Abstract
The unusual discovery of associated cranial and postcranial elements from a single Middle Pleistocene fossil human allows us to calculate body proportions and relative cranial capacity (encephalization quotient) for that individual rather than rely on estimates based on sample means from unassociated specimens. The individual analyzed here (Jinniushan) from northeastern China at 260,000 years ago is the largest female specimen yet known in the human fossil record and has body proportions (body height relative to body breadth and relative limb length) typical of cold-adapted populations elsewhere in the world. Her encephalization quotient of 4.15 is similar to estimates for late Middle Pleistocene humans that are based on mean body size and mean brain size from unassociated specimens.
Keywords: climatic adaptation, paleoanthropology, Pleistocene
Individually associated cranial and postcranial human fossils from the Middle Pleistocene are extremely rare. Thus, evaluation of temporal changes during this period in important characteristics such as relative brain size (encephalization) have relied on either comparisons of group means for brain and body size (1) or indirect methods of evaluating body size from cranial dimensions, such as orbital size (2, 3). In addition, analyses of geographic or temporal variation during this period in aspects of body shape that are of adaptive and phylogenetic significance, such as body breadth relative to height or limb length (4, 5), have been hampered by a lack of individually associated elements from critical areas of the skeleton, again necessitating more indirect methods for evaluating these characteristics (6).
We present data here from a Middle Pleistocene human fossil skeleton from northeastern China, Jinniushan, dated to ≈260,000 B.P. The specimen includes enough of the postcranial and cranial skeleton to allow estimation of both body mass and brain size in a single individual, as well as body height and body breadth. This is the only such specimen reported to date that falls chronologically between KNM-WT 15000 at ≈1.6 million years ago from East Africa (7) and Tabun 1 at 122,000 ± 16,000 years B.P. from the Levant (8). In fact, even these two specimens are not ideal for such an analysis: KNM-WT 15000 is a juvenile whose adult body mass must be derived through growth extrapolation (9), and Tabun 1 does not include a completely preserved pelvis, so no direct estimation of body breadth is possible. Thus, the Jinniushan specimen represents a nearly unique opportunity to assess key aspects of morphology in an important period of human evolution, when relative brain size appears to have been increasing rapidly (1, 2) and geographic variation in body shape was becoming pronounced (6, 10) as the geographic range of the human species expanded to cover most of the Old World.
Background and Dates
The Jinniushan specimen was discovered near the town of Yinkou in Liaoning Province in northeastern China (N 40° 34′ 40′′, E 122° 26′ 38′′) in an isolated karst prominence in a fissure of a collapsed limestone cave (11, 12). It was excavated in 1984 by a group from the Department of Archaeology at Beijing University directed by L.Z. The human specimen was discovered near the base of 15 m of deposit in the bottom of Layer 7, which also contained the remains of Pleistocene fauna, specifically Macaca robustus, Trogontherium sp., Megaloceros pachyosteus, Dicerorhinus mercki, and Microtus brandtioides (11, 13). Electron-spin resonance and uranium series dating of animal teeth from Layer 7 suggest a date of ≈260,000 years ago, consistent with the faunal assemblage (13). Later dates had been previously reported for this specimen (14, 15), but they represented an average of dates taken from several stratigraphic levels of the site, including some levels that were higher in the section than the human remains. We are therefore comfortable with a date of ≈260,000 years ago for the level containing the Jinniushan hominid specimen.
The Jinniushan site is high in latitude, north (and east) of Beijing, although the climate at 260,000 years ago may have been somewhat more temperate than today based on the presence of M. robustus and D. mercki as well as on sedimentological analysis.
The Jinniushan Specimen
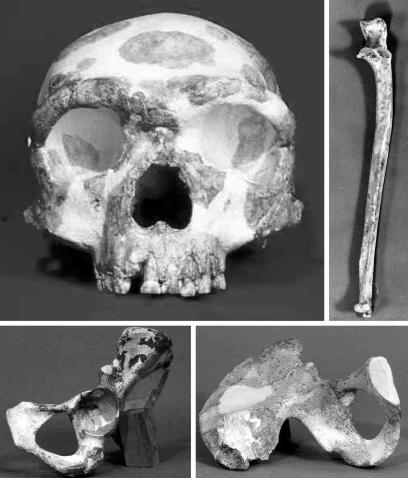
The individual skeletal elements of the Jinniushan specimen were all found within an area of <2 m2 and consist of a cranium with most of the maxillary dentition, six vertebrae (one cervical and five thoracic), two left ribs, a complete left patella, a complete left ulna, numerous articulated bones of both hands and both feet, and a complete left os coxae (Fig. 1). There is no duplication of elements, and the joint surfaces of adjacent bones articulate comfortably, so there is no reason to think that more than one individual is represented.
Fig. 1.
The cranium, left os coxae, and left ulna of the Jinniushan specimen. Note that the different skeletal elements are not reproduced to the same scale.
The sex of the specimen is significant to our interpretation here for at least two reasons. First, estimations of stature and body mass are made by using sex-specific regression equations. Second, interpretation of those estimates must always take sex into account.
The most reliable indicators of sex in humans are found on the pelvis, specifically the pubis. Examination of the traits that Phenice found to be best indicators of sex (16) provides the most convincing evidence that the Jinniushan specimen is female. Because of damage to the medial portion of the pubis, it is not possible to evaluate whether there was a ventral arc present. However, a subpubic concavity is present, and the medial aspect of the ischiopubic ramus is ridged rather than flat. According to Phenice, these latter two features would classify the Jinniushan specimen as female. The sciatic notch, which is generally wider in females than in males in all human populations, is intermediate in breadth. Compared with the Kebara 2 Neandertal specimen (a male), another archaic human that preserves a complete os coxae, the Jinniushan specimen is gracile in features such as the iliac buttress and ischial tuberosity, again compatible with a sex determination of female. The ischiopubic index (length of the superior pubic ramus/height of ischial bone × 100) has long been well known to be greater in females than in males (17). Jinniushan has an ischiopubic index of 132.3, and Kebara 2 has an ischiopubic index of 126.0, consistent with the diagnosis of female for the former specimen and male for the latter. The one piece of evidence that suggests that the Jinniushan specimen might be male is the overall size of its os coxae, which is large in absolute terms.
The cranium is robust compared with modern specimens, which led some researchers in the past to conclude that it was male (18). However, when compared more appropriately with the Dali specimen, a cranium of similar geological (and chronological) age from Shanxi Province in northwestern China, the Jinniushan specimen appears to be gracile and probably female. The Jinniushan specimen has outer dimensions similar to those of Dali but has a thinner cranial vault and therefore larger cranial capacity (Dali is 1,120 cm3, and published estimates of Jinniushan range from 1,260 to 1,400 cm3) (11–15, 18, 19). The Jinniushan supraorbitals are thinner than those of the Dali specimen, and the differences between the two penecontemporaneous specimens are probably explained by sexual dimorphism.
Stature Reconstruction
Stature was reconstructed from the left ulna, the only long bone preserved. The complete ulna has a length of 260 mm. Trotter’s formula (20), based on a modern reference sample of Euroamerican females, was used here given that the Jinniushan specimen derives from a high-latitude population. Reconstructed stature using this formula is 168.78 ± 4.30 cm. [In previous analyses (¶, 22) stature for this specimen was calculated by using both the Trotter and Gleser U.S. white female (23) and “mongoloid” male (24) formulae, which produced an estimate of ≈168 cm.]
Body Proportions and Trunk Width
Because the Jinniushan specimen has no sacrum, it was not possible to measure biiliac (total pelvic mediolateral) breadth or pelvic inlet dimensions directly from a complete pelvic girdle. We used the complete os coxae to reconstruct pelvic inlet shape and biiliac breadth. We estimated total pelvic inlet shape from the “iliac brim index,” which is a measure of pelvic inlet curvature. The iliac brim includes the iliac portion of the linea terminalis, from the edge of the sacral articular surface to a point opposite to the psoas groove (see figure 15 in ref. 25 for illustration). The iliac brim index is calculated as the ratio between the depth of the iliac brim and the iliac brim length, measured between these two end points. The depth of the iliac brim was measured as the subtense of the line drawn between the two end points to the deepest point on the linea terminalis and is 11.1 mm in the Jinniushan specimen. Iliac brim length is 56.8 mm, giving an index of 0.195. The iliac brim index is correlated with the shape of the pelvic inlet in a geographically diverse sample of modern humans (25). Specifically, a higher pelvic brim index (more curved brim) is associated with a pelvic inlet that is wider mediolaterally (M–L) and flatter anteroposteriorly (A–P) than one with a lower index. Although there is error associated with this method, it does successfully predict inlet shape in specimens as disparate geographically, temporally, and taxonomically as an australopithecine (AL 288-1) and a Neandertal (Kebara 2). Data from figure 16 in ref. 25 yield the regression equation {total pelvic inlet index = 1.1677 − 2.2608 × iliac brim index [r = 0.743, standard error of estimate (SEE) = 0.079]} that we used here to estimate a total pelvic inlet index (that is, the ratio of A–P/M–L breadth of the pelvic inlet) for the Jinniushan specimen of 0.73, with a 95% confidence interval of 0.57–0.89. This inlet shape, although quite platypelloid, is not unreasonable: the average inlet shape of four modern Native American female samples (weighted by sample size) is 0.7495, with a range of sample means from 0.685 to 0.830 (26). There is also evidence that earlier Homo had more platypelloid pelves than most modern humans (25).
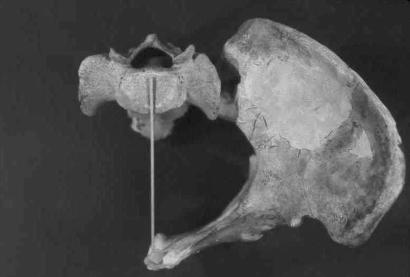
We then positioned the os coxae in anatomical position to achieve this pelvic inlet shape. We first estimated, based on measurements of a series of modern human female pelves, that the A–P inlet distance, measured from the pubis to the anterior edge of the sacral promontory, was 1 cm greater than the A–P inlet distance measured from the pubis to a line drawn between the posterior ends of the linea terminalis on each side. The latter distance, together with the inlet shape index, then established the estimated M–L distance from the midline sagittal plane to the most lateral point on the inlet. The resulting position of the innominate is shown in Fig. 2. The position seemed reasonable in that both pubic and sacroiliac articular surfaces were approximately parallel with the sagittal plane. Biiliac breadth was then measured as twice the M–L distance from the midline to the maximum lateral projection of the iliac crest, which produced a value of 344 mm. We also rotated the os coxae about the pubic symphysis (which would be fixed in vivo) varying sacral breadth to produce A–P/M–L inlet indices equal to the 95% confidence interval extremes given above. This procedure produced a range of estimates of biiliac breadth from 327 to 361 mm. Further varying of the assumed position of the sacral promontory relative to the posterior edge of the linea terminalis by ±5 mm increased this range by only a modest amount, to 325–363 mm. We consider these extreme values to be unlikely based on the overall morphology of the os coxae and the extremely narrow or wide sacra implied by them; however, they are used to bracket our best estimate of biiliac breadth in subsequent analyses of body shape and also body mass estimates that include this dimension.
Fig. 2.
The cast of the Jinniushan os coxae in position for measurement of total pelvic breadth (biiliac breadth) as described in the text. The sacrum is an unassociated modern human sacrum that allowed us to visually determine the position of the midline. Maximum perpendicular distance from the lateral edge of the iliac crest to the midline was taken and then doubled to estimate biiliac breadth. Note that, because of parallax and foreshortening in the photograph, exact dimensions cannot be measured on the figure.
Relative Limb Length
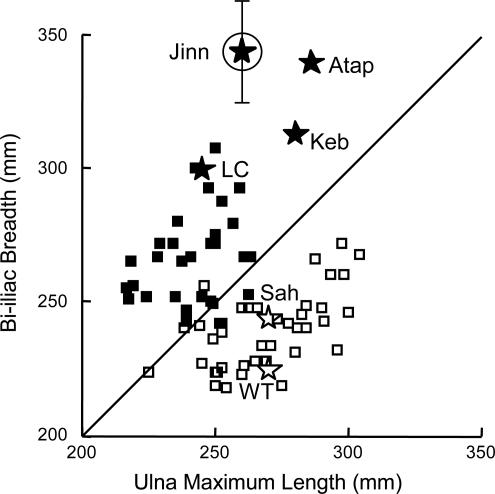
It has been shown that, like other geographically widespread mammalian species, humans conform to ecogeographic rules that relate body shape to climate so that surface area to mass is low in populations that come from cold climates and higher in populations that come from warm climates (10, 27–29). This pattern is manifested in both relative limb length (Allen’s rule) and body breadth. In modern humans, populations from cold regions have relatively shorter limbs and wider bodies than those from warm regions. Fig. 3 shows the relationship between biiliac breadth and ulna length in equatorially adapted East Africans and Arctic-dwelling Inupiats and Aleuts. As expected, cold-adapted modern populations have relatively shorter ulnas for their trunk diameters than warm-adapted populations. Pre-Holocene humans from higher and lower latitudes follow the same pattern as modern humans. Six fossil individuals whose pelvic breadth and ulna length can be measured or estimated (see ref. 10 for details), including Jinniushan, are also plotted in Fig. 3. The four individuals from higher latitudes (the La Chapelle-aux-Saints 1 and Kebara 2 Neandertals and the Middle Pleistocene Atapuerca and Jinniushan specimens) all have very wide pelvic breadths relative to forearm lengths, near or beyond the upper limits for modern high-latitude humans. The Jinniushan specimen is particularly extreme, which may in part be due to its being female, because females have relatively shorter forearms than males (10, 29). The two pre-Holocene African specimens (WT 15000 and Sahaba 16) both have narrow bodies relative to ulna length, as predicted.
Fig. 3.
The relationship between ulna length and biiliac breadth in modern humans. Open symbols represent modern East Africans, and filled symbols represent modern Inupiats/Aleuts. Open stars represent KNM-WT 15000 and Sahaba 16, and filled stars represent Atapuerca, Kebara, La Chapelle-aux-Saints 1, and Jinniushan (circled). The vertical bar shows the 95% confidence interval of the estimate of Jinniushan’s biiliac breadth.
Body Mass
Body mass was estimated in two different ways: using a combination of estimated stature and biiliac breadth and using estimated femoral head diameter. The first approach can be termed “morphometric” (4), and the second can be termed “mechanical” (30). The stature/biiliac method is based on a geographically diverse sample of living humans (4) and has been tested and verified on a number of different living samples of known body mass, including athletic and “normal” mid-lower-latitude humans (31) and high-latitude populations with wide bodies (22). As recently modified (22), the regression equation for females is body mass = 0.504 × stature + 1.804 × biiliac breadth − 72.6 (r = 0.82, SEE = 4.0) (with conversion of skeletal to living biiliac breadth as described in ref. 1). Using the estimates derived above for Jinniushan stature and biiliac breadth in this equation yields an estimated body mass for this specimen of 79.6 kg.
Our second method of estimation of body mass was from the diameter of the femoral head, a joint through which body weight must pass during locomotion and stance. No femur is preserved for the Jinniushan specimen, but we estimated femoral head diameter from the dimensions of the other side of the hip joint, namely acetabular height, using a regression equation based on 39 individuals from a recent archaeological sample: femoral head diameter = 0.9877 × acetabular height − 8.39 (r = 0.95, SEE = 0.3) (E. Trinkaus, personal communication). Acetabular height for the Jinniushan specimen is 59.3 mm, giving an estimated femoral head diameter of 50.2 mm, with a 95% confidence interval of 49.6–50.8 mm. We then used this measurement in two different published regression equations based on appropriate medium- to large-bodied modern populations (32, 33) (see ref. 30 for justification and further details) and obtained body masses of 77.4 and 78.0 kg, respectively (range using 95% confidence intervals for femoral head diameter, 76.0–79.3 kg). The average of these two estimates is 77.6 kg, which is within 2 kg, or ≈2.5%, of our best estimate from stature and biiliac breadth of 79.6 kg (see above). In what follows we have used the mean of these two estimates, 78.6 kg, as the estimated body weight for the Jinniushan specimen. (Note that the closeness of the two estimates also indirectly supports our reconstructed value for biiliac breadth.)
As shown in Table 1, the estimated body mass for Jinniushan is quite large compared with modern population means or other Pleistocene Homo specimens. Data in Table 1 were previously derived (1) using similar methods, except for six new body mass estimates derived for the late Middle Pleistocene Sima de los Huesos Atapuerca specimens (see Table 1). In addition, based on new dates for this site (34), Atapuerca specimens have been placed into the earlier, middle Middle Pleistocene sample, slightly increasing the average body size for this temporal period. Several male Pleistocene specimens, including “Pelvis 1” from Atapuerca (35), significantly exceed Jinniushan in estimated body mass. However, Jinniushan is the largest female yet reported in the pre-Holocene record; the next largest female in our sample is Grotte du Prince, from the early Late Pleistocene (100,000 years B.P.), with an estimated body mass of 74.0 kg. Body size in Homo appears to peak in the Middle Pleistocene with specimens such as Boxgrove and the Atapuerca sample (1, 10), and Jinniushan is consistent with this pattern. Her large estimated body mass is also again consistent with ecogeographic principles (Bergmann’s rule), i.e., a tendency among geographically widespread species to increase in body size in higher latitudes, probably as an adaptation to decrease surface area to body mass in colder climates (4, 28). It is also consistent with the observation that Middle Pleistocene humans, who were presumably less culturally buffered from the environment by such technological adaptations as insulating clothing and fire, may have had a steeper clinal distribution of body proportions than more recent humans (6).
Table 1.
Body masses and encephalization quotients for modern and Pleistocene Homo
| Sample | Temporal period, B.P. | Body mass, kg |
EQ of pooled sex |
||
|---|---|---|---|---|---|
| Pooled sex, mean ± SD (n) | Females, mean ± SD (n) | Associated specimens, mean ± SD (n) | Sample means | ||
| Modern, all | — | 58.2 ± 7.1* (51) | 54.7 ± 5.7* (23) | — | 5.288 |
| Modern, higher latitude | — | 61.2 ± 6.7* (24) | 57.2 ± 5.7* (13) | 5.349 ± 0.555† (29) | — |
| Late Upper Palaeolithic | 10–21,000 | 62.9 ± 7.6 (71) | 57.0 ± 5.9 (23) | 5.479 ± 0.352 (18) | 5.406 |
| Early Upper Palaeolithic | 21–35,000 | 66.6 ± 7.5 (33) | 59.6 ± 6.4 (7) | 5.467 ± 0.449 (10) | 5.352 |
| Late archaic Homo sp. | 36–75,000 | 76.0 ± 5.8 (17) | 67.2 ± 0.4 (2) | 4.984 ± 0.467 (8) | 4.781 |
| Skhul-Qafzeh | 90,000 | 66.6 ± 7.0 (10) | 58.4 ± 4.4 (3) | 5.369 ± 0.166 (4) | 5.293 |
| Early Late Pleistocene | 100–150,000 | 67.7 ± 7.6 (10) | 65.4 ± 5.5 (6) | 4.682 (1) | 4.732 |
| Late Middle Pleistocene | 200–300,000 | 65.6 ± 12.3 (6) | 54.1 (1) | — | 4.198 |
| Jinniushan | 260,000 | 78.6 | 4.150 | — | |
| Middle Middle Pleistocene | 400–550,000 | 71.2 ± 11.3 (11) | 64.6 ± 3.3 (2) | — | 3.770 |
| Late Early to early Middle Pleistocene | 600–1,150,000 | 58.0 ± 7.4 (3) | 66.6 (1) | — | 3.400 |
| Early Pleistocene | 1,200–1,800,000 | 61.8 ± 8.9 (5) | — | 3.064 (1) | 3.458 |
Data are from ref. 1, except for Jinniushan and additional body masses for Atapuerca specimens (Atapuerca specimens also moved to middle Middle Pleistocene period; see text). Body mass for Atapuerca Pelvis 1 was derived as the mean of the mean estimate given in ref. 35 based on estimated stature and biliac breadth (94.2 kg) and that derived from acetabular breadth by using the same technique described in the text for Jinniushan (78.7 kg), i.e., 86.4 kg. Body masses for five other Atapuerca specimens are based on published acetabular breadths (35): AT-800, 75.1 kg; AT-835, 77.5 kg; AT-2350, 75.1 kg; Coxal I, 66.9 kg; AT-1004, 62.2 kg. The first four specimens were judged to be males, and the second two were judged to be females (35).
*Means and SDs of sex/population means, not individuals (see ref. 4).
†Pecos Pueblo (see ref. 1).
Encephalization
Finally, because the Jinniushan specimen preserves both postcranial remains and a fairly complete skull, we have the unusual, if not unique, opportunity to examine the relationship between body size and cranial capacity in this Middle Pleistocene individual. Most estimates of relative brain size have been carried out by using brain size estimates and body size estimates from different specimens or have used body size estimates derived from cranial dimensions, which are less accurate and subject to possible circular reasoning. Jinniushan provides an opportunity to compare an individually derived encephalization quotient (EQ) with previous estimates based on sample means for brain and body size.
To determine EQ in Jinniushan, we used 1,330 cm3, the midpoint of the various estimates of its cranial capacity. We then calculated brain mass following ref. 1, whose equation based on data from 27 primate species is brain mass = 1.147 × cranial capacity0.976 and found a brain mass for Jinniushan of 1,284 g. Using the estimated body mass of 78.6 kg and the equation published by Martin (21) for mammals, EQ = brain mass/11.22 × body mass0.76, we determined an EQ of 4.150 for Jinniushan. This is remarkably close to an estimate based on sample means for body mass and brain mass for the late Middle Pleistocene: 4.198 (Table 1). The latter is derived from the mean brain mass of 13 specimens (1,132 cm3; from ref. 1 but not including the three Atapuerca crania or Jinniushan) and the mean body mass of six specimens (1). The mean estimated EQ for the preceding middle Middle Pleistocene, based on sample mean data, is 3.770, a slight reduction from our earlier estimates (1) because of the inclusion of the Atapuerca specimens. These results further document the rapid increase in encephalization among Middle Pleistocene Homo (1).
Summary
Jinniushan’s geographic location in the far north of China suggests a cold-adapted population. We see this reflected in her large body size, wide trunk, and relatively short limb length. She thus adds to the growing body of evidence for ecogeographic clines in body size and shape among Early and Middle as well as Late Pleistocene Homo (4, 9, 10, 29). Her brain size relative to body size is very similar to that predicted from sample mean data for the late Middle Pleistocene and provides an important data point in verifying the hypothesized increase in encephalization characteristic of this time period.
Acknowledgments
This work was supported by the National Academy of Sciences Committee on Scholarly Communication with the People’s Republic of China, National Program for Advanced Study and Research in China.
Abbreviation
- EQ
encephalization quotient.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
Rosenberg, K. R., Ruff, C. B. & Lü, Z. (1999) Am. J. Phys. Anthropol. 235, Suppl. 28, 235 (abstr.).
References
- 1.Ruff C. B., Trinkaus E., Holliday T. W. Nature. 1997;387:173–176. doi: 10.1038/387173a0. [DOI] [PubMed] [Google Scholar]
- 2.Rightmire P. Am. J. Phys. Anthropol. 2004;124:109–123. doi: 10.1002/ajpa.10346. [DOI] [PubMed] [Google Scholar]
- 3.Aiello L. C., Wood B. A. Am. J. Phys. Anthropol. 1994;95:409–426. doi: 10.1002/ajpa.1330950405. [DOI] [PubMed] [Google Scholar]
- 4.Ruff C. B. Yrbk. Phys. Anthropol. 1994;19:65–107. [Google Scholar]
- 5.Ruff C. B., Trinkaus E., Holliday T. W. In: Portrait of the Artist as a Child: The Gravettian Human Skeleton from the Abrigo do Lagar Velho and its Archaeological Context. Zilhão J., Trinkaus E., editors. Lisbon: Instituto Português de Arqueologia; 2002. pp. 365–391. [Google Scholar]
- 6.Trinkaus E., Stringer C. B., Ruff C. B., Hennessy R. J., Roberts M. B., Parfitt S. A. J. Hum. Evol. 1999;37:1–25. doi: 10.1006/jhev.1999.0295. [DOI] [PubMed] [Google Scholar]
- 7.Walker A., Leakey R. E. F., editors. The Nariokotome Homo erectus Skeleton. Cambridge, MA: Harvard Univ. Press; 1993. [Google Scholar]
- 8.Grün R., Stringer C. J. Hum. Evol. 2000;39:601–612. doi: 10.1006/jhev.2000.0443. [DOI] [PubMed] [Google Scholar]
- 9.Ruff C. B., Walker A. In: The Nariokotome Homo erectus Skeleton. Walker A., Leakey R., editors. Cambridge, MA: Harvard Univ. Press; 1993. pp. 234–265. [Google Scholar]
- 10.Ruff C. B. Annu. Rev. Anthropol. 2002;31:211–232. [Google Scholar]
- 11.Lü Z. L’Anthropologie. 1990;1990:899–902. [Google Scholar]
- 12.Lü Z. In: Current Research in Chinese Pleistocene Archaeology. Shen C., Keates S. G., editors. Oxford: Archaeopress; 2003. pp. 127–136. [Google Scholar]
- 13.Chen T., Yang Q., Wu E. Nature. 1994;368:55–56. [Google Scholar]
- 14.Wolpoff M. Paleoanthropology. 2nd Ed. McGraw–Hill: 1999. [Google Scholar]
- 15.Pope G. Annu. Rev. Anthropol. 1988;17:43–77. doi: 10.1146/annurev.an.17.100188.002333. [DOI] [PubMed] [Google Scholar]
- 16.Phenice T. D. Am. J. Phys. Anthropol. 1969;30:297–302. doi: 10.1002/ajpa.1330300214. [DOI] [PubMed] [Google Scholar]
- 17.Washburn S. L. Am. J. Phys. Anthropol. 1948;6:199–207. doi: 10.1002/ajpa.1330060210. [DOI] [PubMed] [Google Scholar]
- 18.Wu X., Poirier F. Human Evolution in China: A Metric Description of the Fossils and a Review of the Sites. Oxford: Oxford Univ. Press; 1995. [Google Scholar]
- 19.Rosenberg K. R. In: Neandertals and Modern Humans in Western Asia. Akazawa T., Aoki K., Bar-Yosef O., editors. New York: Plenum; 1998. pp. 367–379. [Google Scholar]
- 20.Trotter M. In: Personal Identification in Mass Disasters. Stewart T. D., editor. Washington, DC: Natl. Museum of Natural History; 1970. pp. 71–83. [Google Scholar]
- 21.Martin R. D. Nature. 1981;293:57–60. doi: 10.1038/293057a0. [DOI] [PubMed] [Google Scholar]
- 22.Ruff C. B., Niskanen M., Junno J.-A., Jamison P. J. Hum. Evol. 2005;48:381–392. doi: 10.1016/j.jhevol.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Trotter M., Gleser G. C. Am. J. Phys. Anthropol. 1952;10:463–514. doi: 10.1002/ajpa.1330100407. [DOI] [PubMed] [Google Scholar]
- 24.Trotter M., Gleser G. C. Am. J. Phys. Anthropol. 1958;16:79–123. doi: 10.1002/ajpa.1330160106. [DOI] [PubMed] [Google Scholar]
- 25.Ruff C. B. Am. J. Phys. Anthropol. 1995;98:527–574. doi: 10.1002/ajpa.1330980412. [DOI] [PubMed] [Google Scholar]
- 26.Tague R. G. Am. J. Phys. Anthropol. 1989;80:59–91. doi: 10.1002/ajpa.1330800108. [DOI] [PubMed] [Google Scholar]
- 27.Schreider E. Evolution. 1964;18:1–9. [Google Scholar]
- 28.Roberts D. F. Climate and Human Variability. 2nd Ed. Menlo Park, CA: Cummings; 1978. [Google Scholar]
- 29.Trinkaus E. In: Aspects of Human Evolution. Stringer C. B., editor. London: Taylor and Francis; 1981. pp. 187–224. [Google Scholar]
- 30.Auerbach B. M., Ruff C. B. Am. J. Phys. Anthropol. 2004;125:331–342. doi: 10.1002/ajpa.20032. [DOI] [PubMed] [Google Scholar]
- 31.Ruff C. B. Am. J. Phys. Anthropol. 2000;113:507–517. doi: 10.1002/1096-8644(200012)113:4<507::AID-AJPA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 32.Grine F. E., Jungers W. L., Tobias P. V., Pearson O. M. Am. J. Phys. Anthropol. 1995;97:151–185. doi: 10.1002/ajpa.1330970207. [DOI] [PubMed] [Google Scholar]
- 33.Ruff C. B., Scott W. W., Liu A. Y.-C. Am. J. Phys. Anthropol. 1991;86:397–413. doi: 10.1002/ajpa.1330860306. [DOI] [PubMed] [Google Scholar]
- 34.Bischoff J. L., Shamp D. D., Aramburu A., Arsuaga J. L., Carbonell E., Bermudez de Castro J. M. J. Archaeol. Sci. 2003;30:275–280. [Google Scholar]
- 35.Arsuaga J. L., Lorenzo C., Carretero J.-M., Gracia A., Martinez I., Garcia N., Bermúdez de Castro J.-M., Carbonell E. Nature. 1999;399:255–258. doi: 10.1038/20430. [DOI] [PubMed] [Google Scholar]