Abstract
Inherited mutations in BRCA1 and BRCA2 lead to significantly increased risks of breast and ovarian cancer. We used epidemiologic methods to evaluate the relative risks of breast cancer vs. ovarian cancer among women of Ashkenazi Jewish ancestry with inherited mutations in BRCA1 or BRCA2. The cancer of a family's index case (i.e., breast cancer vs. ovarian cancer) was significantly associated with site-specific risks of cancer in relatives known to carry mutations in BRCA1 or BRCA2. Specifically, breast cancer risks were higher among relatives of breast cancer index cases compared with relatives of ovarian cancer index cases [hazard ratio (HR) = 3.0, P < 0.001 for BRCA1 carriers and HR = 4.8, P = 0.017 for BRCA2 carriers], and ovarian cancer risks were higher among relatives of ovarian cancer index cases compared with relatives of breast cancer index cases (HR = 7.2, P = 0.001 for BRCA1 carriers and HR = 15.8, P = 0.018 for BRCA2 carriers). Breast and ovarian cancer risks also increased with more recent year of birth. For each later decade of birth, risk increased 1.2-fold (P = 0.03). Effects of cancer site of the index case and of birth cohort were independent. These results suggest that both genetic and nongenetic factors modify cancer risks among BRCA1 and BRCA2 mutation carriers, and that genetic modifiers and other familial factors may influence risk specifically for either breast or ovarian cancer.
Keywords: breast cancer, ovarian cancer, penetrance, hereditary cancer
In the decade since BRCA1 (MIM 113705) and BRCA2 (MIM 600185) were identified as susceptibility genes for breast and ovarian cancer, testing of these genes has become part of clinical practice, and thousands of women worldwide have been identified as carriers of mutations in BRCA1 or BRCA2. Women with cancer-predisposing mutations are offered intensive surveillance for early cancer detection and/or preventive measures. Although prevention of breast and ovarian cancer in carriers is highly effective, it primarily involves prophylactic surgery: oophorectomy and risk-reduction mastectomy (refs. 1 and 2 and reviewed in ref. 3). In considering the best course of action for mutation carriers, it is essential to have accurate estimates of possible outcomes that include all genetic and nongenetic factors influencing risk.
Breast cancer risks associated with BRCA1 and BRCA2, and ovarian cancer risk associated with BRCA1, have been shown to be higher in more recent birth cohorts (4–6). These secular (time) trends likely reflect nongenetic influences because risk changed within families among individuals with the same mutations. Meta-analysis further suggested that cancer risk among BRCA1 and BRCA2 carriers was influenced by the site of cancer (i.e., breast cancer or ovarian cancer) in the index case (5). This observation suggested that genetic modifiers, alleles of genes other than BRCA1 or BRCA2, might affect outcome in carriers of BRCA1 or BRCA2 mutations.
The goal of this study was to investigate the influences of cancer site in the index case and of birth cohort on breast and ovarian cancer risks among BRCA1 and BRCA2 mutation carriers. We ascertained Ashkenazi Jewish index cases with breast and/or ovarian cancer who carried one of the three mutations common in this population: BRCA1.185delAG, BRCA1.5382insC, or BRCA2.6174delT. We determined mutation status of all available relatives by molecular genetic testing. We compared breast cancer risks and ovarian cancer risks for women with BRCA1 or BRCA2 mutations ascertained through index cases with breast cancer vs. those ascertained through index cases with ovarian cancer. We also compared cancer risks among women with BRCA1or BRCA2 mutations of different birth cohorts.
Results
One of the three ancient Ashkenazi Jewish cancer-predisposing mutations in BRCA1 or BRCA2 was identified in 498 Israeli women with breast or ovarian cancer. All available adult female relatives of 312 cases were enrolled and genotyped, yielding 458 relatives with one of the three mutations: 233 BRCA1.185delAG, 80 BRCA1.5382insC, and 145 BRCA2.6174delT (Table 1). These included 85 relatives of index cases ascertained through consecutive, hospital-based series of breast or ovarian cancer and 373 relatives of index cases ascertained at cancer genetics clinics.
Table 1.
Numbers of families and adult female relatives with BRCA1 or BRCA2 mutations
| Mutant gene | Cancer site of index case | Country of residence | Families | Relatives with mutations |
|---|---|---|---|---|
| BRCA1 | Breast | Israel | 130 | 180 |
| Breast | U.S. | 68 | 154 | |
| Ovary | Israel | 85 | 133 | |
| Total | 253 | 467 | ||
| BRCA2 | Breast | Israel | 56 | 74 |
| Breast | U.S. | 37 | 58 | |
| Ovary | Israel | 48 | 71 | |
| Total | 141 | 203 |
Risks of breast or ovarian cancer to women with BRCA1 or BRCA2 mutations in the Israeli cohort were significantly associated with the cancer site in the index case. Ovarian cancer risk was higher among relatives of ovarian cancer index cases than among relatives of breast cancer index cases (hazard ratio (HR) = 11.9, P < 0.001), controlling for year of birth, mutation, and method of ascertainment (consecutive series vs. clinic referral). Conversely, breast cancer risk was higher among relatives of breast cancer index cases than among relatives of ovarian cancer index cases (HR = 3.1, P < 0.001), controlling for year of birth, mutation, and method of ascertainment.
We were concerned that an apparent effect of cancer site in the index case could be an artifact of ascertainment bias, if familial risk would more likely be recognized when cancer type was concordant among relatives. To address this issue, we used information from the New York Breast Cancer Study (NYBCS), in which all relatives were ascertained through an index case with breast cancer (4). Of 107 families with BRCA1 or BRCA2 mutations in the NYBCS, 11 families included both breast cancer in the index case and ovarian cancer in the mother of the index case. Six of these 11 families (55%) included additional relatives with ovarian cancer. In contrast, among the 98 NYBCS families in which the index case's mother did not develop ovarian cancer, 14 families (14%) included additional relatives with ovarian cancer. Incidence of ovarian cancer in the NYBCS families was therefore associated with ovarian cancer in the mother (P = 0.018). Clustering of ovarian cancer in a subset of NYBCS families, all of whom were ascertained for breast cancer, suggests that similar clustering in Israel was not likely to be an artifact of self-selection of ovarian cancer families. In subsequent analyses, data from Israel was analyzed with data from New York, controlling for country of residence (see Materials and Methods).
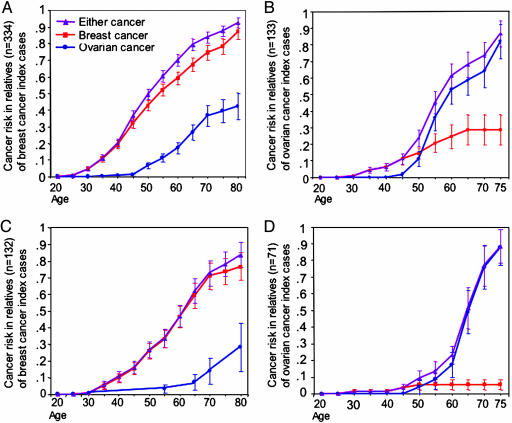
Cumulative risks of breast cancer, ovarian cancer, and cancer at either site for relatives with BRCA1 or BRCA2 mutations, stratified by gene and cancer site of the index case, are shown in Fig. 1. Combined risks of developing either breast or ovarian cancer did not differ by cancer site of the index case. By age 75, combined cancer risk for BRCA1 carriers was 0.87 (SE 0.03) among relatives of breast cancer index cases and 0.87 (SE 0.08) among relatives of ovarian cancer index cases, and for BRCA2 carriers combined risks were 0.78 (SE 0.08) and 0.88 (SE 0.10), respectively. (Note that these risks do not reflect population-based penetrances, because this cohort includes families ascertained both by means of consecutive series and through clinic referrals.)
Fig. 1.
Cumulative risks of breast cancer, ovarian cancer, and cancer of either site for relatives with BRCA1 or BRCA2 mutations, based on cancer site in the index case. (A) Women with BRCA1 mutations, ascertained through index cases with breast cancer. (B) Women with BRCA1 mutations, ascertained through index cases with ovarian cancer. (C) Women with BRCA2 mutations, ascertained through index cases with breast cancer. (D) Women with BRCA2 mutations, ascertained through index cases with ovarian cancer. SEs are shown for risk estimates at each age. For women with mutations in either BRCA1 or BRCA2, risks of breast cancer are higher for relatives of index cases with breast cancer, whereas risks of ovarian cancer are higher for relatives of index cases with ovarian cancer. Ascertainment of families is from consecutive series of cases in Israel and the U.S (4) and from genetics clinics in Israel, so absolute risks may not represent those for families with the same mutations in the general population.
However, cancer site-specific risks differed significantly for relatives of breast cancer patients vs. relatives of ovarian cancer patients (Fig. 1). Ovarian cancer risks were higher among relatives of index cases with ovarian cancer, and breast cancer risks were higher among relatives of index cases with breast cancer. Cancer risk in carriers reflected a complex interaction between cancer site of the index case and the age of the woman at risk. Before age 50, all mutation carriers, regardless of cancer site of the index case, were at higher risk for breast cancer than for ovarian cancer. After age 50, breast cancer risk remained higher than ovarian cancer risk among relatives of index cases with breast cancer (Fig. 1 A and C), but ovarian cancer risk was higher than breast cancer risk among relatives of ovarian cancer index cases (Fig. 1 B and D). Hazard ratios summarizing these comparisons are indicated in Table 2. Effects of cancer site of the index cases were similar for relatives with BRCA1 or BRCA2 mutations. Effects of cancer site in the index case were significant for women with mutations in either BRCA1 or BRCA2 and for risks of either breast cancer or ovarian cancer.
Table 2.
Effects of cancer site in index cases on cancer risks among relatives with mutations
| Mutant gene | Cancer site of index case | Cancer risk in relatives | Hazard ratios (95% C.I.)* | P value |
|---|---|---|---|---|
| BRCA1 | Breast vs. ovarian | Breast | 3.0 (1.7–5.2) | <0.001 |
| Ovarian vs. breast | Ovary | 7.2 (2.1–24.1) | 0.001 | |
| BRCA2 | Breast vs. ovarian | Breast | 4.9 (1.3–17.5) | 0.02 |
| Ovarian vs. breast | Ovary | 15.8 (1.6–155) | 0.02 |
*Hazard ratios and 95% confidence intervals (C.I.) controlled for mutation, year of birth, method of ascertainment (consecutive series vs. clinic referral), and place of residence (Israel vs. U.S.).
It is particularly important to identify a subset of women at especially high risk of ovarian cancer, because ovarian cancer is more lethal than breast cancer and is less frequent in the general population. Being a carrier relative of an index case with ovarian cancer was associated both with absolute risk of ovarian cancer (Fig. 1) and with increased risk of ovarian cancer vs. breast cancer both among families ascertained in consecutive series (HR = 4.7, P = 0.045) and among families ascertained at genetics clinics (HR = 20.8, P = 0.003). Indeed, among BRCA2 carriers who are relatives of index cases with ovarian cancer, the risk of breast cancer was no higher than the general population, whereas the risk of ovarian cancer was >0.80 by age 75 (Fig. 1D).
We also investigated whether the cancer site effect could be an artifact of ascertainment or testing bias. That is, because we analyzed only carriers confirmed by molecular testing, would relatives with the same type of cancer as the index case more frequently consent to testing? To evaluate this possibility, we estimated risks based on all first- and second-degree relatives in the Israeli families, whether tested and not tested. Relatives who were not tested were assigned a Bayesian probability of being carriers based on their position in the pedigree, disease status, and age. The a priori probability was based on position in the pedigree (e.g., 0.5 for the first-degree relative of a known carrier), and the conditional probability was based on age-specific risks for breast and ovarian cancer among Israeli carriers in this study and age-specific rates of breast and ovarian cancer in the Israeli population (Israel Cancer Registry: www.health.gov.il/icr/; Statistical Abstract of Israel: http://www1.cbs.gov.il/reader/shnatonenew_site.htm). This analysis also showed a significantly higher breast cancer risk for relatives of breast cancer index cases [HR = 1.4, 95% confidence interval (C.I.) 1.0–2.0, P = 0.05] and a significantly higher ovarian cancer risk for relatives of ovarian cancer index cases (HR = 3.6, 95% C.I. 1.9–6.8, P < 0.001), suggesting that the effect of cancer site of the index case is not the result of a testing bias.
We also evaluated the effect of birth year on risks among carriers of BRCA1 and BRCA2 mutations. Birth cohort has been shown to be associated with breast cancer risk among women with BRCA1 or BRCA2 mutations, with higher risks among women born in later years (4–6). A very similar effect for breast cancer was observed in the Israeli subjects. For carriers of either BRCA1 or BRCA2 mutations, age-specific breast cancer risks and age-specific ovarian cancer risks were higher among women born in later years. We found that, for each later decade of birth, cancer risk increased 1.2-fold (P = 0.03), controlled for mutation, ascertainment method, and cancer site of index case.
Discussion
The expression of any disease caused by mutations in a major gene may be modified, in principle, by other genes and by nongenetic factors. We suggest such effects for expression of breast and ovarian cancer among women with mutations in BRCA1 or BRCA2. Previous studies demonstrated that breast and ovarian cancer risks associated with mutations in BRCA1 and BRCA2 are high, as expected, for a monogenic Mendelian trait (3, 4, 7). Furthermore, among women with mutations in these genes, risk may be modified by genetic or nongenetic factors.
The effect of birth cohort on cancer risk offers the most compelling indication of nongenetic influences on cancer risk among BRCA1 and BRCA2 carriers. In several cohorts, age-specific breast cancer risks associated with BRCA1 and BRCA2 have been shown to be higher among women born more recently (4, 5). A similar effect has been observed for ovarian cancer risk among BRCA1 carriers (6). Our results confirm these observations.
The effect of cancer site in the index case on site-specific cancer risks among carrier relatives is most consistent with modifying genetic influences among BRCA1 and BRCA2 carriers. Familial clustering of site-specific cancer outcomes has been previously suggested for breast cancer based on family histories (5). In a small group of BRCA1 families ascertained through incident ovarian cancer, female relatives were at higher risk for ovarian cancer than for breast cancer (8). Based on direct genotyping of relatives, we observed such clustering for both BRCA1 and BRCA2 carriers, and for both breast and ovarian cancer. The cancer site effect appears robust, having been observed among carriers from two different countries (Israel and the U.S.) and using different ascertainment schemes (referral cases and consecutive breast cancer index cases).
A familial cancer-site effect could theoretically result from either shared genetic modifiers or shared environmental risk factors. However, in multiple-generation families with recent immigration histories, like the families in this study, intrafamilial differences in environment are large even within one generation. Shared family environment is therefore less likely than shared genes to be responsible for familial clustering of cancer site, but nongenetic familial effects cannot be ruled out.
The effect of cancer in the index case can probably be generalized to families with other mutations in BRCA1, because the effect was similar in relatives with BRCA1.185delAG and relatives with BRCA1.5382insC. However, generalizing from families with BRCA2.6174delT to families with other BRCA2 mutations is more complex, because 6174delT is located in the 3.6-kb “ovarian cancer cluster region” (OCCR) of BRCA2 (9). Women with mutations in this region of BRCA2 are at higher risk of ovarian cancer than are women with mutations in the 5′ or 3′ regions of the gene. An effect of ovarian cancer in the index case might be more detectable in families with mutations in the OCCR of BRCA2, and, in turn, such BRCA2 mutations might confer higher risk because of different interaction with modifiers.
Genes modifying breast or ovarian cancer risk due to BRCA1 and BRCA2 have been proposed (reviewed in ref. 10). Our results suggest that genetic modifiers would influence risks for either breast or ovarian cancer, but not both. Site-specific effects have been observed for several modifier genes. Rare alleles of the oncogene HRAS1 increase risk for ovarian cancer, but not breast cancer, among BRCA1 carriers (11). A polymorphism in the 5′ UTR of the RAD51 gene (135 G→C, rs1801320) increases risk for breast cancer, but not ovarian cancer, among BRCA2 carriers (12–14). The magnitude of the RAD51 effect (HR = 3.2–5.5) is consistent with that observed for breast cancer in relatives of breast cancer index cases. The length of the polyglutamine repeat of the androgen receptor has not consistently been associated with risk among BRCA1 carriers (15–17), but studies reporting positive effects found opposite associations for breast and ovarian cancer, with long repeats associated with younger age at breast cancer diagnosis (18) and short repeats associated with younger age at ovarian cancer diagnosis (19). The length of the polyglutamine repeat in the AIB1/NCOA3 gene has been associated with younger age at breast cancer diagnosis among BRCA1 carriers, but did not affect ovarian cancer diagnosis (20, 21). The magnitudes of effects due to cancer site in the index case are consistent with significant effects of genetic modifiers. Because such effects cluster in families, modifiers are most likely to be identified by using family-based studies, not only case-control designs.
Although the influence of cancer of an index case on relatives' risk of ovarian vs. breast cancer has been reported in other studies (5, 8), the magnitude of the effect in this series is considerably greater than we anticipated. With the sample sizes of this study, confidence intervals are quite wide, so lower limits could reflect the biological effect in some populations. It will be interesting to learn whether effects are similar in population-based studies, in populations with different background rates of breast and ovarian cancer, and for women with other BRCA2 mutations. Our results underscore the complexity of genetics for BRCA1- and BRCA2-associated cancers, as additional factors are found to influence risk. Clinical perception of risk among BRCA1 and BRCA2 carriers reflects an averaging of many families, which may have different risk profiles. In all carriers, breast cancer surveillance and/or prevention are paramount in younger women (<50 yr). However, among BRCA2 carriers and all still-unaffected carriers identified at older ages, our results suggest that it may be justified to focus preventive measures more specifically on ovarian cancer or breast cancer, based on family history.
Materials and Methods
Israeli Subjects.
The Israel Breast Cancer Genetics Consortium was formed to evaluate families known to harbor BRCA1 or BRCA2 mutations common in the Jewish population. Participating centers were Sheba Tel Hashomer, Sourasky-Ichilov, and Rabin-Beilinson Medical Centers in the Tel Aviv metropolitan area, Shaare Zedek Medical Center in Jerusalem, and Rambam Medical Center in Haifa. Together, these centers carry out most BRCA1 and BRCA2 testing in Israel. All participants gave written informed consent. The study was approved by the institutional review boards of each center and by the Israeli National Genetics Committee.
Index cases were female breast or ovarian cancer patients of Ashkenazi Jewish origin, found to carry one of the three ancient BRCA1 or BRCA2 mutations. Risk was evaluated among relatives of these index cases who also carried the familial mutation. Index cases and relatives were ascertained from January 1996 to December 2003. At all centers, pedigrees were drawn to include as many family members as possible. Relatives were contacted and asked to join the study. Clinical information collected for each participant included year of birth, age at last follow-up, age at cancer diagnoses, age at prophylactic oophorectomy, and age at prophylactic mastectomy.
Because there are only three common BRCA1 and BRCA2 mutations among Ashkenazi women with breast or ovarian cancer, screening these genes is more widespread in Israel than in the United States, and referral for cancer genetic counseling is not necessarily based on high-risk family history. Index cases in the series reported an average of 1.15 (SD 1.17) persons with breast or ovarian cancer among their mothers and sisters. Nonetheless, because ascertainment methods varied among centers, we assessed the impact of ascertainment on risk estimates by evaluating both the entire series of subjects (n = 373) and the subset of subjects ascertained through incident cases regardless of family history (n = 85). We also compared risks estimated from genotyped subjects with risks estimated from analyses of all family members, whether tested or not tested.
Genetic Analysis in Israeli Subjects.
DNA was extracted from peripheral blood samples and tested as previously published for the three ancient mutations common in the Ashkenazi Jewish population: BRCA1.185delAG, BRCA1.5382insC, and BRCA2.6174delT (22–24). Genotypes of relatives were determined either by direct genetic testing or by identifying obligate carriers based on family structure and relatives' genotypes.
New York Subjects.
The NYBCS characterized risks of breast and ovarian cancer among women of Ashkenazi Jewish ancestry with mutations in BRCA1 or BRCA2 who were identified by means of index cases with breast cancer (4). Among families ascertained through index cases with breast cancer in Israel vs. New York, there was no interaction between the effect of cancer site on site-specific risks and country of residence (U.S. or Israel), and the magnitude of the effect was similar in Israeli and American families. Therefore, analyses of families ascertained by means of index cases with breast cancer included both relatives of index cases from Israel and relatives of index cases from New York, controlled for country of residence (Table 1). Details of ascertainment and genotyping of the NYBCS subjects have been previously published (4).
Statistical Analyses.
Breast and ovarian cancer risks were estimated by using Kaplan–Meier survival and Cox proportional hazard regression (25). Index cases were excluded from these analyses. Risks for unaffected women were censored at age of last follow-up, at prophylactic surgery, or at death, whichever came first. Risks for women with breast cancer were censored at age of breast cancer diagnosis. Risks for women with ovarian cancer were censored at age of ovarian cancer diagnosis. Combined risks of either breast or ovarian cancer were censored at age of first cancer diagnosis among affected women, and at age of last follow-up, prophylactic surgery, or death, whichever came first, among unaffected women. Hazard ratios for effects of cancer site in the index case were calculated by using Cox proportional regression, controlling for mutation, year of birth, country of residence, and ascertainment. The effect of birth cohort was analyzed by using Cox proportional regression controlling for mutation, cancer site in the index case, and ascertainment method, with birth year as the independent variable.
Acknowledgments
We thank Paul Renbaum for BRCA1/BRCA2 genotyping and Ming K. Lee for statistical analysis and advice. This study was supported by grants from the Israel Cancer Association (to the Israel Breast Cancer Consortium), the Breast Cancer Research Foundation, and the Israel Cancer Research Foundation and by a gift from the Basker family, in loving memory of Eileen Basker.
Abbreviations
- HR
hazard ratio
- NYBCS
New York Breast Cancer Study
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Kauff N. D., Satagopan J. M., Robson M. E., Scheuer L., Hensley M., Hudis C. A., Ellis N. A., Boyd J., Borgen P. I., Barakat R. R., et al. N. Engl. J. Med. 2002;346:1609–1615. doi: 10.1056/NEJMoa020119. [DOI] [PubMed] [Google Scholar]
- 2.Rebbeck T. R., Lynch H. T., Neuhausen S. L., Narod S. A., Van’t Veer L., Garber J. E., Evans G., Isaacs C., Daly M. B., Matloff E., et al. N. Engl. J. Med. 2002;346:1616–1622. doi: 10.1056/NEJMoa012158. [DOI] [PubMed] [Google Scholar]
- 3.Narod S. A., Offit K. J. Clin. Oncol. 2005;23:1656–1663. doi: 10.1200/JCO.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 4.King M. C., Marks J. H., Mandell J. B., New York Breast Cancer Study Group Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 5.Antoniou A., Pharoah P. D., Narod S., Risch H. A., Eyfjord J. E., Hopper J. L., Loman N., Olsson H., Johannsson O., Borg A., et al. Am. J. Hum. Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kroiss R., Winkler V., Bikas D., Fleischmann E., Mainaum C., Frommlet F., Muhr D., Fuerhauser C., Tea M., Bittner, et al. Hum. Mutat. 2005;26:583–589. doi: 10.1002/humu.20261. [DOI] [PubMed] [Google Scholar]
- 7.Antoniou A. C., Pharoah P. D., Narod S., Risch H. A., Eyfjord J. E., Hopper J. L., Olsson H., Johannsson O., Borg A., Pasini B., et al. J. Med Genet. 2005;42:602–603. doi: 10.1136/jmg.2004.024133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antoniou A. C., Gayther S. A., Stratton J. F., Ponder B. A., Easton D. F. Genet. Epidemiol. 2000;18:173–190. doi: 10.1002/(SICI)1098-2272(200002)18:2<173::AID-GEPI6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 9.Gayther S. A., Mangion J., Russell P., Seal S., Barfoot R., Ponder B. A., Stratton M. R., Easton D. Nat. Genet. 1997;15:103–105. doi: 10.1038/ng0197-103. [DOI] [PubMed] [Google Scholar]
- 10.Narod S. A. Nat. Rev. Cancer. 2002;2:113–123. doi: 10.1038/nrc726. [DOI] [PubMed] [Google Scholar]
- 11.Phelan C. M., Rebbeck T. R., Weber B. L., Devilee P., Ruttledge M. H., Lynch H. T., Lenoir G. M., Stratton M. R., Easton D. F., Ponder B. A., et al. Nat. Genet. 1996;3:309–311. doi: 10.1038/ng0396-309. [DOI] [PubMed] [Google Scholar]
- 12.Wang W. W., Spurdle A. B., Kolachana P., Bove B., Modan B., Ebbers S. M., Suthers G., Tucker M. A., Kaufman D. J., Doody M. M., et al. Cancer Epidemiol. Biomarkers Prev. 2001;9:955–960. [PubMed] [Google Scholar]
- 13.Levy-Lahad E., Lahad A., Eisenberg S., Dagan E., Paperna T., Kasinetz L., Catane R., Kaufman B., Beller U., Renbaum P., Gershoni-Baruch R. Proc. Natl. Acad. Sci. USA. 2001;98:3232–3236. doi: 10.1073/pnas.051624098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadouri L., Kote-Jarai Z., Hubert A., Durocher F., Abeliovich D., Glaser B., Hamburger T., Eeles R. A., Peretz T. Br. J. Cancer. 2004;90:2002–2005. doi: 10.1038/sj.bjc.6601837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadouri L., Easton D. F., Edwards S., Hubert A., Kote-Jarai Z., Glaser B., Durocher F., Abeliovich D., Peretz T., Eeles R. A. Br. J. Cancer. 2001;85:36–40. doi: 10.1054/bjoc.2001.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dagan E., Friedman E., Paperna T., Carmi N., Gershoni-Baruch R. Eur. J. Hum. Genet. 2002;10:724–728. doi: 10.1038/sj.ejhg.5200880. [DOI] [PubMed] [Google Scholar]
- 17.Spurdle A. B., Antoniou A. C., Duffy D. L., Pandeya N., Kelemen L., Chen X., Peock S., Cook M. R., Smith P. L., Purdie D. M., et al. Breast Cancer Res. 2005;7:R176–R183. doi: 10.1186/bcr971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rebbeck T. R., Kantoff P. W., Krithivas K., Neuhausen S., Blackwood M. A., Godwin A. K., Daly M. B., Narod S. A., Garber J. E., Lynch H. T., et al. Am. J. Hum. Genet. 1999;64:1371–1377. doi: 10.1086/302366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine D. A., Boyd J. Cancer Res. 2001;61:908–911. [PubMed] [Google Scholar]
- 20.Rebbeck T. R., Wang Y., Kantoff P. W., Krithivas K., Neuhausen S. L., Godwin A. K., Daly M. B., Narod S. A., Brunet J. S., Vesprini D., et al. Cancer Res. 2001;61:5420–5424. [PubMed] [Google Scholar]
- 21.Kadouri L., Kote-Jarai Z., Easton D. F., Hubert A., Hamoudi R., Glaser B., Abeliovich D., Peretz T., Eeles R. A. Int. J. Cancer. 2004;108:399–403. doi: 10.1002/ijc.11531. [DOI] [PubMed] [Google Scholar]
- 22.Levy-Lahad E., Catane R., Eisenberg S., Kaufman B., Hornreich G., Lishinsky E., Shohat M., Weber B. L., Beller U., Lahad A., et al. Am. J. Hum. Genet. 1997;60:1059–1067. [PMC free article] [PubMed] [Google Scholar]
- 23.Gershoni-Baruch R., Dagan E., Fried G., Kepten I., Robinson E. Eur. J. Hum. Genet. 1999;7:833–836. doi: 10.1038/sj.ejhg.5200371. [DOI] [PubMed] [Google Scholar]
- 24.Shiri-Sverdlov R., Oefner P., Green L., Baruch R. G., Wagner T., Kruglikova A., Haitchick S., Hofstra R. M., Papa M. Z., Mulder I., et al. Hum. Mutat. 2000;16:491–501. doi: 10.1002/1098-1004(200012)16:6<491::AID-HUMU6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 25.Kalbfleisch J. D., Prentice R. L. The Statistical Analysis of Failure Time Data. New York: Wiley; 1980. [Google Scholar]