Abstract
Mesp2 is a transcription factor that plays fundamental roles in somitogenesis, and its expression is strictly restricted to the anterior presomitic mesoderm just before segment border formation. The transcriptional on–off cycle is linked to the segmentation clock. In our current study, we show that a T-box transcription factor, Tbx6, is essential for Mesp2 expression. Tbx6 directly binds to the Mesp2 gene upstream region and mediates Notch signaling, and subsequent Mesp2 transcription, in the anterior presomitic mesoderm. Our data therefore reveal that a mechanism, via Tbx6-dependent Notch signaling, acts on the transcriptional regulation of Mesp2. This finding uncovers an additional component of the interacting network of various signaling pathways that are involved in somitogenesis.
Keywords: enhancer, transgenic mouse, RBPJκ, luciferase assay
Somitogenesis not only is an important morphogenic process that generates metameric structures in vertebrates, but it is also a intriguing model system for the study of the interactions among various signaling cascades that facilitate periodic pattern formation. The segmental boundary of each somite forms at the anterior end of the presomitic mesoderm (PSM) or unsegmented paraxial mesoderm, which is supplied from the primitive streak or tailbud at a later stage of development.
Notch signaling plays fundamental roles in segmental pattern formation by means of oscillating the activity in the tailbud, its forward movement through the PSM as traveling waves, and its stabilization at the anterior end of the PSM (1, 2). A segment border forms at the posterior limit of the stabilized stripe of Notch signaling activity (2). The oscillation of the Notch signals in the tailbud region is regulated by the transcription factor Hes7 (3), a glycosyltransferase Lunatic fringe (2), and by Wnt signaling (4). In contrast, the positioning of segment formation by a determination wavefront is thought to be defined by antagonistic interactions between gradients of Fgf signals from the posterior end (5) and retinoic acid (RA) from anterior end of the PSM (6). On the other hand, mutant analyses identified a T-box protein, Tbx6, as an indispensable component for correct PSM differentiation and segmentation (7). However, the direct molecular relationships between these factors have not yet been well characterized.
A basic helix–loop–helix transcription factor, Mesp2, has a crucial role both in somite segment border formation and in the establishment of the rostrocaudal patterning of each somite (8). Mesp2 shows dynamic and periodical expression in the anterior PSM, which defines the positioning of the forming somite by suppressing Notch signaling, partly through the activation of lunatic fringe (2). Genetic analyses have revealed that Mesp2 expression itself is controlled by Notch signaling, which indicates the presence of a complicated feedback circuitry (9, 10). However, the molecular mechanisms that control Mesp2 expression remain largely unknown. In our present study, we show that Tbx6 directly binds to upstream elements of the Mesp2 gene and is essential for the activation of Mesp2 expression. Furthermore, we demonstrate that Notch signaling strongly enhances Mesp2 activation by Tbx6, and we identify the sequences that are important for this enhancement. Hence, we identify a Tbx6-mediated Notch signaling pathway as a mechanism underlying the regulation of Mesp2 expression.
Results and Discussion
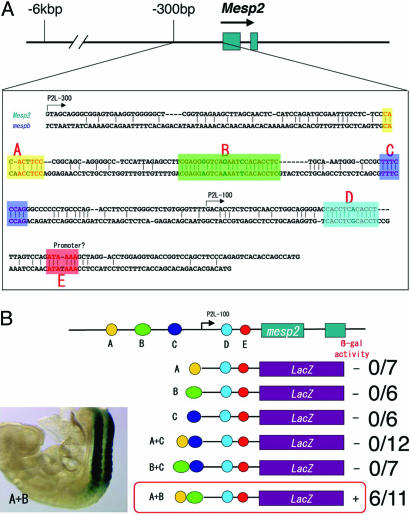
Evolutionally Conserved Sites in the Upstream Region of the Mesp2 Gene Promote Strong Reporter Activity in Forming Somites.
The distinct expression patterns of Mesp2 expression during somitogenesis are strictly regulated. As we previously reported (11), a transgenic approach has revealed that a 300-bp portion of the 5′-adjoining sequence of the Mesp2 ORF induces lacZ reporter activity in forming somites. This finding reflects the Mesp2 expression pattern in the anteriormost PSM, suggesting that this 5′ region includes cis elements that regulate PSM-specific Mesp2 expression. We performed comparisons of the genomic sequences of mouse Mesp2 and its putative ortholog in zebrafish, mespb, and identified five conserved sites (A–E) in this 300-bp segment (Fig. 1A). Each of these sites was then independently examined for enhancer properties by using a transgenic strategy. We previously showed that one of our transgenic constructs, P2L-100, containing sites D and E, which cover the 100 bp upstream of the Mesp2 ATG start codon, did not activate the lacZ reporter gene (11). We thus concentrated our analysis on sites A–C in our current experiments by ligating them with the P2L-100 construct. None of these three sites could individually promote lacZ reporter activity in somites (Fig. 1B). However, the combination of sites A and B (designated as “site A+B” hereafter) induced strong β-gal expression in the somite region (Fig. 1B Left). This result suggests that specific transcription factors required for somite-specific Mesp2 expression may bind to site A+B.
Fig. 1.
Characterization of the Mesp2 enhancer region. (A) Comparisons between the genomic sequences of mouse Mesp2 and zebrafish mespb (an ortholog of mouse Mesp2) reveal five conserved sites in the 300-bp proximal promoter region. These conserved sites are denoted as A–E. The numbers above the genomic sequences indicate the base count from the ATG transcriptional start site of the Mesp2 ORF. (B) Summary of the transient transgenic assay results with different combinations of conserved sites from the Mesp2 upstream region (sites A–C). Each site was tested either alone or in combination with other sites for somite-specific enhancer activity. The presence (+) or absence (−) of β-gal activity and the incidence of this among transgene-positive embryos is shown schematically on the right of each reporter construct. The combination of site A and site B (shown as A+B) resulted in strong somite-specific enhancer activity (Left).
Tbx6 Binds to Cis-Regulatory Elements of the Mesp2 Gene and Activates Its Expression.
To identify transcription factors that bind to the cis-regulatory elements of the Mesp2 gene, we performed yeast one-hybrid screening. Using site A+B sequences as the “bait,” we isolated a T-box transcription factor, Tbx6, as a candidate binding protein. T-box proteins have been shown to recognize and bind to nucleotide sequences of 10–11 bp in length that possess a conserved CACAC motif (12). Significantly, sites A, B, and D in the upstream sequences of the Mesp2 gene contain this motif (Fig. 2A). EMSA subsequently revealed that FLAG-Tbx6 binds to both site B and site D, in addition to the T (Brachyury) binding consensus sequence (12) (Fig. 6A, which is published as supporting information on the PNAS web site). By using site B sequences as a probe for FLAG-Tbx6 binding, EMSA experiments produced two band shifts, a distinct band with a lower mobility and a weaker band with a higher mobility. These two species presumably represent the binding of two and one Tbx6 molecule(s), respectively, because palindromic repeats or spaced tandem repeats of two half fragments of site B generated band shift patterns identical to those of site B (Fig. 6B). Tbx6 binding to site B and site D was successfully competed for by oligonucleotides containing the T binding consensus sequence and could be supershifted by incubation of the forming complexes with an anti-FLAG antibody (Fig. 6A). We conclude therefore that Tbx6 binds to the upstream region of the Mesp2 gene by means of the DNA binding activity of its T-box domain.
Fig. 2.
Tbx6 binds to the Mesp2 enhancer and promotes gene expression. (A) Wild-type and mutant sequences of site B and site D. The bases highlighted in lowercase denote the mutation sites. (B) Tbx6 binds to site B and site D. Digoxigenin-labeled oligonucleotide probes containing site B or site D were subjected to EMSA with (+) and without (−) Tbx6. Mutation of the CACAC motif in site B (lane mB1) resulted in the loss of both the wild-type band shifts, whereas mutations in GGGTC (lane mB2) abolished only the upper band. Mutations in the CACAC motif from site D also eliminated the band shift (lane mD). (C) β-Gal reporter expression analysis in transgenic mouse embryos with constructs containing either wild-type or mutated Mesp2 upstream regions. Each of the images is a lateral view with the anterior region toward the top. The numbers of β-gal-positive transgenic embryos are shown in each image (β-gal-positive/transgene-positive).
We next introduced nucleotide substitutions to the conserved CACAC motifs in sites B and D and examined the binding ability of Tbx6 to these mutated oligonucleotide probes. Interestingly, a mutation in site B, designated mB1 (Fig. 2A), eliminated both of the wild-type site B band shifts in an EMSA (Fig. 2B). Because T-box transcription factors can recognize palindromic sequences and bind to these sites as dimers (13, 14), we introduced nucleotide substitutions to the GGGTC sequence in site B, which is situated in the 5′ region adjacent to the CACAC motif (Fig. 2A, mB2). In subsequent EMSA analysis, the mB2 substitution was found to have eliminated only the upper site B band shift, indicating that this mutant oligonucleotide can bind only one Tbx6 molecule (Fig. 2B). These results suggest that site B is a partial palindrome that associates with two Tbx6 molecules and that the initial binding depends on the CACAC motif. The site D probe generated one EMSA band, and mutation of the CACAC motif in this site (mD) eliminated this band shift (Fig. 2B).
T-box proteins constitute a large family of transcription factors (15). Tbx18 (16) and Brachyury (Bra) (17) are expressed in segmented somites and in the tailbud, respectively. Mga is a ubiquitous transcriptional repressor that possesses both T-box and basic helix–loop–helix motifs (18), and the T-box motifs of Mga and Tbx6 show similarities (19). In our present experiments, we examined the DNA-binding abilities of Tbx6, Bra, Mga, and Tbx18 to upstream Mesp2 sequences using EMSA experiments (Fig. 6D). Bra and Tbx18 showed no binding activity to either site B or site D, whereas Mga bound weakly to site B. Taken together, we conclude from these data that Tbx6 is the most likely factor, among the T-box-containing proteins expressed in the PSM, that binds to site B and site D of the Mesp2 gene.
To examine the function of these upstream Mesp2 cis elements on gene expression, we performed transient transgenic mouse analyses using a lacZ reporter with mutated cis elements in 6-kb upstream sequences of the Mesp2 ORF. The nucleotide substitutions that eliminate the binding of Tbx6 to sites B and D of the Mesp2 promoter (P2EmB1D) diminished gene reporter activity in these assays (Fig. 2C). Furthermore, targeted disruption of sites B and D eliminated Mesp2 expression in the forming somites of homozygous embryos (data not shown), demonstrating that these cis-regulatory elements are essential for somite-specific Mesp2 expression.
In mouse embryo, Mesp2 mRNA emerges in anterior PSM, at the position of S-1 (8, 9). Tbx6 protein exists also in S-1 (20). Mesp2 is not expressed in the PSM of Tbx6-null mouse embryos (7), suggesting that it is a downstream target of Tbx6. Although the distinct Mesp2 signal overlaps only in the anteriormost part of Tbx6, the initial Mesp2 mRNA emerges in the more posterior region, overlapping with the Tbx6 signal (Fig. 6E). These results suggest that Tbx6 is necessary at least for initiation of Mesp2 expression.
In zebrafish, fused somite (fss), which encodes Tbx24, is known as a distant homolog of mouse Tbx6, and the corresponding mutant embryos have neither segmented somite nor mespb expression (21). The cis-regulatory elements are also well conserved between the upstream regions of Mesp2 and mespb (Fig. 1A), and Tbx24 also binds to the Mesp2 upstream region (data not shown). Recently, Davidson et al. (22) reported that, during heart development in the simple chordate Ciona intestinalis, a Mesp homolog is also expressed in a Tbx6-dependent manner. Comparing genomic sequences among Ciona, mouse, and zebrafish, the authors identified multiple Tbx6 binding sites in the upstream sequence of Ciona Mesp homolog. Taken together, we speculate from these findings that Tbx6-mediated activation of the Mesp genes is an evolutionally conserved mechanism in Chordata.
The Notch Intracellular Domain (NICD) Activates a Mesp2 Reporter Construct in a Tbx6-Dependent Manner.
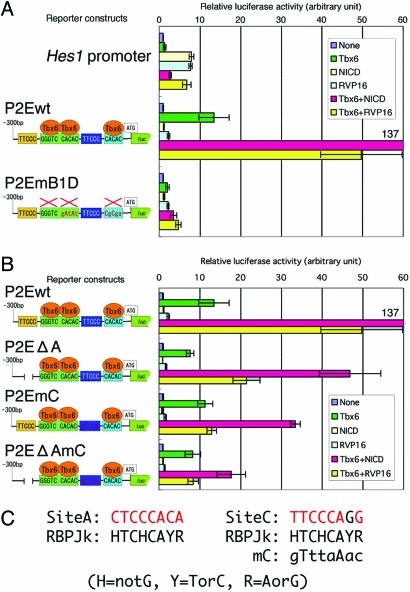
To analyze the detailed regulatory mechanisms underlying the control of Mesp2 expression, we constructed a Mesp2 reporter system comprising a firefly luciferase reporter and Mesp2 cis elements. Cotransfection of a Tbx6 expression vector with the Mesp2 reporter increased luciferase activity by 10-fold (Fig. 3), indicating that Tbx6 functions as a transcriptional activator of Mesp2. In somite-stage embryos, Tbx6 is expressed throughout the PSM and also in the tailbud region (20, 23), whereas Mesp2 expression is restricted to the anterior PSM just before somite formation, and the expression overlaps only in the anterior limit of the Tbx6 expression domain (Fig. 6E). The discrepancy between these expression patterns strongly indicates that other unknown factor(s) participate in the pathways that restrict the Mesp2 expression domain to the anterior PSM. Because Notch signaling plays crucial roles in many aspects of somitogenesis, and given that Mesp2 expression is known to depend on Dll1-Notch signaling (10), we examined the involvement of Notch signaling in the Tbx6-mediated transactivation of Mesp2.
Fig. 3.
Mesp2 expression is activated by Notch signaling in a Tbx6-dependent manner. For each set of analyses, the luciferase activity was normalized to the values obtained in the absence of an expression vector (None). Error bars represent the standard deviation from six independent experiments. RVP16, RBPJκ-VP16. (A) Tbx6 activates a Mesp2–luciferase reporter gene construct synergistically with the NICD or RBPJκ-VP16. Mutation of site B and site D (denoted as P2EmB1D) eliminates this transactivation. (B) Notch signal activates the Mesp2 reporter construct via site A and site C. The reporter constructs are indicated to the left of the graph. (C) Nucleotide sequences of the possible RBPJκ binding sites in site A (Left) and site C (Right) and the comparison between these regions and the RBPJκ binding consensus sequence (denoted as RBPJk) (27). The nucleotides matching the consensus sequence are shown in red for site A and site C. Nucleotide substitutions in site C (denoted as mC) are indicated in lowercase.
The typical Notch signaling pathway is composed of ligands known as DSL (Delta, Serrate, and Lag-2), Notch receptors, effectors known as CSL (CBF-1, Suppressor of Hairless, and Lag-1), and a number of other proteins that modulate the functions of each component of the pathway (24). Once the DSL ligands bind to the Notch receptor, the NICD is proteolytically cleaved, translocates into the nucleus, and binds to its CSL effector (RBPJκ in the case of mouse) to activate the transcription of downstream target genes (24). We transiently introduced expression vectors for NICD and RBPJκ-VP16 (dominant–active RBPJκ) (25), in conjunction with Tbx6, into cultured cells bearing the Mesp2 reporter. As a positive control, we used the Hes1 promoter, which is known to be a downstream target of Notch signaling (26). Transfection of the Hes1 reporter construct produced significant luciferase activity even in the absence of NICD (data not shown), reflecting the endogenous NICD activity, and the reporter activity increased further in the presence of either NICD or RBPJκ-VP16. In contrast, neither NICD nor RBPJκ-VP16 was found to activate the Mesp2 reporter (Fig. 3A). However, when NICD and Tbx6 were cotransfected, significant increases in luciferase activity were detected (Fig. 3A). RBPJκ-VP16 also can activate the Mesp2 promoter when cotransfected with Tbx6 (Fig. 3A), suggesting that RBPJκ-dependent Notch signaling activated Mesp2 reporter in a Tbx6-dependent manner. Consistent with this finding, mutations in site B and site D, which eliminate Tbx6 binding to the Mesp2 upstream region, greatly reduced Mesp2 reporter activation by NICD or RBPJκ-VP16 (Fig. 3A).
To identify the Notch signaling responsive site within the Mesp2 upstream region, we analyzed the activity of two additional reporter constructs bearing either a deletion or a mutation in the conserved sites A and C, because these regions contain sequences that have some similarity to the RBPJκ consensus binding site (24, 27) (Fig. 3C). We speculated that these sites may play an important role in the regulation of Mesp2 expression based on our observation that site A is essential for somite-specific expression in combination with site B (Fig. 1). Moreover, reporter activity in forming somites is lost when sequential deletion of the upstream region of the Mesp2 gene removes a part of site C (11). In our current experiments, the deletion of site A reduced the levels of synergistic activation of the Mesp2 reporter by both Notch signaling and Tbx6 by up to 50% (Fig. 3B, P2EΔA). Reporter activation was also remarkably diminished when we introduced mutations into both site A and site C (Fig. 3B, P2EΔAmC), suggesting that the binding of RBPJκ is required for the Tbx6-dependent transduction of Notch signaling. In contrast to the Hes family genes, no direct interaction between the Notch signaling pathway and the Mesp2 regulatory region had been previously identified. Our current findings thus provide the first evidence that Mesp2 is a direct target of Notch signaling. Furthermore, we identified a regulatory mechanism underlying the Notch signaling pathway that is based on the binding of Tbx6 to transcriptional regulatory sequences (summarized in Fig. 4 A and B).
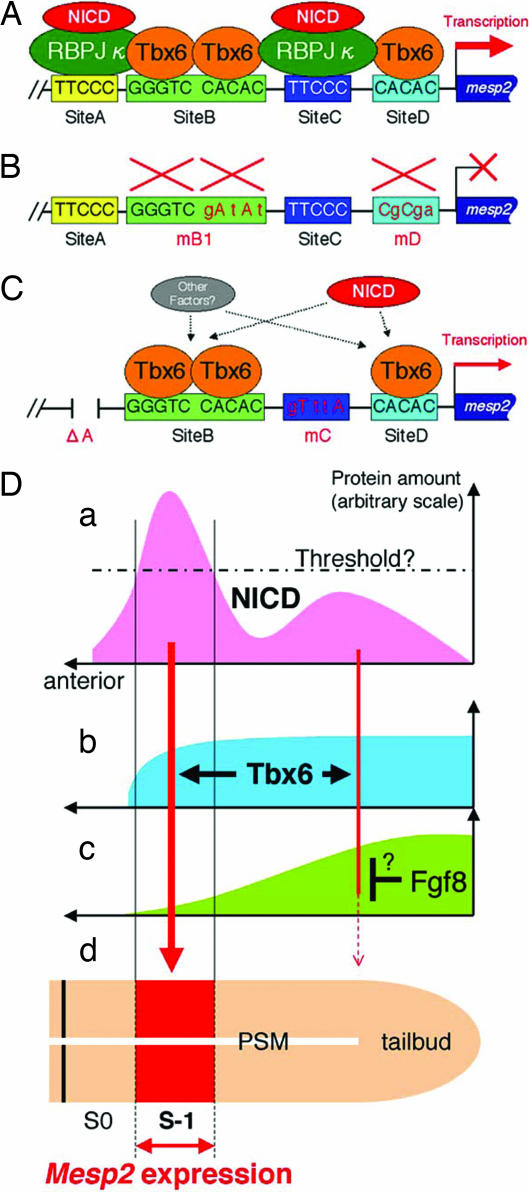
Fig. 4.
Proposed mechanisms underlying the control of Mesp2 expression. Tbx6 and NICD (colored ovals) interact with the conserved upstream sites in the Mesp2 gene, sites A–D (represented by boxes). Tbx6 binds to site B (two molecules) and site D (single molecule). Site A and site C interact with RBPJκ to achieve a significant increase in Mesp2 expression levels in the presence of Notch signals (A). This activation fully depends on the binding of Tbx6 to site B or site D (B). Tbx6 may activate Mesp2 expression without site A and site C, presumably through an RBPJκ-independent Notch signaling pathway and via other signals (C). (D) Schematic representation of a proposed model that may explain developmentally regulated Mesp2 expression in the anterior PSM. (a) NICD is highly accumulated in the anterior PSM and less in the posterior (1, 2) to activate Mesp2 expression (red arrows). There may be a threshold level of NICD accumulation to initiate Mesp2 activation (broken line). (b) Tbx6 protein is distributed in the tailbud and PSM (20) and facilitates Mesp2 activation by NICD. (c) It is possible that the activation of Mesp2 expression in the tailbud and posterior PSM, if any, is repressed by other factor(s), such as Fgf8 (36), via an unknown mechanism. (d) As a result, Mesp2 expression is restricted in the anterior PSM (red box).
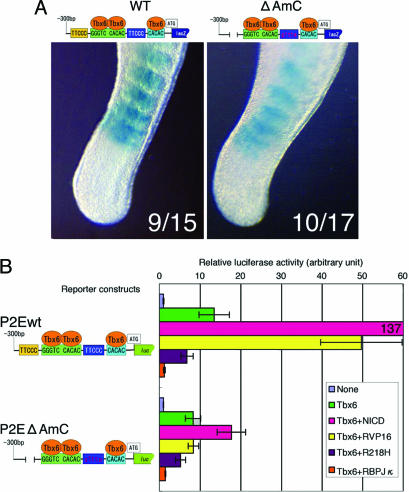
We next conducted transient transgenic assays using our lacZ reporters with mutations in sites A and C. Surprisingly, the coexistence of the site A deletion and site C mutation (P2EΔAmC) in our reporter system showed somite-specific β-gal expression, although the activity was slightly weaker than normal (Fig. 5A). One possibility that might explain this disparity is that there may be a redundant, RBPJκ-independent pathway of Notch signaling that activates Mesp2 expression. Consistent with this hypothesis, the P2EΔAmC reporter retained the ability to respond to the coexpression of NICD and Tbx6, although this activity was only 13% of wild-type levels (Fig. 3B). Notably, the P2EΔAmC reporter showed no synergistic activation after the coexpression of Tbx6 and RBPJκ-VP16 (Fig. 3B), indicating that the ability to respond to RBPJκ-dependent Notch signaling is eliminated by the disruption of sites A and C. These results suggest that Notch signaling activates Mesp2 expression in both RBPJκ-dependent and RBPJκ-independent manners (Fig. 4C). Although most of the Notch signals are mediated by CSL effectors, such as RBPJκ, there is some reported evidence that suggests the existence of RBPJκ-independent Notch signal transduction pathways (28, 29). The molecular components involved in RBPJκ-independent Notch signaling are still poorly understood, but our present data suggest the possibility that Tbx6 not only facilitates RBPJκ-dependent Notch signaling but also acts as a component of an RBPJκ-independent Notch signaling pathway.
Fig. 5.
The expression of Mesp2 is not achieved solely by RBPJκ-dependent Notch signaling. (A) Transgenic analyses reveal that somite-specific reporter expression can still be observed by using the P2EΔAmC construct, which contains a deletion of site A and mutations in site C. The numbers of β-gal-positive embryos are indicated for each image (β-gal-positive/transgene-positive). (B) The expression of a dominant-negative RBPJκ diminishes reporter activation by Tbx6 for both the wild-type (wt) and P2EΔAmC (Tbx6+R218H, purple bars) vectors. Wild-type RBPJκ also strongly suppressed reporter activity driven by Tbx6 (Tbx6+RBPJκ, orange bars). Error bars represent the standard deviation in six independent experiments.
Another possible mechanism of somite-specific reporter expression that we observed in our P2EΔAmC transgenic embryos is the involvement of Notch-independent signals (Fig. 4C). Although it is clear that Notch signaling is genetically upstream of Mesp2 activation (9, 10), Psen1 knockout mouse embryos, which are deficient in Notch proteolysis and therefore do not produce NICD (30), show only moderate decreases in Mesp2 expression levels (10). Together with our present findings, these observations may indicate that the controlling mechanism for Mesp2 gene expression is a redundant and robust system and is composed of a number of signaling cascades. Regardless of this possibility, Tbx6 is likely to be essential for all of the signaling pathways involved in Mesp2 expression, because mutation of the Tbx6 binding sites in the upstream regions of the Mesp2 gene completely eliminates reporter expression in forming somites (Fig. 2C).
Because Tbx6 mRNA (Fig. 6E) and protein (20) are distributed throughout the tailbud and posterior PSM, the factors that restrict the expression domain of Mesp2 in anterior PSM remain to be identified. Notably, although Tbx6 seems to activate reporter expression in cultured cells by itself, dominant-negative RBPJκ(R218H), which retains NICD binding activity but has lost any DNA binding ability (31), inhibits the Tbx6-dependent reporter activation by 50% (Fig. 5B). This finding suggests that Tbx6 itself has only weak transactivation properties, if any, and needs to cooperate with other signals such as Notch for full activity. We speculate that reporter activation by Tbx6 itself (Figs. 3 and 5) may be accomplished by cooperation with Notch signaling, presumably driven by endogenous NICD in cultured cells. Endogenous NICD concentration in cells or tissues is very low and biochemically undetectable (32). However, cultured fibroblast cells express mature Notch protein (33) and show γ-secretase-like activity that generates NICD from Notch protein (32). Furthermore, NICD activates Hes1 reporter at very low concentrations, below the level of biochemical detection (32). Consistent with these data, Hes1 reporter showed higher basal activity than Mesp2 reporters or control reporter with no promoter/enhancer: 100 times higher in COS-7 cells and 60 times higher in NIH/3T3 cells in our observation (data not shown). We suppose that endogenous NICD affects the expression of Notch downstream genes in cultured cells.
NICD accumulation is observed as a strong band-like pattern in the anterior PSM and as a weak diffused signal in the posterior PSM (1, 2). Mesp2 is initially detectable in the middle of a distinct band of NICD in the anterior PSM (2), consistent with the importance of Notch signaling in Mesp2 expression indicated by our present study. However, the weak Notch signaling activity observed in the posterior PSM may activate Mesp2 expression, whereas Mesp2 transcripts appear only in the anterior PSM. One possibility is that there is a “threshold” of NICD levels that is required to trigger Tbx6-dependent Mesp2 activation (Fig. 4D). Because RBPJκ is expressed ubiquitously in the developing embryo (34) and strongly represses Tbx6-dependent activation of the Mesp2 reporters (Fig. 5B), it may also function as a suppressor in the posterior PSM that prevents inadequate expression of Mesp2.
Recent reports also indicate that there are two gradients of mutually inhibitory signals, Fgf8 and RA, that have important roles in the positional determination of segment formation (35). It is likely therefore that the Fgf8 and RA signals also participate in the regulation of Mesp2 expression. Recently, Delfini et al. (36) reported an intriguing result suggesting that Fgf signaling represses Mesp expression. Using in ovo electroporation, they demonstrated that the up-regulation of Fgf in the PSM diminishes the endogenous expression of cMeso, the chick Mesp homolog. It is plausible therefore that Fgf8, which is strongly expressed in the tailbud and posterior PSM, prevents the inadequate expression of Mesp2 in posterior region. The involvement of RA in Mesp2 expression remains elusive, however, because the disruption of CYP26 (37), a degradation enzyme for RA, does not severely affect Mesp2 expression levels (2). In the zebrafish embryo, FGF signaling up-regulates a basic helix–loop–helix transcription factor, her13.2, which maintains the oscillation of the Notch signals in both the tailbud and PSM by repressing the Notch-regulated genes her1 and her7 (38). RA and Fgf signals may thus contribute to the positioning of Mesp2 expression by coordinating the regular oscillation of Notch signals in the tailbud and PSM.
Interestingly, it has been revealed that Tbx6 is one of the direct targets of RBPJκ-dependent Notch signaling (39). During somitogenesis, Notch signals may first activate Tbx6 expression in the tailbud and posterior PSM region and then activate Mesp2 expression in the anterior PSM in cooperation with Tbx6. Furthermore, Tbx6 also works upstream of the Notch signaling pathway. In embryos of Tbx6 hypomorphic mutant mice, Dll1 expression in the tailbud and posterior PSM is greatly reduced (40). Promoter analyses of Dll1 have demonstrated that Tbx6, in synergy with Wnt signaling, activates Dll1 expression by binding to T-binding consensus sequences (20, 41). Taken together, our present results demonstrate that Tbx6 and Notch signaling constitute a regulatory network that controls somite formation via the regulation of Mesp2 expression.
Materials and Methods
Transgenic Analyses.
DNA fragments, with and without mutations in conserved upstream sites, were generated from a Mesp2 genomic fragment by using a standard PCR-based protocol. Transgene inserts were digested from the corresponding plasmids, purified, and injected into the male pronucleus of a fertilized egg (42). The injected embryos were then transferred into pseudopregnant recipients and allowed to develop until 9.5–10.5 days postcoitum. Embryos were then analyzed for lacZ expression by X-gal staining (43) and subsequently examined for the presence of the transgene by PCR analysis (44).
Yeast One-Hybrid Screening.
Synthetic oligonucleotides corresponding to contiguous sequences of conserved site A (nucleotides −199 to −191 from first ATG of Mesp2 ORF) and site B (nucleotides −162 to −140) were inserted into the vectors pHISi-1 and placZi (Clontech), immediately upstream of the HIS3 and lacZ reporter genes, respectively. The resulting constructs were then linearized and introduced simultaneously into Saccharomyces cerevisiae YM4271 (Clontech) to generate the bait strain. The bait strain was then transformed by using 80 μg of 11.5 days postcoitum mouse tail cDNA library plasmid (45) to screen up to 2 million independent clones. We obtained hundreds of positive clones (HIS3+ and LacZ+) and recovered library plasmid from 77 of these. Fifty-one of these 77 clones were sequenced and found to encode Tbx6.
EMSA.
The full-length Tbx6 ORF was obtained from the pACT-Tbx6 construct, which was isolated from the yeast one-hybrid screening. After ligation to a 3XFLAG tag (Sigma), the tagged Tbx6 insert was cloned into pCS2+ (46). In vitro transcription/translation was then performed with a TNT in vitro translation kit (Promega) following the manufacturer’s protocol. Oligonucleotide probes were labeled with digoxigenin-11-dideoxy UTP by using recombinant TdT (Roche Diagnostics). Crude in vitro translated product (5 μl) was subjected to EMSA as a protein sample. As a negative control, reticulocyte lysate without Tbx6 template was used. EMSA was performed by using the DIG Gel Shift Kit, 2nd Generation (Roche Diagnostics), following the manufacturer’s protocol. The band shifts were detected by using LumiImager LAS-1000 (Fuji).
Luciferase Assay.
Segments (356 bp) corresponding to the 5′-adjoining sequence of the Mesp2 ORF, with and without mutations in the conserved binding sites, were subcloned into the pGL3-Basic (Promega) vector to generate luciferase reporter constructs. The expression vectors for the proteins to be assessed were constructed in the same way as that used in the EMSAs described above. COS-7 cells were routinely and regularly passaged in DMEM supplemented with 10% FBS. Cells were seeded at 2.5 × 104 cells per well in 24-well plates, and, after 24 h of cultivation, they were transfected with a total of 350 ng of DNA containing the reporter plasmids and expression vectors for the proteins under analysis (50 ng of each expression vector and 200 ng of reporter construct, adjusted to 350 ng by the addition of empty vector). Twenty-four hours after transfection, the cells were lysed by Passive Lysis Buffer (Promega) and subjected to a luciferase assay by using the Dual Luciferase System (Promega). In all experiments, 5 ng of the sea pansy luciferase expression vector phRL-TK (Promega) was used per well as the internal control. Luciferase activity was normalized to the phRL-TK internal control activity (sea pansy luciferase). The experiments were performed in triplicate for each assay and repeated at least twice.
Supplementary Material
Acknowledgments
We are grateful to Tasuku Honjo (Kyoto University, Kyoto) for providing cDNA clones of RBPJκ, RBPJκ-VP16, and dnRBPJκ (R218H) and to Mariko Ikumi, Eriko Ikeno, and Shinobu Watanabe for technical assistance. We also thank Hiroyuki Takeda, Mitsuru Morimoto, and Masayuki Oginuma for helpful discussions and for their comments on the manuscript. This work was supported by the Organized Research Combination System of the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Abbreviations
- PSM
presomitic mesoderm
- NICD
Notch intracellular domain
- RA
retinoic acid.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Huppert S. S., Ilagan M. X., De Strooper B., Kopan R. Dev. Cell. 2005;8:677–688. doi: 10.1016/j.devcel.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Morimoto M., Takahashi Y., Endo M., Saga Y. Nature. 2005;435:354–359. doi: 10.1038/nature03591. [DOI] [PubMed] [Google Scholar]
- 3.Bessho Y., Hirata H., Masamizu Y., Kageyama R. Genes Dev. 2003;17:1451–1456. doi: 10.1101/gad.1092303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aulehla A., Wehrle C., Brand-Saberi B., Kemler R., Gossler A., Kanzler B., Herrmann B. G. Dev. Cell. 2003;4:395–406. doi: 10.1016/s1534-5807(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 5.Sawada A., Shinya M., Jiang Y. J., Kawakami A., Kuroiwa A., Takeda H. Development. 2001;128:4873–4880. doi: 10.1242/dev.128.23.4873. [DOI] [PubMed] [Google Scholar]
- 6.Moreno T. A., Kintner C. Dev. Cell. 2004;6:205–218. doi: 10.1016/s1534-5807(04)00026-7. [DOI] [PubMed] [Google Scholar]
- 7.Chapman D. L., Papaioannou V. E. Nature. 1998;391:695–697. doi: 10.1038/35624. [DOI] [PubMed] [Google Scholar]
- 8.Saga Y., Hata N., Koseki H., Taketo M. M. Genes Dev. 1997;11:1827–1839. doi: 10.1101/gad.11.14.1827. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi Y., Koizumi K., Takagi A., Kitajima S., Inoue T., Koseki H., Saga Y. Nat. Genet. 2000;25:390–396. doi: 10.1038/78062. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi Y., Inoue T., Gossler A., Saga Y. Development. 2003;130:4259–4268. doi: 10.1242/dev.00629. [DOI] [PubMed] [Google Scholar]
- 11.Haraguchi S., Kitajima S., Takagi A., Takeda H., Inoue T., Saga Y. Mech. Dev. 2001;108:59–69. doi: 10.1016/s0925-4773(01)00478-6. [DOI] [PubMed] [Google Scholar]
- 12.Conlon F. L., Fairclough L., Price B. M., Casey E. S., Smith J. C. Development. 2001;128:3749–3758. doi: 10.1242/dev.128.19.3749. [DOI] [PubMed] [Google Scholar]
- 13.Mitani Y., Takahashi H., Satoh N. Development. 2001;128:3717–3728. doi: 10.1242/dev.128.19.3717. [DOI] [PubMed] [Google Scholar]
- 14.Muller C. W., Herrmann B. G. Nature. 1997;389:884–888. doi: 10.1038/39929. [DOI] [PubMed] [Google Scholar]
- 15.Wilson V., Conlon F. L. Genome Biol. 2002;3:REVIEWS3008. doi: 10.1186/gb-2002-3-6-reviews3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraus F., Haenig B., Kispert A. Mech. Dev. 2001;100:83–86. doi: 10.1016/s0925-4773(00)00494-9. [DOI] [PubMed] [Google Scholar]
- 17.Koseki H., Wallin J., Wilting J., Mizutani Y., Kispert A., Ebensperger C., Herrmann B. G., Christ B., Balling R. Development. 1993;119:649–660. doi: 10.1242/dev.119.3.649. [DOI] [PubMed] [Google Scholar]
- 18.Hurlin P. J., Steingrimsson E., Copeland N. G., Jenkins N. A., Eisenman R. N. EMBO J. 1999;18:7019–7028. doi: 10.1093/emboj/18.24.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lardelli M. Dev. Genes Evol. 2003;213:519–522. doi: 10.1007/s00427-003-0348-2. [DOI] [PubMed] [Google Scholar]
- 20.White P. H., Chapman D. L. Genesis. 2005;42:193–202. doi: 10.1002/gene.20140. [DOI] [PubMed] [Google Scholar]
- 21.Nikaido M., Kawakami A., Sawada A., Furutani-Seiki M., Takeda H., Araki K. Nat. Genet. 2002;31:195–199. doi: 10.1038/ng899. [DOI] [PubMed] [Google Scholar]
- 22.Davidson B., Shi W., Levine M. Development. 2005;132:4811–4818. doi: 10.1242/dev.02051. [DOI] [PubMed] [Google Scholar]
- 23.Chapman D. L., Agulnik I., Hancock S., Silver L. M., Papaioannou V. E. Dev. Biol. 1996;180:534–542. doi: 10.1006/dbio.1996.0326. [DOI] [PubMed] [Google Scholar]
- 24.Mumm J. S., Kopan R. Dev. Biol. 2000;228:151–165. doi: 10.1006/dbio.2000.9960. [DOI] [PubMed] [Google Scholar]
- 25.Furriols M., Bray S. Dev. Biol. 2000;227:520–532. doi: 10.1006/dbio.2000.9923. [DOI] [PubMed] [Google Scholar]
- 26.Jarriault S., Brou C., Logeat F., Schroeter E. H., Kopan R., Israel A. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- 27.Barolo S., Walker R. G., Polyanovsky A. D., Freschi G., Keil T., Posakony J. W. Cell. 2000;103:957–969. doi: 10.1016/s0092-8674(00)00198-7. [DOI] [PubMed] [Google Scholar]
- 28.Matsuno K., Go M. J., Sun X., Eastman D. S., Artavanis-Tsakonas S. Development. 1997;124:4265–4273. doi: 10.1242/dev.124.21.4265. [DOI] [PubMed] [Google Scholar]
- 29.Hori K., Fostier M., Ito M., Fuwa T. J., Go M. J., Okano H., Baron M., Matsuno K. Development. 2004;131:5527–5537. doi: 10.1242/dev.01448. [DOI] [PubMed] [Google Scholar]
- 30.Koizumi K., Nakajima M., Yuasa S., Saga Y., Sakai T., Kuriyama T., Shirasawa T., Koseki H. Development. 2001;128:1391–1402. doi: 10.1242/dev.128.8.1391. [DOI] [PubMed] [Google Scholar]
- 31.Kato H., Taniguchi Y., Kurooka H., Minoguchi S., Sakai T., Nomura-Okazaki S., Tamura K., Honjo T. Development. 1997;124:4133–4141. doi: 10.1242/dev.124.20.4133. [DOI] [PubMed] [Google Scholar]
- 32.Schroeter E. H., Kisslinger J. A., Kopan R. Nature. 1998;393:382–386. doi: 10.1038/30756. [DOI] [PubMed] [Google Scholar]
- 33.De Strooper B., Annaert W., Cupers P., Saftig P., Craessaerts K., Mumm J. S., Schroeter E. H., Schrijvers V., Wolfe M. S., Ray W. J., et al. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 34.Oka C., Nakano T., Wakeham A., de la Pompa J. L., Mori C., Sakai T., Okazaki S., Kawaichi M., Shiota K., Mak T. W., Honjo T. Development. 1995;121:3291–3301. doi: 10.1242/dev.121.10.3291. [DOI] [PubMed] [Google Scholar]
- 35.Dubrulle J., Pourquie O. Development. 2004;131:5783–5793. doi: 10.1242/dev.01519. [DOI] [PubMed] [Google Scholar]
- 36.Delfini M. C., Dubrulle J., Malapert P., Chal J., Pourquie O. Proc. Natl. Acad. Sci. USA. 2005;102:11343–11348. doi: 10.1073/pnas.0502933102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakai Y., Meno C., Fujii H., Nishino J., Shiratori H., Saijoh Y., Rossant J., Hamada H. Genes Dev. 2001;15:213–225. doi: 10.1101/gad.851501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawamura A., Koshida S., Hijikata H., Sakaguchi T., Kondoh H., Takada S. Genes Dev. 2005;19:1156–1161. doi: 10.1101/gad.1291205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White P. H., Farkas D. R., Chapman D. L. Genesis. 2005;42:61–70. doi: 10.1002/gene.20124. [DOI] [PubMed] [Google Scholar]
- 40.White P. H., Farkas D. R., McFadden E. E., Chapman D. L. Development. 2003;130:1681–1690. doi: 10.1242/dev.00367. [DOI] [PubMed] [Google Scholar]
- 41.Hofmann M., Schuster-Gossler K., Watabe-Rudolph M., Aulehla A., Herrmann B. G., Gossler A. Genes Dev. 2004;18:2712–2717. doi: 10.1101/gad.1248604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hogan B., Beddington R., Costantini F., Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. Woodbury, NY: Cold Spring Harbor Lab. Press; 1994. [Google Scholar]
- 43.Saga Y., Yagi T., Ikawa Y., Sakakura T., Aizawa S. Genes Dev. 1992;6:1821–1831. doi: 10.1101/gad.6.10.1821. [DOI] [PubMed] [Google Scholar]
- 44.Sasaki H., Hogan B. L. Genes Cells. 1996;1:59–72. doi: 10.1046/j.1365-2443.1996.04004.x. [DOI] [PubMed] [Google Scholar]
- 45.Ohara O., Nagase T., Mitsui G., Kohga H., Kikuno R., Hiraoka S., Takahashi Y., Kitajima S., Saga Y., Koseki H. DNA Res. 2002;9:47–57. doi: 10.1093/dnares/9.2.47. [DOI] [PubMed] [Google Scholar]
- 46.Rupp R. A., Snider L., Weintraub H. Genes Dev. 1994;8:1311–1323. doi: 10.1101/gad.8.11.1311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.