Abstract
The ubiquitously expressed molecular chaperone GRP78 (78 kDa glucose-regulated protein) generally localizes to the ER (endoplasmic reticulum). GRP78 is specifically induced in cells under the UPR (unfolded protein response), which can be elicited by treatments with calcium ionophore A23187 and sarcoplasmic/endoplasmic reticulum Ca2+-ATPase inhibitor TG (thapsigargin). By using confocal microscopy, we have demonstrated that GRP78 was concentrated in the perinuclear region and co-localized with the ER marker proteins, calnexin and PDI (protein disulphide-isomerase), in cells under normal growth conditions. However, treatments with A23187 and TG led to diminish its ER targeting, resulting in redirection into a cytoplasmic vesicular pattern, and overlapping with the mitochondrial marker MitoTracker. Cellular fractionation and protease digestion of isolated mitochondria from ER-stressed cells suggested that a significant portion of GRP78 is localized to the mitochondria and is protease-resistant. Localizations of GRP78 in ER and mitochondria were confirmed by using immunoelectron microscopy. In ER-stressed cells, GRP78 mainly localized within the mitochondria and decorated the mitochondrial membrane compartment. Submitochondrial fractionation studies indicated further that the mitochondria-resided GRP78 is mainly located in the intermembrane space, inner membrane and matrix, but is not associated with the outer membrane. Furthermore, radioactive labelling followed by subcellular fractionation showed that a significant portion of the newly synthesized GRP78 is localized to the mitochondria in cells under UPR. Taken together, our results indicate that, at least under certain circumstances, the ER-resided chaperone GRP78 can be retargeted to mitochondria and thereby may be involved in correlating UPR signalling between these two organelles.
Keywords: calcium disturbance, chaperone, endoplasmic reticulum stress response, 78 kDa glucose-regulated protein (GRP78), mitochondrial targeting, unfolded protein response (UPR)
Abbreviations: [Ca2+]c, cytosolic Ca2+ concentration; [Ca2+]ER, endoplasmic reticulum Ca2+ concentration; COXIV, cytochrome c oxidase subunit IV; ER, endoplasmic reticulum; GRP78, 78 kDa glucose-regulated protein; HSP, heat-shock protein; IMS, intermembrane space; PDI, protein disulphide-isomerase; SERCA, sarcoplasmic/endoplasmic reticulum Ca2+-ATPase; TG, thapsigargin; UPR, unfolded protein response; VDAC, voltage-dependent anion-selective channel
INTRODUCTION
The ER (endoplasmic reticulum) is a multifunctional organelle that controls important cellular processes, including Ca2+ homoeostasis, protein synthesis, protein trafficking and apoptosis [1,2]. [Ca2+]ER (ER Ca2+ concentration) is regulated by the concerted actions of Ca2+-binding proteins, Ca2+ pumps and Ca2+ release channels [3], and it is known that a constantly high luminal level of Ca2+ is essential for protein folding [4]. Under physiologically or pharmacologically adverse conditions that perturb Ca2+ homoeostasis, accumulation of unfolded or misfolded proteins in the ER lumen occurs, referred to as ER stress, and the cells will activate a series of signal transduction cascades collectively termed the UPR (unfolded protein response) [5,6]. The UPR includes translational attenuation of global protein synthesis [7], ERAD (ER-associated protein degradation) [8] and transcriptional induction of genes coding for ER chaperones and protein-folding enzymes [9]. However, if ER stress is severe and prolonged, the cells will eventually activate the cell death pathway. ER stress contributes to neuronal apoptosis and excitotoxicity, and is associated with the pathogenesis of several different neurodegenerative disorders, including Alzheimer's disease and stroke [10,11].
One characteristic of the UPR is the induction of the ER-resident stress proteins referred to as the glucose-regulated proteins. The best characterized, GRP78 (78 kDa glucose-regulated protein), also known as BiP (immunoglobulin heavy-chain-binding protein), is thought to function in Ca2+ sequestration or as a molecular chaperone in the folding and assembly of membrane or secreted proteins [12–14]. Additionally, it is an anti-apoptosis protein. Overexpression and antisense approaches in cell systems show that GRP78 can protect cells against cell death caused by disturbances of ER homoeostasis. GRP78 can suppress elevations of intracellular Ca2+ levels following exposure of neurons to glutamate, and this effect of GRP78 apparently results from decreased release of Ca2+ from ryanodine-sensitive stores [15].
Mitochondria are central integrators and transducers for proapoptotic signals. However, emerging evidence suggests that mitochondria are important components of the ER-stress-induced apoptotic pathway [16]. Increased [Ca2+]c (cytosolic Ca2+ concentration) caused by [Ca2+]ER mobilization leads to a mitochondrial Ca2+ overload, which in turn causes the release of cytochrome c from the IMS (intermembrane space) of mitochondria into the cytosol. The cytosolic cytochrome c then binds to Apaf-1 (apoptotic protease-activating factor-1), initiating a proteolytic cascade that ultimately results in cell death [17]. The released cytochrome c may also bind to Ins(1,4,5)P3 receptor, which mediates further Ca2+ release from the ER, resulting in augmented cytochrome c release that amplifies the apoptotic signal [18]. Based on the facts that both ER and mitochondria are Ca2+-storage sites and that [Ca2+]c plays a pivotal role in cell death signalling, it is conceivable that apoptosis elicited by Ca2+ disturbance results from the concerted actions of ER and mitochondria [19–21].
Mammalian ER luminal chaperones, including GRP94 (94 kDa glucose-regulated protein), GRP78 and PDI (protein disulphide-isomerase) contain a C-terminal tetrapeptide sequence KDEL (Lys-Asp-Glu-Leu) that serves as an ER-retention signal and therefore is always regarded as an ER lumen protein [22]. However, some exceptions have been reported. For example, GRP94, which is normally confined to the ER, has been shown to escape the KDEL-mediated retention system to serve as a surface protein [23,24], secrete to the extracellular space [24] and associate with the pre-Golgi intermediate compartment [25]. Upon induction, GRP78 has been shown to be associated with keratin 8 in the cytoplasm [26], secreted to the extracellular space [24] and expressed on the cell surface [27]. Furthermore, ER stress can cause subpopulations of GRP78 to redistribute from the ER lumen to the cytosol and the ER membrane [28]. PDI is also found in the rest of the endomembrane system, including the Golgi, secretory vesicles, plasma membrane and mitochondria [29,30]. Additionally, calreticulin, a Ca2+-binding chaperone in the lumen of the ER, has been implicated in other cellular activities that occur in locations outside the ER, including the cytosol, cell surface and nucleus [31,32].
Experimentally, ER stress is routinely induced by pharmacological agents that disrupt ER Ca2+ storage, such as A23187, a Ca2+ ionophore, and TG (thapsigargin), an inhibitor of the SERCA (sarcoplasmic/endoplasmic reticulum-Ca2+ ATPase) pumps. Treatment with these drugs results in a decrease of [Ca2+]ER with a concurrent increase of [Ca2+]c, which in turn activates the UPR coupled with a potent induction of GRP78 [33–35]. The present study was performed to address the cellular distribution of GRP78 in cells under UPR induced by A23187 and TG. For the first time, we provide several lines of direct evidence indicating that GRP78 targets to the mitochondria in the ER-stress response. Moreover, proteinase K digestion experiments combined with submitochondrial localization studies suggest that a significant proportion of mitochondrial GRP78 is associated with the inner membrane and the matrix. These data are discussed in terms of the role of mitochondrial targeting of GRP78 in the apoptosis signalling pathway(s) involving the ER and the mitochondria.
MATERIALS AND METHODS
Cell culture and drug treatments
9L rat brain tumour cells were maintained in Eagle's minimum essential medium supplemented with 10% (v/v) foetal bovine serum, 100 units/ml penicillin G and 100 μg/ml streptomycin in a humidified 5% CO2 atmosphere at 37 °C. To elicit UPR, cells were incubated in the presence of 2 μM A23187 or 300 nM TG. Unless otherwise mentioned, cells were treated for 8 h before being harvested.
Metabolic labelling and gel electrophoresis
To investigate the global de novo protein synthesis in cells under UPR, the cells were metabolically labelled with [35S]methionine at 20 μCi/ml for 1 h before being harvested. UPR was induced by treatment with the drugs, and the cells were harvested at 2 h intervals to produce time-dependent response curves. For labelling, the cells were washed with PBS and cultured in methionine-free medium for 1 h before the addition of [35S]methionine, with drugs present during starvation and absent during labelling. At the end of the labelling period, the cells were washed twice with PBS and lysed in sample buffer. The samples were boiled and then resolved by SDS/12.5% PAGE. Radiolabelled proteins were visualized by autoradiography, and the signals were quantified by densitometric scanning (Molecular Dynamics).
Immunofluorescence microscopy
For immunofluorescence analysis, cells growing on glass coverslips were washed with PBS and then fixed with 4% (w/v) paraformaldehyde for 20 min at 37 °C. The cells were washed with PBS, permeabilized with 0.1% (v/v) Triton X-100 for 1 h, and blocked for 30 min in medium containing serum. After another wash with PBS, immunostaining was performed by incubating the cells with the rabbit anti-GRP78 (Affinity BioReagents; 1:100), mouse anti-PDI (Affinity BioReagents; 1:400) and rabbit anti-calnexin (Santa Cruz Biotechnology; 1:100) primary antibodies for 2 h at room temperature (25 °C). Primary antibodies were diluted in 0.1% (v/v) Triton X-100, 0.2% (w/v) BSA, 0.5 mM PMSF and 1 mM dithiothreitol in PBS. After washing with PBS, the bound primary antibodies were detected using FITC-conjugated goat anti-rabbit antibodies, Cy5-conjugated goat anti-mouse antibodies or Cy5-conjugated donkey anti-rabbit antibodies (Molecular Probes; 1:200) for 2 h at room temperature. To stain mitochondria, 25 nM MitoTracker Red CMXRos (Molecular Probes) was added to the medium and incubated for 20 min before paraformaldehyde fixation. Samples were examined with a Zeiss laser-scanning microscope LSM510 confocal imaging system, and all images were recorded under a Plan-Neofluor 100× [NA (numerical aperture) 1.3] objective.
Differential centrifugation
The cells were washed with PBS, scraped from the dishes and centrifuged at 600 g for 5 min. The pellet was resuspended in 1 ml of cold mitochondria isolation buffer consisting of 0.3 M sucrose, 1 mM EGTA, 5 mM Mops, 5 mM KH2PO4 and 0.1% (w/v) BSA (pH 7.4), and then homogenized in a glass homogenizer. Disrupted cells were centrifuged at 2600 g for 5 min to pellet the unlysed cells and nuclei. The supernatant was centrifuged further at 15000 g for 10 min at 4 °C to obtain the crude mitochondria fraction. The resulting supernatant was centrifuged at 100000 g for 1 h to separate the microsomal fractions (ER) from the cytosol. Subfractionation of mitochondria was conducted as described in [36]. Briefly, mitochondria were resuspended in 70 mM sucrose and 10 mM Tes/KOH (pH 7.5) and then incubated on ice for 15 min. The osmotic strength was adjusted to 0.3 M sucrose, and after a further 15 min incubation on ice, the suspension was centrifuged at 18000 g for 10 min, yielding a supernatant containing IMS, outer membrane and a mitoplast pellet. The soluble IMS and outer membrane pellet were recovered by centrifugation at 200000 g for 10 min. The pelleted mitoplasts were ruptured by freeze–thawing. The sample was then centrifuged at 18000 g for 15 min to obtain the matrix and inner membrane fractions.
Density gradient centrifugation
The crude mitochondria were resuspended in mitochondria isolation buffer, laid over a 1–1.5 M sucrose step gradient in 10 mM Tris/HCl (pH 7.5) and 1 mM EDTA and then centrifuged at 60000 g for 20 min at 4 °C. The enriched mitochondrial fractions were collected from the sucrose gradient interface and were precipitated with trichloroacetic acid. The precipitated proteins were collected by centrifugation at 13000 g for 10 min, washed with acetone, recentrifuged at 13000 g for 10 min and solubilized in SDS sample buffer.
Protease accessibility assays
Equal amounts of crude mitochondria were resuspended in digestion buffer (250 mM sucrose and 10 mM Mops/KOH, pH 7.2) with or without 1% (v/v) Triton X-100. Proteinase K was added to a final concentration of 250 μg/ml. The samples were incubated on ice for 30 or 45 min, and proteolysis was stopped by the addition of 4 mg/ml PMSF. The samples were collected by centrifugation at 15000 g for 10 min at 4 °C and analysed by Western blotting.
Western blot and antibodies
Protein samples were separated by SDS/12.5% PAGE and transferred on to nitrocellulose membrane (Hybond C Extra; Amersham Biosciences). Immunoblotting was performed using the following antibodies: anti-GRP78 (1:1000), anti-HSP90 (heat-shock protein 90) (Santa Cruz Biotechnology; 1:1000), anti-calnexin (1:200), anti-actin (Santa Cruz Biotechnology; 1:2000) anti-HSP60 (Santa Cruz Biotechnology; 1:500), anti-COXIV (cytochrome c oxidase subunit IV) (Stressgen; 1:500), anti-VDAC (voltage-dependent anion-selective channel) protein (Affinity Bioreagents; 1:500) and anti-(cytochrome c) (Clontech Laboratories; 1:100). Horseradish-peroxidase-conjugated anti-goat or anti-rabbit antibodies were purchased from Amersham Biosciences and Santa Cruz Biotechnology. The blots were developed using an enhanced chemiluminescence method (PerkinElmer Life Sciences).
Immunoelectron microscopy
Cells were pre-fixed with 2.5% (w/v) glutaraldehyde in PBS for 1 h followed by post-fixation with 1% (w/v) osmium tetroxide in PBS containing 1.5% (w/v) potassium ferricynide for 1 h, dehydrated in graded ethanol, and infiltrated and polymerized with Spurr's resin overnight. Ultrathin sections were cut using a ultramicrotome and were collected on nickel grids covered with a Formvar-carbon film. After etching with H2O2 for 10 min, the sections were rinsed with PBS and incubated with rabbit anti-GRP78 antibody (1:100) for 2 h. After extensive washing, the sections were incubated with goat anti-rabbit antibodies conjugated with 10 nm gold particles at 1:100 for 2 h and rinsed in distilled water. The grids were double-stained with uranyl acetate and lead citrate and were observed using a Hitachi H7500 transmission electron microscope at 100 kV.
RESULTS
Time course for GRP78 expression during UPR
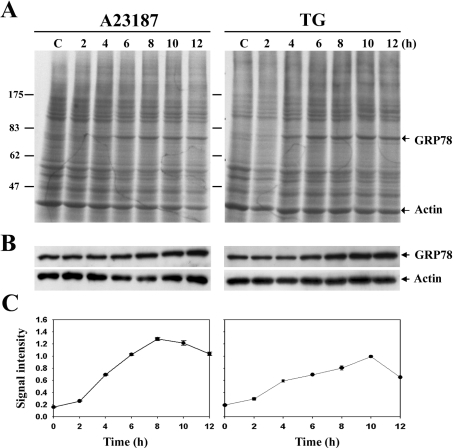
UPR was elicited by treatments of cells with 2 μM A23187 or 300 nM TG. Metabolic labelling experiments indicated that the general protein synthesis profile remained relatively unchanged, except that a single protein was greatly induced (Figure 1A). The induced protein was identified as GRP78 by Western blotting (Figure 1B), and the kinetics of its time-dependent induction was determined. Induction of GRP78 was evident after 4 h of treatment and continued to increase thereafter. Maximal induction was 8-fold at 8 h and 5-fold at 10 h after treatments with A23187 and TG respectively (Figure 1C). Treatment with 2 μM A23187 or 300 nM TG for 8 h was established as the condition that was optimal for GRP78 induction, which was used for all subsequent experiments, unless kinetic studies were to be performed.
Figure 1. Induction of GRP78 in ER-stressed 9L RBT cells.
(A) Cells were treated with A23187 and TG for the durations indicated and were metabolically labelled with [35S]methionine for 1 h before being harvested. Protein samples prepared from equivalent amounts of cells were resolved by SDS/PAGE and were visualized by autoradiography. Molecular-mass sizes are given in kDa. C, control. (B) Protein samples were separated by SDS/PAGE and were subjected to Western blotting with anti-GRP78 and anti-actin antibodies. (C) Relative signal intensities of GRP78 in the autoradiography were determined by densitometric scanning and were normalized for actin signals. Results are means±S.E.M. for three independent experiments.
Subcellular localization of GRP78
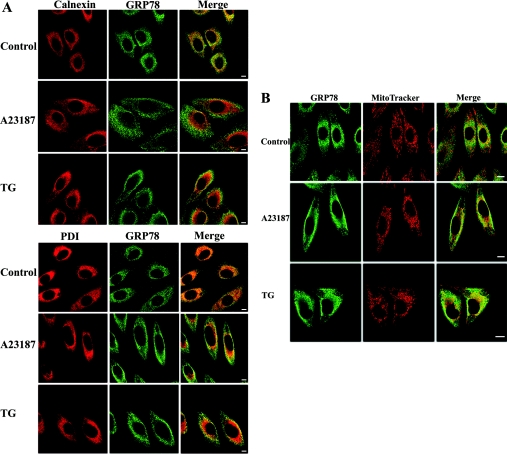
Previous reports of GRP78 in different cellular compartments prompted us to examine and compare the changes of GRP78 in intracellular distribution patterns in response to ER stress, specifically under Ca2+ disturbance. Immunostaining of GRP78 coupled with confocal microscopy demonstrated the granular and perinuclear expression as normal ER distribution in control cells. The co-localization of GRP78 with two kinds of ER marker proteins, membrane protein calnexin and lumen protein PDI, were sustained, and only when the cells were exposed to A23187 or TG did the greater proportion of GRP78 display a diffused distribution throughout the cytoplasm at a slightly higher intensity (Figure 2A). However, the staining patterns of calnexin and PDI did not vary in the treated cells. In parallel experiments, co-staining with the mitochondria-selective marker MitoTracker showed that the GRP78 staining manifested into the diffused cytoplasmic patterns and partially overlapped with the MitoTracker in ER-stressed cells. Overlapping of GRP78 and mitochondria marker apparently depicted worm-shaped strings, suggesting that there is an increase in the level of co-localization and that this might occur from an increase in the level of targeting (Figure 2B).
Figure 2. Intracellular distribution of GRP78 before and after ER stress.
(A) Cells were stained with anti-calnexin or anti-PDI antibodies followed by Cy5-conjugated secondary antibodies, and anti-GRP78 antibodies followed by FITC-conjugated secondary antibodies. The yellow signals in the merge images indicate the co-localization of GRP78 and ER marker protein. Scale bar, 10 μm. (B) Cells were incubated with MitoTracker, fixed and stained with anti-GRP78 antibodies followed by FITC-conjugated secondary antibodies. The yellow signals in the merge images indicate the co-localization of GRP78 and mitochondria. Scale bar, 10 μm.
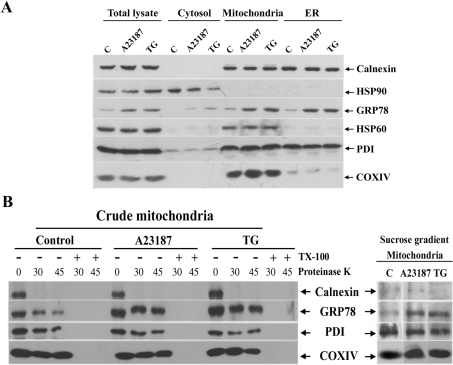
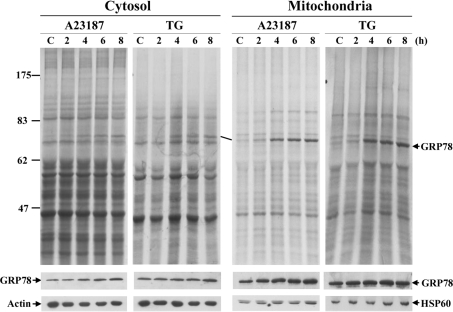
Distribution change of GRP78 in cells under UPR revealed by microscopic studies was re-examined by cellular fractionation experiments. Cytosolic, crude mitochondrial and ER fractions were prepared by differential centrifugation from cell homogenates. Total lysate, cytosol, crude mitochondria and ER fractions were analysed by Western blotting with antibodies specific for GRP78 and organelle marker proteins (Figure 3A). Following ER stress, an increase in GRP78 protein level was detected in all fractions analysed. Both ER proteins calnexin and PDI were also recovered from the crude mitochondrial fraction, raising a concern that GRP78 might co-fractionate with ER contaminants rather than with mitochondria in this fraction.
Figure 3. Subcellular localization of GRP78 in normal and ER-stressed cells.
Cells were fractionated by differential centrifugation, and the samples were analysed using Western blotting using antibodies against the indicated proteins. HSP90, HSP60 or COXIV, and calnexin or PDI were used as specific marker proteins for cytosol, mitochondria and ER respectively. C, control. (A) The amounts of protein loaded were 20 μg for total lysate, cytosol, crude mitochondria and ER fractions. (B) Proteinase K digestion of crude mitochondrial fractions (20 μg) for 30 or 45 min in the presence or absence of detergent (Triton X-100). Right-hand panel, purified mitochondria from sucrose-density gradient.
In order to elucidate further the localization of GRP78 in mitochondria after ER stress, the crude mitochondrial fractions collected from control and ER-stressed cells were subjected to proteinase K digestion. As shown in Figure 3(B), approx. 70% of GRP78 in the control cells was degraded by proteinase K treatment. Interestingly, GRP78 in the ER-stressed cells exhibited higher proteinase K resistance. Blotting with antibodies against the N-terminus of calnexin, which faced the ER lumen, demonstrated nearly complete disappearance of calnexin after proteinase K treatment, suggesting that GRP78 co-fractionation with crude mitochondria did not result solely from ER contamination. Western blot analysis of PDI showed that a portion of PDI was protected and resistant to protease digestion. The resistance of COXIV against protease implied that the mitochondrial membranes remained intact during proteinase K treatment. Permeabilization of the membranes using Triton X-100 rendered the proteins susceptible to protease action, resulting in effective proteinase K digestion. These results show that ER stress led to the targeting of a subpopulation of GRP78 into the mitochondria and therefore became protease-resistant. To achieve better separation of the mitochondria and the ER, the crude mitochondria were fractionated further on a sucrose-density gradient. The enriched mitochondrial fraction was almost devoid of calnexin, but contained COXIV, indicating a complete separation between the mitochondrial and ER fractions (Figure 3B, right-hand panel). Surprisingly, PDI was also found in this fraction, suggesting the existence of a small fraction of mitochondrial PDI in 9L cells. Electron microscopy was further employed to reveal the PDI-stained mitochondria (results not shown). Western blot analysis of GRP78 showed that ER stress increased levels of mitochondrial GRP78.
Submitochondrial localization of GRP78
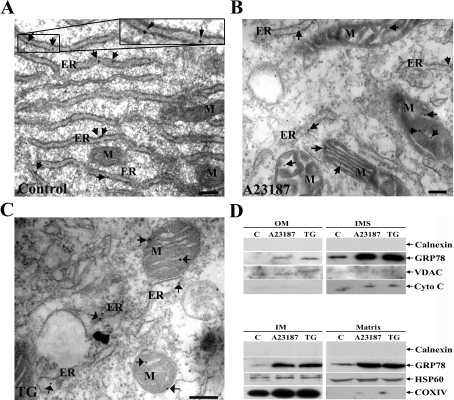
To examine the submitochondrial localization of GRP78 in more detail, the ultralocalization of GRP78 was monitored by electron microscopy. The gold particles were found to be associated with cisternae of the ER and ER membrane in control cells (Figure 4A). In contrast, GRP78-stained mitochondria from ER-stressed cells yielded significant labelling, with grains mainly decorating the inner mitochondrial membrane, although some grains were located within the organelle (Figures 4B and 4C). The appearance of little GRP78 staining in ER might be due to limited exposure of the antigen and the broad spread of ER. Secondary antibody alone and rabbit IgG gave only background staining (results not shown). Next, the crude mitochondria were fractionated further into various submitochondrial fractions (Figure 4D). The protein samples in outer membrane, IMS, inner membrane and matrix were confirmed by co-fractionation of a specific marker proteins VDAC, cytochrome c, COXIV and HSP60 respectively. Before drug treatment, only a little GRP78 was found to be associated with the outer membrane; while there was pre-existing mitochondrial-localized GRP78, the level increased significantly in the ER-stressed cells.
Figure 4. Submitochondrial localization of GRP78 in normal and ER-stressed cells.
(A) Control, (B) A23187-treated and (C) TG-treated cells were processed for immunoelectron microscopy. Rabbit polyclonal anti-GRP78 antibodies and colloidal-gold-conjugated goat anti-rabbit IgG were used as the primary and secondary antibodies respectively. Arrows point to the localization of GRP78. Scale bars, 200 nm. M, mitochondrion. (D) The crude mitochondrial fractions were fractionated further into outer membrane (OM), IMS, inner membrane (IM) and matrix as described in the Materials and methods section. The protein samples were subjected to Western blotting. VDAC, cytochrome c (Cyto C), COXIV and HSP60 were used as markers for the submitochondrial fractions. Calnexin was used as a marker for the ER fraction.
Mitochondrial localization of newly synthesized GRP78
Drug-treated cells were metabolically labelled with [35S]methionine before fractionation analysis. The cells were fractionated into cytosolic and crude mitochondrial fractions, which were then subjected to gel electrophoresis followed by autoradiography and Western blotting. The expression and distribution of newly synthesized GRP78 were followed in a time-course study. The level of newly synthesized GRP78 in cytosol and mitochondria became readily detectable after 4 h of treatment (Figure 5), indicating that a significant amount of newly synthesized GRP78 localized to mitochondria in cells under UPR, but it could not be ruled out that the crude mitochondrial fraction may contain a minor amount of ER.
Figure 5. Mitochondrial localization of newly synthesized GRP78.
Cells were treated with A23187 or TG for different durations and then labelled with [35S]methionine for 1 h before being harvested. The cells were separated into cytosolic and mitochondrial fractions by differential centrifugation as described in the Materials and methods section. Each extract (20 μg of protein) was analysed by SDS/10% PAGE and was visualized by autoradiography. Western blotting with the antibodies against actin and HSP60 were used as markers for the cytosol and mitochondrial fractions respectively. Molecular-mass sizes are given in kDa. C, control.
DISCUSSION
Proper temporospatial controls are fundamentally important for a protein to exercise its function(s). We have demonstrated that, under certain circumstances, GRP78 localizes to the mitochondria, an intracellular compartment in which GRP78 has never been reported to reside. Additionally, the de novo synthesized subpopulations of GRP78 localized to the mitochondria in cells under UPR during which the protein is greatly induced. By using a combination of techniques, including metabolic labelling, confocal microscopy, immunoelectron microscopy and cellular fractionation, we have provided several lines of direct evidence for the mitochondrial targeting of GRP78, which is generally deemed as an ER-residing molecular chaperone. Extra-ER localization of GRP78 has been observed. Immunostaining coupled with confocal microscopy often demonstrate its perinuclear expression in control cells. After treatment, GRP78 staining became more intense, appeared granular predominantly in the ER and also displayed a diffused distribution throughout the cytoplasm [20,28]. In the present study, several organelle-specific markers, including calnexin and PDI for ER, and MitoTracker for mitochondria, have been used and their signals are correlated with those of GRP78 in order to track the intracellular distribution before and after ER stress. Cellular fractionation and protease-susceptible assays revealed that a significant portion of mitochondrial-localized GRP78 remains resistant to proteinase K digestion.
GRP78, and many ER lumen proteins, carry a specific sorting signal that attributes to their own intercellular distribution. The characteristic tetrapeptide KDEL is required for ER retention and is thought to be recognized by a membrane-bound receptor that continually retrieves the proteins from a later compartment of the secretory pathway and returns them to the ER [37]. As mentioned previously, GRP78 has been found to be located in the cytoplasm and associated with the cytoskeletal protein, keratin 8 [26]. More frequently, the protein has been shown to be secreted [24], as well as associated with the endomembrane system [28] and cell surface [38,39]. It is noteworthy that the extra-ER localization of GRP78 is always augmented when the synthesis of GRP78 is induced in the ER-stressed cells [24,25,28,39]. One of the potential explanations for the presence of GRP78 outside the ER is that the KDEL receptors may be saturated or functionally impaired, hence allowing some of the newly synthesized KDEL proteins to target elsewhere. Consequently, a number of newly synthesized molecular chaperones avoid capture and reach the destinations outside the ER [40]. It is also possible that release is caused by the fidelity and efficiency of compartmentalization through the secretory pathway. Proteins destined for the secretory pathway are translocated into the ER by signal sequence. However, many signal-containing proteins have been implicated in functional and pathological roles at sites outside the secretory pathway. The alternative localizations of secretory pathway proteins are generated by a combination of mechanisms, including inefficient translocation into the ER and leaky ribosome scanning [41]. One such example is the ER luminal chaperone calreticulin, which is compartmentalized into two functional populations: the major in the ER and the minor in the cytosol. The cytosolic form of calreticulin was found to arise by an aborted translocation mechanism dependent on its signal sequence and factors in the ER and membrane. Moreover, different ER-targeting signals result in different amounts of precursor remaining in the cytosol [42]. Hence, the ER-stress-induced GRP78 may favour the redirection into the mitochondria. Additionally, comprehensive database searching gave no clue on potential mitochondrial-localization signals in GRP78, indicating that the appearance of GRP78 in mitochondria may be due to the existence of unconventional mitochondrial-sorting signal. The correlation between intracellular trafficking of GRP78 and its possible associations with other proteins remains to be investigated.
Compartmental co-ordination is progressively recognized with the discovery of the activation and translocation of cell death modulators and effectors in cell death pathways. The translocation of signal proteins and effector molecules, both pro-apoptotically and anti-apoptotically among four major cellular compartments, mitochondria, cytoplasm, nucleus and ER, has become one of the striking features in apoptosis signalling [16,43,44]. At or early after the onset of apoptosis, a number of signal proteins including pro-apoptotic p53 [45], Bcl-2 family proteins Bax and Bim [45,46], and anti-apoptotic Raf-1 [47,48] translocate from the cytoplasm to the mitochondria. Another apoptosis effector, Abl tyrosine kinase, has also been shown to relocate from ER to mitochondria. The translocation of Abl is further suggested to promote the ER-stress-induced release of cytochrome c, which in turn will promote further release of Ca2+ from the ER. This process is deemed as a feed-forward mechanism for amplifying the death signals, as well as a direct cross-talk between ER and mitochondria [20]. Moreover, GRP78 is also shown to prevent apoptosis that arises from disturbance of intracellular Ca2+, suggesting a critical role of GRP78 in regulating cellular Ca2+ homoeostasis. Given the facts that GRP78 is a Ca2+-binding protein as well as a molecular chaperone, it is conceivable that mitochondrial localization of GRP78 may also be involved in the above process or that it performs unique intracellular functions.
The results of the present study demonstrate that the induced GRP78 localizes to mitochondria in response to ER stress. Although it is not yet clear whether the mitochondrial localization of GRP78 is due to the ability to modulate Ca2+ buffering store or other functions, the targeting of GRP78 may allow the change of functions and thus the integration of Ca2+ sensing into different cellular pathways. Nevertheless, the possible mechanism and biological function of mitochondrial targeting of GRP78 involved in the cellular protection warrants further study and the results allow the prospect that such localization may occur as part of GRP78 activation, helping to specify its diverse distributions in response to ER stress.
Acknowledgments
This work was supported by National Science Council, R.O.C. grant NSC-94-2311-B-007-002. We thank Shang-Fang Chang for advice with electron microscopy and Dr Margaret Dah-Tsyr Chang for a critical review of the manuscript.
References
- 1.Brostrom M. A., Brostrom C. O. Calcium dynamics and endoplasmic reticular function in the regulation of protein synthesis: implications for cell growth and adaptability. Cell Calcium. 2003;34:345–363. doi: 10.1016/s0143-4160(03)00127-1. [DOI] [PubMed] [Google Scholar]
- 2.Sitia R., Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature (London) 2003;426:891–894. doi: 10.1038/nature02262. [DOI] [PubMed] [Google Scholar]
- 3.Meldolesi J., Pozzan T. The heterogeneity of ER Ca2+ stores has a key role in nonmuscle cell signaling and function. J. Cell Biol. 1998;142:1395–1398. doi: 10.1083/jcb.142.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berridge M. J. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman R. J., Scheuner D., Schroder M., Shen X., Lee K., Liu C. Y., Arnold S. M. The unfolded protein response in nutrient sensing and differentiation. Nat. Rev. Mol. Cell Biol. 2002;3:411–421. doi: 10.1038/nrm829. [DOI] [PubMed] [Google Scholar]
- 6.Ma Y., Hendershot L. M. The unfolding tale of the unfolded protein response. Cell. 2001;107:827–830. doi: 10.1016/s0092-8674(01)00623-7. [DOI] [PubMed] [Google Scholar]
- 7.Brostrom C. O., Brostrom M. A. Regulation of translational initiation during cellular responses to stress. Prog. Nucleic Acid Res. Mol. Biol. 1998;58:79–125. doi: 10.1016/s0079-6603(08)60034-3. [DOI] [PubMed] [Google Scholar]
- 8.Hiller M. M., Finger A., Schweiger M., Wolf D. H. ER degradation of a misfolded luminal protein by the cytosolic ubiquitin-proteasome pathway. Science. 1996;273:1725–1728. doi: 10.1126/science.273.5282.1725. [DOI] [PubMed] [Google Scholar]
- 9.Okada T., Yoshida H., Akazawa R., Negishi M., Mori K. Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem. J. 2002;366:585–594. doi: 10.1042/BJ20020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katayama T., Imaizumi K., Manabe T., Hitomi J., Kudo T., Tohyama M. Induction of neuronal death by ER stress in Alzheimer's disease. J. Chem. Neuroanat. 2004;28:67–78. doi: 10.1016/j.jchemneu.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Mattson M. P., LaFerla F. M., Chan S. L., Leissring M. A., Shepel P. N., Geiger J. D. Calcium signaling in the ER: its role in neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2000;23:222–229. doi: 10.1016/s0166-2236(00)01548-4. [DOI] [PubMed] [Google Scholar]
- 12.Lievremont J. P., Rizzuto R., Hendershot L., Meldolesi J. BiP, a major chaperone protein of the endoplasmic reticulum lumen, plays a direct and important role in the storage of the rapidly exchanging pool of Ca2+ J. Biol. Chem. 1997;272:30873–30879. doi: 10.1074/jbc.272.49.30873. [DOI] [PubMed] [Google Scholar]
- 13.Little E., Ramakrishnan M., Roy B., Gazit G., Lee A. S. The glucose-regulated proteins (GRP78 and GRP94): functions, gene regulation, and applications. Crit. Rev. Eukaryotic Gene Expression. 1994;4:1–18. doi: 10.1615/critreveukargeneexpr.v4.i1.10. [DOI] [PubMed] [Google Scholar]
- 14.Nigam S. K., Goldberg A. L., Ho S., Rohde M. F., Bush K. T., Sherman M. A set of endoplasmic reticulum proteins possessing properties of molecular chaperones includes Ca2+-binding proteins and members of the thioredoxin superfamily. J. Biol. Chem. 1994;269:1744–1749. [PubMed] [Google Scholar]
- 15.Yu Z., Luo H., Fu W., Mattson M. P. The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis. Exp. Neurol. 1999;155:302–314. doi: 10.1006/exnr.1998.7002. [DOI] [PubMed] [Google Scholar]
- 16.Breckenridge D. G., Germain M., Mathai J. P., Nguyen M., Shore G. C. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003;22:8608–8618. doi: 10.1038/sj.onc.1207108. [DOI] [PubMed] [Google Scholar]
- 17.Strasser A., O'Connor L., Dixit V. M. Apoptosis signaling. Annu. Rev. Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- 18.Boehning D., Patterson R. L., Sedaghat L., Glebova N. O., Kurosaki T., Snyder S. H. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat. Cell Biol. 2003;5:1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- 19.Calfon M., Zeng H., Urano F., Till J. H., Hubbard S. R., Harding H. P., Clark S. G., Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature (London) 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 20.Ito Y., Pandey P., Mishra N., Kumar S., Narula N., Kharbanda S., Saxena S., Kufe D. Targeting of the c-Abl tyrosine kinase to mitochondria in endoplasmic reticulum stress-induced apoptosis. Mol. Cell. Biol. 2001;21:6233–6242. doi: 10.1128/MCB.21.18.6233-6242.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitamura Y., Miyamura A., Takata K., Inden M., Tsuchiya D., Nakamura K., Taniguchi T. Possible involvement of both endoplasmic reticulum- and mitochondria-dependent pathways in thapsigargin-induced apoptosis in human neuroblastoma SH-SY5Y cells. J. Pharmacol. Sci. 2003;92:228–236. doi: 10.1254/jphs.92.228. [DOI] [PubMed] [Google Scholar]
- 22.Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 23.Altmeyer A., Maki R. G., Feldweg A. M., Heike M., Protopopov V. P., Masur S. K., Srivastava P. K. Tumor-specific cell surface expression of the KDEL containing, endoplasmic reticular heat shock protein gp96. Int. J. Cancer. 1996;69:340–349. doi: 10.1002/(SICI)1097-0215(19960822)69:4<340::AID-IJC18>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 24.Takemoto H., Yoshimori T., Yamamoto A., Miyata Y., Yahara I., Inoue K., Tashiro Y. Heavy chain binding protein (BiP/GRP78) and endoplasmin are exported from the endoplasmic reticulum in rat exocrine pancreatic cells, similar to protein disulfide-isomerase. Arch. Biochem. Biophys. 1992;296:129–136. doi: 10.1016/0003-9861(92)90554-a. [DOI] [PubMed] [Google Scholar]
- 25.Ying M., Sannerud R., Flatmark T., Saraste J. Colocalization of Ca2+-ATPase and GRP94 with p58 and the effects of thapsigargin on protein recycling suggest the participation of the pre-Golgi intermediate compartment in intracellular Ca2+ storage. Eur. J. Cell. Biol. 2002;81:469–483. doi: 10.1078/0171-9335-00266. [DOI] [PubMed] [Google Scholar]
- 26.Liao J., Price D., Omary M. B. Association of glucose-regulated protein (grp78) with human keratin 8. FEBS Lett. 1997;417:316–320. doi: 10.1016/s0014-5793(97)01315-x. [DOI] [PubMed] [Google Scholar]
- 27.Delpino A., Castelli M. The 78 kDa glucose-regulated protein (GRP78/BIP) is expressed on the cell membrane, is released into cell culture medium and is also present in human peripheral circulation. Biosci. Rep. 2002;22:407–420. doi: 10.1023/a:1020966008615. [DOI] [PubMed] [Google Scholar]
- 28.Rao R. V., Peel A., Logvinova A., del Rio G., Hermel E., Yokota T., Goldsmith P. C., Ellerby L. M., Ellerby H. M., Bredesen D. E. Coupling endoplasmic reticulum stress to the cell death program: role of the ER chaperone GRP78. FEBS Lett. 2002;514:122–128. doi: 10.1016/s0014-5793(02)02289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rigobello M. P., Donella-Deana A., Cesaro L., Bindoli A. Isolation, purification, and characterization of a rat liver mitochondrial protein disulfide isomerase. Free Radical Biol. Med. 2000;28:266–272. doi: 10.1016/s0891-5849(99)00237-3. [DOI] [PubMed] [Google Scholar]
- 30.Turano C., Coppari S., Altieri F., Ferraro A. Proteins of the PDI family: unpredicted non-ER locations and functions. J. Cell. Physiol. 2002;193:154–163. doi: 10.1002/jcp.10172. [DOI] [PubMed] [Google Scholar]
- 31.Coppolino M. G., Dedhar S. Calreticulin. Int. J. Biochem. Cell Biol. 1998;30:553–558. doi: 10.1016/s1357-2725(97)00153-2. [DOI] [PubMed] [Google Scholar]
- 32.Michalak M., Corbett E. F., Mesaeli N., Nakamura K., Opas M. Calreticulin: one protein, one gene, many functions. Biochem. J. 1999;344:281–292. [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L. Y., Chiang A. S., Hung J. J., Hung H. I., Lai Y. K. Thapsigargin-induced grp78 expression is mediated by the increase of cytosolic free calcium in 9L rat brain tumor cells. J. Cell. Biochem. 2000;78:404–416. doi: 10.1002/1097-4644(20000901)78:3<404::aid-jcb6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 34.Drummond I. A., Lee A. S., Resendez E., Jr, Steinhardt R. A. Depletion of intracellular calcium stores by calcium ionophore A23187 induces the genes for glucose-regulated proteins in hamster fibroblasts. J. Biol. Chem. 1987;262:12801–12805. [PubMed] [Google Scholar]
- 35.Treiman M., Caspersen C., Christensen S. B. A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca2+-ATPases. Trends Pharmacol. Sci. 1998;19:131–135. doi: 10.1016/s0165-6147(98)01184-5. [DOI] [PubMed] [Google Scholar]
- 36.Sweetlove L. J., Mowday B., Hebestreit H. F., Leaver C. J., Millar A. H. Nucleoside diphosphate kinase III is localized to the inter-membrane space in plant mitochondria. FEBS Lett. 2001;508:272–276. doi: 10.1016/s0014-5793(01)03069-1. [DOI] [PubMed] [Google Scholar]
- 37.Pelham H. R. The retention signal for soluble proteins of the endoplasmic reticulum. Trends Biochem. Sci. 1990;15:483–486. doi: 10.1016/0968-0004(90)90303-s. [DOI] [PubMed] [Google Scholar]
- 38.Arap M. A., Lahdenranta J., Mintz P. J., Hajitou A., Sarkis A. S., Arap W., Pasqualini R. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell. 2004;6:275–284. doi: 10.1016/j.ccr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 39.Xiao G., Chung T. F., Pyun H. Y., Fine R. E., Johnson R. J. KDEL proteins are found on the surface of NG108-15 cells. Mol. Brain Res. 1999;72:121–128. doi: 10.1016/s0169-328x(99)00188-6. [DOI] [PubMed] [Google Scholar]
- 40.Wiest D. L., Bhandoola A., Punt J., Kreibich G., McKean D., Singer A. Incomplete endoplasmic reticulum (ER) retention in immature thymocytes as revealed by surface expression of “ER-resident” molecular chaperones. Proc. Natl. Acad. Sci. U.S.A. 1997;94:1884–1889. doi: 10.1073/pnas.94.5.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levine C. G., Mitra D., Sharma A., Smith C. L., Hegde R. S. The efficiency of protein compartmentalization into the secretory pathway. Mol. Biol. Cell. 2005;16:279–291. doi: 10.1091/mbc.E04-06-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaffer K. L., Sharma A., Snapp E. L., Hegde R. S. Regulation of protein compartmentalization expands the diversity of protein function. Dev. Cell. 2005;9:545–554. doi: 10.1016/j.devcel.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Ferri K. F., Kroemer G. Organelle-specific initiation of cell death pathways. Nat. Cell Biol. 2001;3:E255–E263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- 44.Wang N. S., Unkila M. T., Reineks E. Z., Distelhorst C. W. Transient expression of wild-type or mitochondrially targeted Bcl-2 induces apoptosis, whereas transient expression of endoplasmic reticulum-targeted Bcl-2 is protective against Bax-induced cell death. J. Biol. Chem. 2001;276:44117–44128. doi: 10.1074/jbc.M101958200. [DOI] [PubMed] [Google Scholar]
- 45.Marchenko N. D., Zaika A., Moll U. M. Death signal-induced localization of p53 protein to mitochondria: a potential role in apoptotic signaling. J. Biol. Chem. 2000;275:16202–16212. doi: 10.1074/jbc.275.21.16202. [DOI] [PubMed] [Google Scholar]
- 46.Gotoh T., Terada K., Oyadomari S., Mori M. hsp70–DnaJ chaperone pair prevents nitric oxide- and CHOP-induced apoptosis by inhibiting translocation of Bax to mitochondria. Cell Death Differ. 2004;11:390–402. doi: 10.1038/sj.cdd.4401369. [DOI] [PubMed] [Google Scholar]
- 47.Wang H. G., Reed J. C. Bc1-2, Raf-1 and mitochondrial regulation of apoptosis. Biofactors. 1998;8:13–16. doi: 10.1002/biof.5520080103. [DOI] [PubMed] [Google Scholar]
- 48.Zhong J., Troppmair J., Rapp U. R. Independent control of cell survival by Raf-1 and Bcl-2 at the mitochondria. Oncogene. 2001;20:4807–4816. doi: 10.1038/sj.onc.1204614. [DOI] [PubMed] [Google Scholar]