Abstract
Quinones permeate our biotic environment, contributing to both homeostasis and cytotoxicity. All quinones generate reactive oxygen species through redox cycling, while partially substituted quinones also undergo arylation (Michael adduct formation) yielding covalent bonds with nucleophiles such as cysteinyl thiols. In contrast to reactive oxygen species, the role of arylation in quinone cytotoxicity is not well understood. We found that the arylating quinones, including unsubstituted 1,4-benzoquinone (1,4-BzQ) and partially substituted vitamin E congener γ-tocopherol quinone (γ-TQ), were cytotoxic, with γ-TQ > 1,4-BzQ, whereas the fully substituted nonarylating vitamin E congener α-tocopherol quinone was not. In vitro, both arylating quinones formed Michael adducts with the thiol nucleophile N-acetylcysteine (NAC) at rates where 1,4-BzQ > γ-TQ. In cultured cells, concurrent addition of NAC eliminated 1,4-BzQ caused toxicity, but preincubation was required for the same NAC detoxification effect on γ-TQ. These data clearly established the role of arylation in quinone toxicity and revealed that arylating quinone structure affects cytotoxicity by governing detoxification through the rate of adduct formation. Furthermore, arylating quinones induced endoplasmic reticulum (ER) stress by activating the pancreatic ER kinase (PERK) signaling pathway including elF2α, ATF4, and C/EBP homologous protein (CHOP). Detoxification by NAC greatly attenuates CHOP induction in arylating quinone-treated cells, suggesting that ER stress is a cellular mechanism for arylating quinone cytotoxicity.
Keywords: quinone adduction, thiol nucleophiles, tocopherols, CHOP, cytotoxicity
Quinones and their phenolic precursors are present throughout the biotic environment and include polyphenols and tocopherols in the diet, drugs in medicine, environmental pollutants such as polycyclic aromatic hydrocarbons, and their metabolic products (1–8). They are involved in a wide variety of biological and chemical processes, including electron transport in animals and plants, photosynthesis, posttranslational modification of proteins, metabolism of cellular signaling molecules such as estrogens and catecholamines, metabolism of antioxidant and signaling tocopherol congeners (vitamin E), and the elimination of polycyclic aromatic hydrocarbons introduced by combustion processes associated with our petroleum-based chemical environment.
Quinones are a class of highly reactive compounds. Although all quinones are redox cycling agents that generate reactive oxygen species (ROS), partially substituted quinones also function as arylating agents (1–3, 5, 6). The arylating quinones react with cellular nucleophiles such as thiols on cysteine residues of proteins, glutathione (GSH), and detoxifying agents such as N-acetylcysteine (NAC), forming covalently linked quinone–thiol Michael adducts (1–3, 5, 6) that retain the ability to function as redox cycling agents (4, 9). In contrast to well studied ROS generation and consequent oxidative stress in living cells (1–3), the role of Michael adduct formation in quinone toxicity is not well understood. This lack of understanding is largely because of the fact that ROS generation by redox cycling is inherent to both quinones and their adducts, making it difficult to separate the biological effects caused by Michael adduct formation from that of ROS generation (1–3).
Arylating quinones do have unique biologic properties, such as high cytotoxicity, that are not always shared by nonarylating quinones and arylating quinone–thiol adducts (4–6, 9–12). Cellular mechanisms responsible for this cytotoxicity are not well established. Recent findings that Michael adduct formation between arylating quinones and endoplasmic reticulum (ER) protein disulfide isomerases (13), induction of C/EBP homologous protein (CHOP) by hydroquinones (14), and the essential role of ER chaperone BiP in protection against quinone toxicity (15), lead us to propose that ER stress is a cellular mechanism for arylating quinone toxicity. ER is the subcellular organelle in which secretory proteins are folded, stabilized by disulfide bonds, posttranslationally modified, oligomerized, and ultimately exported. This process is tightly monitored by ER quality control mechanisms that sense any disruption and retain unfolded proteins in the ER, triggering ER stress and initiating a complex series of cellular responses (16–18). Three main signaling pathways, pancreatic ER kinase (PERK), ATF6, and IRE1, mitigate ER stress by altering various aspects of cellular metabolism. Persistent ER stress prevails over the cellular defensive mechanisms and causes cell death (19, 20), which has been implicated in various diseases (21–25). We reasoned that Michael adduct formation between arylating quinone and cysteine residues on secretory proteins could disrupt the formation of correct disulfide bond, cause protein misfolding, induce ER stress, and, ultimately, lead to cell toxicity.
In this study, we investigated the role of Michael adduct formation in quinone toxicity and the induction of ER stress by arylating quinones. Comparisons among two vitamin E congener quinones, partially substituted arylating γ-tocopherol quinone (γ-TQ) and fully substituted nonarylating α-tocopherol quinone (α-TQ), and the simplest arylating quinone, unsubstituted 1,4-benzoquinone (1,4-BzQ), allow us to estimate contributions from quinone structure to the extent and rate of Michael adduct formation, to quinone cytotoxicity, and to cellular mechanisms associated with it. A unique contribution of Michael adduct formation to quinone toxicity was clearly established. In addition, we found that the induction of ER stress is tightly coupled to arylating quinone toxicity, indicating ER stress as a cellular mechanism for arylating quinone toxicity. Furthermore, we also found that the high chemical reactivity of 1,4-BzQ leads to rapid detoxification and lower cytotoxicity compared with γ-TQ, suggesting chemical reactivity is not directly coupled to toxicity in a biotic system.
Results
Higher Cytotoxicity Associated with Arylating γ-TQ.
We compared cytotoxicity induced by two members of the vitamin E family, α- and γ-tocopherol (α-T and γ-T), and their oxidation products, α-TQ and γ-TQ (Fig. 1A). The α-TQ and γ-TQ quinones, which are found in animals after the ingestion of their parent phenols (26), are an excellent model system to separate the biological effects caused by ROS generation and Michael adduct formation. Both are redox cycling agents with similar redox potentials (12) and are identical in chemical structure except that position 5 is methylated in nonarylating α-TQ but available for Michael adduct formation in arylating γ-TQ.
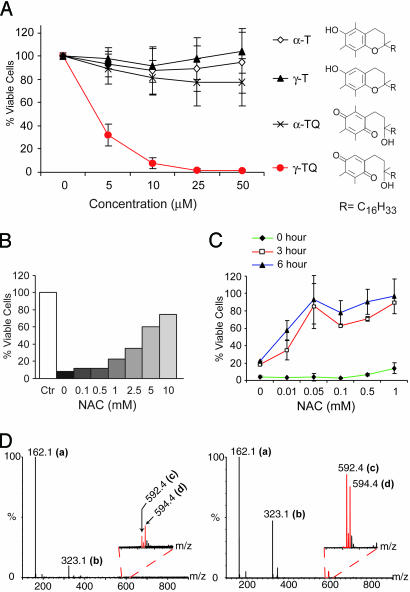
Fig. 1.
Prior adduct formation alleviates arylating γ-TQ caused cytotoxicity. (A) N2A cells were incubated with increasing concentrations of tocopherols and their quinones for 24 h. Cell viability measured by the MTT assay and results are reported as percent relative to untreated cells (mean ± SD). (B) N2A cells were incubated for 24 h with 10 μM γ-TQ with increasing concentrations of NAC. Ctr, control cells treated only with solvent. Cell viability was measured as in A. (C) N2A cells were incubated for 24 h with 10 μM γ-TQ and increasing concentrations of NAC added immediately or preincubated at room temperature for 3 and 6 h, and relative viability was measured as described in A. (D) Equal volumes of 1 mM γ-TQ in ethanol and 5 mM NAC in water were mixed and analyzed by TOF-MS in the negative ion mode with (Right) and without (Left) a 3-h preincubation at room temperature. The m/z values represent unreacted NAC (a) at 162.1, oxidized NAC disulfide (b) at 323.1, adduct in quinone form (c) at 592.4, and adduct in hydroquinone form (d) at 594.4, respectively. Insets represent expanded spectral regions to show assignment of the adduct peaks.
Neuro 2a (N2A) murine neuroblastoma cells were incubated for 24 h with α- and γ-TQ and their phenolic precursors, α-T and γ-T. Cells were kept in regular cell culture medium supplied with 10% FBS during incubation to minimize any stress caused by changing growth conditions. After incubation, cell viability was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. It is clear from the results shown in Fig. 1A that severe cytotoxicity was only observed in cells treated with the arylating quinone, γ-TQ. As little as 5 μM γ-TQ is sufficient to cause 70% of the cells to lose viability. In contrast, little change in viability was observed in cells treated with as high as 50 μM α-TQ or with either phenolic precursor. The same experiment was performed in two different cell lines derived from different origins, Monkey kidney epithelial COS cells and mouse fibroblast 3T3 cells (see Fig. 5, which is published as supporting information on the PNAS web site). An almost identical result was obtained in COS cells with arylating γ-TQ as the only agent causing toxicity after a 24-h treatment. In 3T3 cells, γ-T, the phenolic precursor of γ-TQ, also caused some loss of cell viability, correlating with a recent report that γ-T decreases cell viability at high concentrations in certain cell lines (27). Nonetheless, the arylating quinone, γ-TQ, has the most potent cytotoxic effect in 3T3 cells as in other cell types. Together with our previous results that α- and γ-TQ have similar redox potentials in cultured cells (12), these results demonstrate that arylation has a specific role in quinone cytotoxicity.
Alleviation of Arylating Quinone Toxicity by Prior Michael Adduct Formation.
Arylating quinones differ from nonarylating quinones in their ability to form Michael adducts, particularly with nucleophilic thiol groups in intracellular biomolecules (5, 6). To elucidate whether Michael adduct formation is responsible for the severe γ-TQ cytotoxicity observed, we investigated the role of NAC, a simple and cell-permeable thiol nucleophile, in the alleviation of cytotoxicity. A direct correlation was observed between the improvement of cell viability and increasing concentrations of NAC added concurrently with 10 μM γ-TQ (Fig. 1B). Because NAC itself in the concentrations and incubation times used here had no effect on cell viability (data not shown), this result suggests that the thiol nucleophile protected cells against arylating quinone cytotoxicity.
NAC is both a thiol nucleophile and an antioxidant (28). Therefore, it is not clear whether the NAC protective effect is because of its formation of a Michael adduct with γ-TQ or its antioxidant property that reduces the biological effect caused by ROS generation during γ-TQ redox cycling. To distinguish between these two possibilities, we performed the same experiment with a variation in the order of NAC addition. The γ-TQ and NAC mixture was preincubated at room temperature for 3 and 6 h to allow Michael adduct formation before its addition to cells. Notably, a significant increase in cell viability was observed with an equimolar mixture after preincubation of NAC with γ-TQ (Fig. 1C). Overall, preincubation enhanced the protective effect of NAC by >100-fold. The same protective effect was obtained with another thiol-containing agent, DTT, whereas antioxidants without a thiol group have no effect on γ-TQ-caused toxicity (see Fig. 6, which is published as supporting information on the PNAS web site). Because quinone–thiol Michael adducts also function as redox cycling agents (4, 9), these data strongly suggest that NAC or DTT abolished γ-TQ toxicity by eliminating its ability to form Michael adduct with intracellular nucleophiles.
We performed time-of-flight mass spectroscopy (TOF-MS) analyses to determine whether the time delay in the amelioration of cytotoxicity was correlated with the kinetics of adduct formation. Indeed, only trace amounts of adduct were formed immediately after mixing γ-TQ and NAC, but clearly detectable amounts of adduct were observed after a 3-h preincubation (Fig. 1D). The direct correlation of prior Michael adduct formation and the loss of γ-TQ toxicity led us to conclude that loss of thiol function through Michael adduct formation, but not the ubiquitous ROS generation, is critical for its cytotoxicity.
Induction of ER Stress Is Tightly Associated with Arylating Quinone Toxicity.
An important function of thiols in cysteinyl proteins is the formation of disulfide bonds that are essential for many secretory proteins to achieve their correct conformations (29, 30). Disruption of disulfide bonding by Michael adduct formation should lead to protein misfolding and ER stress. To test whether ER stress is associated with arylating quinone toxicity, we performed a time-course analysis of phosphorylated PERK, a proximal ER stress sensor (16, 18), in cells treated with γ-TQ. Increased levels of phosphorylated PERK were observed after 30 min and continued to increase at later time points (Fig. 2A), suggesting that arylating γ-TQ induces ER stress. To elucidate further the molecular changes along the PERK signaling pathway, we compared the levels of phosphorylated elF2α, ATF4, and CHOP in cells incubated for 24 h with, respectively, α- or γ-TQ and their phenolic precursors, α- or γ-T. Phosphorylated elF2α was increased significantly in cells treated with arylating γ-TQ, but not in cells treated with the nonarylating congener, α-TQ, or with either phenolic precursor (Fig. 2B). Increasing phosphorylated elF2α shuts down general protein translation and leads to the preferential expression of the transcription factor ATF4 and the induction of CHOP (16, 18). Indeed, both ATF4 and CHOP were induced significantly in cells treated with arylating γ-TQ, but not in cells treated with other reagents (Fig. 2B). Because nonarylating α-TQ is capable of redox cycling (12, 31), the specific activation of the PERK signaling pathway by arylating γ-TQ suggests that Michael adduct formation by arylating quinones, not ROS generation by redox cycling, governs the induction of ER stress in our system.
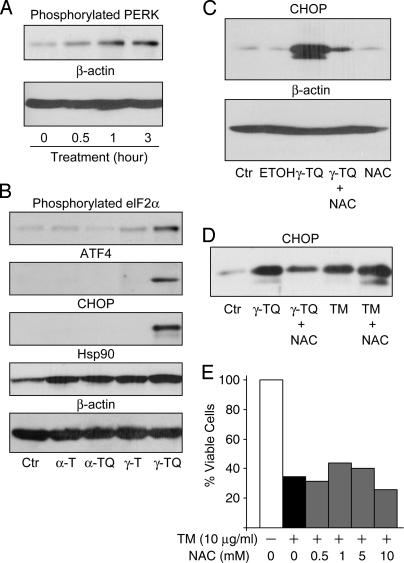
Fig. 2.
ER stress induced by arylating γ-TQ is coupled to cytotoxicity. (A) N2A cells were incubated with 10 μM γ-TQ for indicated time periods. Detergent cell lysates were normalized according to protein concentrations, separated by SDS/PAGE, transferred to a poly(vinylidene difluoride) membrane, and probed with an Ab against phosphorylated PERK. Equal loading was verified by probing the same blot with an Ab against β-actin. (B) N2A cells were incubated for 24 h with ethanol as a control (Ctr), 10 μM α-T, α-TQ, γ-T, or γ-TQ. Normalized cell lysates were separated by SDS/PAGE, and the presence of Ser-51 phosphorylated elF2α, ATF4, CHOP, and Hsp90 was detected by immunoblot analyses with Abs against phosphorylated elF2α, ATF4, CHOP, and Hsp90. Equal loading was verified by immunoblot analysis with an Ab against β-actin. (C) N2A cells were incubated for 24 h with no treatment as a control (Ctr), ethanol only (ETOH), 10 μM γ-TQ, 10 μM γ-TQ and 50 μM NAC preincubated for 3 h, or 50 μM NAC alone. Normalized cell lysates were separated by SDS/PAGE and CHOP was detected by immunoblot analysis. The same blot was probed with an Ab against β-actin to verify equal loading. (D) N2A cells were incubated for 24 h with ethanol as a control (Ctr), 10 μM γ-TQ, 10 μM γ-TQ plus 10 mM NAC without preincubation, 5 μg/ml tunicamycin (TM) alone, or 5 μg/ml tunicamycin and 10 mM NAC without preincubation. Normalized (protein concentration) cell lysates were separated by SDS/PAGE, and CHOP was detected by immunoblot analysis. (E) N2A cells were incubated for 24 h with 10 μg/ml tunicamycin and increasing concentrations of NAC. Relative cell viability was measured by the MTT assay as described in Fig. 1.
To determine whether γ-TQ induced general cellular stress, we performed immunoblot analysis to measure the level of Hsp90 in these cells. Hsp90, a cytosolic stress-responsive protein (32), was slightly increased in cells treated with any of these redox cycling reagents (Fig. 2B, Hsp90). However, we did not detect a significant difference in Hsp90 levels among cells treated with γ- or α-TQ or precursor tocopherols. This observation further supports the specific correlation between arylating γ-TQ and ER stress induction.
Among ER stress induced proteins, CHOP is involved in making the cell death decision associated with ER stress (19, 20). The induction of CHOP by γ-TQ led us to ask whether γ-TQ-caused cytotoxicity (Fig. 1A) is associated with CHOP induction. Taking advantage of the cytoprotective effect of NAC, we compared CHOP levels in cells treated with γ-TQ alone with cells treated with a preincubated γ-TQ and NAC mixture. CHOP was induced in cells treated with γ-TQ alone, and the induction was significantly diminished in cells treated with the γ-TQ and NAC mixture (Fig. 2C). Notably, under our experimental conditions, NAC itself did not influence CHOP expression (Fig. 2C, lane 5), indicating that decrease in CHOP induction does not result from the direct effect of NAC on cell metabolism. Similar results were obtained from a different cell line, COS cells, suggesting that the observation is not cell-line specific (see Fig. 7, which is published as supporting information on the PNAS web site). Again, in COS cells, the dramatic changes of CHOP levels was not accompanied with fluctuations of cytosolic stress-responsive Hsp70 or Hsp90, suggesting a specific correlation between arylating γ-TQ-caused toxicity and CHOP induction (see Fig. 7).
As mentioned above, ER stress is a complicated cellular response involving multiple signaling pathways (16–18). It is not clear whether NAC had any effect on ER stress signaling processes, which led to the reduction of CHOP levels during ER stress instead of influencing γ-TQ. To rule out this possibility, we analyzed the influence of NAC on CHOP induction caused by tunicamycin, a well known ER stress-inducing agent that acts through inhibition of N-linked glycosylation (Fig. 2 D and E). NAC had no effect on either CHOP induction (Fig. 2D, compare lanes 4 and 5) or cytotoxicity (Fig. 2E) caused by tunicamycin. Thus, NAC does not directly influence ER stress signaling processes. Instead, it inhibits γ-TQ-induced ER stress and cytotoxicity by eliminating its ability to form Michael adducts with intracellular nucleophiles. These results confirm that Michael adduct formation is one of the mechanisms for inducing CHOP expression associated with cytotoxicity.
Lower Cytotoxicity of Arylating 1,4-BzQ Because of Its Rapid Michael Adduct Formation.
To determine whether our observations apply to other arylating quinones or were specific to γ-TQ, we analyzed arylating 1,4-BzQ, a major oxidative product of environmental-polluting aromatic hydrocarbons (33). As with γ-TQ, 1,4-BzQ induced cell death in N2A cells after a 24-h incubation (Fig. 3A). Our results, however, showed that the cytotoxicity of γ-TQ (LD50 < 5 μM) was significantly greater than that of 1,4-BzQ (LD50 > 25 μM) (compare Fig. 3A with Fig. 1A). Compared with γ-ΤQ, 1,4-BzQ is chemically more reactive with a greater variety of thiol nucleophiles including cysteinyl proteins such as albumin that has a hidden thiol group (33). The seemingly paradoxical data with 1,4-BzQ, higher chemical reactivity and lower cytotoxicity, could be explained by rapid detoxification of 1,4-BzQ with many different thiols, including those in FBS in the cell culture medium and the cellular thiol containing peptide, glutathione (GSH), a well-known defensive agent against quinone toxicity (1–5). This hypothesis is supported by the striking difference in the inhibitory effect of NAC against 1,4-BzQ compared with γ-TQ. We found that an equal amount of NAC was sufficient to restore cell viability to 80%, and, more importantly, NAC protection against the cytotoxicity of 1,4-BzQ was achieved without preincubation (Fig. 3B), suggesting a rapid reaction between 1,4-BzQ and NAC.
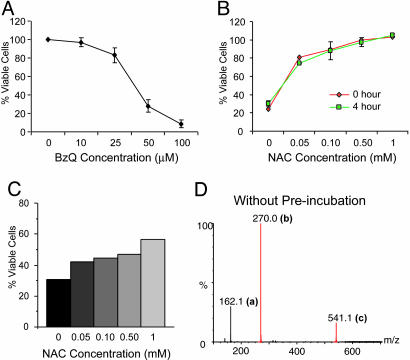
Fig. 3.
Rapid detoxification of 1,4-BzQ correlates with rapid adduct formation. (A) N2A cells were incubated for 24 h with increasing concentrations of 1,4-BzQ, and relative viability was measured by the MTT assay as described in Fig. 1. (B) N2A cells were incubated for 24 h with 50 μM 1,4-BzQ and increasing concentrations of NAC added immediately or preincubated for 4 h. Cell viability was measured as described. (C) N2A cells were treated with NAC with indicated concentrations for 4 h. Cells were washed with PBS, and fresh medium with 50 μM 1,4-BzQ was added. After 24 h, cell viability was measured as described. (D) TOF-MS analysis of adduct formation in a 1:5 molar ratio of 1,4-BzQ and NAC without preincubation. The m/z values in the negative ion mode were assigned to 162.1 for unreacted NAC (a) and 270 (b) and 540.1 (c) for the quinone-NAC adduct and its dimer, respectively.
The rapid reaction between 1,4-BzQ and NAC allows us to test whether the presence of NAC within the cell, instead of in the medium, is sufficient for the detoxification. We pretreated N2A cells with NAC for 4 h, rinsed cells with PBS, and added fresh medium to the cells. Notably, NAC-pretreated cells had a significantly higher resistance to 1,4-BzQ-caused toxicity (Fig. 3C), indicating that increasing initial intracellular thiol content can detoxify 1,4-BzQ through rapid Michael adduct formation.
The rapid Michael adduct formation between 1,4-BzQ and NAC was confirmed by TOF-MS analysis. Unlike γ-TQ, where significant adduct formation was detectable only after a 3 h preincubation (Fig. 1D), 1,4-BzQ reacted immediately with NAC, resulting in large adduct peaks detected by TOF-MS (Fig. 3D and compare with Fig. 1D), demonstrating the high chemical reactivity of this arylating quinone. Together, these results suggest that higher cytotoxicity associated with γ-TQ is because of its lower detoxification rate compared with 1,4-BzQ, allowing free γ-TQ to more readily reach intracellular target proteins. Thus, high reactivity in abiotic systems does not necessarily imply high cytotoxicity in biotic systems.
Induction of ER Stress by the Arylating 1,4-BzQ.
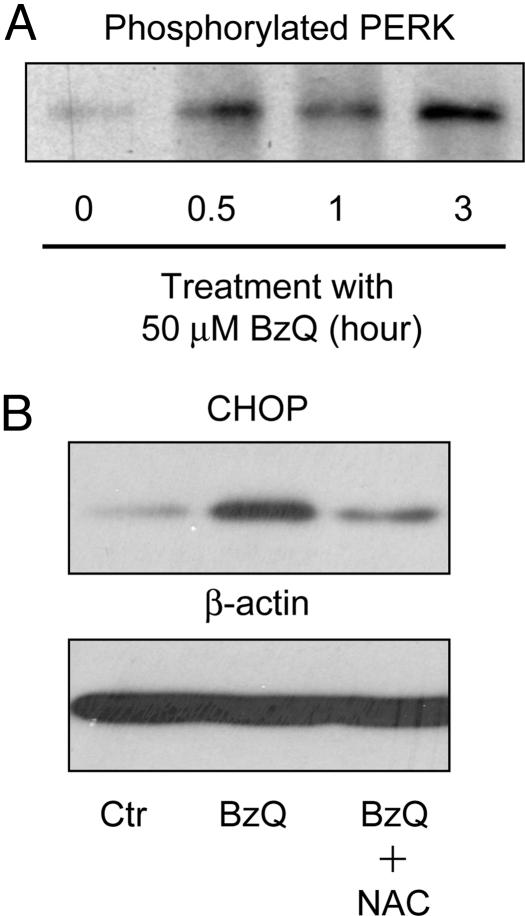
To determine whether the arylating 1,4-BzQ, as with γ-TQ, induces ER stress, we analyzed the level of phosphorylated PERK and the induction of CHOP in cells treated with 1,4-BzQ. As expected, a time-course analysis showed that phosphorylated PERK increased with incubation time in N2A cells treated with 1,4-BzQ (Fig. 4A). Equal loading was verified by total protein stain (see Fig. 8, which is published as supporting information on the PNAS web site). CHOP expression also was induced in cells treated with 1,4-BzQ, and the induction was attenuated on addition of NAC (Fig. 4B). All together, the results of 1,4-BzQ and γ-TQ demonstrate a correlation between CHOP induction and cytotoxicity with radically different quinone structures that retain the ability to function as arylating electrophiles.
Fig. 4.
1,4-BzQ induces ER stress. (A) N2A cells were incubated with 50 μM 1,4-ΒzQ for specified time periods. Cell lysates were normalized according to protein concentration. The amount of phosphorylated PERK was determined by immunoblot analysis with an Ab against phosphorylated PERK. Equal loading was verified by Coomassie blue staining of the poly(vinylidene difluoride) blot (Fig. 8). (B) N2A cells were incubated for 24 h with ethanol as a control (Ctr), 50 μM 1,4-BzQ, or 50 μM 1,4-BzQ plus 50 μM NAC without preincubation. Cell lysates were normalized according to protein concentrations. The levels of CHOP in the cell lysates were determined by immunoblot analysis with an Ab against CHOP. The same blot was probed with an Ab against β-actin to verify equal loading.
Discussion
The contribution from either ROS generation or Michael adduct formation to quinone toxicity is difficult to differentiate, because redox cycling is inherent to all quinones. Our results, higher toxicity associated with arylating γ-TQ in contrast to its nonarylating congener, α-TQ, and detoxification by prior formation of a Michael adduct with the thiol nucleophile NAC, unambiguously reveal the important role of Michael adduct formation in quinone toxicity.
In addition, we have demonstrated that arylating quinone toxicity is directly coupled to the induction of ER stress. This effect is likely due to the disruption of disulfide bond formation by arylating quinone electrophiles. Disulfide bonds are formed in the ER through a series of exchange reactions between cysteinyl proteins and ER thiol–disulfide oxidoreductases (29). Free thiols on both cysteinyl proteins and oxidoreductases should be available to react with arylating quinones during disulfide shuffling. In addition, disulfide bonds are formed during the folding process of secretory proteins. Compared with completely folded proteins, thiol groups in unfolded or partially folded proteins should be more accessible for arylating quinone adduction. Two ER protein disulfide isomerases were found to form Michael adducts with arylating quinone electrophiles, 1,4-BzQ and 1,4-naphthoquinone (13), directly supporting our hypothesis that disulfide shuffling during protein folding in the ER provides an opportunity for Michael adduct formation. As a consequence, formation of disulfide bonds will be disrupted, causing accumulation of misfolded proteins and ER stress. This concept is supported by the observation that arylating γ-TQ treatment decreased the secretion of a monoclonal antibody (Ab) (see Fig. 9, which is published as supporting information on the PNAS web site), which requires correct disulfide bond formation that could be helped by ER protein disulfide isomerases (34).
Our finding, that arylating quinones induce ER stress, provides explanations for several previous observations in this field (14, 15). For example, CHOP induction was observed in renal proximal tubular epithelial cells (LLC-PK1 cells) treated with 2-bromo-bis-(glutathion-S-yl)hydroquinone, an agent that generates ROS during redox cycling to its arylating quinone (14). Because DNA damage also was observed in these cells, CHOP induction was attributed to ROS-induced DNA damage (14). Other work has shown that CHOP is more responsive to ER stress than to DNA damage or growth arrest (35). Thus, CHOP induction in this case could be caused by arylating quinone-induced ER stress as well as DNA damage due to ROS.
A recent study revealed the essential role of ER chaperone BiP induction in 11-deoxy-16,16-dimethyl PGE2-mediated cytoprotection against 2,3,5-Tris-(glutathion-S-yl)hydroquinone, which also converts to its arylating quinone during redox cycling (15). Notably, BiP is involved in the detection of ER stress by various proximal ER stress sensors (16–18). Increased level of BiP expression attenuates ER stress response, decreases CHOP induction, and increases the survival of cells (35, 36). Therefore, the cellular mechanism of this PGE2-mediated cytoprotective effect against arylating quinone could be through the attenuation of ER stress responses.
Two quinones compared in this study, α-and γ-TQ, are oxidation products of α- and γ-T, members of the vitamin E family that are synthesized by plants. Plants synthesize a number of phenolic antioxidants, α-, β-, γ-, and δ-T congeners in the vitamin E family (37), which are oxidized to nonarylating α-TQ, and arylating β-, γ-, and δ-TQ. Interestingly, tocopherol congeners, which are precursors of arylating quinones, β-, γ-, and δ-T, are the major components of most vegetable oils, including corn (85%), soy (95%), flax (99%), and borage (98%) (38). Animals, however, selectively retain the only phenolic antioxidant precursor in the vitamin E family that produces a nonarylating quinone, α-T, as ≈85% of tissue tocopherol (39, 40). We showed in this work that arylating quinones have profound biologic effects, ER stress and cytotoxicity, in animal cells. The role of ER stress in the pathogenesis of various diseases has been revealed by many studies (21–25, 41). Is it possible, as we have suggested (40), that the selection of the nonarylating quinone precursor α-T confers an evolutionary benefit in animal cells?
Materials and Methods
Materials.
Materials and specific vendors were as follows: FBS, MEM, and 3T3, COS, and N2A cells (American Type Culture Collection); DMEM, penicillin, and streptomycin (Invitrogen); α-T, NAC, 1,4-BzQ, tunicamycin, and anti-β-actin monoclonal Ab (Sigma-Aldrich); γ-T (Tama, Tokyo); anti-phospho-PERK Ab (Cell Signaling Technology, Beverly, MA); anti-phsopho-elF2α, anti-CHOP, and anti-ATF4 Abs (Santa Cruz Biotechnology); anti-Hsp90 and anti-Hsp70 Abs (Stressgen Biotechnologies, Victoria, Canada); horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG and HRP-conjugated goat anti-rabbit IgG Abs (Bio-Rad); poly(vinylidene difluoride) membrane and enhanced chemiluminescence plus detection reagent (Amersham Pharmacia and GE Healthcare); and complete protease inhibitor (Roche). General chemicals were purchased from Sigma-Aldrich or Amresco (Euclid, OH).
Cell Lines and Culture.
N2A cells were maintained in MEM supplemented with 10% FBS, penicillin, and streptomycin. COS and 3T3 cells were maintained in DMEM supplemented with 10% FBS, 100 units/ml penicillin, and 100 mg/ml streptomycin. Cells were grown as monolayer cultures in a water-saturated 95% air/5% CO2 atmosphere at 37°C. Three different cell lines derived from different species were used to verify that effects caused by arylating quinone are not specific to a particular type of cells.
Synthesis of Tocopherol Quinone.
γ-TQ was synthesized from the parent tocopherol, γ-T, by FeCl3 oxidation, purified, and characterized as described in ref. 12. α-TQ was synthesized from α-T by using the same protocol.
Cytotoxicity.
Cells were treated with α-T, α-TQ, γ-T, γ-TQ, 1,4-BzQ, or solvent for 24 h. Cell viability was measured by the MTT assay. The detailed procedure is described in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
NAC Detoxification.
For detoxification without preincubation, increasing concentrations of NAC and 10 μM γ-TQ or 50 μM 1,4-BzQ were added to cell cultures together. For detoxification with preincubation, the NAC/quinone mixture was incubated at room temperature for indicated time periods, then added to cell cultures for a 24-h incubation period. As a control, quinones were preincubated in water or PBS to ensure that decomposition did not occur.
Electrospray Ionization TOF-MS (ESI-TOF-MS) of Adducts.
ESI-TOF-MS was performed on an LCT (Micromass, Manchester, U.K.) in the direct infusion mode. The instrument was operated at a capillary voltage of 3,000 V and a cone voltage of 55 V. The desolvation temperature was set at 100°C using nitrogen as the desolvation gas. Equal volumes of 1 mM γ-TQ in ethanol and 5 mM NAC in water were used for the experiments. Zero-time data were obtained by mixing the two solutions and spraying immediately into the MS. Data with 3-h preincubation were obtained by vigorously mixing the two solutions on a vibratory shaker for 3 h before spraying into the MS. Zero time data for 1,4-BzQ–NAC adduct were obtained in a similar way by mixing equal volumes of 1 mM 1,4-BzQ in ethanol and 5 mM NAC in water.
Immunoblot Analysis.
Detergent cell lysates were normalized according to protein concentrations. Samples with equal amounts of proteins were separated in SDS/PAGE and transferred to poly(vinylidene difluoride) membrane for immunoblot analyses. The detailed procedure is described in Supporting Materials and Methods.
Supplementary Material
Acknowledgments
We thank Russ Hille, Arthur Burghes, and Craig Hemann for critically reading this manuscript and Kenneth Jones for helpful discussions. This work was supported by National Science Foundation Environmental Molecular Science Institute Grant CHE-0089147 and the Large Interdisciplinary Grant Program in the Office of Research at Ohio State University. This work was conducted while J.M. was a recipient of a new scholar award from the Ellison Medical Foundation and a Pfizer/AFAR Innovations in Aging Research Grant.
Abbreviations
- ER
endoplasmic reticulum
- T
tocopherol
- TQ
T quinone
- 1,4-BzQ
1,4-benzoquinone
- ROS
reactive oxygen species
- NAC
N-acetylcysteine
- CHOP
C/EBP homologous protein
- PERK
pancreatic ER kinase
- N2A
Neuro 2a
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Bolton J. L., Trush M. A., Penning T. M., Dryhurst G., Monks T. J. Chem. Res. Toxicol. 2000;13:135–160. doi: 10.1021/tx9902082. [DOI] [PubMed] [Google Scholar]
- 2.Monks T. J., Jones D. C. Curr. Drug. Metab. 2002;3:425–438. doi: 10.2174/1389200023337388. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien P. J. Chem. Biol. Interact. 1991;80:1–41. doi: 10.1016/0009-2797(91)90029-7. [DOI] [PubMed] [Google Scholar]
- 4.Monks T. J., Lau S. S. Annu. Rev. Pharmacol. Toxicol. 1998;38:229–255. doi: 10.1146/annurev.pharmtox.38.1.229. [DOI] [PubMed] [Google Scholar]
- 5.Cornwell D. G., Kim S., Mazzer P. A., Jones K. H., Hatcher P. G. Lipids. 2003;38:973–979. doi: 10.1007/s11745-003-1151-4. [DOI] [PubMed] [Google Scholar]
- 6.Sachdeva R., Thomas B., Wang X., Ma J., Jones K. H., Hatcher P. G., Cornwell D. G. Chem. Res. Toxicol. 2005;18:1018–1025. doi: 10.1021/tx0496441. [DOI] [PubMed] [Google Scholar]
- 7.Cavalieri E. L., Rogan E. G. Ann. N.Y. Acad. Sci. 2004;1028:247–257. doi: 10.1196/annals.1322.029. [DOI] [PubMed] [Google Scholar]
- 8.Aitken M. D., Long T. C. In: Soil Biology. Singh A., Ward O. P., editors. Vol. 2. Heidelberg: Springer; 2004. pp. 83–124. [Google Scholar]
- 9.Mezick J. A., Settlemire C. T., Brierley G. P., Barefield K. P., Jensen W. N., Cornwell D. G. Biochim. Biophys. Acta. 1970;219:361–371. doi: 10.1016/0005-2736(70)90213-0. [DOI] [PubMed] [Google Scholar]
- 10.Rossi L., Moore G. A., Orrenius S., O’Brien P. J. Arch. Biochem. Biophys. 1986;251:25–35. doi: 10.1016/0003-9861(86)90047-0. [DOI] [PubMed] [Google Scholar]
- 11.Lindsey J. A., Zhang H. F., Kaseki H., Morisaki N., Sato T., Cornwell D. G. Lipids. 1985;20:151–157. doi: 10.1007/BF02534247. [DOI] [PubMed] [Google Scholar]
- 12.Thornton D. E., Jones K. H., Jiang Z., Zhang H., Liu G., Cornwell D. G. Free Radical Biol. Med. 1995;18:963–976. doi: 10.1016/0891-5849(94)00210-b. [DOI] [PubMed] [Google Scholar]
- 13.Lame M. W., Jones A. D., Wilson D. W., Segall H. J. Proteomics. 2003;3:479–495. doi: 10.1002/pmic.200390062. [DOI] [PubMed] [Google Scholar]
- 14.Jeong J. K., Stevens J. L., Lau S. S., Monks T. J. Mol. Pharmacol. 1996;50:592–598. [PubMed] [Google Scholar]
- 15.Jia Z., Person M. D., Dong J., Shen J., Hensley S. C., Stevens J. L., Monks T. J., Lau S. S. Am. J. Physiol. 2004;287:F1113–F1122. doi: 10.1152/ajprenal.00138.2004. [DOI] [PubMed] [Google Scholar]
- 16.Harding H. P., Calfon M., Urano F., Novoa I., Ron D. Annu. Rev. Cell Dev. Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 17.Rutkowski D. T., Kaufman R. J. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Schroder M., Kaufman R. J. Mutat. Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 19.Zinszner H., Kuroda M., Wang X., Batchvarova N., Lightfoot R. T., Remotti H., Stevens J. L., Ron D. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marciniak S. J., Yun C. Y., Oyadomari S., Novoa I., Zhang Y., Jungreis R., Nagata K., Harding H. P., Ron D. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harding H. P., Ron D. Diabetes. 2002;51(Suppl. 3):S455–S461. doi: 10.2337/diabetes.51.2007.s455. [DOI] [PubMed] [Google Scholar]
- 22.Scheuner D., Mierde D. V., Song B., Flamez D., Creemers J. W., Tsukamoto K., Ribick M., Schuit F. C., Kaufman R. J. Nat. Med. 2005;11:757–764. doi: 10.1038/nm1259. [DOI] [PubMed] [Google Scholar]
- 23.Ozcan U., Cao Q., Yilmaz E., Lee A. H., Iwakoshi N. N., Ozdelen E., Tuncman G., Gorgun C., Glimcher L. H., Hotamisligil G. S. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 24.Ryu E. J., Harding H. P., Angelastro J. M., Vitolo O. V., Ron D., Greene L. A. J. Neurosci. 2002;22:10690–10698. doi: 10.1523/JNEUROSCI.22-24-10690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakagawa T., Zhu H., Morishima N., Li E., Xu J., Yankner B. A., Yuan J. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 26.Kiyose C., Saito H., Ueda T., Igarashi O. J. Nutr. Sci. Vitaminol. (Tokyo) 2001 doi: 10.3177/jnsv.47.102. [DOI] [PubMed] [Google Scholar]
- 27.Jiang Q., Wong J., Fyrst H., Saba J. D., Ames B. N. Proc. Natl. Acad. Sci. USA. 2004;101:17825–17830. doi: 10.1073/pnas.0408340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillissen A., Nowak D. Respir. Med. 1998;92:609–623. doi: 10.1016/s0954-6111(98)90506-6. [DOI] [PubMed] [Google Scholar]
- 29.Ellgaard L. Biochem. Soc. Trans. 2004;32:663–667. doi: 10.1042/BST0320663. [DOI] [PubMed] [Google Scholar]
- 30.Ellgaard L., Ruddock L. W. EMBO Rep. 2005;6:28–32. doi: 10.1038/sj.embor.7400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gavino V. C., Miller J. S., Ikharebha S. O., Milo G. E., Cornwell D. G. J. Lipid Res. 1981;22:763–769. [PubMed] [Google Scholar]
- 32.Nollen E. A., Morimoto R. I. J. Cell Sci. 2002;115:2809–2816. doi: 10.1242/jcs.115.14.2809. [DOI] [PubMed] [Google Scholar]
- 33.Rappaport S. M., Waidyanatha S., Qu Q., Shore R., Jin X., Cohen B., Chen L. C., Melikian A. A., Li G., Yin S., et al. Cancer Res. 2002;62:1330–1337. [PubMed] [Google Scholar]
- 34.Borth N., Mattanovich D., Kunert R., Katinger H. Biotechnol. Prog. 2005;21:106–111. doi: 10.1021/bp0498241. [DOI] [PubMed] [Google Scholar]
- 35.Wang X. Z., Lawson B., Brewer J. W., Zinszner H., Sanjay A., Mi L. J., Boorstein R., Kreibich G., Hendershot L. M., Ron D. Mol. Cell. Biol. 1996;16:4273–4280. doi: 10.1128/mcb.16.8.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris J. A., Dorner A. J., Edwards C. A., Hendershot L. M., Kaufman R. J. J. Biol. Chem. 1997;272:4327–4334. doi: 10.1074/jbc.272.7.4327. [DOI] [PubMed] [Google Scholar]
- 37.Sattler S. E., Cahoon E. B., Coughlan S. J., DellaPenna D. Plant Physiol. 2003;132:2184–2195. doi: 10.1104/pp.103.024257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Firestone D. Physical and Chemical Characteristics of Oil, Fats, and Waxes. Champaign, IL: Am. Oil Chemists’ Soc. Press; 1999. [Google Scholar]
- 39.Gallo-Torres H. E. In: Vitamin E: A Comprehensive Treatise. Machlin L. J., editor. New York: Marcel Dekker; 1980. pp. 193–267. [Google Scholar]
- 40.Cornwell D. G., Williams M. V., Wani A. A., Wani G., Shen E., Jones K. H. Nutr. Cancer. 2002;43:111–118. doi: 10.1207/S15327914NC431_13. [DOI] [PubMed] [Google Scholar]
- 41.Nishitoh H., Matsuzawa A., Tobiume K., Saegusa K., Takeda K., Inoue K., Hori S., Kakizuka A., Ichijo H. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.