Abstract
T cell helper type 2 (Th2) differentiation is driven by a source of IL-4 receptor (IL-4R) that mobilizes IL-4R signaling pathways and the transcription factor GATA-3. Naïve CD4 cells can secrete IL-4 independently of IL-4R signals, but how this secretion is regulated is not understood. Here we demonstrate that costimulation through the tumor necrosis factor receptor family molecule OX40, in synergy with CD28, is essential for high levels of nuclear factor of activated T cells c1 to accumulate in the nucleus of a recently activated naïve T cell. This action is not dependent on either IL-4R or IL-2R signals and results in OX40 controlling initial naïve T cell IL-4 transcription. OX40 signals subsequently enhance nuclear GATA-3 accumulation through an IL-4R-dependent action, leading to Th2 differentiation. These data show that, in the absence of an exogenous source of IL-4, OX40 provides a critical synergistic and temporal signal with other noncytokine receptors to modulate nuclear factor of activated T cells c1 and to promote optimal Th2 generation.
Keywords: IL-4, GATA-3
T cell helper type 2 (Th2) cells secrete IL-4, IL-5, and IL-13 and are responsible for protection against intestinal helminths and biting arthropods, and they have a central role in allergic diseases. How Th2 cells arise is an area of intense interest. It has been argued that Th2 differentiation is a default fate that occurs in the absence of T cell helper type 1 (Th1)-inducing stimuli, such as IL-12, that are derived from antigen-presenting cells (APCs) (1). However, an IL-12 deficiency does not necessarily yield a Th2 response (2). This finding suggests that, in addition to a lack of Th1-favoring signals, other signals that specifically instruct the formation of Th2 cells exist. In line with this conclusion, IL-4 can strongly influence Th2 differentiation or at least Th2 expansion. The IL-4 receptor (IL-4R) induces phosphorylation and nuclear translocation of STAT6, and STAT6 binds to the IL-4 promoter and up-regulates GATA-3, which functions by stabilizing the differentiated Th2 phenotype (3). In accordance, a conditional deletion of GATA-3 abolishes Th2 responses in vivo (4, 5).
Over the years, the origin of IL-4 that might drive Th2 development has been debated. Potential sources include basophils and mast cells (6) and natural killer T cells (7). However, along with these cells, a number of reports have shown that responding naïve CD4 cells can transcribe IL-4 under specific conditions of stimulation in the apparent absence of another source of IL-4 (8–12) and without prior IL-4R signaling (11, 13, 14). Although the initial amount of IL-4 made by a naïve T cell is low, it can be sufficient to promote the T cell’s development into a high IL-4 producer through an autocrine pathway (9, 11, 12). These latter results thus suggest that, in the absence of an exogenous source of IL-4, rather than simply a default pathway, a two-step model of Th2 differentiation operates whereby initial instructive signals during antigen presentation lead to IL-4R-independent early IL-4 transcription followed by IL-4R-dependent amplification to support optimal Th2 generation. The membrane receptors and their intracellular signaling pathways, which control naïve T cell IL-4 transcription, are thus of high significance.
A member of the tumor necrosis factor receptor (TNFR) family, OX40 (CD134), is a principal costimulatory receptor that is not constitutively expressed on naïve T cells but is induced 12 h or more after antigen recognition (15). Triggering OX40 on CD4 T cells can increase clonal expansion and enhance memory T cell development (16). Interestingly, OX40 costimulation has also been reported to promote Th2 generation or Th2 cytokine production under certain conditions in vitro (15, 17–19), and a critical role for OX40–OX40 ligand (OX40L) interactions in Th2 responses in vivo has been shown in experimental models of asthma (20) and Leishmania major (21) and Heligmosomoides polygyrus (22) infections.
In this work, we show that, under physiological conditions of antigen stimulation, ligation of OX40 is critical for a naïve T cell to initially transcribe IL-4; that this ligation controls differentiation into effector T cells, which make Th2 cytokines; and that this differentiation is mediated by targeting nuclear translocation of nuclear factor of activated T cells c1 (NFATc1), a molecule important to Th2 development (23, 24).
Results
Early IL-4 Production and Th2 Differentiation Are Impaired in OX40-Deficient Naïve CD4 T Cells.
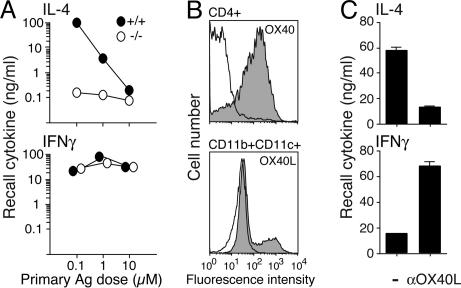
To examine whether OX40–OX40L interactions control IL-4 production under physiological conditions, we cultured naïve wild-type or OX40-deficient CD4 cells from OT-II T cell antigen receptor (TCR) transgenic mice with wild-type APCs and various doses of ovalbumin (OVA) peptide for 7 days. Strong Th2 development (IL-4 shown and IL-5/IL-13 not shown) occurred with a moderate/low dose of antigen (Ag), whereas the Th2 response was lost as the Ag dose was increased (Fig. 1A). In contrast, Th2 differentiation was impaired in the absence of OX40, such that 100- to 1,000-fold lower recall IL-4 levels were detected (Fig. 1A), correlating with OX40 and OX40L expression on CD4 cells and APCs, from 12 h to 2 days after T cell–APC interaction (Fig. 1B). Similarly, an OX40L blocking antibody (Ab) with wild-type T cells mimicked the OX40-deficient phenotype (Fig. 1C). IFN-γ recall responses were unaltered or enhanced, suggesting a selective effect on the Th2 lineage.
Fig. 1.
OX40–OX40L interactions are required for Th2 differentiation. Naïve CD4 T cells from wild-type (filled circles) or OX40-deficient (open circles) OT-II mice were cultured with APCs and indicated doses of OVA-323–339. (A) IL-4 and IFN-γ recall responses measured at day 7 after Ag/APC restimulation of equal numbers of effector T cells for 24 h. (B) OX40 and OX40L expression on wild-type CD4 and CD11b+CD11c+ APCs after stimulation with 0.1 μM Ag for 36 h. The blank histograms indicate isotype control. The shaded histograms indicate positive staining. (C) Effect of OX40L blocking on recall cytokine responses of wild-type T cells cultured with 0.1 μM Ag. αOX40L, anti-OX40L Ab. All data are representative of at least three individual experiments.
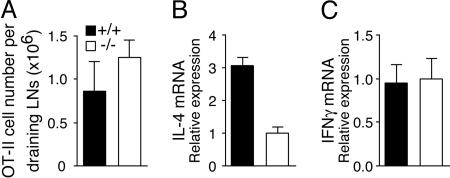
To show a similar role of OX40 in vivo, wild-type or OX40−/− OT-II T cells were adoptively transferred into syngeneic mice, followed by immunization with Ag. Transferred OT-II T cells were recovered 2–4 days after immunization. The number of OX40-deficient T cells was similar to that of wild-type cells, suggesting no survival defect at this time point (Fig. 2A). IL-4 mRNA levels in OX40−/− OT-II cells were reduced compared with wild-type OT-II cells, whereas no difference in IFN-γ mRNA was found (Fig. 2B and C). This finding confirms a critical role for OX40 in Th2 lineage commitment, correlating with defective Th2 immunity in the absence of OX40–OX40L interactions (20–22).
Fig. 2.
Defective Th2 differentiation in vivo in OX40-deficient CD4 T cells. Naïve wild-type or OX40−/− OT-II CD4 cells were transferred into Thy1.1 B6.PL hosts. Mice were immunized with OVA/alum, and draining lymph node cells were taken at 4 days. (A) The number of OT-II T cells measured by staining for Thy1.2. Data are the mean ± SE of three mice. (B and C) IL-4 and IFN-γ mRNA levels in purified Thy1.2+ cells measured by quantitative RT-PCR, normalized to hypoxanthine phosphoribosyltransferase (HPRT). Data are relative values with SD of triplicate PCR wells. Similar results were obtained at day 2.
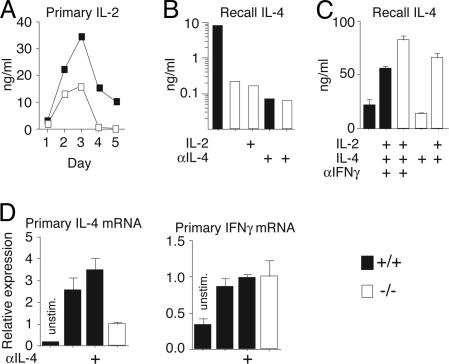
STAT5 signaling from IL-2 augments polarization of naïve CD4 T cells to the Th2 phenotype (25). To address whether defects in IL-2 accounted for the requirement for OX40, within 24 h we found that there was no difference in IL-2 between wild-type and OX40−/− T cells (Fig. 7, which is published as supporting information on the PNAS web site). OX40−/− T cells produced high levels of IL-2 after 2 or more days, although less than wild-type T cells (Fig. 3A). However, exogenous IL-2 did not restore defective Th2 differentiation (Fig. 3B).
Fig. 3.
IL-4 is a critical factor for OX40-driven Th2 differentiation. Naïve CD4 T cells from wild-type (filled symbols) or OX40-deficient (open symbols) OT-II mice were cultured with APCs and 0.1 μM OVA-323–339 as in Fig. 1.(A) Primary IL-2 production from naïve T cells stimulated for 1–5 days. (B and C) Recall IL-4 production at 7 days in the presence of IL-2 (10 ng/ml), IL-4 (10 ng/ml), anti-IL-4 or anti-IFN-γ (10 μg/ml), added at days 0 and 3. (D) Primary IL-4 and IFN-γ mRNA induction, in the presence or absence of anti-IL-4, 36 h after naïve T cell activation. mRNA levels in purified CD4 cells by RT-PCR, normalized to HPRT, are shown. Data are relative values with SD of triplicate PCR wells. Results are representative of at least two experiments.
IL-4 is also a crucial cytokine for Th2 differentiation. Accordingly, neutralization of endogenous IL-4 from wild-type T cells resulted in a complete block in Th2 generation, mimicking the phenotype with OX40−/− T cells (Fig. 3B). Supporting this result, exogenous IL-4 restored Th2 differentiation to levels seen with wild-type T cells under neutral conditions (Fig. 3C). Exogenous IL-2 synergized with IL-4 for optimal Th2 generation in both wild-type and OX40−/− T cells (Fig. 3C). This finding suggested that early IL-4, produced after OX40–OX40L interactions during naïve T cell encounters with APCs, was a crucial factor for Th2 development. Correlating with this conclusion, we observed IL-4-independent IL-4 mRNA up-regulation in wild-type T cells responding to Ag 36 h after initial activation (Fig. 3D), as well as IL-4 secretion within 2 days of naïve T cell activation (data not shown), and this response was strongly reduced in the absence of OX40.
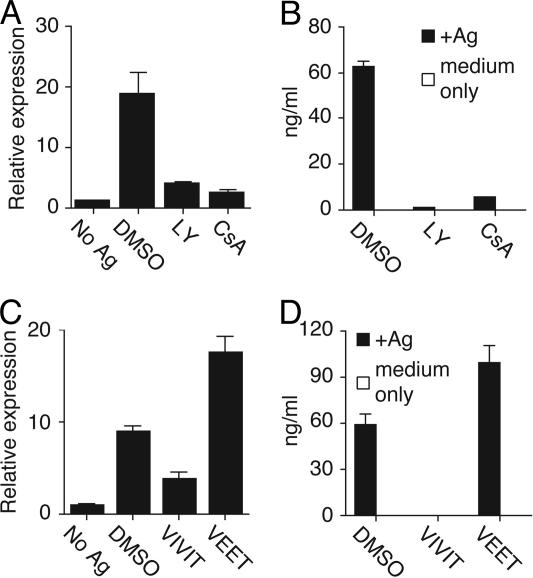
Our previous data showed that phosphatidylinositol 3-kinase (PI3K) is a downstream mediator of OX40 signals (26). LY294002 (LY), an inhibitor of PI3K, was added 12 h after stimulation to coincide with OX40 expression but also to allow initial signaling to proceed normally. LY greatly inhibited IL-4 transcription in wild-type T cells (Fig. 4A) and resulted in dramatically impaired generation of effector cells producing IL-4 in recall responses (Fig. 4B). These results collectively indicate that OX40 signals enhance IL-4 transcription at an early stage, and this then leads to a feedback loop through the IL-4R that further supports Th2 differentiation.
Fig. 4.
PI3K, CN, and NFAT control OX40-mediated IL-4 and Th2 differentiation. Naïve CD4 T cells from wild-type OT-II mice were stimulated with 0.1 μM Ag/APCs as in Fig. 1. After 12 h of activation, LY (PI3K inhibitor), cyclosporin A (CsA) (CN inhibitor) (A and B), VIVIT peptide (NFAT inhibitor), VEET control peptide (C and D), or DMSO were added. T cells were harvested at 36 h to measure IL-4 mRNA (A and C) and at day 7 to measure IL-4 recall responses after Ag restimulation as in Fig. 1 (B and D).
OX40 Signals Facilitate Nuclear Translocation of NFATc1.
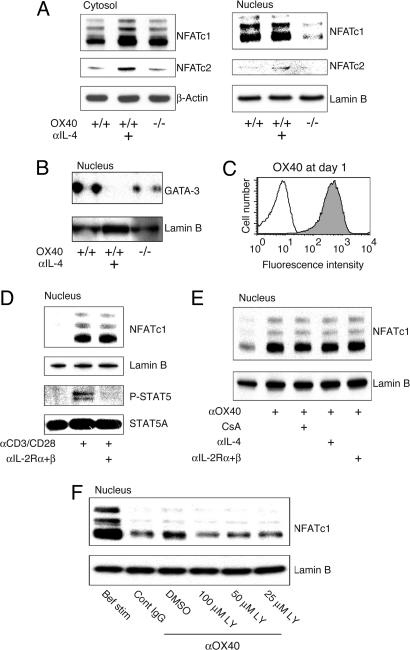
NFATc1 (NFATc, NFAT2) resides in the cytoplasm of resting cells. Upon appropriate stimulation, NFATc1 translocates to the nucleus, where it can activate IL-4 (27). The phosphatase calcineurin (CN) can dephosphorylate NFAT in a calcium-dependent manner, resulting in NFAT’s nuclear entry, and cyclosporin A (CsA) inhibits this activity of CN. Additionally, a peptide, VIVIT, can interfere selectively with the interaction of CN with NFAT and block NFAT nuclear entry without affecting CN phosphatase activity (28, 29). Correlating with a potential role for these molecules in early IL-4 production by naïve T cells, CsA and VIVIT peptide inhibited both primary IL-4 mRNA and recall IL-4 protein expression (Fig. 4). To evaluate whether NFATc1 was targeted by OX40, we prepared nuclear extracts from naive T cells 36 h after activation. Impaired nuclear accumulation of NFATc1 was observed in T cells lacking OX40 (Fig. 5A). Cytoplasmic NFATc1 was either unaltered or reduced, indicating that OX40 specifically promoted nuclear accrual. NFATc1 accumulation after antigen triggering was not reduced by anti-IL-4, indicating that this was an IL-4R-independent event (Fig. 5A). GATA-3 is also a critical transcription factor for Th2 differentiation (4, 5), and the accumulation of GATA-3 in the nucleus was decreased in the absence of OX40 (Fig. 5B). However, this costimulation-induced nuclear GATA-3 expression was inhibited by anti-IL-4 (Fig. 5B), distinguishing it from the IL-4-independent increase in NFATc1. Excluding a role for IL-2, blocking IL-2R-α and -β had no effect on accumulation of NFATc1 (Fig. 5D).
Fig. 5.
OX40 directs IL-4-independent nuclear accumulation of NFATc1. Naïve CD4 T cells from wild-type and OX40−/− OT-II mice were stimulated with 0.1 μM Ag/APCs in the presence or absence of anti-IL-4, as in Fig. 1. (A and B) Levels of NFATc1, NFATc2, and GATA-3 were examined in purified CD4 cells at 36 h by blotting cytoplasmic and nuclear extracts. Lamin B, control nucleus-specific protein, is shown. (C–F) Naïve wild-type CD4 cells were stimulated with anti-CD3/CD28 and IL-2 for 24 h. (C) OX40 expression at 24 h. (D) Nuclear NFATc1 at 24 h. Anti-IL-2R-α and anti-β blocking mAbs were added from the beginning of culture. (E and F) Nuclear NFATc1 at 28 h. At 24 h, CD4 cells were recultured without stimulation or with anti-OX40 (100 μg/ml) for 4 h in the presence of blocking Abs, CsA, or LY. Data are representative of at least two independent experiments.
Our previous data showed that OX40 acts on naïve T cells in combination with CD28 and that OX40 action is in large part dependent on initial CD28 signaling (15, 30). To more easily dissect the effect of OX40 away from TCR and CD28 signals, naïve T cells were stimulated for 24 h with CD3/CD28 to express OX40 (Fig. 5C) and were then recultured with agonist anti-OX40 in isolation for 4 h. OX40 triggering strongly enhanced the levels of nuclear NFATc1 (Fig. 5 E and F), and this was independent of IL-2R, IL-4R, and, in this case, calcium/CN signals (Fig. 5E) but was dependent on PI3K (Fig. 5F). When analyzing the level of nuclear NFATc1 at 24 h before reculture, compared with 4 h after (Fig. 5F), it was observed that OX40 maintained NFATc1 over this time period, as opposed to inducing its entry, suggestive of an effect on suppressing nuclear export.
These results demonstrate that OX40 signals synergistically cooperate with TCR and CD28 pathways to induce NFATc1 to accumulate in the nucleus to drive initial IL-4 transcription from naïve T cells.
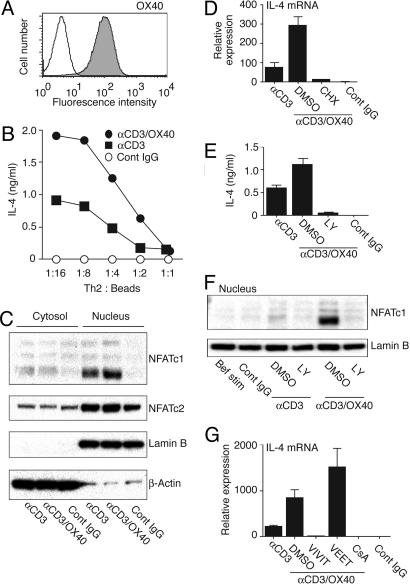
OX40 Signals Up-Regulate IL-4 Through NFATc1 in Th2 Cells.
Finally, to assess whether OX40 targets the IL-4 transcriptional machinery in differentiated T cells, we generated effector Th2 cells and ligated OX40 with antibody in the presence of anti-CD3. A differentiation period of 6 days in the presence of IL-4 leads to effector T cells that retain surface OX40 (Fig. 6A), have only low levels of nuclear NFATc1 (Fig. 6C and F), and do not secrete IL-4 without further stimulation (Fig. 6B and E). Upon cross-linking of both OX40 and CD3, substantially elevated levels of IL-4, mRNA (Fig. 6D and G), and protein (Fig. 6B and E) were induced compared with CD3 alone. Cross-linking OX40 in the absence of CD3 had no effect (data not shown). Thus, with more differentiated T cells, unlike naïve T cells, CD28 signals were not required for OX40 to signal effectively.
Fig. 6.
OX40 triggering up-regulates IL-4 and nuclear NFATc1 in effector Th2 cells. CD4 T cells were cultured in Th2 skewing conditions. At day 6, live effector cells were restimulated with beads coated with anti-CD3 and anti-OX40. (A) OX40 expression at day 6. (B and E) IL-4 protein and (D and G) IL-4 mRNA, 4 h after stimulation. (C and F) Nuclear NFATc1 levels at 4 h. Th2 cells were preincubated for 2 h with cycloheximide (D), LY (E and F), CsA, VIVIT peptide, or VEET peptide (G), then restimulated with Ab-coated beads for 4 h.
Consistent with naïve T cells (Fig. 5), the level of NFATc1 preferentially increased in the nucleus of effector cells when OX40 was engaged (Fig. 6 C and F), and this increase depended on PI3K (Fig. 6F). In contrast to naïve T cells that were previously activated for 24 h (Fig. 5 E and F), effector Th2 cells did not already express high levels of nuclear NFATc1, suggesting that, here, OX40, in combination with CD3, signals promoted nuclear import and potentially suppressing export. Accordingly, the CN/NFAT inhibitor peptide VIVIT inhibited production of IL-4 protein (data not shown) and mRNA (Fig. 6G). CsA also inhibited IL-4 (mRNA shown and protein not shown) (Fig. 6G). IL-4 mRNA up-regulation was further blocked by cycloheximide (Fig. 6D). These data confirm a positive regulatory role for OX40 signals in controlling both nuclear accumulation of NFATc1 and IL-4 transcription.
Discussion
Major strides have been made in delineating the cytokines, such as IL-4 and IL-12, and their transcription factors, such as GATA-3, c-maf, and T-bet, which can regulate commitment to Th2/Th1 lineages (31). However, there have been few reports providing strong evidence of factors that might precede and control, or at least synergize with, these cytokine signals and hence instruct Th2/Th1 divergence. In the absence of exogenous sources of IL-4 or IL-12, the affinity or dose of antigen can differentially promote Th1 versus Th2 differentiation (32–35). These data have implied that TCR signal strength might be a factor in regulating early events of differentiation. Thus, one idea is that signaling through the TCR is important both for inducing molecules necessary for later events in lineage commitment and for allowing other signals from distinct T cell membrane receptors. Our data now show that OX40 triggering is absolutely critical for Th2 development where antigen presentation determines the Th2/Th1 balance.
The OX40 signal was transmitted through increasing the level of NFATc1 that accumulates and/or persists in the nucleus, thereby promoting early IL-4 transcription by naïve T cells. At the same time, the level of nuclear NFATc2 (NFATp/NFAT1) was low and not increased, consistent with a counteracting role for this molecule on IL-4 (36). This finding correlates with data showing that presentation of low-affinity peptide, leading to Th2 development, also results in early induction of high levels of nuclear NFATc1 but low levels of NFATc2 (37). Collectively, these results suggest that regulation of the NFATc1/NFATc2 balance in naïve T cells is thus central to Th2 programming. In line with these results, NFATc2-deficient T cells show sustained IL-4 mRNA expression after CD3 triggering (38). Interestingly, we found that nuclear accumulation of NFATc1 was terminated at 48–60 h after stimulation of naïve cells (data not shown), suggesting this termination could be an important late regulatory event that additionally controls T cell differentiation. This event might be significant in light of surprising data that showed that a retroviral vector containing constitutively active NFATc1, and hence sustaining nuclear NFATc1 after its normal time of export, resulted in Th1 and not Th2 differentiation in wild-type T cells (39). Thus, high-level, early but transient accumulation of NFATc1 in the nucleus appears to be critical for ultimately producing a Th2 cell.
Although we show that OX40 cosignals with TCR signals can regulate NFATc1 and IL-4 in an effector T cell with a naïve T cell, both TCR and CD28 signals were initially necessary for OX40 to promote IL-4. Stimulation of naïve T cells with TCR/CD28 results, within 1–3 h, in the STAT-independent induction of IL-4 and IFN-γ transcription (40) and in nonselective histone modifications at both IL4 and IFNG loci (41). In addition, NFAT has been suggested to be a critical transcription factor for cytokines at this stage (42). Our data now complement these studies and demonstrate that signals from OX40 act synergistically with these early signals, also in a STAT-independent manner, to maintain and further up-regulate IL-4 mRNA within 24–36 h after naive T cell activation. Supporting the notion of a combined synergistic and temporal action of CD28 and OX40, other data have shown that a CD28 deficiency also results in impaired Th2 responses (43) and that CD28 signals can up-regulate nuclear NFATc1 levels above those present after TCR triggering (44). Thus, several signals control early Th2 divergence in a naïve T cell but through a common target, NFATc1.
Ca2+/CN are required for allowing NFATc1 entry into the nucleus (27), and we observed an absolute requirement for CN activity for IL-4 induction in both naïve and effector T cells. This result is in agreement with previous data showing CN was essential for early IL-4 mRNA expression in response to weak peptide stimulation (37) and that T cells from dominant negative CN transgenic mice were impaired in Th2 development (45). OX40 signals did not regulate phosphorylation of phospholipase C-γ (data not shown) or induce Ca2+ influx (Fig. 8, which is published as supporting information on the PNAS web site) in effector Th2 cells, suggesting that it is unlikely that OX40 directly targets Ca2+/CN. Moreover, ionomycin could not restore defective Th2 differentiation of OX40−/− T cells (data not shown), demonstrating that although Ca2+/CN are essential for Th2 development, they are not the primary factors that dictate the Th2/Th1 balance.
A critical requirement for PI3K was found in blocking studies where suppressed IL-4 mRNA and protein expression and NFATc1 nuclear translocation were observed. These data possibly suggest that nuclear export of NFATc1 could be one target of OX40, which is supported by data in naïve T cells previously stimulated for 24 h (Fig. 5). This type of action might be mediated through glycogen synthase kinase (GSK)3β by way of PI3K-regulated activation of Akt, a pathway postulated to regulate such export. Activation of Akt and phosphorylation of GSK3β does occur after ligation of OX40 (ref. 26 and data not shown). But, in effector Th2 cells, we failed to confirm a role for Akt in OX40-controlled IL-4, in that retroviral expression of dominant negative Akt had no effect on IL-4 transcription (data not shown). This finding implies that, at least with effector cells, nuclear import of NFATc1 might be the principal function controlled by OX40 or that OX40 can control both import and export, depending on the stage of differentiation of the T cell, or perhaps that signaling pathways other than through Akt are active. In support of regulating nuclear import, a peptide that inhibits NFAT–CN interaction suppressed OX40-induced IL-4. However, further experiments will be needed to fully understand how TCR, CD28, and OX40 signals synergize to control NFATc1 nuclear accumulation and the upstream signaling pathways that are involved.
In addition to CD28 and OX40 controlling Th2 differentiation, two other noncytokine receptors have been implicated in this process. Inducible costimulator (ICOS)-deficient mice are impaired in generating asthmatic and nematode-induced Th2 responses (46, 47), but there is some controversy regarding the target of ICOS action. Dong and colleagues (48) reported that ICOS can induce IL-4-independent early IL-4 transcription, also targeting NFATc1. However, Abe and colleagues (49) reported normal initial IL-4 transcription and nuclear NFATc1 levels in ICOS-deficient TCR transgenic T cells and suggested that ICOS enhances IL-4R sensitivity and control of STAT6 phosphorylation and GATA-3 induction. Notch signaling has also been shown to regulate IL-4R/STAT6-independent IL-4 (50). After ligand binding, Notch undergoes proteolytic processing by γ-secretase to release its intracellular signaling peptide, which translocates to the nucleus. Flavell and colleagues (50) proposed that Notch regulates the IL-4 gene by binding to RBPJκ sites in the IL-4 enhancer, theoretically separating its action from CD28, OX40, and ICOS. Future studies will be important to truly understand the relationship between these molecules and the molecular basis for their apparent commonality in control of IL-4.
In conclusion, we show that OX40 signals are intimately associated with early accumulation of high levels of NFATc1 in the nucleus of recently activated naïve T cells and that this controls initial IL-4R-independent transcription of IL-4. Collectively, these data highlight the stringent control of IL-4 production by naïve T cells and its regulation by the combined action of several membrane receptors.
Materials and Methods
Mice.
The studies reported here conform to the principles outlined by the Animal Welfare Act and the National Institutes of Health guidelines for use of animals in research. All experiments were done in compliance with the regulations and guidelines of the Association for Assessment of Laboratory Animal Care. BL/6 and B6.PLThy1a (Thy1.1) mice were from The Jackson Laboratory. OT-II TCR transgenic mice, bred on the BL/6 background, were a gift from W. Heath (The Walter and Elizabeth Hall Institute, Melbourne) and used as a source of Vβ5/Vα2/Thy1.2 CD4 T cells responsive to the peptide OVA-323–339. OX40−/− OT-II mice were produced by intercrossing with OX40−/− mice on the BL/6 background.
Peptides, Abs, and Cytokines.
OVA-323–339, VIVIT (RRRRRRRRRRR-GGG-MAGPVIVITGPHEE), and VEET (RRRRRRRRRRR-GGG-MAGPPHIVEETGPHVI) were synthesized by A&A Laboratories (San Diego). Abs against CD3 (145–2C11), CD28 (37N51), OX40 (OX86), OX40L (RM134L), IFN-γ (XMG1.2), and IL-4 (11B11) were produced in-house. Abs against IL-2R-α (PC61.5), IL-2R-β (TM-b1), and OX40L-PE were from eBioscience (San Diego, CA). GATA-3 (HG3–31), NFATc1 (7A6), NFATc2 (4G6-G5), and lamin B (M20) were from Santa Cruz Biotechnology. STAT5A was from R & D Systems. Phospho-STAT5 was from Cell Signaling Technology (Beverly, MA). Actin (C4) was from ICN. CD4-FITC, CD11b-FITC, CD11c-APC, biotinylated OX40, and streptavidin-PE were from BD Pharmingen. IL-2 and IL-4 were from PeproTech (Rocky Hill, NJ).
T Cells and APCs.
CD4 T cells were purified from spleen and lymph nodes. Briefly, whole cells, passed over nylon columns, were subjected to complement lysis using antibodies to CD8 (3.155), heat-stable antigen (J11D), class II MHC (M5/114, Y17, and CA-4.A12), B cells (RA3.6B2), macrophage (M1/70), natural killer cells (PK136), and dendritic cells (33D1). Any residual APCs and any in vivo-activated T cells were removed by isolating high-density cells spun through a Percoll gradient. Purified T cell populations were phenotypically naïve and contained ≈1% CD44highCD62low T cells (Fig. 9, which is published as supporting information on the PNAS web site). APCs were made by depleting T cells with complement and antibodies to Thy1.2 (F7D5 and HO.13.4), CD4 (RL172.4), and CD8 (3.155) and were irradiated with 3,000 rad before use.
Ab-Coated Beads.
Ten million streptavidin-coated beads (CELLection Biotin Binder Kit; Invitrogen) were incubated with biotinylated anti-CD3 (1 μg)/control rat IgG (2 μg), anti-CD3 (1 μg)/anti-OX40 (2 μg), or control rat IgG (3 μg) at 30 μl total volume for 30 min with a mild vortex.
T Cell Culture.
Cells were cultured in RPMI medium 1640 (Invitrogen) with penicillin, streptomycin, glutamine, 2-mercaptoethanol, and 7% FCS (Omega Scientific, Tarzana, CA). CD4 T cells (5 × 105 cells per ml) were stimulated with 5 μg/ml plate-bound anti-CD3, 5 μg/ml soluble anti-CD28. For Th2 polarization, CD4 T cells were cultured with anti-CD3/CD28, 10 μg/ml anti-IFN-γ (XMG1.2), 10 ng/ml IL-2, and 10 ng/ml IL-4. Cells were initially stimulated for 3 days then transferred to new plates containing new Th2-skewed culture media and expanded for an additional 3 days without anti-CD3 stimulation. For stimulation of OT-II CD4 T cells, cultures were plated at 5 × 105 cells per ml with 6 × 106 cells per ml T cell-depleted splenic APCs and various concentrations of OVA-323–339. At day 3, whole cells were transferred to plates containing new media and expanded for an additional 4 days. Equivalent numbers of live effector cells were restimulated at 1.5 × 106 cells per ml with 1.5 × 107 cells per ml splenic APCs and 20 μM OVA-323–339 to assess differentiation into Th2/Th1 phenotypes. For blocking experiments, anti-OX40L (50 μg/ml), anti-IL-4 (10 μg/ml), anti-IFN-γ (10 μg/ml), anti-IL-2R-α plus -β (5 μg/ml each), LY (Calbiochem; 3 μM), CsA (Calbiochem; 0.1 μM), VIVIT, or control VEET peptide (25 μM) were added to the OT-II cell cultures, or VIVIT (25 μM), VEET (25 μM), LY (20 μM), CsA (1 μM), or cycloheximide (CHX, 10 μM; Calbiochem) were added to the Th2 cell cultures. For anti-OX40 stimulation of activated primary CD4 T cells, 200 μl of anti-OX40 in PBS (0.5 mg/ml) was added to polystyrene 5-ml round-bottom tubes and incubated overnight at 4°C. Twenty million CD4 T cells in 0.8 ml of culture medium were directly added to the tubes and cultured for 4 h.
In Vivo Priming of CD4 T Cells.
Nonirradiated syngeneic B6.PL recipient mice (Thy1.1) were injected i.v. with 3 × 106 naïve Vβ5/Vα2/Thy1.2 CD4 T cells from wild-type or OX40−/− OT-II transgenic donors. One day after cell transfer, mice were immunized s.c. with 10 μg of OVA protein (Sigma) and 2 mg of aluminum hydroxide (ImjectAlum; Pierce) in PBS. Inguinal and periaortic draining lymph node cells were harvested 2 and 4 days after immunization. FACS staining with anti-Thy1.2-FITC was used to enumerate responding T cells, and OT-II cells were purified by magnetic cell sorting Thy1.2 microbeads (Miltenyi Biotech, Auburn, CA).
ELISA.
Culture supernatants were assessed for cytokine content by enhanced sandwich ELISA protocols: JES6–1A12 and biotin-JES6–5H4 for IL-2, 11B11 and biotin-BVD6–24G2 for IL-4, and R46A-2 and biotin-XMG1.2 for IFN-γ (BD Pharmingen). ExtrAvidin, biotinylated monoclonal anti-avidin (WC19.10), and ExtrAvidin-peroxidase conjugate were from Sigma.
Real-Time RT-PCR.
Quantitative RT-PCR was performed by using SYBR green I dye and an ABI GeneAmp 5700 sequence BioDetector (PE Biosystems, Foster City, CA) according to the manufacturer’s instructions. Total RNA was extracted by using TRIzol (Invitrogen) and reverse transcribed by using oligo(dT)12–18 (Super Script II; Invitrogen). Each transcript was analyzed concurrently on the same plate with HPRT, and levels of mRNA were normalized to HPRT abundance. Sequence-specific primers for murine IL-4 (forward primer: 5′-AGATCATCGGCATTTTGAACG-3′; reverse primer: 5′-TTTGGCACATCCATCTCCG-3′); IFN-γ (forward primer: 5′-GGATGCATTCATGAGTATTGC-3′; reverse primer: 5′-CCTTTTCCGCTTCCTGAGG-3′); HPRT (forward primer: 5′-CTGGTGAAAAGGACCTCTCG-3′; reverse primer: 5′-TGAAGTACTCATTATAGTCAAGGGCA-3′) were used.
Western Blotting.
Nuclear extracts were prepared by using NE-PER Nuclear and Cytoplasmic Extraction Reagent (Pierce). Protein content was determined by bicinchoninic acid assay (Pierce). The extract was resolved on NuPAGE Novex 4–12% [bis(2-hydroxyethyl)amino]tris(hydroxymethyl)methane gel (Invitrogen). After transfer to poly(vinylidene difluoride), the membranes were blotted with primary antibodies, then the protein levels were visualized by enhanced chemiluminescence (PerkinElmer) by using horseradish peroxidase-conjugated anti-mouse IgG, anti-rabbit IgG (Jackson ImmunoResearch), and anti-goat IgG (Bio-Rad).
Supplementary Material
Acknowledgments
We thank Mary Cheng, Kate Harms, and Xiaohong Tang for technical assistance. This work was supported by National Institutes of Health Grants AI49453 and AI50498 (to M.C.). This is manuscript no. 679 from the La Jolla Institute for Allergy and Immunology.
Abbreviations
- Ab
antibody
- Ag
antigen
- APC
antigen-presenting cell
- CN
calcineurin
- CsA
cyclosporin A
- HPRT
hypoxanthine phosphoribosyltransferase
- IL-4R
IL-4 receptor
- LY
LY294002
- NFAT
nuclear factor of activated T cells
- OVA
ovalbumin
- OX40L
OX40 ligand
- PI3K
phosphatidylinositol 3-kinase
- TCR
T cell antigen receptor
- Th1
T cell helper type 1
- Th2
T cell helper type 2.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Moser M., Murphy K. M. Nat. Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 2.Jankovic D., Kullberg M. C., Hieny S., Caspar P., Collazo C. M., Sher A. Immunity. 2002;16:429–439. doi: 10.1016/s1074-7613(02)00278-9. [DOI] [PubMed] [Google Scholar]
- 3.Kurata H., Lee H. J., O’Garra A., Arai N. Immunity. 1999;11:677–688. doi: 10.1016/s1074-7613(00)80142-9. [DOI] [PubMed] [Google Scholar]
- 4.Zhu J., Min B., Hu-Li J., Watson C. J., Grinberg A., Wang Q., Killeen N., Urban J. F., Guo L., Paul W. E. Nat. Immunol. 2004;5:1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 5.Pai S. Y., Truitt M. L., Ho I. C. Proc. Natl. Acad. Sci. USA. 2004;101:1993–1998. doi: 10.1073/pnas.0308697100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seder R. A., Paul W. E., Dvorak A. M., Sharkis S. J., Kagey-Sobotka A., Niv Y., Finkelman F. D., Barbieri S. A., Galli S. J., Plaut M. Proc. Natl. Acad. Sci. USA. 1991;88:2835–2839. doi: 10.1073/pnas.88.7.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshimoto T., Paul W. E. J. Exp. Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamogawa Y., Minasi L. A., Carding S. R., Bottomly K., Flavell R. A. Cell. 1993;75:985–995. doi: 10.1016/0092-8674(93)90542-x. [DOI] [PubMed] [Google Scholar]
- 9.Croft M., Swain S. L. J. Immunol. 1995;154:4269–4282. [PubMed] [Google Scholar]
- 10.Demeure C. E., Yang L. P., Byun D. G., Ishihara H., Vezzio N., Delespesse G. Eur. J. Immunol. 1995;25:2722–2725. doi: 10.1002/eji.1830250950. [DOI] [PubMed] [Google Scholar]
- 11.Noben-Trauth N., Hu-Li J., Paul W. E. J. Immunol. 2000;165:3620–3625. doi: 10.4049/jimmunol.165.7.3620. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z., Liu Q., Hamed H., Anthony R. M., Foster A., Finkelman F. D., Urban J. F., Jr., Gause W. C. J. Immunol. 2005;174:2242–2249. doi: 10.4049/jimmunol.174.4.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitz J., Thiel A., Kuhn R., Rajewsky K., Muller W., Assenmacher M., Radbruch A. J. Exp. Med. 1994;179:1349–1353. doi: 10.1084/jem.179.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkelman F. D., Morris S. C., Orekhova T., Mori M., Donaldson D., Reiner S. L., Reilly N. L., Schopf L., Urban J. F., Jr. J. Immunol. 2000;164:2303–2310. doi: 10.4049/jimmunol.164.5.2303. [DOI] [PubMed] [Google Scholar]
- 15.Gramaglia I., Weinberg A. D., Lemon M., Croft M. J. Immunol. 1998;161:6510–6517. [PubMed] [Google Scholar]
- 16.Croft M. Nat. Rev. Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 17.Flynn S., Toellner K. M., Raykundalia C., Goodall M., Lane P. J. Exp. Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohshima Y., Yang L. P., Uchiyama T., Tanaka Y., Baum P., Sergerie M., Hermann P., Delespesse G. Blood. 1998;92:3338–3345. [PubMed] [Google Scholar]
- 19.Ito T., Wang Y. H., Duramad O., Hori T., Delespesse G. J., Watanabe N., Qin F. X., Yao Z., Cao W., Liu Y. J. J. Exp. Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jember A. G., Zuberi R., Liu F. T., Croft M. J. Exp. Med. 2001;193:387–392. doi: 10.1084/jem.193.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akiba H., Miyahira Y., Atsuta M., Takeda K., Nohara C., Futagawa T., Matsuda H., Aoki T., Yagita H., Okumura K. J. Exp. Med. 2000;191:375–380. doi: 10.1084/jem.191.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ekkens M. J., Liu Z., Liu Q., Whitmire J., Xiao S., Foster A., Pesce J., VanNoy J., Sharpe A. H., Urban J. F, et al. J. Immunol. 2003;170:384–393. doi: 10.4049/jimmunol.170.1.384. [DOI] [PubMed] [Google Scholar]
- 23.Ranger A. M., Hodge M. R., Gravallese E. M., Oukka M., Davidson L., Alt F. W., de la Brousse F. C., Hoey T., Grusby M., Glimcher L. H. Immunity. 1998;8:125–134. doi: 10.1016/s1074-7613(00)80465-3. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida H., Nishina H., Takimoto H., Marengere L. E., Wakeham A. C., Bouchard D., Kong Y. Y., Ohteki T., Shahinian A., Bachmann M., et al. Immunity. 1998;8:115–124. doi: 10.1016/s1074-7613(00)80464-1. [DOI] [PubMed] [Google Scholar]
- 25.Zhu J., Cote-Sierra J., Guo L., Paul W. E. Immunity. 2003;19:739–748. doi: 10.1016/s1074-7613(03)00292-9. [DOI] [PubMed] [Google Scholar]
- 26.Song J., Salek-Ardakani S., Rogers P. R., Cheng M., Van Parijs L., Croft M. Nat. Immunol. 2004;5:150–158. doi: 10.1038/ni1030. [DOI] [PubMed] [Google Scholar]
- 27.Todd M. D., Grusby M. J., Lederer J. A., Lacy E., Lichtman A. H., Glimcher L. H. J. Exp. Med. 1993;177:1663–1674. doi: 10.1084/jem.177.6.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aramburu J., Yaffe M. B., Lopez-Rodriguez C., Cantley L. C., Hogan P. G., Rao A. Science. 1999;285:2129–2133. doi: 10.1126/science.285.5436.2129. [DOI] [PubMed] [Google Scholar]
- 29.Noguchi H., Matsushita M., Okitsu T., Moriwaki A., Tomizawa K., Kang S., Li S. T., Kobayashi N., Matsumoto S., Tanaka K., et al. Nat. Med. 2004;10:305–309. doi: 10.1038/nm994. [DOI] [PubMed] [Google Scholar]
- 30.Rogers P. R., Song J., Gramaglia I., Killeen N., Croft M. Immunity. 2001;15:445–455. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 31.Ho I. C., Glimcher L. H. Cell. 2002;109:S109–S120. doi: 10.1016/s0092-8674(02)00705-5. [DOI] [PubMed] [Google Scholar]
- 32.Hosken N. A., Shibuya K., Heath A. W., Murphy K. M., O’Garra A. J. Exp. Med. 1995;182:1579–1584. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfeiffer C., Stein J., Southwood S., Ketelaar H., Sette A., Bottomly K. J. Exp. Med. 1995;181:1569–1574. doi: 10.1084/jem.181.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tao X., Constant S., Jorritsma P., Bottomly K. J. Immunol. 1997;159:5956–5963. [PubMed] [Google Scholar]
- 35.Rogers P. R., Croft M. J. Immunol. 1999;163:1205–1213. [PubMed] [Google Scholar]
- 36.Hodge M. R., Ranger A. M., de la Brousse F. C., Hoey T., Grusby M. J., Glimcher L. H. Immunity. 1996;4:397–405. doi: 10.1016/s1074-7613(00)80253-8. [DOI] [PubMed] [Google Scholar]
- 37.Brogdon J. L., Leitenberg D., Bottomly K. J. Immunol. 2002;168:3825–3832. doi: 10.4049/jimmunol.168.8.3825. [DOI] [PubMed] [Google Scholar]
- 38.Kiani A., Viola J. P., Lichtman A. H., Rao A. Immunity. 1997;7:849–860. doi: 10.1016/s1074-7613(00)80403-3. [DOI] [PubMed] [Google Scholar]
- 39.Porter C. M., Clipstone N. A. J. Immunol. 2002;168:4936–4945. doi: 10.4049/jimmunol.168.10.4936. [DOI] [PubMed] [Google Scholar]
- 40.Grogan J. L., Mohrs M., Harmon B., Lacy D. A., Sedat J. W., Locksley R. M. Immunity. 2001;14:205–215. doi: 10.1016/s1074-7613(01)00103-0. [DOI] [PubMed] [Google Scholar]
- 41.Avni O., Lee D., Macian F., Szabo S. J., Glimcher L. H., Rao A. Nat. Immunol. 2002;3:643–651. doi: 10.1038/ni808. [DOI] [PubMed] [Google Scholar]
- 42.Ansel K. M., Lee D. U., Rao A. Nat. Immunol. 2003;4:616–623. doi: 10.1038/ni0703-616. [DOI] [PubMed] [Google Scholar]
- 43.Rulifson I. C., Sperling A. I., Fields P. E., Fitch F. W., Bluestone J. A. J. Immunol. 1997;158:658–665. [PubMed] [Google Scholar]
- 44.Dong C., Yang D. D., Wysk M., Whitmarsh A. J., Davis R. J., Flavell R. A. Science. 1998;282:2092–2095. doi: 10.1126/science.282.5396.2092. [DOI] [PubMed] [Google Scholar]
- 45.Yamashita M., Katsumata M., Iwashima M., Kimura M., Shimizu C., Kamata T., Shin T., Seki N., Suzuki S., Taniguchi M., et al. J. Exp. Med. 2000;191:1869–1879. doi: 10.1084/jem.191.11.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kopf M., Coyle A. J., Schmitz N., Barner M., Oxenius A., Gallimore A., Gutierrez-Ramos J. C., Bachmann M. F. J. Exp. Med. 2000;192:53–61. doi: 10.1084/jem.192.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonzalo J. A., Tian J., Delaney T., Corcoran J., Rottman J. B., Lora J., Al-garawi A., Kroczek R., Gutierrez-Ramos J. C., Coyle A. J. Nat. Immunol. 2001;2:597–604. doi: 10.1038/89739. [DOI] [PubMed] [Google Scholar]
- 48.Nurieva R. I., Duong J., Kishikawa H., Dianzani U., Rojo J. M., Ho I., Flavell R. A., Dong C. Immunity. 2003;18:801–811. doi: 10.1016/s1074-7613(03)00144-4. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe M., Watanabe S., Hara Y., Harada Y., Kubo M., Tanabe K., Toma H., Abe R. J. Immunol. 2005;174:1989–1996. doi: 10.4049/jimmunol.174.4.1989. [DOI] [PubMed] [Google Scholar]
- 50.Amsen D., Blander J. M., Lee G. R., Tanigaki K., Honjo T., Flavell R. A. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.