Cl in full medium did modify IκBα. This we attribute to chlorine exchange with other amines in the medium to form more permeable chloramines. Oxidation by Gly-Cl prevented IκBα degradation in cells treated with TNFα (tumour necrosis factor α) and inhibited nuclear translocation of NF-κB. IκBα modification was reversed by methionine sulphoxide reductase, with both A and B forms required for complete reduction. Oxidized IκBα persisted intracellularly for up to 6 h. Reversion occurred in the presence of cycloheximide, but was prevented if thioredoxin reductase was inhibited, suggesting that it was due to endogenous methionine sulphoxide reductase activity. These results show that cell-permeable chloramines, either directly or when formed in medium, could regulate NF-κB activation via reversible IκBα oxidation.
Keywords: chloramine, hypochlorous acid (HOCl), inhibitor of nuclear factor κB (IκB), methionine oxidation, methionine sulphoxide reductase (Msr), nuclear factor κB (NF-κB)
Abbreviations: AP, alkaline phosphatase; DNCB, -chloro-2,4-dinitrobenzene; DTT, dithiothreitol; EMSA, electrophoretic mobility-shift assay; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Gly-Cl, glycine chloramine; HBSS, Hanks balanced salt solution; IκB, inhibitor of nuclear factor κB; Msr, methionine sulphoxide reductase; NF-κB, nuclear factor κB; NP-40, Nonidet P40; Tau-Cl, taurine chloramine; TNFα, tumour necrosis factor α
INTRODUCTION
NF-κB (nuclear factor κB) is a major transcription factor that regulates expression of a large number of genes that code for cytokines, adhesion molecules and other components of the inflammatory response [1,2]. In unstimulated cells, NF-κB is retained in the cytoplasm as a complex with inhibitory proteins known as IκB (inhibitor of NF-κB) [3]. Upon stimulation, IκBα is phosphorylated, ubiquitinated and degraded by the proteasome [4–6]. The degradation of IκBα unmasks the nuclear localization sequence of NF-κB, allowing translocation into the nucleus and subsequent initiation of gene expression. There has been considerable interest in the activation of NF-κB by oxidants, but the physiological importance of this mechanism remains controversial [7,8]. Another oxidative process that inhibited NF-κB activation by stimuli such as TNFα (tumour necrosis factor α) was observed in cells treated with taurine chloramine (Tau-Cl) [9]. The inhibitory action of Tau-Cl was consistent with it oxidizing IκBα at Met45, rendering it resistant to degradation [9,10].
Tau-Cl is formed from taurine and hypochlorous acid (HOCl), which is generated by the neutrophil enzyme myeloperoxidase. HOCl is a strong oxidant and an effective bactericidal agent that can react with a wide range of biological targets.
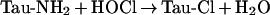 |
(1) |
Chloramines formed by reaction with amines (reaction 1) retain the oxidizing capability of HOCl, but are much less reactive and more selective for thiols and methionine residues [11,12]. Neutrophils contain high concentrations of taurine, and this has fuelled interest in the formation of Tau-Cl and its reactivity. However, amino groups on other amino acids react at least as rapidly as taurine with HOCl [13], so a range of chloramines could be generated at inflammatory sites. These chloramines are variably toxic, depending on their cell permeability, as well as on their reactivity [14,15]. They are also able to interact with cell signalling pathways, with monochloramine being shown to inhibit the expression of adhesion molecules [16] and Tau-Cl to down-regulate the production of various inflammatory cytokines and adhesion molecules [17–19]. This effect of Tau-Cl could be the result of inhibition of NF-κB [17]. Such inhibition also provides a mechanism whereby production of neutrophil oxidants could dampen down the inflammatory response.
The findings of Kanayama et al. [9] showing that Tau-Cl is able to oxidize IκBα, an intracellular target, are surprising since we and others have shown that Tau-Cl does not enter cells to any appreciable extent [20,21]. In their experiments, IκBα oxidation required high concentrations of Tau-Cl, and this was added to complete cell culture medium. Chloramines undergo chlorine exchange with other amines and amino acids [22], and it is therefore possible that Tau-Cl oxidized IκBα indirectly via a more permeable chloramine generated by chlorine exchange with an amino compound that is present in the medium (reaction 2).
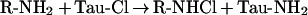 |
(2) |
Therefore we hypothesized that chlorine exchange from Tau-Cl to other amines in medium leads to more permeable chloramines that are responsible for the IκB oxidation and that permeable chloramines in the absence of medium would be much more effective oxidants.
To test this proposal, we compared the effects of Tau-Cl, glycine chloramine (Gly-Cl) and HOCl, on the NF-κB system in Jurkat cells, in HBSS (Hanks balanced salt solution) and in complete cell medium. We show that cell permeability is critical, that low concentrations of Gly-Cl modify IκBα and prevent NF-κB translocation to the nucleus, and that Tau-Cl is effective only in media where chlorine exchange can occur. We have also examined the ability of the cells to reverse IκBα modification and the role of Msr (methionine sulphoxide reductase).
MATERIALS AND METHODS
Reagents
Cell culture media and supplies were from Gibco BRL, supplied by Invitrogen. Antibodies directed against IκBα and p65 of NF-κB were purchased from Santa Cruz Biotechnology. Hoechst 33342 stain was from Molecular Probes, FITC-conjugated anti-rabbit F(ab′)2 from Jackson ImmunoResearch Laboratories, and AP (alkaline phosphatase) and Complete™ protease inhibitors were from Roche Diagnostics. Reagent HOCl was supplied by Sara Lee Corp. Other chemicals were purchased from Sigma, Chemical Co.
Preparation of Tau-Cl and Gly-Cl
Chloramines were freshly prepared by mixing taurine or glycine with HOCl at the molar ratio of 5:1 in HBBS. The 5-fold excess of amino acid ensured that only monochloramine and no dichloramine was formed [15]. Chloramine concentrations were determined spectrophotometrically using ϵ252 415 M−1·cm−1 [15].
Cell culture and treatment with chloramines
Jurkat T-lymphocytes (A.T.C.C., Manassas, VA, U.S.A.) were maintained in RPMI 1640 medium supplemented with 10% (w/v) heat inactivated foetal calf serum, 100 units/ml penicillin, and 100 μg/ml streptomycin maintained at 37 °C in a humidified atmosphere of 5% CO2. Cells were not made quiescent by the removal of serum before treatment. Before treatment with HOCl or chloramines, cells were washed three times with HBSS to remove any potential oxidant scavengers and suspended in HBSS at 2×106 cells/ml. The indicated concentration of oxidant was added to 1 ml of cells which was incubated at 37 °C for the indicated times. For incubations in medium, chloramines were added into complete medium containing 2×106 cells/ml and immediately mixed. Reactions were stopped by the addition of excess methionine or 3,3′-thiodipropionic acid to remove unreacted chloramines.
GAPDH (glyceraldehyde-3-phosphate dehydrogenase) assay
Cells were lysed by the addition of 0.025% (v/v) NP-40 (Nonidet P40) in PBS and centrifuged at 13000 g for 10 min at 4 °C to obtain a cytoplasmic extract. GAPDH activity was assayed in an enzymatically linked reaction with phosphoglycerate kinase by following the oxidation of NADH at 340 nm [23].
GSH measurements
After oxidant exposure, Jurkat cells were treated with 1 mM monobromobimane for 20 min in the dark to derivatize GSH, and were analysed using HPLC using a SPHERI-5 ODS 5 micron column (Brownlee Columns, Applied Biosystems) with fluorescence detection [24]. The retention times and peak areas from the cell extracts were compared with a standard GSH solution, derivatized and separated as above.
Western blotting of IκBα
After oxidant treatment, cells were washed three times with ice-cold PBS and lysed with NP-40 lysis buffer (25 mM Hepes, pH 7.4, 100 mM NaCl, 2 mM EDTA, 1.6 mg/ml Complete™ protease inhibitors and 0.5% NP-40) for 15 min on ice. Insoluble material was removed by centrifugation at 13000 g for 5 min. Equal amounts of protein from cell lysates were separated by SDS/15% PAGE. To detect the small change in the molecular mass of IκBα due to oxidation, the gel was overrun until IκBα came close to the bottom of the gel. The proteins were then transferred on to a PVDF membrane, and the blots were probed with antibodies against IκBα, according to the manufacturer's instructions. A horseradish-peroxidase-conjugated secondary antibody was used to visualize the immunoblotted proteins using the ECL® (enhanced chemiluminescence) technique (Amersham Biosciences). Protein concentrations were determined by the Bradford assay using BSA as a standard (Bio-Rad).
Treatment of cell lysates with AP and Msr
After exposure to 150 μM Gly-Cl for 15 min in HBSS, cells were collected, centrifuged at 13000 g for 10 min at 4 °C and resuspended in NP-40 lysis buffer. For treatment with AP, cell lysates (25 μl each) were adjusted to pH 9.8 with 0.1 M NaOH then mixed with 10 units of AP and incubated at 37 °C for 30 min. To treat with MsrA/B, a sample of the supernatant (25 μl) was supplemented with 20 mM DTT (dithiothreitol) and treated with 6 μM MsrA/B (recombinant Staphylococcus aureus MsrA and MsrB prepared as reported previously [25]). Cell lysates were incubated for 15 min at 37 °C. After these enzyme treatments, each sample was mixed with loading gel buffer, boiled for 5 min, separated on a 15% polyacrylamide gel and Western blotting was performed as described above.
EMSA (electrophoretic mobility-shift assay)
Nuclear extracts were prepared as described previously [26]. An oligonucleotide probe containing the binding site of NF-κB (5′-AGTTGAGGGGACTTTCCCAGGC-3′) labelled with biotin (custom-synthesized by Invitrogen) was incubated with 2.5 μg of nuclear extract for 20 min at room temperature (20 °C). The protein–DNA complexes were fractionated on a 6% native polyacrylamide gel run in 0.5× TBE (Tris/borate/EDTA) buffer and visualized by the Light Shift EMSA kit (Pierce) following the manufacturer's instructions.
Immunofluorescence assay for NF-κB activation in Jurkat cells
Cells in 100 μl aliquots were cytospun on to glass slides, fixed with 4% (w/v) buffered paraformaldehyde (pH 7.4) for 30 min followed by blocking and permeabilization for 1 h in PBS with 1% (w/v) BSA and 0.1% (w/v) saponin at room temperature [27]. Each cytospin spot was incubated overnight at 4 °C with anti-(NF-κB p65) antibody (Santa Cruz Biotechnology) in a humidified chamber [27]. After three washes with PBS/1% (w/v) BSA, the cells were blocked further with 10% goat serum for 30 min followed by incubation with FITC-conjugated goat anti-rabbit antibody at room temperature. After rinsing with PBS, the slides were counterstained with Hoechst 33342 (10 μg/ml) for 5 min. The slides were then washed, air-dried and mounted with glycerol and examined using fluorescence microscopy at 40× magnification.
RESULTS
Permeability and intracellular thiol oxidation by chloramines
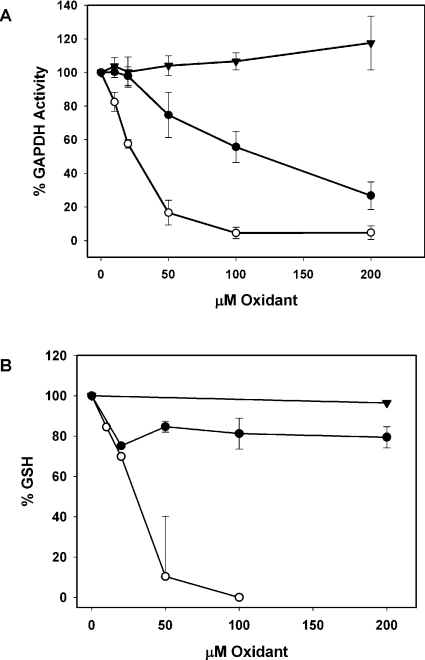
Tau-Cl is a charged molecule that has been shown for various cell types to have low permeability, whereas HOCl and other chloramines, such as Gly-Cl, can cross cell membranes [15,20]. To confirm that this is the case with Jurkat cells, the activity of GAPDH, an intracellular thiol enzyme that is particularly sensitive to inactivation by HOCl and chloramines [28], was monitored. There was no detectable decrease in GAPDH activity on treating cells for 15 min in HBSS with up to 200 μM Tau-Cl (Figure 1A). This contrasts with HOCl, which, under the same conditions, caused complete inactivation of GAPDH, and with Gly-Cl, where over 80% of the activity was inhibited at 200 μM. These results are similar to those reported for endothelial cells treated with chloramines [21]. At these concentrations of Tau-Cl and Gly-Cl, cells show no signs of necrosis or lysis, whereas, at the higher HOCl concentration (40 μM), cells are targeted for subsequent apoptosis [29,30].
Figure 1. (A) GAPDH inactivation and (B) GSH loss in Jurkat cells treated with Tau-Cl (▼) Gly-Cl (●) or HOCl (○) in HBSS for 15 min.
To stop the reaction, methionine was added to scavenge any unreacted oxidant. Cytoplasmic extracts were assayed for GAPDH activity or GSH as described in the Materials and methods section. Results are means±S.D. for at least three experiments. The GSH concentration in the control cells was 5.9 nmol.
The intracellular concentration of GSH was also monitored. There was a dramatic loss of GSH when Jurkat cells were reacted with low concentrations of HOCl (Figure 1B). Up to 200 μM Gly-Cl treatment caused an approx. 20% loss of GSH, while only minor losses were seen with 1 mM Tau-Cl.
Chloramine-induced modification of IκBα
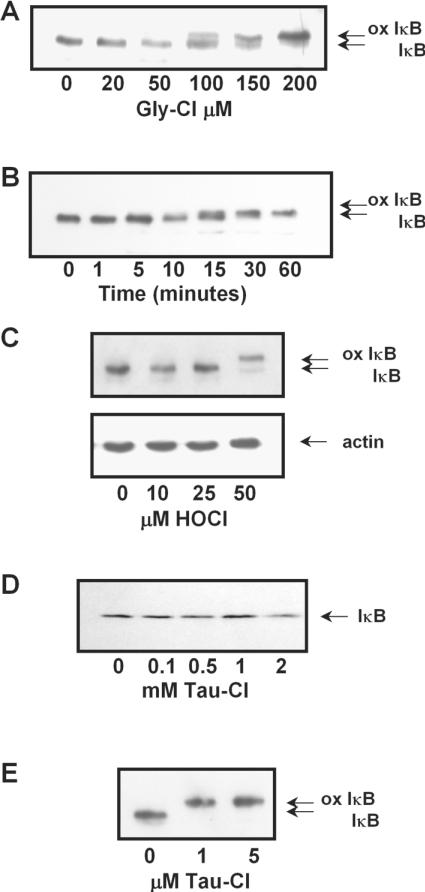
The reported oxidation of IκBα on Met45 was shown to retard the oxidized protein when run on SDS/PAGE, to form a doublet with the unoxidized protein [9]. To determine whether the retardation is chloramine-specific, we characterized the dose-dependent effects of Gly-Cl, Tau-Cl and HOCl in HBSS. Gly-Cl at 50–200 μM in HBSS altered the IκBα mobility (Figure 2A), with significant retardation of IκBα being detected after 10 min with 100 μM Gly-Cl, and a complete shift to the fully oxidized form within 60 min (Figure 2B). Oxidation of IκBα was also seen when cells were treated with 50 μM HOCl (Figure 2C). Tau-Cl in HBSS caused no change in the IκBα band (Figure 2D) even at the higher concentrations (1 mM) (Figure 2D) reported previously to induce oxidation [9,10]. However, if Jurkat cells were lysed before treatment, a complete shift to the fully oxidized form of IκBα was seen even with 1 μM Tau-Cl (Figure 2E).
Figure 2. Concentration-dependent oxidation of IκBα in Jurkat cells treated in HBSS.
Concentration-dependent oxidation of IκBα in Jurkat cells treated in HBSS with (A) Gly-Cl (C) HOCl or (D) Tau-Cl, assessed after 15 min, the lower panel shows actin as a loading control; (B) time course of IκBα oxidation by 100 μM Gly-Cl; (E) concentration dependence for cells that were lysed before treating with Tau-Cl for 10 min. All reactions were stopped by the addition of 1 mM methionine. All cell lysates were assessed for oxidation of IκBα by running samples by SDS/15% PAGE for 2.5 h and blotting for IκBα, as described in the Materials and methods section. Oxidized IκB (ox IκB) is evident as a slower migrating band.
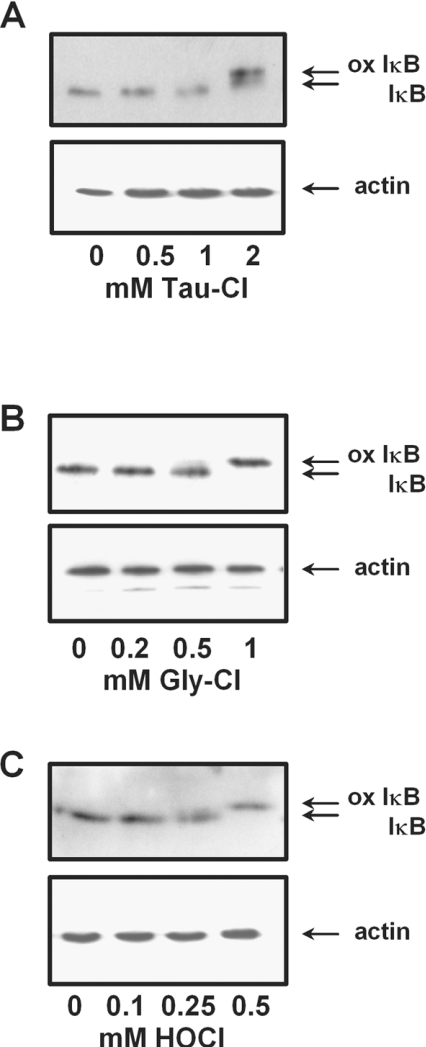
When the above experiments were repeated with cells in medium containing foetal calf serum, Tau-Cl at 2 mM induced the oxidation of IκBα (Figure 3A). Gly-Cl and HOCl treatment also induced the IκBα shift in medium (Figures 3B and 3C), but only at concentrations 5–10-fold higher than those shown in Figure 2 to induce changes in HBSS.
Figure 3. Concentration-dependent oxidation of IκBα in Jurkat cells treated in complete medium with (A) Tau-Cl (B) Gly-Cl or (C) HOCl for 30 min.
Analyses were performed as in Figure 2 with the lower panel showing actin as a loading control. ox IκB, oxidized IκB.
Characterization of chloramine-induced modification of IκBα
The band shift caused by chloramine treatment of IκBα was similar to that associated with the TNFα-stimulated phosphorylation of Ser32 and Ser36. However, reacting lysates from Gly-Cl pre-treated cells with AP did not affect the altered electrophoretic mobility of the IκBα (Figure 4, lane 5), indicating that it was not due to serine phosphorylation. Instead, treatment with Msr, which selectively reduces free or protein-bound oxidized methionine residues, reversed the band shift completely (Figure 4, lane 10). There are cysteine residues within IκBα that are potential targets for oxidation by chloramines. However, addition of DTT to the cell extract was unable to reverse the band shift, indicating that this was not due to reversible cysteine oxidation (Figure 4, lanes 2 and 4).
Figure 4. Reversal of IκBα oxidation by Msr.
Cells were treated with 150 μM Gly-Cl for 15 min. Lysates from Gly-Cl-treated cells containing 20 mM DTT were treated with 5 μg of MsrA, 5 μg of MsrB or both enzymes for 15 min at 37 °C, or with 10 units of AP for 30 min. Blots were analysed with anti-IκBα antibodies as in Figure 2. Similar results were obtained from three independent experiments. ox IκB, oxidized IκB.
Non-enzymatic methionine oxidation generates a mixture of the (R)- and (S)-isomers of the sulphoxide. In the Msr family of enzymes, MsrA is specific for the (S)-isomer and MsrB for the (R)-isomer [25,31,32]. A combination of the two enzymes (MsrA/B) (Figure 4) was required for complete reduction of the IκB from Gly-Cl-pre-treated cells, while treatment with either MsrA or MsrB only showed partial reduction (Figure 4B, lanes 8 and 9). These results show that IκBα is oxidized on a methionine by Gly-Cl and that both (S)- and (R)-methionine sulphoxide are formed.
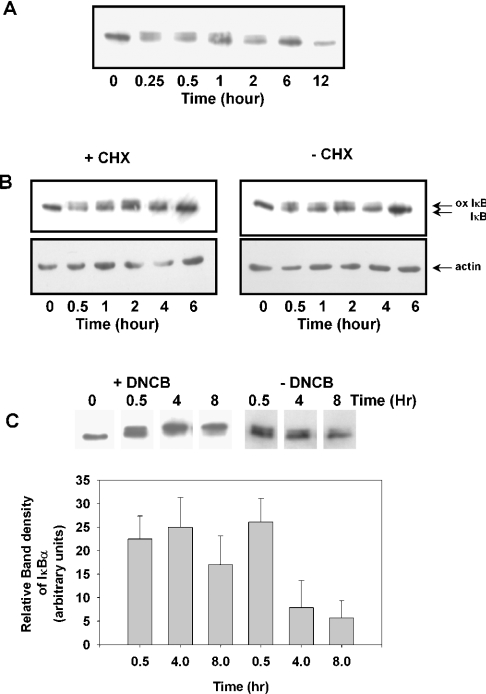
Time course of chloramine-induced oxidation of IκBα
To determine how long the IκBα remained oxidized, cells were treated with Gly-Cl, then the reaction was stopped by the addition of methionine, and the cells were placed back into fresh medium. The oxidized form of IκBα was evident up to 6 h (Figure 5A). To determine whether the return of unoxidized IκBα was due to re-synthesis following degradation of the oxidized IκBα or to reversal of methionine oxidation, cells were pre-treated with cycloheximide to stop all protein synthesis before treating with Gly-Cl and placing back into medium (Figure 5B). Cycloheximide made no difference to the ability of IκBα to revert to the unoxidized form, suggesting that it was not due to re-synthesis. To ascertain whether the gradual disappearance of the retarded band was due to endogenous Msr activity, DNCB (1-chloro-2,4-dinitrobenzene) was used to inhibit thioredoxin reductase [33], which, along with thioredoxin, is required to maintain Msr activity. When the cells were pre-treated for 10 min with DNCB before exposure to Gly-Cl, the oxidized IκBα was not reduced, even after 8 h (Figure 5C).
Figure 5. Intracellular persistence of oxidized IκBα.
Jurkat cells were pre-treated with 150 μM Gly-Cl and, after quenching the chloramine by the addition of 1 mM methionine, were returned to complete medium for the indicated times. Cell lysates were assessed for oxidation of IκBα as in Figure 2. (A) Time course for recovery of IκBα. (B) Effects of pre-treatment with 20 μM cycloheximide (CHX) for 1 h. ox IκB, oxidized IκB. (C) Effects of pre-treatment with 30 μM DNCB for 10 min. The histogram shows relative band densities of cells treated or not with DNCB from four independent experiments.
Chloramine-dependent inhibition of IκBα degradation induced by TNFα
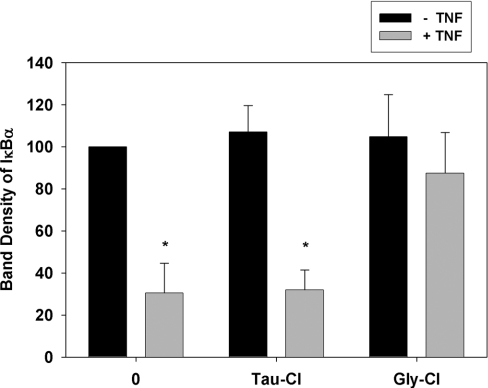
Activation of the NF-κB pathway with agonists such as TNFα results in serine phosphorylation of IκBα. This initiates the degradation of IκBα and nuclear translocation of NF-κB [34,35]. The phosphorylated band is retarded on SDS/PAGE, but it is rapidly degraded and does not accumulate. This was evident as a decrease in intensity of the IκBα band after Jurkat cells were stimulated with TNFα in HBSS (Figure 6). Pre-treatment with Tau-Cl in HBSS did not affect the ability of TNFα to induce the degradation of the IκBα band. In contrast, the retarded band formed in cells exposed to Gly-Cl was not degraded following TNFα treatment (Figure 6).
Figure 6. Effects of Gly-Cl and Tau-Cl on the response of IκBα to TNFα.
Jurkat cells were pre-treated with 150 μM Tau-Cl or Gly-Cl for 15 min in HBSS then stimulated with 20 ng/ml TNFα for 30 min. Analyses were performed as in Figure 2. Band intensities were measured by densitometry and normalized to the untreated control, which was set to 100%. Results are means±S.D. for four experiments *P<0.001, compared with the untreated cells; all other treatments were not statistically significant.
Chloramine-induced inhibition of nuclear translocation and activation of NF-κB after TNFα stimulation
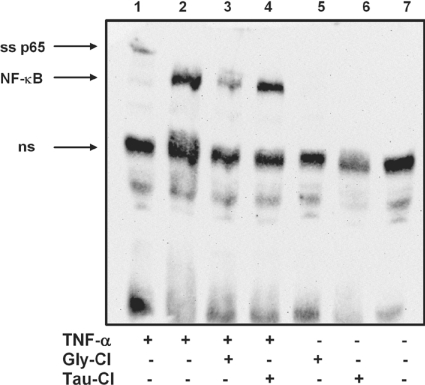
To determine the effect of Gly-Cl-induced inhibition of IκBα degradation on NF-κB activity, nuclear extracts were prepared and an EMSA was carried out. Cells treated with 20 ng/ml TNFα gave a strong signal indicative of NF-κB binding to the probe (Figure 7, lane 2). This was not affected by pre-treating the cells with Tau-Cl (Figure 7, lane 4). In contrast, the nuclear transfer of NF-κB was markedly suppressed by pre-treatment for 15 min with Gly-Cl (Figure 7, lane 3). Tau-Cl and Gly-Cl on their own caused no stimulation of NF-κB activity (Figure 7, lanes 5 and 6).
Figure 7. Suppression of TNFα-induced nuclear transfer of NF-κB by Gly-Cl.
Cells were treated with either Gly-Cl or Tau-Cl, as indicated, for 15 min at 37 °C. Fresh medium containing TNFα (20 ng/ml) (lanes 1–4) or medium alone (lanes 5 and 6) was added and cells were incubated for a further 45 min. Nuclear extracts were prepared for EMSA. Lanes 2, 3 and 4 were all from TNFα-stimulated cells. Lane 3, pre-treated with Gly-Cl. Lane 4, pre-treated with Tau-Cl. Anti-p65 was added to the extract in lane 1, where ss p65 indicates the supershifted p65 subunit of NF-κB, and ns indicates non-specific bands. Lane 7 represents control cells. Similar results were obtained in three independent experiments.
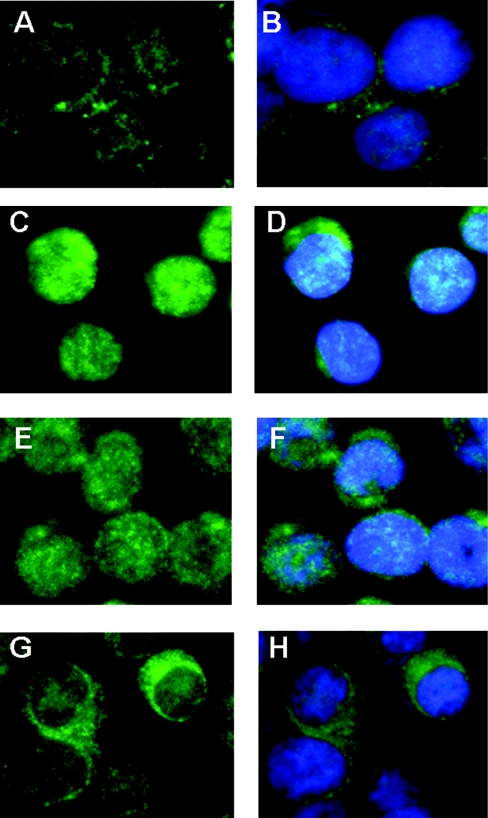
Translocation of NF-κB was also examined using immunofluorescence. As expected, TNFα stimulation induced NF-κB activation, as shown by the translocation of the p65 subunit from the cytoplasm (Figures 8A and 8B) to the nucleus (Figures 8C and 8D). Pre-treatment with Tau-Cl for 15 min before stimulation with TNFα also showed p65 translocation into the nucleus (Figures 8E and 8F). However, when cells were pre-treated with Gly-Cl (Figures 8G and 8H) and then incubated with TNFα, nuclear staining was drastically decreased, indicating that NF-κB was not activated.
Figure 8. Effects of Gly-Cl and Tau-Cl on TNFα-mediated translocation of NF-κB to the nucleus in Jurkat cells.
After 30 min of treatment with 100 μM Gly-Cl or 1 mM Tau-Cl, cells were stimulated with TNFα (20 ng/ml) for 30 min and analysed for translocation of NF-κB to the nucleus with anti-p65 antibody. (B, D, F and H) show cells that were counterstained with Hoechst 33342 to visualize the nuclei. (A, B) Jurkat cells without TNFα-stimulation. (C, D) TNFα-stimulated Jurkat cells. (E, F) TNFα-stimulated cells after pre-treatment with Tau-Cl. (G, H) TNFα-stimulated cells after pre-treatment with Gly-Cl. In (D) and (F), but not (B) and (H), there is co-localization of the fluorescence-conjugated antibody and nuclear stain.
DISCUSSION
We have shown using SDS/PAGE and Western blotting of cell extracts that treatment of Jurkat cells with Gly-Cl causes a shift in the position of the IκBα band. The shift was apparent with 50 μM Gly-Cl after 15 min, and the proportion of the retarded band increased with both time and concentration, as more chloramine entered the cells. A similar band shift was observed with HOCl, although this occurred at a concentration that was close to being toxic to the cells [29]. The retarded band was not degraded in cells stimulated with TNFα, and pre-treatment of the cells with Gly-Cl prevented NF-κB migration into the nucleus. In contrast, no shift of the IκBα band or inhibition of NF-κB activation was seen when cells were treated with Tau-Cl in HBSS, even at a 10-fold higher concentration. The important difference between these oxidants is that Gly-Cl and HOCl, but not Tau-Cl, are able to oxidize intracellular targets as demonstrated by the inactivation of GAPDH. HOCl and chloramines readily inactivate GAPDH, and loss of intracellular activity provides a sensitive index of cell permeability to these oxidants [21,22]. Our findings with Jurkat cells are similar to those with endothelial cells and fibroblasts, which also consume Gly-Cl, albeit slowly, but not Tau-Cl [21,36]. We therefore conclude from our results that HOCl and chloramines can modify IκBα and prevent NF-κB activation, provided that they can penetrate the cells.
The IκBα mobility changes we observed are the same as reported by Kanayama et al. [9]. However, in contrast with our results, they saw these effects in cells treated with Tau-Cl. The key difference between the two studies is that they added Tau-Cl to cells in culture medium that contains other amino acids, whereas we used HBSS. We also saw IκBα oxidation with high concentrations of Tau-Cl in medium. These observations can be explained by the ability of chloramines to undergo trans-chlorination reactions with other amines in the medium. In the case of Tau-Cl, this produces more permeable chloramines that are able to access IκBα and cause modifications. Trans-chlorination was demonstrated directly by Peskin et al. [22] who showed that mixtures of Tau-Cl and glycine form Gly-Cl within a few minutes. They also saw oxidation of GAPDH when Tau-Cl was added to cells in medium, indicating that a cell-permeable oxidant is formed. Gly-Cl and HOCl were also able to modify IκBα when added to cells in medium, but at much higher concentrations than were effective in HBSS. This could partly be a result of exchange reactions that gave less-reactive chloramines. Scavenging of the oxidants by medium components such as methionine and thiols would also have decreased their effectiveness.
The mobility shift of the IκBα band after Gly-Cl exposure was reversed by treating cell extracts with Msr. This provides further support for the findings of Kanayama et al. [9], who observed a similar shift in the mobility of recombinant IκBα when treated with Tau-Cl. They used deletion mutants in which methionine residues were replaced by alanine to show that Met45 was necessary for the shift and showed by MALDI (matrix-assisted laser-desorption ionization) MS that Tau-Cl converted it into the sulphoxide. This shift was able to be reversed when extracts were treated with MsrA [10]. We found that complete reversal was possible, but that it required a combination of MsrA and MsrB. This indicates that Met45 was oxidized to both the (S)- and (R)-isomers of the sulphoxide. Our observation that a high concentration of DTT in treated extracts did not reverse the band shift substantiates the conclusion of Kanayama et al. [9] that oxidation of a cysteine residue was not responsible for the change in IκBα gel mobility.
All of the evidence suggests that methionine sulphoxide formation on IκBα prevents its subsequent ubiquitin-mediated degradation. We showed that AP treatment had no effect on the mobility of the oxidized protein, and oxidized IκBα was not degraded when the cells were activated with TNFα. The susceptible methionine residue is close to the phosphorylation sites at Ser32 and Ser36. Methionine sulphoxide formation, particularly if it occurs within helices, can cause major conformational rearrangements [37], and it seems most likely that oxidation of Met45 causes structural disruption that makes the protein no longer a target for kinase action.
The oxidized IκBα band persisted for many hours in the Jurkat cells, but was eventually converted into a single band in the original position. As cycloheximide did not prevent this conversion, we conclude that it was due to reversal of oxidation rather than to degradation and resynthesis. Our findings with DNCB-treated cells are consistent with reversal being due to endogenous Msr activity. By inhibiting thioredoxin reductase, DNCB would prevent recycling of the thioredoxin used by Msr as a co-substrate, thereby preventing the reductase from reducing the sulphoxide on IκBα.
Methionine is one of the most favoured biological targets for HOCl and chloramines [11,38]. Met45 of IκBα appears to be particularly susceptible. Oxidation occurred over the same concentration range of Gly-Cl that inactivated GAPDH, when less than 20% of the GSH was oxidized. This implies that the rate of reaction of IκBα methionine in the cell is higher than that of GSH. As the rates of reaction of Gly-Cl with GSH and free methionine are approximately equal [11], this facile oxidation suggests that the environment of Met45 in the IκB protein enhances its reactivity. Furthermore, our results show that, although GSH is one of the best antioxidants against HOCl and chloramines, it does not protect IκBα against oxidation. The sensitivity of IκBα to oxidants such as chloramines, and its ability to be reduced by Msr raise the possibility of IκBα being a critical cell target for redox regulation. Although involvement of cysteine residues in redox signalling is well documented, selective oxidation of methionine residues as a signalling mechanism has received less attention. Initial views were that methionine residues act as an oxidant sink, with Msr activity enabling regeneration [39]. However, as more proteins are shown to undergo functional changes as a result of methionine oxidation [40,41], a role for methionine in redox regulation is becoming more likely. Regulation of the NF-κB pathway via IκBα oxidation is a potential candidate for this mechanism.
Our results also have significant implications for experimental studies of chloramines. The cellular effects of a particular chloramine will depend not only on its ability to cross cell membranes, but also on the presence of reactive molecules in the extracellular medium. In the more complex systems such as full medium or serum, where multiple chlorine exchange reactions could occur, the initial chloramine may not have a major impact on outcome. Tau-Cl in culture medium has been shown to inhibit NF-κB-dependent gene expression of iNOS (inducible nitric oxide synthase) and cytokine production induced by TNFα and to generally down-regulate the inflammatory process [16–19]. These effects are likely to be due to secondary chloramines generated from the medium. They should also occur with other chloramines and cell-permeable compounds such as Gly-Cl or monochloramine. Our results demonstrate further that chloramines generated by neutrophils at inflammatory sites may play a role in down-regulating the NF-κB response in an oxidant-mediated feedback mechanism.
Acknowledgments
This study was supported by the Health Research Council of New Zealand and a National Heart Foundation Postgraduate Scholarship to R.G.M. F.-C.C. was a holder of the University of Otago 125th Jubilee International Postgraduate Scholarship Award.
References
- 1.Baeuerle P. A., Henkel T. Function and activation of NF-κB in the immune system. Annu. Rev. Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin A. S., Jr Series introduction: the transcription factor NF-κB and human disease. J. Clin. Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeuerle P. A., Baltimore D. IκB: a specific inhibitor of the NF-κB transcription factor. Science. 1988;242:540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- 4.Brown K., Gerstberger S., Carlson L., Franzoso G., Siebenlist U. Control of IκB-α proteolysis by site-specific signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 5.Henkel T., Machleidt T., Alkalay I., Kronke M., Ben-Neriah Y., Baeuerle P. A. Rapid proteolysis of IκB-α is necessary for activation of transcription factor NF-κB. Nature (London) 1993;365:182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- 6.Traenckner E. B., Pahl H. B., Henkel T., Schmidt K. N., Wilk S., Baeuerle P. A. Phosphorylation of human IκBα on serines 32 and 36 controls IκBα proteolysis and NF-κB activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowie A., O'Neill L. A. Oxidative stress and nuclear factor-κB activation: a reassessment of the evidence in the light of recent discoveries. Biochem. Pharmacol. 2000;59:13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- 8.Hayakawa M., Miyashita H., Sakamoto I., Kitagawa M., Tanaka H., Yasuda H., Karin M., Kikugawa K. Evidence that reactive oxygen species do not mediate NF-κB activation. EMBO J. 2003;22:3356–3366. doi: 10.1093/emboj/cdg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanayama A., Inoue J., Sugita-Konishi Y., Shimizu M., Miyamoto Y. Oxidation of IκBα at methionine 45 is one cause of taurine chloramine-induced inhibition of NF-κB activation. J. Biol. Chem. 2002;277:24049–24056. doi: 10.1074/jbc.M110832200. [DOI] [PubMed] [Google Scholar]
- 10.Mohri M., Reinach P. S., Kanayama A., Shimizu M., Moskovitz J., Hisatsune T., Miyamoto Y. Suppression of the TNFα-induced increase in IL-1α expression by hypochlorite in human corneal epithelial cells. Invest. Ophthalmol. Visual Sci. 2002;43:3190–3195. [PubMed] [Google Scholar]
- 11.Peskin A. V., Winterbourn C. C. Kinetics of the reactions of hypochlorous acid and amino acid chloramines with thiols, methionine, and ascorbate. Free Radical Biol. Med. 2001;30:572–579. doi: 10.1016/s0891-5849(00)00506-2. [DOI] [PubMed] [Google Scholar]
- 12.Peskin A. V., Winterbourn C. C. Histamine chloramine reactivity with thiol compounds, ascorbate, and methionine and with intracellular glutathione. Free Radical Biol. Med. 2003;35:1252–1260. doi: 10.1016/s0891-5849(03)00502-1. [DOI] [PubMed] [Google Scholar]
- 13.Pattison D. I., Davies M. J. Absolute rate constants for the reaction of hypochlorous acid with protein side chains and peptide bonds. Chem. Res. Toxicol. 2001;14:1453–1464. doi: 10.1021/tx0155451. [DOI] [PubMed] [Google Scholar]
- 14.Thomas E. L. Myeloperoxidase-hydrogen peroxide-chloride antimicrobial system: effect of exogenous amines on antibacterial action against Escherichia coli. Infect. Immun. 1979;25:110–116. doi: 10.1128/iai.25.1.110-116.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas E. L., Grisham M. B., Jefferson M. M. Preparation and characterization of chloramines. Methods Enzymol. 1986;132:569–585. doi: 10.1016/s0076-6879(86)32042-1. [DOI] [PubMed] [Google Scholar]
- 16.Omori M., Ogino T., Than T. A., Okada S. Monochloramine inhibits the expression of E-selectin and intercellular adhesion molecule-1 induced by TNF-α through the suppression of NF-κB activation in human endothelial cells. Free Radical Res. 2002;36:845–852. doi: 10.1080/1071576021000005276. [DOI] [PubMed] [Google Scholar]
- 17.Barua M., Liu Y., Quinn M. R. Taurine chloramine inhibits inducible nitric oxide synthase and TNF-α gene expression in activated alveolar macrophages: decreased NF-κB activation and IκB kinase activity. J. Immunol. 2001;167:2275–2281. doi: 10.4049/jimmunol.167.4.2275. [DOI] [PubMed] [Google Scholar]
- 18.Park E., Jia J., Quinn M. R., Schuller-Levis G. Taurine chloramine inhibits lymphocyte proliferation and decreases cytokine production in activated human leukocytes. Clin. Immunol. 2002;102:179–184. doi: 10.1006/clim.2001.5160. [DOI] [PubMed] [Google Scholar]
- 19.Chorazy M., Kontny E., Marcinkiewicz J., Maslinski W. Taurine chloramine modulates cytokine production by human peripheral blood mononuclear cells. Amino Acids. 2002;23:407–413. doi: 10.1007/s00726-002-0204-0. [DOI] [PubMed] [Google Scholar]
- 20.Grisham M. B., Jefferson M. M., Melton D. F., Thomas E. L. Chlorination of endogenous amines by isolated neutrophils: ammonia-dependent bactericidal, cytotoxic, and cytolytic activities of the chloramines. J. Biol. Chem. 1984;259:10404–10413. [PubMed] [Google Scholar]
- 21.Midwinter R. G., Peskin A. V., Vissers M. C. M., Winterbourn C. C. Extracellular oxidation by taurine chloramine activates ERK via the epidermal growth factor receptor. J. Biol. Chem. 2004;279:32205–32211. doi: 10.1074/jbc.M402070200. [DOI] [PubMed] [Google Scholar]
- 22.Peskin A. V., Midwinter R. G., Harwood D. T., Winterbourn C. C. Chlorine transfer between glycine, taurine and histamine: reaction rates and impact on cellular reactivity. Free Radical Biol. Med. 2004;15:1622–1630. doi: 10.1016/j.freeradbiomed.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Beutler E. Red Cell Metabolism: a Manual of Biochemical Methods. Orlando: Grune and Stratton; 1984. [Google Scholar]
- 24.Cotgreave I. A., Moldeus P. Methodologies for the application of monobromobimane to the simultaneous analysis of soluble and protein thiol components of biological systems. J. Biochem. Biophys. Methods. 1986;13:231–249. doi: 10.1016/0165-022x(86)90102-8. [DOI] [PubMed] [Google Scholar]
- 25.Moskovitz J., Singh V. K., Requena J., Wilkinson B. J., Jayaswal R. K., Stadtman E. R. Purification and characterization of methionine sulfoxide reductases from mouse and Staphylococcus aureus and their substrate stereospecificity. Biochem. Biophys. Res. Commun. 2002;290:62–65. doi: 10.1006/bbrc.2001.6171. [DOI] [PubMed] [Google Scholar]
- 26.Vile G. F., Rothwell L. A., Kettle A. J. Hypochlorous acid activates the tumor suppressor protein p53 in cultured human skin fibroblasts. Arch. Biochem. Biophys. 1998;359:51–56. doi: 10.1006/abbi.1998.0881. [DOI] [PubMed] [Google Scholar]
- 27.Janssen Y. M., Sen C. K. Nuclear factor κB activity in response to oxidants and antioxidants. Methods Enzymol. 1999;300:363–374. doi: 10.1016/s0076-6879(99)00141-x. [DOI] [PubMed] [Google Scholar]
- 28.Pullar J. M., Winterbourn C. C., Vissers M. C. M. Loss of GSH and thiol enzymes in endothelial cells exposed to sublethal concentrations of hypochlorous acid. Am. J. Physiol. 1999;277:H1505–H1512. doi: 10.1152/ajpheart.1999.277.4.H1505. [DOI] [PubMed] [Google Scholar]
- 29.Midwinter R. G., Vissers M. C. M., Winterbourn C. C. Hypochlorous acid stimulation of the MAP-kinase pathway enhances cell survival. Arch. Biochem. Biophys. 2001;394:13–20. doi: 10.1006/abbi.2001.2530. [DOI] [PubMed] [Google Scholar]
- 30.Vissers M. C. M., Pullar J. M., Hampton M. B. Hypochlorous acid causes caspase activation and apoptosis or growth arrest in human endothelial cells. Biochem. J. 1999;344:443–449. [PMC free article] [PubMed] [Google Scholar]
- 31.Moskovitz J., Poston J. M., Berlett B. S., Nosworthy N. J., Szczepanowski R., Stadtman E. R. Identification and characterization of a putative active site for peptide methionine sulfoxide reductase (MsrA) and its substrate stereospecificity. J. Biol. Chem. 2000;275:14167–14172. doi: 10.1074/jbc.275.19.14167. [DOI] [PubMed] [Google Scholar]
- 32.Sharov V. S., Schoneich C. Diastereoselective protein methionine oxidation by reactive oxygen species and diastereoselective repair by methionine sulfoxide reductase. Free Radical Biol. Med. 2000;29:986–994. doi: 10.1016/s0891-5849(00)00400-7. [DOI] [PubMed] [Google Scholar]
- 33.Arner E. S., Bjornstedt M., Holmgren A. 1-Chloro-2,4-dinitrobenzene is an irreversible inhibitor of human thioredoxin reductase: loss of thioredoxin disulfide reductase activity is accompanied by a large increase in NADPH oxidase activity. J. Biol. Chem. 1995;270:3479–3482. doi: 10.1074/jbc.270.8.3479. [DOI] [PubMed] [Google Scholar]
- 34.Blackwell T. S., Christman J. W. The role of nuclear factor-κB in cytokine gene regulation. Am. J. Respir. Cell Mol. Biol. 1997;17:3–9. doi: 10.1165/ajrcmb.17.1.f132. [DOI] [PubMed] [Google Scholar]
- 35.Kopp E. B., Ghosh S. NF-κB and rel proteins in innate immunity. Adv. Immunol. 1995;58:1–27. doi: 10.1016/s0065-2776(08)60618-5. [DOI] [PubMed] [Google Scholar]
- 36.Vile G. F., Rothwell L. A., Kettle A. J. Initiation of rapid, p53-dependent growth arrest in cultured human skin fibroblasts by reactive chlorine species. Arch. Biochem. Biophys. 2000;377:122–128. doi: 10.1006/abbi.2000.1706. [DOI] [PubMed] [Google Scholar]
- 37.Bigelow D. J., Squier T. C. Redox modulation of cellular signaling and metabolism through reversible oxidation of methionine sensors in calcium regulatory proteins. Biochim. Biophys. Acta. 2005;1703:121–134. doi: 10.1016/j.bbapap.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Winterbourn C. C. Comparative reactivities of various biological compounds with myeloperoxidase-hydrogen peroxide-chloride, and similarity of the oxidant to hypochlorite. Biochim. Biophys. Acta. 1985;840:204–210. doi: 10.1016/0304-4165(85)90120-5. [DOI] [PubMed] [Google Scholar]
- 39.Levine R. L., Moskovitz J., Stadtman E. R. Oxidation of methionine in proteins: roles in antioxidant defense and cellular regulation. IUBMB Life. 2000;50:301–307. doi: 10.1080/713803735. [DOI] [PubMed] [Google Scholar]
- 40.Davis D. A., Newcomb F. M., Moskovitz J., Wingfield P. T., Stahl S. J., Kaufman J., Fales H. M., Levine R. L., Yarchoan R. HIV-2 protease is inactivated after oxidation at the dimer interface and activity can be partly restored with methionine sulphoxide reductase. Biochem. J. 2000;346:305–311. [PMC free article] [PubMed] [Google Scholar]
- 41.Moskovitz J. Methionine sulfoxide reductases: ubiquitous enzymes involved in antioxidant defense, protein regulation, and prevention of aging-associated diseases. Biochim. Biophys. Acta. 2005;1703:213–219. doi: 10.1016/j.bbapap.2004.09.003. [DOI] [PubMed] [Google Scholar]