Abstract
Foods high in resistant starch have the potential to improve human health and lower the risk of serious noninfectious diseases. RNA interference was used to down-regulate the two different isoforms of starch-branching enzyme (SBE) II (SBEIIa and SBEIIb) in wheat endosperm to raise its amylose content. Suppression of SBEIIb expression alone had no effect on amylose content; however, suppression of both SBEIIa and SBEIIb expression resulted in starch containing >70% amylose. When the >70% amylose wheat grain was fed to rats in a diet as a wholemeal, several indices of large-bowel function, including short-chain fatty acids, were improved relative to standard wholemeal wheat. These results indicate that this high-amylose wheat has a significant potential to improve human health through its resistant starch content.
Keywords: genetic engineering, nutrition, starch
Diet-related noninfectious chronic diseases, such as coronary heart disease, certain cancers (especially of the colon and rectum), and diabetes, are major causes of morbidity and mortality in both affluent industrialized countries and emerging nations. Increased consumption of whole-grain cereal foods can reduce the risk of these conditions substantially (1–3) and also correlates negatively with obesity (4). Resistant starch (RS) and nonstarch polysaccharides (NSP) are major components of dietary fiber and contribute to these benefits. NSP resist digestion by enzymes in the human small intestine completely, helping to explain their fecal bulking and laxative action (5), which may underpin the protection against colorectal cancer (6) afforded by greater fiber consumption. Although starch can be digested (theoretically to completion) in the human small intestine, amylolysis varies by rate and extent for many foods. Reduction of the rate of starch digestion in the small intestine has the potential to lower the rate of entry of glucose into the circulation and, thus, reduce demand for insulin (7). This is measured as the glycemic index (GI), and lowering the GI is emerging as an important mechanism for managing the incidence and severity of type II diabetes. The fraction of the ingested starch that escapes into the human large bowel is known as RS. Short-chain fatty acids (SCFA) are major end products of the fermentation of NSP and RS by the microflora (5), and they promote important aspects of large bowel function. One of the principal SCFA, butyrate, may also play a role in promoting a normal phenotype in colonocytes and lowering the risk of colorectal cancer (8). It is emerging that RS contributes substantially to some of the benefits that have been ascribed solely to dietary fiber (5). RS intakes are low in populations at high risk of the diseases of affluence, and there is a case for increasing RS consumption as an effective means of improving nutrition for public health at the population level.
Starches consist of glucose monomers polymerized through α1,4 and α1,6 linkages into two classes of polymers, amylose and amylopectin. Amylopectin is a large highly branched polysaccharide [degree of polymerization (DP) >5,000], whereas amylose has infrequent α1,6 linkages and a lower DP (<2,000). In cooked foods, amylose molecules reassociate rapidly on cooling, forming complexes that resist digestion, whereas amylopectin molecules reassociate slowly and are more readily digested. This difference explains the higher RS content of high-amylose products. The synthesis of amylose and amylopectin occurs via two pathways. Amylose synthesis requires an active granule-bound starch synthase (GBSS). Amylopectin is synthesized by a complex pathway involving, among others, several isoforms of SS, starch-branching enzymes (SBE), and starch-debranching enzymes (9). Monocots and dicots differ in the isoforms of SBE they contain. In potato, two isoforms of SBE are present, SBEI and SBEII. Suppression of both SBEI and SBEII was required to generate starch with amylose content of >50% (10). In monocots, SBEI and two forms of SBEII, SBEIIa and SBEIIb, are found. In maize, suppression of SBEIIb results in the “amylose-extender” phenotype, with amylose contents from 50% to 90% (11), whereas suppression of SBEI or SBEIIa had no impact on endosperm amylose content (12, 13). Amylose-extender maize has been used to increase the RS content of processed foods (14); however, additional sources of RS are needed.
Of the cereal grains cultivated widely, wheat supplies ≈20% of the food calories for the world population and is the preferred base for most cereal-based processed products, such as bread, pasta, and noodles. Wheat consumption is increasing world-wide with increasing affluence. Modification of the starch composition of wheat by raising its RS content presents an opportunity for a potentially large-scale improvement in public health. Wheat is a hexaploid having three different genomes, A, B, and D. The hexaploid nature of the wheat genome makes finding and combining gene mutations in genes, such as the SBE genes, which are found in each of the three genomes, a challenging proposition. In contrast, gene silencing through RNA interference (RNAi) offers the potential to suppress simultaneously the expression of a target gene from each locus. In this study, RNAi technology was used to silence the expression of SBEIIa and SBEIIb, resulting in a high-amylose (>70%) phenotype. A nutritional experiment using high-amylose wheat in rats confirmed that its consumption as a wholemeal raised large-bowel digesta mass and SCFA, entirely consistent with a higher content of RS.
Results
Transgenic Wheats with Highly Reduced Levels of Expression of SBEIIa and SBEIIb Were Generated by Using RNAi Constructs.
Constructs directing the expression of hairpin-RNA molecules were constructed by cloning fragments of the wheat SBEIIa and SBEIIb cDNAs (corresponding to the exon 1–3 region of the genes involved) in inverted repeats, separated by intron 3 of the respective genes. An endosperm-specific high-molecular-weight glutenin promoter (Dx5 subunit gene, GenBank accession no. X12928) was positioned at the 5′ end of the inverted repeat region, and a nopaline synthase termination region was placed at the 3′ end (see Fig. 5, which is published as supporting information on the PNAS web site). The promoter-inverted repeat-terminator constructs were transferred to binary transformation vectors derived from pSB11 and pSB1 (15) and used for Agrobacterium-mediated transformation. Positive wheat transgenic lines of SBEIIa (hp-SBEIIa lines) and SBEIIb (hp-SBEIIb lines) were identified by both PCR and Southern blot hybridization (data not shown). Approximately one to six copies of SBEIIa transgenes and one to seven copies of SBEIIb transgenes were present in the respective positive transgenic lines.
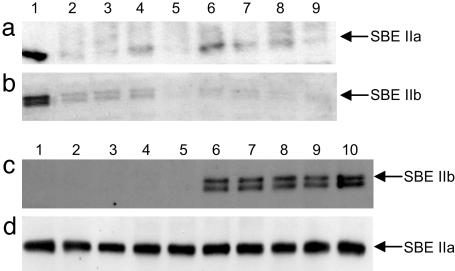
T2 endosperms from hp-SBEIIa and hp-SBEIIb T1 plants (see Materials and Methods) were analyzed for protein expression by immunoblotting with antisera specific for SBEIIa or SBEIIb. Twelve hp-SBEIIa plants contained endosperms with reduced levels of SBEIIa protein ranging from <1% to 10% compared with wild type. The SBEIIa expression levels in a representative subset of samples are shown in Fig. 1a. Of the nine hp-SBEIIb lines analyzed, three showed no detectable SBEIIb expression in the developing grains; two had uniform wild-type expression; and four were segregating for no expression, reduced expression, or wild type. Fig. 1c shows the SBEIIb expression levels in a subset of samples. SBEIIb expression was also reduced in seeds containing the hp-SBEIIa transgene and reduced expression of SBEIIa (Fig. 1b). SBEIIb expression was unaltered in lines containing the hp-SBEIIa transgene and unaltered expression of SBEIIa. However, the converse effect was not observed in hp-SBEIIb transgenic seeds, where SBEIIb was essentially completely silenced (Fig. 1d) but SBEIIa expression was unaltered.
Fig. 1.
Immunodetection of SBEII isoforms in wheat endosperm. Segregation of SBEIIa (a) and SBEIIb (b) expression in three developing endosperms from the T2 wheat hp-SBEIIa transgenic line 087 (lanes 2–4) and five developing endosperms from the T2 hp-SBEIIa transgenic line 104 (lanes 5–9), as shown by immunoblotting using anti-SBEIIa and -SBEIIb antibodies, respectively. Lane 1 is nontransformed NB1. Segregation of SBEIIb (c) and SBEIIa (d) expression in five developing endosperms from the T2 wheat hp-SBEIIb transgenic line 008 (lanes 1–5) and four developing endosperms from the T2 hp-SBEIIb transgenic line 009 (lanes 6–9), as shown by immunoblotting using anti-SBEIIb and -SBEIIa antibodies, respectively. Lane 10 is nontransformed NB1.
Starch Granule Morphology Is Significantly Altered in Endosperm with Reduced SBEIIa Expression.
Scanning electron microscopy was used to identify gross changes in granule size and structure (Fig. 2). Compared with the untransformed control (Fig. 2a), starch granules from endosperms with reduced SBEIIa expression displayed significant morphological alterations (Fig. 2b). The granules in reduced SBEIIa expression endosperms were highly irregular in shape, and a large number of A granules (>10-μm diameter) appeared to be sickle-shaped. On the other hand, endosperm starch granules from plants with reduced SBEIIb expression had smooth spherical-to-ellipsoidal A and B (<10-μm diameter) granules typical of a nontransformed control wheat starch (Fig. 2c).
Fig. 2.
Scanning electron micrographs of isolated starch granules. NB1 (nontransformed control wheat) (a), 087 (hp-SBEIIa wheat) (b), and 008 (hp-SBEIIb wheat) (c).
Under polarized light, wild-type starch granules typically show a strong birefringence pattern; however, birefringence is typically strongly reduced in granules containing high-amylose starch. Starch granules from lines with reduced SBEIIa expression had both altered granule morphology and a significant loss of birefringence when visualized under polarized light (Table 1). Over 90% of the starch granules were nonbirefringent. For lines with essentially no SBEIIb expression, no change in birefringence was observed compared with nontransformed controls. In both wild-type and SBEIIb-suppressed lines, ≈94% of the starch granules exhibited full birefringence.
Table 1.
Starch characteristics of wheat transgenic lines of SBEII
| Line identification | Enzyme targeted | Birefringence* |
Amylose content estimated iodometrically,* % | Amylose content determined by SEC, % | 100 grain weight,*† g | Starch content,* % | ||
|---|---|---|---|---|---|---|---|---|
| Nil, % | Partial, % | Full, % | ||||||
| NB1 | Nontransformed | 1.6 | 3.5 | 94.9 | 31.8 | 25.5 | 3.8 | 52.0 |
| 087 | SBEIIa | 94.6 | 4.0 | 1.5 | 88.5 | 74.4 | 3.8 | 43.4 |
| 008 | SBEIIb | 0.6 | 5.21 | 94.1 | 27.3 | 32.8 | 3.6 | 50.3 |
| LSD (5%) | — | 9.02 | 3.3 | 9.9 | 7.7 | ND | NS | 4.9 |
ND, not determined. NS, not significant.
*Mean of three replicates.
†Dry weight of 100 kernels.
Amylose Content Is Increased to >70% in Starch from Endosperm with Reduced SBEIIa Expression.
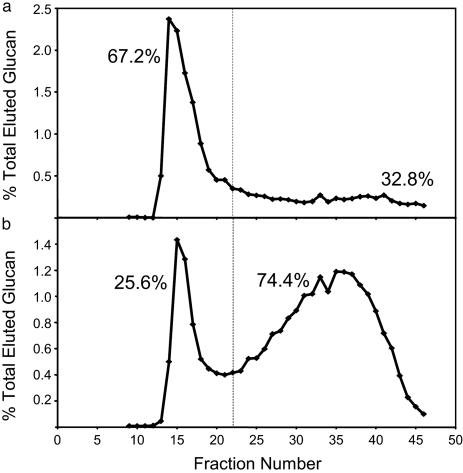
The amylose content of wheat transgenic lines was estimated by two independent methods: an iodometric method and a size exclusion chromatography (SEC) method. The iodometric determination of amylose content is based on measuring the color change induced when iodine binds to linear regions of α-1,4 glucan against a standard curve generated using known concentrations of purified potato amylose and amylopectin. The SEC method is based on the column chromatography separation of undebranched amylose and amylopectin (see Fig. 3). Table 1 shows data for representative lines from each of three classes of plants; first, plants containing the hp-SBEIIa construct with very low levels of SBEIIa expression; second, plants containing the hp-SBEIIb construct and no detectable expression of SBEIIb; and third, the nontranformed wild-type control. The low SBEIIb expression line (008) had an amylose content (27.3% iodometric, 32% SEC) that was similar to the nontransformed control line NB1 (31.8% iodometric, 25.5% SEC) However, in the reduced SBEIIa expression line (087), the amylose content was significantly elevated (88.5% iodometric, 74.4% SEC). Results from both analytical methods are reported, because they are independent measures of amylose content; however, the iodometric method tends to overestimate the amylose content of cereal starches and in this report, the amylose contents quoted refer to the values determined by SEC.
Fig. 3.
Size distribution analysis of wheat endosperm starch. Sepharose CL 2B gel chromatogram of undebranched starch from transgenic lines 008 (hp-SBEIIb wheat) (a) and 087 (hp-SBEIIa wheat) (b). The starch content of fractions was assayed by using a starch assay kit (Sigma). Amylopectin (first peak) and amylose (second peak) content estimated by this method as a percentage of total starch is shown on respective graphs.
Starch from Endosperm with Reduced SBEIIa Expression Has a Decreased Proportion of Shorter Glucan Chains.
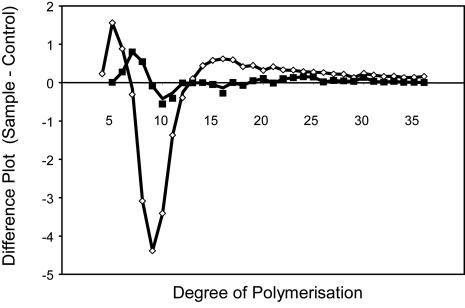
Chain-length distribution of isoamylase debranched starch was determined by fluorophore-assisted carbohydrate electrophoresis. This technique provides a high-resolution analysis of the distribution of chains in the range from DP 1 to 50. The molar difference plot in which the normalized chain-length distributions of the nontransformed control were subtracted from the normalized distribution of the transgenic lines is shown in Fig. 4. There is a marked decrease in the proportion of chain lengths of DP 4–12 and a corresponding increase in the chain lengths greater than DP 12 in starch from SBEIIa-reduced expression lines. No statistically significant alteration in the chain-length distribution of starch from hp-SBEIIb lines was observed when compared with wild-type.
Fig. 4.
Chain length distribution analysis of wheat endosperm starch by fluorophore-assisted carbohydrate electrophoresis. Chain length profile of debranched starches from wheat transgenic lines was compared with that of nontransformed control NB1. The percentage of total mass of individual oligosaccharides from starch from NB1 is subtracted from the corresponding values from starches from transgenic lines. Samples are hp-SBE IIa line, 085 (◇), and hp-SBE IIb line, 008 (■).
Total Starch Content in Endosperm of Wheat with Reduced SBEIIa Expression Is Lowered only Slightly.
One impact of modifying the starch composition of lines is a yield penalty incurred through either reduced agronomic performance or decreased grain size. Analysis of glasshouse-grown plants revealed no statistically significant difference in kernel weight between hp-SBEIIa or hp-SBEIIb wheat lines compared with nontransformed control (Table 1), despite the very marked changes in starch properties in the transgenic and control lines. However, the analysis of total starch content revealed a slight reduction in the endosperm starch content of the hp-SBEIIa line (43.4%) compared with 52% in the control and 50.3% in the hp-SBEIIb line (Table 1).
High-Amylose Grain from Wheats with Reduced SBEIIa Expression Improves Indices of Gastrointestinal Health in Rats.
A nutritional trial was carried out in rats to investigate the effects of high-amylose wheat on bowel-health indices. The initial body weight of rats did not differ between the groups (overall mean of 193 g; n = 12, SE = 3). Diets were well accepted and supported rates of food consumption and weight gain (average 6.5 g per day) that were appropriate for rats of this age. There was no effect of dietary treatment on final body weight with a mean of 278 (SE = 7, n = 6) and 282 (SE = 6, n = 6) for the low- and high-amylose wheat groups, respectively. Daily food intake averaged 20 g per day for each of the two groups (P > 0.05) during the metabolism cage phase of the study.
Large-bowel tissue weight was generally higher in rats fed the high-amylose wheat (data not shown for colon). Only in the cecum did the effect near significance (P < 0.07) and in that viscus, the weights were 0.91 g (SE = 0.07, n = 6) and 1.23 g (SE = 0.16, n = 6) for the low- and high-amylose wheats, respectively. The wet weight of digesta was higher in rats fed high-amylose wheat, but the effect was significant only in the cecum, where it was >110% larger than in rats fed the low-amylose wheat diet (Table 2). The dry weight of digesta was also significantly higher in the cecum (Table 2).
Table 2.
Large-bowel digesta wet and dry weight of rats consuming experimental diets
| Low-amylose wheat | High-amylose wheat | |
|---|---|---|
| Cecum | ||
| Wet weight, g | 1.47 (0.12)* | 3.14 (0.34)* |
| Dry weight, g | 0.33 (0.03)* | 0.67 (0.10)* |
| Proximal colon | ||
| Wet weight, g | 0.29 (0.12) | 0.48 (0.09) |
| Dry weight, g | 0.06 (0.03) | 0.11 (0.02) |
| Distal colon | ||
| Wet weight, g | 0.83 (0.17) | 1.10 (0.08) |
| Dry weight, g | 0.30 (0.08) | 0.35 (0.04) |
All values are shown as the mean and SE (in parentheses) of six animals. Values in a row with like symbols are significantly different.
*, P < 0.01.
The high-amylose wheat produced a lower pH value (pH 5.90) in cecal contents compared with the low-amylose wheat (pH 6.23) (Table 3). Although there were no significant differences in concentration (Table 3) or molar proportions of SCFA (acetate, 48.8 vs. 49.8; propionate, 14.7 vs. 18.0; and butyrate, 32.0 vs. 29.8 for low- and high-amylose wheat diets, respectively), cecal digesta pools for the total and individual acids were all significantly larger in rats fed the high-amylose wheat diet than in controls (Table 4). Fecal total SCFA excretion was also significantly higher (P < 0.02) in rats fed the high-amylose wheat with a mean value of 46.1 (SE = 5) μmol per day compared with 24.7 (SE = 5) μmol per day by rats fed the standard wheat (data not shown).
Table 3.
Cecal digesta pH and SCFA concentrations of rats consuming experimental diets
| Diet | pH | SCFA concentration, mmol/kg |
|||
|---|---|---|---|---|---|
| Acetate | Propionate | Butyrate | Total | ||
| Low-amylose wheat | 6.23 (0.05)* | 38.6 (1.9) | 11.9 (1.7) | 25.8 (3.3) | 79.6 (3.1) |
| High-amylose wheat | 5.90 (0.14)* | 43.6 (7.8) | 15.8 (3.1) | 23.0 (2.5) | 84.1 (8.6) |
All values are shown as the mean and SE (in parentheses) of six animals. Values in any column with like symbols are significantly different.
*, P < 0.05.
Table 4.
Cecal SCFA pools of rats consuming experimental diets
| Diet | SCFA pools, μmol |
|||
|---|---|---|---|---|
| Acetate | Propionate | Butyrate | Total | |
| Low-amylose wheat | 44 (4)* | 14 (2)† | 31 (6)‡ | 88 (10)* |
| High-amylose wheat | 106 (18)* | 38 (7)† | 57 (8)‡ | 202 (25)* |
All values are shown as the mean and SE (in parentheses) of six animals. Values in any column with like symbols are significantly different.
*, P < 0.01;
†, P < 0.02;
‡, P < 0.05.
Discussion
The objective of this research was to define a genetic mechanism for the production of high-amylose wheat capable of delivering RS effectively in the diet. This study addressed two issues: first, the definition of which member of the branching enzyme gene family has to be suppressed to generate a high amylose starch; and second, the work demonstrated that the high-amylose wheat generated by this mechanism induced beneficial changes in indices of gastrointestinal health in a rat-feeding trial.
RNAi Experiments Demonstrate Suppression of SBEIIa, Not SBEIIb, Is Critical for High Amylose in Wheat.
There are three known mechanisms for increasing amylose content in plants. The first is to increase granule-bound starch synthase (GBSS) activity. Overexpression of GBSS has recently been reported to yield a rice starch with slightly increased amylose content (16); however, the lack of reports concerning this mechanism precludes making an assessment of its general utility at this time. The second mechanism is to decrease amylopectin synthesis by suppression of the activity of starch synthases and/or isoamylases leading to a net increase in amylose content. Generally, this approach yields only modest increases in amylose content. For example, amylose contents of 35–45% have been in reported in maize sugary-2, su1, du-1 (17), and wheat Sgp-1 (18) mutants. However, the one exception is the much higher levels of amylose (>70%) present in a barley lacking SSIIa activity (19).
In this study, the third mechanism for increasing amylose content, suppression of the activity of SBE, has been applied. RNAi technology was used to investigate the roles of SBEIIa and SBEIIb, having previously shown that reduction of SBEI has no impact on amylose content in wheat (20). This latter result is consistent with studies in potato, maize, and rice (12, 21, 22). Near-complete suppression of SBEIIb alone in wheat achieved through RNAi (<1% expression level compared with control) did not cause any marked alteration in starch structure. This result contrasts with previous findings in maize (11) and rice (23), where a reduction in SBEIIb resulted in increases in amylose content. As discussed below, transformation with a hairpin construct targeting SBEIIa led to suppression of both SBEIIa and SBEIIb, resulting in a starch with an amylose content of >70% with concomitant changes in starch granule morphology, starch composition, and starch fine structure. One explanation for the difference in the nature of gene suppression event leading to high-amylose starches between maize and wheat lies in the relative expression levels of SBEIIa and SBEIIb in each species. Although SBEIIb is expressed at ≈50 times the level of SBEIIa in maize endosperm (24), in wheat, there is more abundant SBEIIa than SBEIIb in the amyloplast stroma (25, 26). We conclude that SBEIIa and SBEIIb may have highly complementary or essentially redundant roles, and the determining factor is the combined expression level of SBEII isoforms rather than a situation where one or the other isoform has a specific independent role.
The hp-SBEIIa construct suppressed the expression of both SBEIIa and SBEIIb, whereas the hp-SBEIIb construct suppressed only the expression of SBEIIb. The mechanism of cross suppression of SBEIIb by the hp-SBEIIa construct is unknown. The region spanning exons 1, 2, and 3 of the homeologous SBEIIa and SBEIIb genes in wheat, the regions included in the constructs used in this study, have a sequence identity of 70%. The intron 3 regions of wheat SBEIIa and SBEIIb used in constructs in this study have only <50% identity. There is one region of 21 nucleotides of 100% identify between the hp-SBEIIb construct and the wheat SBEIIa cDNA, and three regions of 21 nucleotides with 75–80% identity (see Fig. 6, which is published as supporting information on the PNAS web site). Similarly, the sequence of SBEIIa used in the hp-SBEIIa construct has one stretch of 21 nucleotides with 100% identity with wheat SBEIIb cDNA and five regions with 70–80% identity. Despite these sites representing possible targets for the RNAi mechanism to induce cross suppression, Northern blot analysis (Fig. 7, which is published as supporting information on the PNAS web site) provided evidence that only the transcript of the targeted isoform was affected; the transcript of the other isoform was unaffected. This indicates that the cross suppression of SBEIIb mRNA accumulation by hp-SBEIIa is not due to the RNAi mechanism directly. There are several possible explanations for this phenomenon. First, in hp-SBEIIa lines, translation of SBEIIb mRNA may be inhibited through a mechanism that does not involve mRNA degradation. Second, inhibition of as-yet-incompletely characterized phosphorylation or complexation mechanisms involving the various starch biosynthetic enzymes could result in decreased SBEIIb stability in the hp-SBEIIa lines (26).
Rat Nutritional Study Demonstrates High-Amylose Wheat Has Positive Effects on Indices of Gastrointestinal Health.
A major driver for the development of high-amylose wheats has been to generate a source of RS that can be readily deployed through the food supply to benefit large numbers of consumers. The analytical data show conclusively that approach was effective in raising the amylose content of the starch of the wheat grain. A key outcome was to confirm that these compositional changes translated to desired nutritional attributes. There was no evidence for an adverse impact on growth and performance in the current study in animals fed transgenic high-amylose wheat. Limitations in the quantities of grain meant that the first trial had to be carried out in rats for a relatively short period. Nevertheless, the data show conclusively that indices of large-bowel fermentation were all significantly higher in rats fed the high-amylose wheat, consistent with more RS, compared with those fed the standard wheat. Thus large-bowel digesta wet weight and SCFA pools and fecal SCFA excretion were all ≈100% higher in rats fed the novel wheat compared with those fed the control diet. pH values were also significantly lower, again consistent with greater fermentation. That these differences were due to starch, and not NSP, was ensured by balancing the fiber content of the diets. These data in rats fed the high-amylose wheat were generally similar to those noted previously in rats fed Himalaya 292, a barley cultivar with high RS arising through altered starch synthesis (27–29). Interestingly, the molar ratios of the major SCFA in rats fed the novel wheat were rather different from Himalaya 292 with considerably more butyrate in the present study. This is of some interest in view of the apparent importance of butyrate in promoting large-bowel function. Collectively, the data support the potential of the new high-amylose wheat to produce foods high in RS and with a low glycemic index. The data justify further investigation of the health potential of high-amylose wheats, especially in processed foods, as an important additional mechanism to deliver significant health benefits to large numbers of consumers through their diet.
Materials and Methods
Wheat Transformation Vector.
DNA fragments containing sequence corresponding to exons 1, 2, and 3 and intron 3 of the wheat SBEIIa gene, wSBEII-DA1 (GenBank accession no. AF338431), and the wheat SBEIIb gene, wSBEII-DB1 (25), were ligated as detailed in Supporting Text, which is published as supporting information on the PNAS web site, to generate SBEIIa and SBEIIb RNAi constructs, respectively. The respective ligated sequence was introduced into an intermediate vector pDV03000 containing a high-molecular-weight glutenin promoter sequence from wheat (30) and the terminator sequence of nopaline synthase gene from Agrobacterium (31) to generate SBEIIa (pDV03-IIa) and SBEIIb (pDV03-IIb) intermediate vectors. Each of the expression cassettes from pDV03-IIa and pDV03-IIb was then inserted into binary transformation vectors derived from pSB1 and pSB11 (15) to generate hp-SBEIIa and hp-SBEIIb constructs, as detailed in Supporting Text.
Transformation of Wheat.
hp-SBEIIa and hp-SBEIIb constructs were transformed into wheat by using an Agrobacterium tumefaciens transformation system (32).
Expression Analyses of SBEIIa and SBEIIb.
Seven T2 endosperms each from 13 T1 hp-SBEIIa and 9 T1 hp-SBEIIb-positive transgenic plants were analyzed for SBEIIa and SBEIIb expression. Endosperm-soluble proteins from wheat plants were extracted from developing endosperms (18 days after anthesis) by using 50 mM potassium phosphate buffer, pH 7.5 (3 ml/g of endosperm), containing 5 mM EDTA, 5 mM DTT, and 1 mM pefabloc. Nondenaturing polyacrylamide gel electrophoresis and immunoblotting were carried out as described (33). The primary antibodies used were wheat SBEIIa [anti-WBEIIa (34)] and wheat SBEIIb [anti-WBEIIb (25)]. Densitometry analysis of immunoblots was done by using the software uvi photo (UVItec, Cambridge, U.K.).
Microscopic Analyses of Starch Granules.
Starch from the endosperm half of single grains was extracted as detailed in Supporting Text. Purified starch suspension in water was visualized under both normal and polarized light by using a Leica-DMR compound microscope (Leica, Deerfield, IL) to determine the starch granule morphology. Scanning electron microscopy was carried out on a JEOL JSM 35C instrument. Purified starches were sputter-coated with gold and scanned at 15 kV at room temperature.
Iodometric Estimation of Amylose Content.
Amylose content of wheat endosperm starch was estimated by the iodometric method, as described (20).
Estimation of Amylose Content by Sepharose CL-2B Gel Filtration.
Starch molecules were separated by the Sepharose CL-2B column based on their molecular weight and assayed as detailed in Supporting Text. The chromatogram revealed two peaks of elution. Amylose content was estimated as a percentage of the total amount of starch within the second peak of elution to the total amount of starch within both the peaks.
Fluorophore-Assisted Carbohydrate Electrophoresis Chain-Length Distribution.
Chain-length distribution of wheat endosperm starch was analyzed as described (35) by using a P/ACE 5510 capillary electrophoresis system (Beckman) with argon-LIF detection.
Animal Experimentation and Ethical Considerations.
All procedures involving the growth, importation, transportation, processing, diet preparation, and residue disposal of the transgenic wheat were conducted under approvals and procedures mandated by the Office of the Gene Technology Regulator (Canberra, Australia). All procedures relating to the use of experimental animals were approved by and carried out under the oversight of the Commonwealth Scientific and Industrial Research Organization Health Sciences and Nutrition Animal Ethics Committee and conformed to published guidelines (36).
Care of Animals.
Twelve 4-week-old male Sprague–Dawley rats were used. They were purchased from the Animal Resource Facility Western Australia and housed in groups in standard wire-bottomed cages at the Animal Services Unit of Commonwealth Scientific and Industrial Research Organization Health Sciences and Nutrition in a room of controlled temperature (22 ± 1°C) and lighting (lights on 0800–2000 h).
Diets and Feeding.
After arrival, the rats were adapted to a nonpurified commercial diet for 7 days. They were then allocated randomly to two dietary treatment groups of six animals. The composition of the basal diet, which was based on AIN 93G (37) specifications and prepared from standard ingredients, is shown in Table 5. The proximate composition of the high- and low-amylose wheats, wheat bran, maize starch, and casein was determined by using standard analytical techniques (38). The diets were designed to provide 350 g/kg of starch from each wheat source and formulated to contain 200 g of protein/kg, 570 g of carbohydrate/kg (as 450 g of starch and 120 g of sucrose), 70 g of fat/kg, and 90 g of NSP/kg. The high- and low-amylose wheat diets contained 576 and 481 g/kg of the novel and conventional wheats, respectively, both as wholemeal flour. Low-amylose maize starch was used to ensure uniform starch content for the two diets. Processed wheat bran (comprising 183 g of starch and 302 g of NSP/100 g), safflower oil, and casein were used to obtain the desired macronutrient profile. Diets were prepared as a powder and were freely available, as was drinking water. The experimental diets were fed for 13 days, the last 9 days of which the rats were kept in individual metabolism cages to allow accurate estimation of feed and water intake and total collection of feces, which were retained for analysis. Rats were observed daily and weighed weekly. Diet consumption of rats when housed individually in metabolism cages was recorded daily, as was the weight of feces. Representative samples of the diets were taken during the study and analyzed for macronutrients and NSP according to standard procedures (38).
Table 5.
Formulation and chemical composition of the experimental diets
| Ingredient | Low-amylose wheat, g/kg of diet | High-amylose wheat, g/kg of diet |
|---|---|---|
| Casein | 113 | 72 |
| Sucrose | 116 | 120 |
| Safflower oil | 44 | 38 |
| Wheat bran | 121 | 65 |
| Low-amylose wheat flour | 481 | |
| High-amylose wheat flour | 576 | |
| Maize starch* | 83 | 86 |
| Vitamin premix† | 8 | 9 |
| Mineral premix† | 29 | 30 |
| Choline | 2 | 2 |
| l-cysteine | 2 | 2 |
| As analyzed, % of dry matter | ||
| Total starch | 42.6 | 41.9 |
| Protein | 20.4 | 21.6 |
| Lipid | 11.5 | 8.0 |
| Fiber (as nonstarch polysaccharide) | 7.8 | 8.7 |
*Conventional (low-amylose) starch (3401C) (Penford Australia Lane Cove, New South Wales, Australia).
†Pharmamix P169 (Propharma Australia Dandenong, Victoria, Australia), which contained, per kg of mix, 1.5 g of retinyl acetate, 25 mg of cholecalciferol, 20 g of α-tocopherol, 2 g of riboflavin, 7.5 mg of cyanocobalamin, 5.6 g of Ca pantothenate, 50 mg of biotin, 10 g of nicotinamide, 1 g of menadione, 50 g of FeSO4·7H2O, 10 g of MnO2, 50 g of ZnO, 5 g of CuSO4·7H2O, 0.25 g of CoSO4, 0.5 g of KI, 0.1 g of Na2SeO4, and 31 g of antioxidant (Oxicap E2; Novus Nutrition, Melbourne, Australia).
Sampling and Analytical Procedures.
Sampling procedures have been described in detail (39). Information specific to the experiments described here is given in Supporting Text.
Statistical Methods.
Data from the rat-feeding trial are shown as the mean ± SE for six observations per group. They were analyzed by t test, and a value of P < 0.05 was taken as the criterion of significance.
Supplementary Material
Acknowledgments
We acknowledge Rudi Appels and Jim Peacock for the scientific support provided at various stages of the inception and execution of this work; Gulay Mann for the milling of transgenic wheats; Oscar Larroque for starch-chain length analysis; Rosemary White for microscopy; Michael Mular for compositional analyses; Debbie Davies for animal care and digesta analysis; and Paul Orchard, Julie Dallimore, Mark Cmiel, Anne Tassie, and Xueqin Wang for technical assistance. We also acknowledge the financial support provided by Biogemma UK for this work.
Abbreviations
- NSP
nonstarch polysaccharides
- RS
resistant starch
- SCFA
short-chain fatty acid
- DP
degree of polymerization
- SBE
starch-branching enzyme
- RNAi
RNA interference
- SEC
size exclusion chromatography.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Richardson D. P. Proc. Nutr. Soc; 2003. pp. 161–169. [DOI] [PubMed] [Google Scholar]
- 2.Slavin J. L. J. Am. Col. Nutr. 2000;19(Suppl):300S–307S. doi: 10.1080/07315724.2000.10718964. [DOI] [PubMed] [Google Scholar]
- 3.Venn B. J., Mann J. I. Eur. J. Clin. Nutr. 2004;58:1443–1461. doi: 10.1038/sj.ejcn.1601995. [DOI] [PubMed] [Google Scholar]
- 4.Koh-Banerjee P., Franz M., Sampson L., Liu S., Jacobs D. R., Jr., Spiegelman D., Willett W., Rimm E. Am. J. Clin. Nutr. 2004;80:1237–1245. doi: 10.1093/ajcn/80.5.1237. [DOI] [PubMed] [Google Scholar]
- 5.Topping D. L., Clifton P. M. Physiol. Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 6.Bingham S. A., Day N. E., Luben R., Ferrari P., Slimani N., Norat T., Clavel-Chapelon F., Kesse E., Nieters A., Boeing H., et al. Lancet. 2003;361:1496–1501. doi: 10.1016/s0140-6736(03)13174-1. [DOI] [PubMed] [Google Scholar]
- 7.Kendall C. W., Emam A., Augustin L. S., Jenkins D. J. J. AOAC Int. 2004;87:769–774. [PubMed] [Google Scholar]
- 8.Brouns F., Kettlitz B., Arrigoni E. Trends Food Sci. Technol. 2002;13:252–261. [Google Scholar]
- 9.Ball S. G., Morell M. K. Annu. Rev. Plant Biol. 2003;54:207–233. doi: 10.1146/annurev.arplant.54.031902.134927. [DOI] [PubMed] [Google Scholar]
- 10.Schwall G. P., Safford R., Westcott R. J., Jeffcoat R., Tayal A., Shi Y.-C., Gidley M. J., Jobling S. A. Nat. Biotechnol. 2000;18:551–554. doi: 10.1038/75427. [DOI] [PubMed] [Google Scholar]
- 11.Garwood D. L., Shannon J. C., Creech R. G. Cereal Chem. 1976;53:355–364. [Google Scholar]
- 12.Blauth S. L., Kim K.-N., Klucinec J., Shannon J. C., Thompson D., Guiltinan M. Plant Mol. Biol. 2002;48:287–297. doi: 10.1023/a:1013335217744. [DOI] [PubMed] [Google Scholar]
- 13.Blauth S. L., Yao Y., Klucinec J. D., Shannon J. C., Thompson D. B., Guilitinan M. J. Plant Physiol. 2001;125:1396–1405. doi: 10.1104/pp.125.3.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown I. L., Macnamara S., Power L. J., Hazzard K., McNaught K. J. Food Australia. 2000;52:22–26. [Google Scholar]
- 15.Komari T., Hiei Y., Saito Y., Murai N., Kumashiro T. Plant J. 1996;10:165–174. doi: 10.1046/j.1365-313x.1996.10010165.x. [DOI] [PubMed] [Google Scholar]
- 16.Itoh K., Ozaki H., Okada K., Hori H., Takeda Y., Mitsui T. Plant Cell Physiol. 2003;44:473–480. doi: 10.1093/pcp/pcg068. [DOI] [PubMed] [Google Scholar]
- 17.Gao M., Wanat J., Stinard P. S., James M. G., Myers A. M. Plant Cell. 1998;10:399–412. doi: 10.1105/tpc.10.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamori M., Fujita S., Hayakawa K., Matsuki J. Theor. Appl. Genet. 2000;101:21–29. [Google Scholar]
- 19.Morell M. K., Kosar-Hashemi B., Cmiel M., Samuel M. S., Chandler P., Rahman S., Buléon A., Batey I. L., Li Z. Plant J. 2003;34:173–185. doi: 10.1046/j.1365-313x.2003.01712.x. [DOI] [PubMed] [Google Scholar]
- 20.Regina A., Kosar-Hashemi B., Li Z., Rampling L., Cmiel M., Gianibelli C., Konik-Rose C., Larroque O., Rahman S., Morell M. K. Funct. Plant Biol. 2004;31:591–601. doi: 10.1071/FP03193. [DOI] [PubMed] [Google Scholar]
- 21.Safford R., Jobling S. A., Sidebottom C. M., Westcott R. J., Cooke D., Tober K. J., Strongitharm B. H., Russel A., Gidley M. J. Carbohydr. Poly. 1998;35:155–168. [Google Scholar]
- 22.Satoh H., Nishi A., Yamashita K., Takemoto Y., Tanaka Y., Hosaka Y., Sakurai A., Naoko F., Nakamura Y. Plant Physiol. 2003;133:1–11. doi: 10.1104/pp.103.021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizuno K., Kawasaki T., Shimada H., Satoh H., Kobayashi E., Okumura S., Arai Y., Baba T. J. Biol. Chem. 1993;268:19084–19091. [PubMed] [Google Scholar]
- 24.Gao M., Fisher D. K., Kim K-N, Shannon J. C., Guiltinan M. J. Plant Physiol. 1997;114:69–78. doi: 10.1104/pp.114.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regina A., Kosar-Hashemi B., Li Z., Pedler A., Mukai Y., Yamamoto M., Gale K., Sharp P. J., Morell M. K., Rahman S. Planta. 2006;222:899–909. doi: 10.1007/s00425-005-0032-z. [DOI] [PubMed] [Google Scholar]
- 26.Tetlow I. J., Wait R., Lu Z., Akkasaeng R., Bowsher C. G., Esposito S., Kosar-Hashemi B., Morell M. K., Emes M. J. Plant Cell. 2004;16:694–708. doi: 10.1105/tpc.017400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bird A. R., Jackson M., Davies D. A., Usher S., Topping D. L. J. Nutr. 2003;134:831–835. doi: 10.1093/jn/134.4.831. [DOI] [PubMed] [Google Scholar]
- 28.Bird A. R., Jackson M., Davis D. A., Usher S., Topping D. L. Br. J. Nutr. 2004;92:607–615. doi: 10.1079/bjn20041248. [DOI] [PubMed] [Google Scholar]
- 29.Topping D. L., Morell M. K., King R. A., Li Z., Bird A. R., Noakes M. Starch/Stärke. 2003;53:539–545. [Google Scholar]
- 30.Anderson O. D., Greene F. C., Yip R. E., Halford N. G., Shewry P. R., Malpica-Romero J. M. Nucleic Acids Res. 1989;17:461–462. doi: 10.1093/nar/17.1.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Depicker A., Stachel S., Dhaese, Zambryski P., Goodman H. M. J. Mol. Appl. Genet. 1982;1:561–573. [PubMed] [Google Scholar]
- 32.Risacher T., Craze M. International Patent Appl. 2000 PCT/EO00/04177, published as WO00/63398. [Google Scholar]
- 33.Morell M. K., Blennow A., Kosar-Hashemi B., Samuel M. S. Plant Physiol. 1997;113:201–208. doi: 10.1104/pp.113.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahman S., Regina A., Li Z., Mukai Y., Yamamoto M., Kosar-Hashemi B., Abrahams S., Morell M. K. Plant Physiol. 2001;125:1314–1324. doi: 10.1104/pp.125.3.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Shea M. G., Samuel M. S., Konik C. M., Morell M. K. Carbohydr. Res. 1998;307:1–12. [Google Scholar]
- 36.National Health and Medical Research Council, CSIRO and Australian Agricultural Council . Code of Practice for the Care and Use of Animals for Experimental Purposes. Canberra, Australia: Australian Government Publishing Service; 1985. [Google Scholar]
- 37.Reeves P. G., Nielsen F. H., Fahey G. C., Jr J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 38.AOAC International . Official Methods of Analysis. 17th Ed. Washington, DC: Association of Official Analytical Chemists; 2002. [Google Scholar]
- 39.Bird A. R., Hayakawa T., Marsono Y., Gooden J. M., Record I. R., Correll R. L., Topping D. L. J. Nutr. 2000;130:1780–1787. doi: 10.1093/jn/130.7.1780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.