Abstract
The following test of the circadian phase-shift hypothesis for patients with winter depression (seasonal affective disorder, or SAD) uses low-dose melatonin administration in the morning or afternoon/evening to induce phase delays or phase advances, respectively, without causing sleepiness. Correlations between depression ratings and circadian phase revealed a therapeutic window for optimal alignment of circadian rhythms that also appears to be useful for phase-typing SAD patients for the purpose of administering treatment at the correct time. These analyses also provide estimates of the circadian component of SAD that may apply to the antidepressant mechanism of action of appropriately timed bright light exposure, the treatment of choice. SAD may be the first psychiatric disorder in which a physiological marker correlates with symptom severity before, and in the course of, treatment in the same patients. The findings support the phase-shift hypothesis for SAD, as well as suggest a way to assess the circadian component of other psychiatric, sleep, and chronobiologic disorders.
Keywords: chronobiology, circadian rhythms, melatonin, seasonal affective disorder, dim light melatonin onset (DLMO)
The two phase-resetting agents for treating circadian rhythm disorders are bright light and melatonin (1). The latter is the only option for totally blind people who cannot synchronize to the day/night cycle or do so at an abnormal time (2, 3). Both bright light and melatonin are used to treat the circadian disorders of sighted people [reviewed by Bunney et al. (4)]; these include shift-work maladaptation and jet lag, as well as advanced and delayed sleep phase syndromes. However, the condition in which bright light is used most often is one in which a circadian component has not yet been fully established: winter depression [seasonal affective disorder (SAD)] (5). In females of childbearing age, SAD is perhaps the most common mood disturbance unremittingly experienced year after year during the 6 months between the autumnal and vernal equinoxes at temperate latitudes, such as the northern United States and lower provinces of Canada (6), where there are marked seasonal changes in natural day length (photoperiod). Accordingly, SAD initially was treated with bright light in the early morning and evening to simulate the longer days of spring (7, 8). However, in 1998 evidence for the superiority of morning light (vs. evening light) was established in large numbers of subjects (9–11), a finding that can be interpreted (to a greater or lesser extent) as supportive of a number of biological-rhythm hypotheses (12–14), including the circadian phase-shift hypothesis (PSH) (15).
The PSH is based on the seminal concept that some affective disorders might be at least partly due to a mismatch in circadian rhythms (16, 17), specifically, between those rhythms related to the sleep/wake cycle and those that are more tightly coupled to the endogenous circadian pacemaker (located in the suprachiasmatic nuclei of the hypothalamus). The PSH postulates that most SAD patients become depressed in the winter because of the later dawn, causing their circadian rhythms to delay with respect to clock time and with respect to the sleep/wake cycle (18): through providing a corrective phase advance (1), morning light could be antidepressant by realigning rhythms with the sleep/wake cycle (15, 18, 19). The PSH further postulates that a smaller subgroup of SAD patients become depressed because of a phase advance (perhaps cueing to the early winter dusk) and would preferentially respond to a corrective phase delay from evening light (15, 19–21).
The proportion of the atypical phase-advanced subgroup has been assumed to be minimal (15, 18, 22, 23), stemming in part from some (14, 15, 22, 24), but not all (14, 25, 26), studies that found a small overall delay in the circadian rhythms of patients compared with normal controls studied in the winter. Further support for the PSH has mainly come from the fact that exposure at any other time of day has never been shown to be more antidepressant than morning light alone and from some, but not all (22, 26, 27), studies that found statistically significant correlations (of varying import) in the predicted direction between mood and circadian phase in response to light: many of these studies have been recently reviewed (23), except for one cited above (24); in addition, data from two of the first morning vs. evening light studies (15, 24) have been independently analyzed (28). The largest was reported by Terman et al. in 2001 (23): percent decrease in depression ratings after morning light correlated (r = 0.44, df = 26, P = 0.02) with the magnitude of the phase advance in the dim light melatonin onset (DLMO) (the most commonly used marker for assessing endogenous circadian phase position in humans). {In entrained, sighted people, the DLMO is the interpolated time when the evening rise in melatonin levels [sampled under conditions of dim light to avoid suppression of its production (29)] continues above a certain threshold, operationally defined in plasma as 10 pg/ml (unless otherwise specified).} Even the best of these correlations, however, does not establish causality. For this and other reasons, but most importantly because there is a continued need to provide the most accurate quantitative estimate of the circadian component of SAD (and, by inference, of the antidepressant response to light), the present melatonin treatment study was undertaken. Such a study is also crucial in establishing the PSH.
Induction of phase shifts by some agent other than light is a critical test of the PSH. Melatonin is ideal for this purpose, because this “chemical signal of darkness” is thought to be opposite of light (1, 30). Because the duration of melatonin production is the neurochemical signal for the annual change in night length in seasonally breeding animals (14, 30), administering melatonin in the morning or evening to SAD patients to increase the duration of “the biological night” would not be expected to be of any therapeutic benefit, unless it induced circadian phase shifts similar to those caused by light. To accomplish this goal, melatonin must be taken at the opposite half of the photoperiod than when bright light is scheduled (1): accordingly, most SAD patients (who are phase delayed) should preferentially respond to afternoon/evening (PM) administration (which causes phase advances) compared with morning (AM) administration (which causes phase delays). At doses of 0.1 mg or less, particularly in patients without insomnia (31), melatonin is minimally soporific and cannot be distinguished from inert filler in otherwise identical placebo capsules. In contrast, bright light treatment is accompanied by a potentially large placebo response that varies between studies and individuals (9).
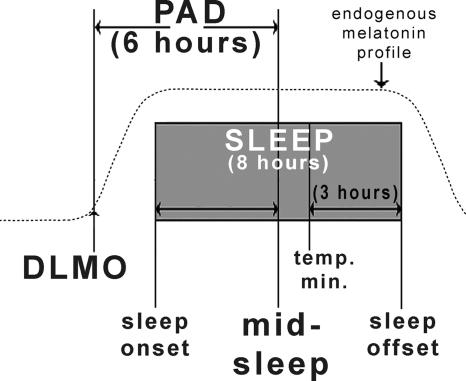
Fig. 1 shows, in healthy controls studied in the winter, the average intervals (in hours rounded to the nearest integer) between sleep times and the DLMO as well as (for reference) the core body temperature minimum, thought to be phase locked with the DLMO (27, 32). The following is our first report using the phase-angle difference (PAD) between the DLMO and midsleep (normally 6 h), which is calculated by dividing the sleep onset-to-offset duration by 2 and then subtracting the quotient from the sleep offset. We have presented or published some preliminary findings (§ , ¶, ‖) using the PAD between the DLMO and sleep phase markers.
Fig. 1.
Schematic diagram of normal phase relationships (rounded to the nearest integer) between sleep phase markers, the 10 pg/ml plasma DLMO (10), and the core body temperature minimum (Tmin) (27, 32) derived from historical controls. The present study used the DLMO/midsleep interval PAD of 6 h as the hypothesized therapeutic window for optimal circadian alignment. Sleep times were determined actigraphically. Plasma melatonin levels were obtained under dim light every 30 min in the evening. The operational definition of the DLMO is the interpolated time of continuous rise above the threshold of 10 pg/ml; for example, if the melatonin level at 8 p.m. was 5 pg/ml and at 8:30 p.m. was 15 pg/ml, the DLMO would be 8:15 p.m.
Results
Phase-Shifting Effects of Melatonin Were Sufficient to Test the PSH.
PM melatonin caused a 0.89-h phase advance in the clock time of the DLMO (t = 7.61, df = 21, P < 0.001) and a 0.69-h increase in PAD (advance in the DLMO with respect to midsleep: t = 4.66, df = 21, P < 0.001). (After PM-melatonin treatment, mean DLMO clock time was 20:18 ± 0:14.) AM melatonin did not significantly delay the DLMO (0.18 h) or decrease PAD (0.01 h). Placebo treatment was associated with nonsignificant trends, an advance in the DLMO and an increase in PAD of both 0.17 h (t = 1.71, df = 23, P = 0.10; and t = 1.76, df = 23, P = 0.09, respectively), consistent with the photoperiod that lengthened throughout the study. Nevertheless, because AM melatonin and placebo treatments did not produce statistically significant changes in circadian phase, these treatment groups were appropriately collapsed in one of the analyses below (see Fig. 6). Shifts in sleep times were mostly small and statistically insignificant and were in a direction consistent with the treatment and the constraints on bedtimes: the largest statistically significant shift was an 18-min delay in sleep onset after AM melatonin (t = 2.57, df = 21, P = 0.02). Baseline Structured Interview Guide for the Hamilton Depression Rating Scale: Seasonal Affective Disorder Version (SIGH-SAD) (33) ratings (27, 28, and 28, respectively) were the same for the placebo, AM- and PM-melatonin treatment groups; there were no significant pretreatment vs. posttreatment percent change differences [ANOVA: F (2, 66) = 1.65, P = 0.20; and Kruskal-Wallis H test: χ2 = 4.09, df = 2, P = 0.13] among the three treatment groups.
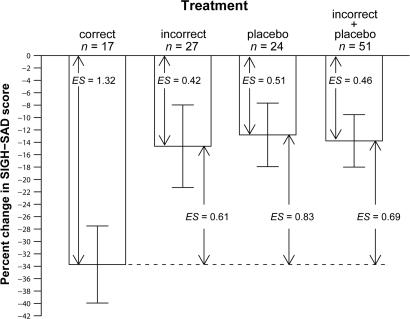
Fig. 6.
Percent change in (SIGH-SAD) depression score after correct treatment, incorrect treatment, and placebo, as well as incorrect treatment and placebo combined (see text for details of the composition of these treatment groups). Baseline SIGH-SAD scores for the three treatment groups (correct treatment, incorrect treatment, and placebo) were 28.9 ± 1.0, 28.8 ± 1.3, and 26.6 ± 1.4, respectively. The Kruskal–Wallis H test (χ2 = 5.83, df = 2, P = 0.05) was statistically significant, but not the one-way ANOVA [F = 2.96 on (2, 65), P = 0.06]. By using the Welch two-sample t test to compare differences in the change scores of the correct-treatment group with those of the other groups, correct treatment significantly decreased depression ratings more than the other groups: incorrect (19.1%: t = 2.09, df = 40.8, P = 0.04); placebo (20.9%: t = 2.60, df = 34.2, P = 0.01); the latter two groups combined (19.9%: t = 2.65, df = 32.1, P = 0.01). Pretreatment to posttreatment percent changes were significant for all groups: correct (t = 5.43, df = 16, P < 0.001), incorrect (t = 2.20, df = 26, P = 0.04), placebo (t = 2.50, df = 23, P = 0.02), and the latter two groups combined (t = 3.25, df = 50, P = 0.002). Effect sizes (ES) are shown for pretreatment to posttreatment percent change scores for each group; also shown are the more conservative ES for differences in change scores between the correct-treatment group and the other groups. [Before phase typing, percent change in the PM-treated group was −28.5 ± 5.6, and percent change in the AM-treated group was −15.5 ± 8.0, although there were no statistically significant differences between the three treatment groups in percent changes in SIGH-SAD scores (see above).]
Pretreatment Phase Typing of SAD Patients and Its Implications.
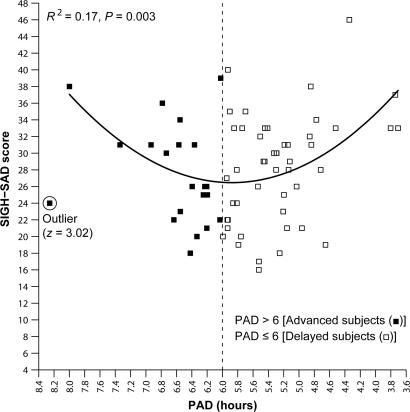
In Fig. 2, the statistically significant parabola [R2 = 0.17, df = (2, 65), P = 0.003] has a minimum at 5.88, which validates the choice of PAD 6 (see Fig. 1) for phase typing these subjects before doing change-score and treatment-response analyses. Furthermore, in the present data set, neither parabolic nor absolute deviation linear plots from the parabolic minimum were statistically significant when any other circadian marker comprising the DLMO and/or sleep times was substituted for PAD. Even the two constituents of the PAD (DLMO and midsleep clock times) had nonsignificant parabolic correlations [R2 = 0.006, F (2, 65) = 0.21, P = 0.81; and R2 = 0.05, F (2, 65) = 1.64, P = 0.20, respectively] with depression ratings.
Fig. 2.
Pretreatment SIGH-SAD depression score as a function of PAD (the interval between the DLMO and midsleep). [The circled data point (from a 36-year-old female subject who was assigned to placebo treatment) was the only one that met outlier criteria (z = 3.02) and was therefore removed from all subsequent analyses and did not substantially affect any of the above findings (no outliers were detected in any other analyses).] The parabolic curve (minimum = 5.88) indicates that PAD accounts for 17% of the variance in SIGH-SAD scores [F (2, 65) = 6.43]. [A significant linear correlation was found for the absolute deviation from the parabolic minimum (r = 0.39, r2 = 0.15, df = 65, P = 0.001), confirming the validity of the parabolic curve fit.]
The implicit phase typing done above was explicitly done before conducting all of the remaining analyses. Those who at baseline had PADs ≤ 6 (n = 48; 71%) were designated as phase-delayed types, and those who had PADs > 6 (n = 20; 29%) were considered phase-advanced. (Unless otherwise specified, the use of the terms advanced and delayed applies to these pretreatment assignments and not to posttreatment phase.) With one exception (see Fig. 5), when the advanced and delayed groups are separated in all of the correlational analyses, statistical significance is found only in the delayed group, and analyses confined to this group are almost always more robust than in those with the advanced and delayed groups combined.
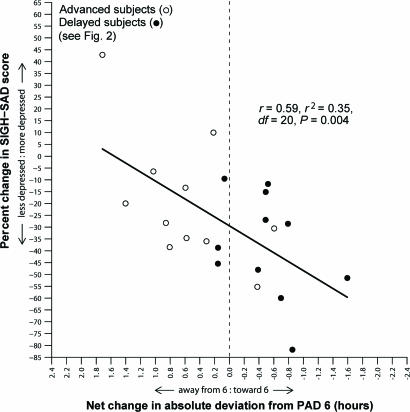
Fig. 5.
Percent change in SIGH-SAD score as a function of net change in absolute deviation toward and away from PAD 6 in PM-melatonin treated advanced and delayed subjects. Pretreatment vs. posttreatment shifts with respect to PAD 6 account for 35% of the variance.
Response to Treatment Correlates with Correcting Circadian Misalignment.
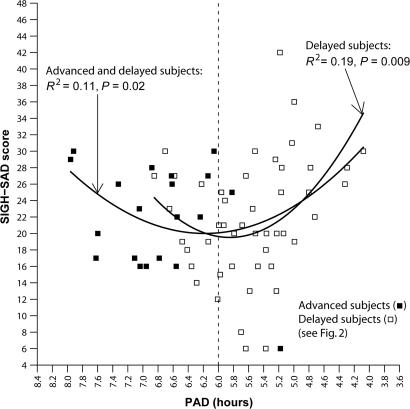
The day after treatment was discontinued, posttreatment (Fig. 3), the statistically significant parabolic relationship remained between depression score and PAD [R2 = 0.11, df = (2, 65), P = 0.02]; a second parabola fitted to the data of the subset of (delayed) subjects with pretreatment PADs ≤ 6 was also significant [R2 = 0.19; df = (2, 45); P = 0.009; minimum = 5.85]. This group is analyzed in more detail in Fig. 4.
Fig. 3.
Posttreatment SIGH-SAD score as a function of PAD. The parabolic curve (minimum = 6.18) indicates that PAD accounts for 11% of the variance in SIGH-SAD scores [F (2, 65) = 3.96] for all subjects and 19% for phase-delayed subjects [F (2, 45) = 5.19]. Absolute deviations from the parabolic minima (6.18 and 5.85, respectively) were statistically significant (advanced and delayed subjects: r = 0.29, r2 = 0.09, df = 65, P = 0.02; delayed subjects: r = 0.48, r2 = 0.23, df = 65, P = 0.001).
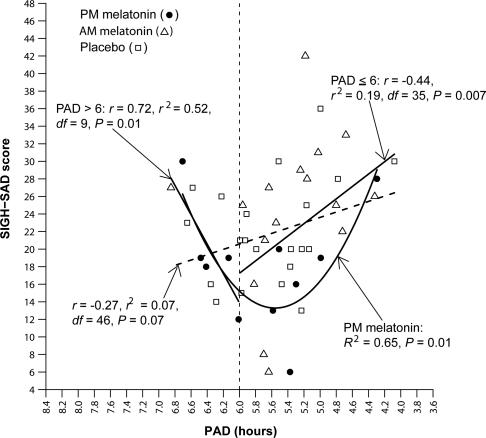
Fig. 4.
Posttreatment SIGH-SAD score as a function of PAD in delayed subjects. (The parabolic curve and related statistics for the delayed subjects are provided in Fig. 3.) The linear correlation between PAD and SIGH-SAD score (diagonal hatched line) did not reach statistical significance, confirming that the parabolic curve in Fig. 3 for delayed subjects (R2 = 0.19, P = 0.009) is the better fit for these data. Directional linear correlations for under- and overshifters (to the right and left of PAD 6, respectively) were both statistically significant. The parabolic curve for subjects receiving PM melatonin indicates that PAD accounts for 65% of the variance in SIGH-SAD scores [F (2, 8) = 7.57; minimum = 5.56]; the correlation between the absolute deviation from the parabolic minimum was also statistically significant (r = 0.75, r2 = 0.56, df = 8, P = 0.01).
Linear regressions restricted to the delayed group appear in Fig. 4, as well as the parabola for delayed subjects treated with PM melatonin. The regression that did not exclude subjects with posttreatment PADs > 6 (overshifters) did not quite reach statistical significance (r = −0.27, r2 = 0.07, df = 46, P = 0.07; however, Spearman's ρ = −0.33, n = 48, and P = 0.02 and Kendall's τ = −0.20, n = 48, and P = 0.04 were statistically significant). [These statistics are reported for comparison with those of the parabolic curves presented in Figs. 3 and 4 (in all of the above analyses, statistical significance was lost when the data fitted to a significant parabolic curve were fitted to a linear regression).] If the overshifters and undershifters (those who remained ≤ PAD 6) are analyzed separately, the linear regressions were statistically significant, despite the reduction in sample size (respectively: r = 0.72, r2 = 0.52, df = 9, P = 0.01; and r = −0.44, r2 = 0.19, df = 35, P = 0.007). Thus, the data in Fig. 4 confirm the therapeutic window of about PAD 6, at least for the phase-delayed group: indeed, the parabolic fit of the data for the delayed subjects who received PM melatonin, the treatment that caused the greatest phase shifts, is remarkably impressive, particularly for such a small sample [R2 = 0.65, df = (2, 8), P = 0.01, minimum = 5.56].
As mentioned above, analyses of the delayed group, rather than the advanced group, appear to be the driving force behind most of the statistically significant findings. This finding also applies to the linear regression (raw data not shown) of pretreatment to posttreatment percent changes in depression scores vs. shifts toward or away from PAD 6 (for delayed subjects, r = 0.35, r2 = 0.12, df = 46, P = 0.01; and for all subjects, r = 0.32, r2 = 0.10, df = 66, P = 0.007); the three delayed subjects who worsened the most received the “incorrect” treatment (AM melatonin), which caused a phase delay away from PAD 6 [it is not surprising that only a few subjects actually became more depressed during the study, given the small, but significant, antidepressant response to placebo (see Fig. 6)]. The plot for this type of analysis appears in Fig. 5 for all phase-advanced and phase-delayed subjects who received PM melatonin, the treatment that caused the greatest phase shifts: the linear regression between percent change in depression ratings and shifts toward or away from PAD 6 was quite robust, despite the relatively small sample size (r = 0.59, r2 = 0.35, df = 20, P = 0.004). {Furthermore, the data in this figure make clear that most of the advanced types (who, in response to PM melatonin, usually advanced further away from PAD 6) had a worse clinical response than the delayed types (for whom PM melatonin would be expected to be the treatment of choice); notably, the subject who shifted away from PAD 6 more than anyone else and whose depression scores worsened more than those of all but two other subjects [phase-delayed types who also received the incorrect treatment (AM-melatonin); data not shown] was from the advanced subgroup for whom PM melatonin would have been predicted to be the incorrect treatment.} The r2 of 0.35 is the largest found in the combined group of phase-advanced and phase-delayed subjects (second only to the R2 of 0.65 in the PM-treated delayed group alone in Fig. 4).
Subjects were retrospectively (and blindly) assigned to correct vs. incorrect treatments: PM melatonin is the correct treatment for delayed types (n = 11) and AM melatonin for advanced types (n = 6); AM melatonin is the incorrect treatment for delayed types (n = 16) and PM melatonin for advanced types (n = 11). Accordingly, 17 subjects received the correct treatment and 27 the incorrect one (24 received placebo). The correct treatment decreased depression ratings by 34%, compared with ≈13–15% for the other treatment groups, separately or combined (Fig. 6). Two ways of calculating effect sizes were considered (Fig. 6); the more conservative ones were based on percent differences in change scores compared with the correct treatment: 0.61 (incorrect treatment), 0.83 (placebo), and 0.69 (the latter two groups combined).
Discussion
Toward Estimating the Circadian Component of SAD and the Antidepressant Response to Light.
SAD may be the first psychiatric disorder in which statistically significant correlations are found between overall symptom severity and a physiological marker before, and in the course of, treatment in the same patients. Including those reviewed by Brody et al. (34), there are a few studies in which significant correlations were found either before treatment (35–37) or in response to treatment. In any event, our correlations compare favorably with these, as well as those reported in light-treatment studies (23, 32). Consistent with Fig. 3 (above), Burgess, Eastman, and coworkers (32) recently found a parabolic correlation [R2 = 0.33, df = (2, 22), P = 0.01] between a corresponding posttreatment therapeutic window (3-h PAD between the temperature minimum and waketime; see Fig. 1) and the change in SIGH-SAD scores in SAD subjects who received morning light, evening light, or placebo. In a somewhat similar analysis to theirs (that is, plotting the change in SIGH-SAD score against posttreatment DLMO/midsleep PAD), we found a statistically significant parabola [R2 = 0.27, F (2, 19) = 3.60, P = 0.05; minimum = 5.55] in delayed subjects treated with PM melatonin (data not shown); however, we think that the more informative analysis in these subjects is posttreatment SIGH-SAD score vs. PAD [plotted above (see Fig. 4): R2 = 0.65, F (2, 8) = 7.57, P = 0.01; minimum = 5.56].
Explaining 65% of the variance in the parabolic correlation of phase-delayed subjects and 35% of the variance in the change scores of the combined group in response to PM melatonin (the treatment that caused the greatest phase shifts) are our best estimates of the circadian component of SAD and the antidepressant effect of melatonin and, by inference, light. As mentioned above, most of the statistical significance in the above findings is driven by the delayed types: to what extent this difference can be explained by greater heterogeneity, smaller size, or greater influence of noncircadian factors in the advanced group is not yet known; also, the phase-shifting effects of AM melatonin (which was intended to be a control rather than an active treatment) were not optimized as much as those of PM melatonin (which corresponds to morning light, the treatment of choice for most patients). Post hoc analyses might identify sources of heterogeneity in the advanced group (and possibly in the delayed group). Iterative analyses may lead to methodological refinements, for example, reduction of the inherent noise in the behavioral/cognitive/mood assessments. These analyses also may reduce the proportion of the advanced group, as well as result in: (i) a therapeutic window with more resolution than our integer of six; (ii) separate therapeutic windows for advanced and delayed groups; and/or (iii) different ways of phase typing. In any event, phase typing alone will probably not be useful in diagnosing SAD patients, because the means and ranges of their circadian phase markers do not appear to markedly differ from those of healthy controls; in other words, as-yet-to-be-identified variables are required for circadian misalignment to result in a winter depression. Perhaps SAD patients are uniquely vulnerable to clinical manifestations of changes in circadian phase. Studies throughout the year may be helpful in determining meaningful normative and ipsative differences (18). Furthermore, although PAD 6 appears to be a useful way to subtype SAD for guiding treatment choices, we would not be surprised if each person had a therapeutic window that differed from the group mean. We also would not be surprised if the therapeutic window had some relevance for healthy individuals.
Integrating and Reconciling Past and Present Findings.
The findings of this study, which phase types large numbers of SAD patients based on a reliable physiological marker with respect to actigraphically documented sleep times, are consistent with those previously reported and provide explanations for most of the discrepancies in the literature. For example, absence of PAD 6 phase typing could be why some studies did not find antidepressant differences between morning and evening light (26, 38) and why these differences were usually more apparent in complete remission rates (9, 11) (perhaps the complete remitters were the ones who received the correct treatment). Failure to consistently find a statistically significant pretreatment phase delay (14, 25, 26) is explained by a phase-advanced subgroup larger than previously assumed.
In the Terman et al. (23) morning vs. evening light cross-over study, the correlation with morning light did not reveal a therapeutic window for optimal circadian alignment (instead, they found a statistically significant linear relationship: the greater the phase advance to morning light, the greater the antidepressant response). We undertook several types of analyses (in addition to those reported above) on our corresponding data, but we were not able to replicate their finding. However, their subjects may not have shifted across the therapeutic window to the same extent as our PM-melatonin-treated subjects, because the average posttreatment DLMO clock time of our PM-melatonin subjects was 37 min earlier than their morning light subjects. Perhaps a more important difference between our conclusions and those of the Terman group is that theirs include a recommendation of earlier morning light exposure for SAD patients who are relatively less phase delayed, whereas we might have considered some of these patients as belonging to the phase-advanced subgroup, for whom, even before the present study was undertaken (19), we would have recommended evening light treatment.
Is Melatonin a Treatment for SAD?
Our study was not designed to assess the optimal potential for melatonin treatment. Nevertheless, the clinical benefit appears to be substantial, although not as robust as light treatment; it should be noted, however, that there is a much less placebo component in the present study. In any event, these effect sizes (see Fig. 6), as well as the 19–21% separations between the correct treatment and the other treatments, are greater than what is usually reported in fixed-dose clinical trials of antidepressants (39–41). Although the delayed group had larger effect sizes when analyzed alone than when combined with the advanced group, separate analyses of these two groups were not statistically meaningful because of reduced sample size. Over the four weeks of SIGH-SAD ratings, only the mean scores for the correct-treatment group steadily improved (28.9 → 23.8 → 20.2 → 18.9). Because AM melatonin did not cause the same magnitude of phase shifts as PM melatonin, the treatment effects found above are probably underestimates, particularly for the advanced group. Although more studies are needed, these data suggest that most SAD patients might benefit from an appropriate low-dose formulation of melatonin taken in the afternoon.
Conclusions
In order of certainty, we conclude that (i) the prototypical SAD patient is phase delayed, whereas a less well defined subgroup may be phase advanced; (ii) the circadian component (at least for the prototypical patients) is substantial, and it is consistent with the PSH and a hypothesized therapeutic window for optimal circadian alignment; and (iii) the work presented here will be useful as a template for reanalyzing extant data sets and for implementing new studies of nonseasonal depression, as well as other sleep and psychiatric disorders, in which a circadian component might be present.
Materials and Methods
For more details on experimental techniques used in this study, see Supporting Materials and Methods and Fig. 7, which are published as supporting information on the PNAS web site. Plasma DLMO assessments (see also Fig. 1) and reliably documented sleep times were done on what turned out to be a total of 68 SAD patients (see below) before and after 3 weeks of taking melatonin or placebo capsules. Depression ratings were done weekly by using the SIGH-SAD (33). Subjects were randomly assigned to morning or afternoon/evening melatonin treatment or to placebo. Seven to eight capsules per day were taken every 2 h, to produce physiological levels of exogenous melatonin to extend the endogenous melatonin profile either earlier or later, to cause either a phase advance or a phase delay, respectively, in the endogenous circadian pacemaker (as marked by a shift to an earlier or later time, respectively, in the DLMO) with respect to sleep times that were held constant.
At least 27 subjects met established SAD screening criteria each year and engaged in 4 weeks of sleeping at home at negotiated sleep onset and offset times (with the first cohort of 9 subjects starting the first or second week of January, and cohorts 2 and 3 starting 2 and 4 weeks later, respectively). Sleep times were based on self-selected weekday schedules that were held constant throughout the protocol and monitored by daily diaries (and, for years 2–4, more reliably by wrist actigraphy). Depression severity (SIGH-SAD score) was assessed weekly, and DLMOs were obtained at the end of the baseline week and after 3 weeks of taking 7–8 capsules per day (one every 2 h beginning at waketime and ending 4 or 2 h before sleep, respectively). Capsules contained placebo or melatonin [0.075 or 0.1 mg (totaling 0.225 or 0.3 mg per day), depending on the year of the study] either in the morning (AM) or in the afternoon/evening (PM), or placebo at all times.
Statistical significance is reported above only if linear regressions had a P value of at least 0.05, by using Pearson's r, confirmed by rank-order Kendall's τ and Spearman's ρ tests, but only Pearson's is reported, unless otherwise specified. A parabolic curve was considered statistically significant only if it was also significant when plotted linearly as a function of the absolute deviation from the parabolic minimum. Means are followed by ±SEMs. For comparisons of means between different groups, we used the more conservative Welch two-sample t test, which does not assume equal variances. Data from the first year of the study were excluded in the following analyses, because of the absence of actigraphic recordings of sleep times. Of the remaining 81 subjects, complete data sets were available for 69, of whom 66 were females and the average age was 39 ± 1.1 (range: 23–59), consistent with the known demographics of SAD (5, 6); the outlier (see Fig. 2) was removed, leaving a sample size of 68. There were no significant differences between treatment groups (placebo: n = 25; AM = PM = 22) in either (age and gender) demographic or baseline (SIGH-SAD and waketime) variables.
Supplementary Material
Acknowledgments
We thank the research subjects, the nursing staff of the Oregon Health & Science University (OHSU) General Clinical Research Center, Neil Cutler, Rick Boney, Krista Yuhas, Angie Koenig, Nancy Stahl, Brant Hasler, Rebecca Bernert, Cathy Evces, Anusha Chhagan, Anju Bhargava, Dr. Les Christianson, Dr. Darian Minkunas, and Dr. Laurie Vessely. We also thank Dr. Keith Parrott, Pharm.D., for preparation of the capsules and Enviro-Med for help in providing bright-light fixtures to the subjects following the study. This work was supported by Public Health Service Grants R01 MH55703, R01 MH56874, R01 AG21826, and R01 HD42125 (to A.J.L.) and 5 M01 RR000334 (to the General Clinical Research Center of OHSU). A.J.L. was supported by the National Alliance for Research on Schizophrenia and Depression 2000 Distinguished Investigator Award. J.S.E. was supported by Public Health Service Grant K23 RR017636-01.
Abbreviations
- SAD
seasonal affective disorder
- PSH
phase-shift hypothesis
- DLMO
dim light melatonin onset
- PAD
phase angle difference
- SIGH-SAD
Structured Interview Guide for the Hamilton Depression Rating Scale–SAD version
Footnotes
Conflict of interest statement: A.J.L. is coinventor on several melatonin use-patents owned by Oregon Health & Science University currently not licensed to any company.
Lewy, A. J., Lefler, B. J., Hasler, B. P., Bauer, V. K., Bernert, R. A. & Emens, J. S. (2003) Chronobiol. Int. 20, 1215–1217 (abstr.).,
Lewy, A. J., Lefler, B. J., Yuhas, K., Hasler, B. P., Bernert, R. A. & Emens, J. S. (2004) Neuropsychopharmacology 29, S103–S104 (abstr.).
Lewy, A. J., Emens, J. S., Lefler, B. J. & Bauer, V. K. (2005) Neuropsychopharmacology 30, S62–S63 (abstr.).
References
- 1.Lewy A. J., Bauer V. K., Ahmed S., Thomas K. H., Cutler N. L., Singer C. M., Moffit M. T., Sack R. L. Chronobiol. Int. 1998;15:71–83. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- 2.Sack R. L., Brandes R. W., Kendall A. R., Lewy A. J. N. Engl. J. Med. 2000;343:1070–1077. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- 3.Hack L. M., Lockley S. W., Arendt J., Skene D. J. J. Biol. Rhythms. 2003;18:420–429. doi: 10.1177/0748730403256796. [DOI] [PubMed] [Google Scholar]
- 4.Bunney B. G., Potkin S. G., Bunney W. E. In: Biology of Depression: From Novel Insights to Therapeutic Strategies. Licinio J., Wong M. L., editors. Vol. 1. Hoboken, NJ: Wiley; 2005. pp. 467–483. [Google Scholar]
- 5.Rosenthal N. E. Winter Blues. New York: Guilford; 1998. [Google Scholar]
- 6.Kasper S., Wehr T. A., Bartko J. J., Gaist P. A., Rosenthal N. E. Arch. Gen. Psychiatry. 1989;46:823–833. doi: 10.1001/archpsyc.1989.01810090065010. [DOI] [PubMed] [Google Scholar]
- 7.Lewy A. J., Kern H. A., Rosenthal N. E., Wehr T. A. Am. J. Psychiatry. 1982;139:1496–1498. doi: 10.1176/ajp.139.11.1496. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal N. E., Sack D. A., Gillin J. C., Lewy A. J., Goodwin F. K., Davenport Y., Mueller P. S., Newsome D. A., Wehr T. A. Arch. Gen. Psychiatry. 1984;41:72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- 9.Eastman C. I., Young M. A., Fogg L. F., Liu L., Meaden P. M. Arch. Gen. Psychiatry. 1998;55:883–889. doi: 10.1001/archpsyc.55.10.883. [DOI] [PubMed] [Google Scholar]
- 10.Lewy A. J., Bauer V. K., Cutler N. L., Sack R. L., Ahmed S., Thomas K. H., Blood M. L., Latham Jackson J. M. Arch. Gen. Psychiatry. 1998;55:890–896. doi: 10.1001/archpsyc.55.10.890. [DOI] [PubMed] [Google Scholar]
- 11.Terman M., Terman J. S., Ross D. C. Arch. Gen. Psychiatry. 1998;55:875–882. doi: 10.1001/archpsyc.55.10.875. [DOI] [PubMed] [Google Scholar]
- 12.Czeisler C. A., Kronauer R. E., Mooney J. J., Anderson J. L., Allan J. S. Psychiatric Clinics North Am. 1987;10:687–709. [PubMed] [Google Scholar]
- 13.Teicher M. H., Glod C. A., Magnus E., Harper D., Benson G., Krueger K., McGreenery C. E. Arch. Gen. Psychiatry. 1997;54:124–130. doi: 10.1001/archpsyc.1997.01830140034007. [DOI] [PubMed] [Google Scholar]
- 14.Wehr T. A., Duncan W. C., Sher L., Aeschbach D., Schwartz P. J., Turner E. H., Postolache T. T., Rosenthal N. E. Arch. Gen. Psychiatry. 2001;58:1108–1114. doi: 10.1001/archpsyc.58.12.1108. [DOI] [PubMed] [Google Scholar]
- 15.Lewy A. J., Sack R. L., Miller S., Hoban T. M. Science. 1987;235:352–354. doi: 10.1126/science.3798117. [DOI] [PubMed] [Google Scholar]
- 16.Kripke D. F., Mullaney D. J., Atkinson M., Wolf S. Biol. Psychiatry. 1978;13:335–351. [PubMed] [Google Scholar]
- 17.Wehr T. A., Wirz-Justice A., Goodwin F. K., Duncan W., Gillin J. C. Science. 1979;206:710–713. doi: 10.1126/science.227056. [DOI] [PubMed] [Google Scholar]
- 18.Lewy A. J., Sack R. L., Singer C. M., White D. M. Psychopharmacol. Bull. 1987;23:349–353. [PubMed] [Google Scholar]
- 19.Lewy A. J. Psychiatric Ann. 1987;17:664–669. [Google Scholar]
- 20.Lewy A. J., Sack R. L., Singer C. M., White D. M., Hoban T. M. In: Seasonal Affective Disorder. Thompson C., Silverstone T., editors. London: CNS; 1989. pp. 205–221. [Google Scholar]
- 21.Lewy A. J., Sack R. L., Singer C. M., White D. M., Hoban T. M. In: Seasonal Affective Disorders and Phototherapy. Rosenthal N. E., Blehar M. C., editors. New York: Guilford; 1989. pp. 295–310. [Google Scholar]
- 22.Avery D. H., Dahl K., Savage M. V., Brengelmann G. L., Larsen L. H., Kenny M. A., Eder D. N., Vitiello M. V., Prinz P. N. Biol. Psychiatry. 1997;41:1109–1123. doi: 10.1016/S0006-3223(96)00210-7. [DOI] [PubMed] [Google Scholar]
- 23.Terman J. S., Terman M., Lo E. S., Cooper T. B. Arch. Gen. Psychiatry. 2001;58:69–75. doi: 10.1001/archpsyc.58.1.69. [DOI] [PubMed] [Google Scholar]
- 24.Sack R. L., Lewy A. J., White D. M., Singer C. M., Fireman M. J., Vandiver R. Arch. Gen. Psychiatry. 1990;47:343–351. doi: 10.1001/archpsyc.1990.01810160043008. [DOI] [PubMed] [Google Scholar]
- 25.Koorengevel K. M., Beersma D. G., den Boer J. A., van den Hoofdakker R. H. J. Biol. Rhythms. 2002;17:463–475. doi: 10.1177/074873002237140. [DOI] [PubMed] [Google Scholar]
- 26.Wirz-Justice A., Kräuchi K., Brunner D. P., Graw P., Haug H.-J., Leonhardt G., Sarrafzadeh A., English J., Arendt J. Acta Neuropsychiatrica. 1995;7:41–43. doi: 10.1017/S0924270800037522. [DOI] [PubMed] [Google Scholar]
- 27.Eastman C. I., Gallo L. C., Lahmeyer H. W., Fogg L. F. Biol. Psychiatry. 1993;34:210–220. doi: 10.1016/0006-3223(93)90074-n. [DOI] [PubMed] [Google Scholar]
- 28.Terman M. In: Light and Biological Rhythms in Man. Wetterberg L., editor. Stockholm: Pergamon; 1993. pp. 421–436. [Google Scholar]
- 29.Lewy A. J., Wehr T. A., Goodwin F. K., Newsome D. A., Markey S. P. Science. 1980;210:1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 30.Axelrod J. Science. 1974;184:1341–1348. doi: 10.1126/science.184.4144.1341. [DOI] [PubMed] [Google Scholar]
- 31.Zhdanova I. V., Wurtman R. J., Regan M. M., Taylor J. A., Shi J. P., LeClair O. U. J. Clin. Endocrinol. Metab. 2001;86:4727–4730. doi: 10.1210/jcem.86.10.7901. [DOI] [PubMed] [Google Scholar]
- 32.Burgess H. J., Fogg L. F., Young M. W., Eastman C. I. Chronobiol. Int. 2004;21:1–17. doi: 10.1081/cbi-200025979. [DOI] [PubMed] [Google Scholar]
- 33.Williams J. B. W., Link M. J., Rosenthal N. E., Terman M. Structured Interview Guide for the Hamilton Depression Rating Scale: Seasonal Affective Disorder Version (SIGH-SAD) New York: New York State Psychiatric Institute; 1988. [Google Scholar]
- 34.Brody A. L., Barsom M. W., Bota R. G., Saxena S. Semin. Clin. Neuropsychiatry. 2001;6:102–112. doi: 10.1053/scnp.2001.21837. [DOI] [PubMed] [Google Scholar]
- 35.Perlis M. L., Giles D. E., Buysse D. J., Thase M. E., Tu X., Kupfer D. J. Biol. Psychiatry. 1997;42:904–913. doi: 10.1016/S0006-3223(96)00439-8. [DOI] [PubMed] [Google Scholar]
- 36.Zobel A. W., Nickel T., Sonntag A., Uhr M., Holsboer F., Ising M. J. Psychiatric Res. 2001;35:83–94. doi: 10.1016/s0022-3956(01)00013-9. [DOI] [PubMed] [Google Scholar]
- 37.Kunzel H. E., Binder E., Nickel T., Ising M., Fuchs B., Majer M. Neuropsychopharmacology. 2003;28:2169–2178. doi: 10.1038/sj.npp.1300280. [DOI] [PubMed] [Google Scholar]
- 38.Thalén B. E., Kjellman B. F., Mørkrid L., Wibom R., Wetterberg L. Acta Psychiatrica Scand. 1995;91:352–360. doi: 10.1111/j.1600-0447.1995.tb09794.x. [DOI] [PubMed] [Google Scholar]
- 39.Khan A., Khan S. R., Walens G., Kolts R., Giller E. L. Neuropsychopharmacology. 2003;28:552–557. doi: 10.1038/sj.npp.1300059. [DOI] [PubMed] [Google Scholar]
- 40.Judd L. L., Akiskal H. S. Pharmacopsychiatry. 2000;33:3–7. doi: 10.1055/s-2000-7967. [DOI] [PubMed] [Google Scholar]
- 41.Walsh B. T., Seidman S. N., Sysko R., Gould M. J. Am. Med. Assoc. 2002;287:1840–1846. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.